PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14359.
To the Editor:
Diabetes represents a pandemic that has been recognized as an independent risk factor for severe infection with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in the context of the coronavirus disease 19 (COVID‐19) pandemic, 1 although it may not actually increase the risk for SARS‐CoV‐2 transmission for patients with diabetes compared with the general population. 2 , 3 Patients with diabetes feature a twofold increase in the odds for severe SARS‐CoV‐2 infection and a two‐ to threefold increase in the odds for death because of disease. 1 , 4 , 5 Patients with diabetes experience a significant increase in the risk for in‐hospital death because of SARS‐CoV‐2 infection, as shown in a recent, large nationwide study. 6 Therefore, optimal treatment strategy for these patients becomes a top priority. 7
Sodium‐glucose co‐transporter‐2 (SGLT‐2) inhibitors, an antidiabetic drug class with multiple, pleiotropic and beneficial effects, especially in patients with concomitant cardiovascular or renal disease, should be used with caution in the context of infection, because of the increased risk for diabetic ketoacidosis. 8 The anti‐inflammatory properties of SGLT‐2 inhibitors might be beneficial for infected patients, as they could hypothetically ameliorate the cytokine storm. 9 , 10 Previous studies have shown the significant reduction in inflammatory markers, such as C‐reactive protein, ferritin and interleukin‐6, with SGLT‐2 inhibitors. 9 In addition, this drug class exerts beneficial effects on endothelial function, a finding that could have potential applicability for the prevention of thrombotic complications among SARS‐CoV‐2 patients. 11
Recently published observational studies support that SGLT‐2 inhibitors are not inferior to incretin‐based agents concerning surrogate COVID‐19 outcomes, 12 while they might decrease the risk for mechanical ventilation. 13 However, more evidence from clinical practice is required, to identify the impact of antidiabetic drugs administered prior to infection on outcomes of interest among infected patients. 14 , 15 In addition, the ongoing DARE‐19 trial, whose protocol has recently been published in Diabetes, Obesity and Metabolism, will provide answers on whether dapagliflozin could prevent COVID‐19–related complications and all‐cause mortality in patients admitted with SARS‐CoV‐2 infection. 16
We sought to determine whether SGLT‐2 inhibitors influence the risk for respiratory tract infections and acute respiratory distress syndrome (ARDS), pooling data from the published cardiovascular and renal outcome, placebo‐controlled trials until November 2020. We utilized data from published reports, also searching relevant supplementary appendices and ‘grey literature’ sources, namely clinicaltrials.gov. Of note, a similar analysis was recently published for dipeptidyl‐peptidase‐4 inhibitors. 17
Two independent reviewers (DP and AB) extracted the data from the eligible reports using a pilot‐tested, data extraction form. We evaluated the following primary outcomes of interest: upper respiratory tract infection, lower respiratory tract infection, viral infection, influenza and ARDS. We also assessed the following secondary outcomes: pharyngitis, bronchitis and pneumonia, as reported across the selected trials.
As we assessed only dichotomous variables, differences were calculated with the use of risk ratio (RR), and with 95% confidence interval (CI), after implementation of the Mantel‐Haenszel random effects formula. Statistical heterogeneity among studies was assessed by using I2 statistics. Heterogeneity was considered to be low if I2 was between 0% and 25%, moderate if I2 was between 25% and 50%, or high if I2 was greater than 75%. 18 All analyses were performed at the .05 significance level, while they were undertaken with RevMan 5.3 software. 19
Two independent reviewers (DP and CP) assessed the quality of the included randomized controlled trials using the revised Cochrane risk of bias tool for randomized trials (RoB 2.0) for the primary efficacy outcome. 20 Discrepancies between reviewers were solved by discussion, consensus or arbitration by a third senior reviewer (MD).
We pooled data from six trials in a total of 47,728 enrolled participants assigned either to SGLT‐2 inhibitor treatment or placebo. 21 , 22 , 23 , 24 , 25 , 26 A summary of participants' baseline characteristics is provided in Table S1. As shown in detail, all trials enrolled subjects aged older than 60 years, overweight or obese, with cardiovascular co‐morbidities; prevalence of cardiovascular disease among these patients ranged from 37% to 99%. Regarding other drug classes with potential implications in the COVID‐19 pandemic, usage rates of renin‐angiotensin‐aldosterone system inhibitors ranged from 80% to 100%, while usage rates of dipeptidyl‐peptidase‐4 inhibitors ranged from 12% to 17%. Risk of bias was evaluated as low across all trials (Table S2). None of the other available trials have reported relevant data of interest.
Concerning the prespecified primary safety outcomes, none of the results achieved statistical significance. More specifically, SGLT‐2 inhibitor treatment compared with placebo resulted in a non‐significant decrease in the risk for upper respiratory tract infection (RR = 0.95, 95% CI; 0.48–1.88, I2 = 0%) and for lower respiratory tract infection (RR = 0.66, 95% CI; 0.39–1.13, I2 = 0%), as shown in Figure 1A,B, respectively. In addition, SGLT‐2 inhibitor treatment produced a non‐significant increase in the risk for viral infection (RR = 1.15, 95% CI; 0.49–2.71, I2 = 0%) and influenza (RR = 1.27, 95% CI; 0.70–2.32, I2 = 0%) specifically, as depicted in Figure 1C,D, respectively. We have also shown that SGLT‐2 inhibitor treatment led to a non‐significant decrease in the risk for ARDS (RR = 0.68, 95% CI; 0.24–1.96, I2 = 0%), as shown in Figure 1E.
FIGURE 1.
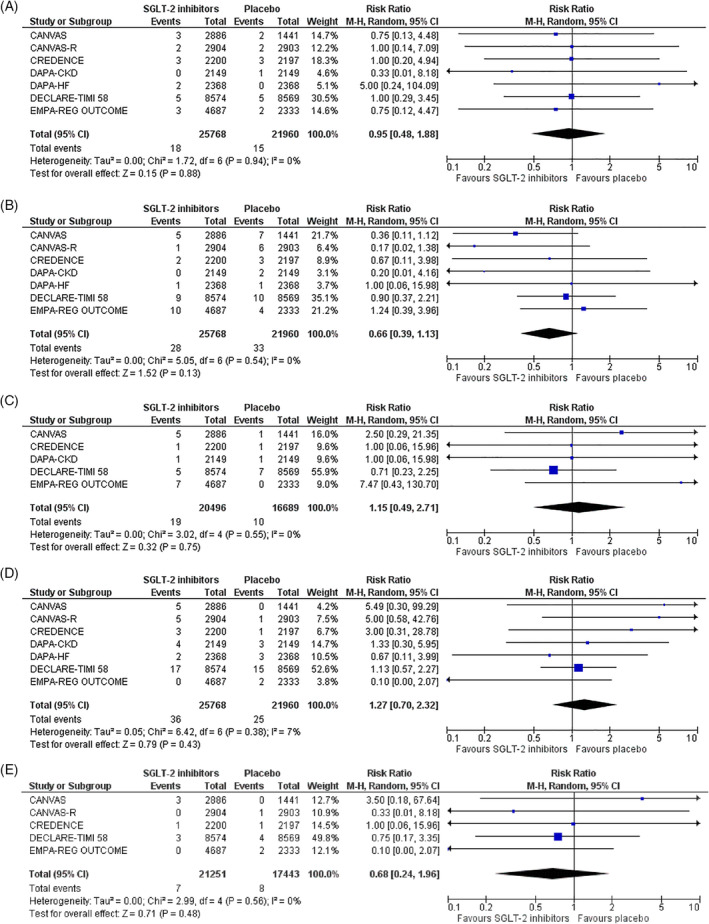
Effect of sodium‐glucose co‐transporter‐2 (SGLT‐2) inhibitors compared with placebo on the risk for A, Upper respiratory tract infection, B, Lower respiratory tract infection, C, Viral infection, D, Influenza and E, acute respiratory distress syndrome (ARDS)
Importantly, when we attempted to test whether different SGLT‐2 inhibitors have a differential effect on the assessed primary safety outcomes, we documented that the only significant results were observed with canagliflozin: however, the latter provided a significant decrease in the risk for lower respiratory tract infection (RR = 0.36, 95% CI; 0.15–0.87, I2 = 0%) (Figure S1a), leading to a significant increase in the risk for influenza (RR = 4.23, 95% CI; 1.07–16.67, I2 = 0%) (Figure S1b). More details regarding the effect of each SGLT‐2 inhibitor on each outcome of interest are provided in Table S3.
Heterogeneity was low for all the evaluated comparisons. The relative frequency of each primary safety outcome was extremely low, as shown in detail in Table S4. The latter is reasonable, because all these events were reported as adverse events during the trials, which were designed to address surrogate cardiovascular and renal outcomes; however, it also represents a limitation of our meta‐analysis.
Of note, as far as the secondary safety outcomes are concerned, we showed that SGLT‐2 inhibitor treatment compared with placebo resulted in a significant decrease in the risk for pneumonia by 15% (RR = 0.85, 95% CI; 0.75–0.97, I2 = 0%), as depicted in Figure 2A, also leading to a non‐significant decrease in the risk for pharyngitis (RR = 0.52, 95% CI; 0.06–4.70, I2 = 0%) and bronchitis (RR = 0.71, 95% CI; 0.41–1.24, I2 = 47%), as shown in Figure 2B,C, respectively. Reporting bias is considered to be unclear for these outcomes, as these trials were designed to assess ‘hard’ cardiovascular and renal endpoints, while the safety outcomes analysed in this meta‐analysis were reported as adverse events. It remains unclear whether diagnosis was based only upon clinical manifestations or if additional laboratory investigation was performed in each case, because diagnosis of upper respiratory tract infections is usually clinical, while that of lower respiratory tract infections and pneumonia may require further investigation. This constitutes, therefore, the major limitation to our analysis.
FIGURE 2.
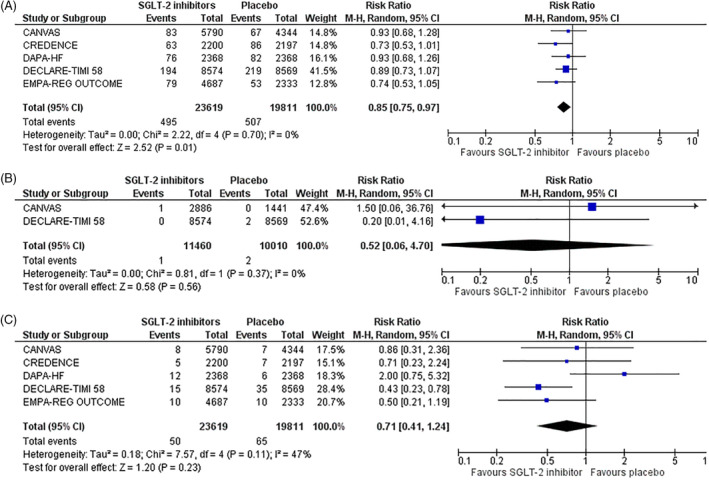
Effect of sodium‐glucose co‐transporter‐2 (SGLT‐2) inhibitors compared with placebo on the risk for A, pneumonia, B, pharyngitis and C, bronchitis
To our knowledge, this is the first meta‐analysis addressing the risk for respiratory tract infections, viral infection and ARDS with SGLT‐2 inhibitors compared with placebo for the very high‐risk patients enrolled in the hallmark cardiovascular and renal outcome trials, showing that this antidiabetic class of drugs do not affect the risk for upper or lower respiratory tract infection, influenza, viral infection or ARDS, despite the low relative frequency of each outcome across the selected trials. Of course, herein we show that SGLT‐2 inhibitor treatment resulted in a significant decrease in the risk for pneumonia, a finding that could be of exceptional value in the context of the COVID‐19 pandemic.
Collectively, patients treated with SGLT‐2 inhibitors do not have an increased risk for respiratory infection compared with patients with diabetes treated with other antidiabetic drug classes, while they might have a decreased risk for pneumonia. These findings have direct implications for the corresponding risk of these patients to acquire a SARS‐CoV‐2 infection. Of course, continuation or not of SGLT‐2 inhibitors among patients with documented infection should always be made upon the treating physician's clinical discretion. Finally, DARE‐19 and other similar trials will provide further insights into the safety and efficacy of SGLT‐2 inhibitors in the inpatient setting during the COVID‐19 pandemic.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
DP and MD conceived and designed the study. DP, CP and AB extracted data of interest and performed the analyses. DP wrote the first draft of the report. CP and MD critically reviewed the draft. All authors approved the final form of the manuscript.
Supporting information
Figure S1. Effect of canagliflozin compared to placebo on the risk for a) lower respiratory tract infection and b) influenza.
Table S1. Summary of participants baseline characteristics of special interest.
Table S2. Risk of bias across the included studies.
Table S3. Subgroup analyses for safety outcomes of interest according to SGLT‐2 inhibitor treatment. Data are presented as risk ratio (95% confidence interval)
Table S4. Relative frequency of safety events of interest across the selected trials.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Aggarwal G, Lippi G, Lavie CJ, Henry BM, Sanchis‐Gomar F. Diabetes mellitus association with coronavirus disease 2019 (COVID‐19) severity and mortality: a pooled analysis. J Diabetes. 2020;12(11):851‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin Res Cardiol. 2020;109(5):531‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS‐CoV‐2. J Endocrinol Invest. 2020;43(6):867‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu J, Zhang J, Sun X, et al. Influence of diabetes mellitus on the severity and fatality of SARS‐CoV‐2 (COVID‐19) infection. Diabetes Obes Metab. 2020;22(10):1907‐1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mantovani A, Byrne CD, Zheng MH, Targher G. Diabetes as a risk factor for greater COVID‐19 severity and in‐hospital death: a meta‐analysis of observational studies. Nutr Metab Cardiovasc Dis. 2020;30(8):1236‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID‐19‐related mortality in England: a whole‐population study. Lancet Diabetes Endocrinol. 2020;8(10):813‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vas P, Hopkins D, Feher M, Rubino F, Whyte MB. Diabetes, obesity and COVID‐19: a complex interplay. Diabetes Obes Metab. 2020;22(10):1892‐1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lim S, Bae JH, Kwon HS, Nauck MA. COVID‐19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17(1):11‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Katsiki N, Ferrannini E. Anti‐inflammatory properties of antidiabetic drugs: a "promised land" in the COVID‐19 era? J Diabetes Complications. 2020;34(12):107723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. 2020;17(12):761‐772. [DOI] [PubMed] [Google Scholar]
- 11. Alshnbari AS, Millar SA, O'Sullivan SE, Idris I. Effect of sodium‐glucose cotransporter‐2 inhibitors on endothelial function: a systematic review of preclinical studies. Diabetes Ther. 2020;11(9):1947‐1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bastrup Israelsen S, Pottegård A, Sandholdt H, Madsbad S, Thomsen RW, Benfield T. Comparable COVID‐19 outcomes with current use of GLP‐1 receptor agonists, DPP‐4 inhibitors or SGLT‐2 inhibitors among patients with diabetes who tested positive for SARS‐CoV‐2 [published online ahead of print, January 27, 2021]. Diabetes Obes Metab. 2021; 10.1111/dom.14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dalan R, Ang LW, Tan WYT, et al. The association of hypertension and diabetes pharmacotherapy with COVID‐19 severity and immune signatures: an observational study [published online ahead of print, August 7, 2020]. Eur Heart J Cardiovasc Pharmacother. 2020; 10.1093/ehjcvp/pvaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pérez‐Belmonte LM, Torres‐Peña JD, López‐Carmona MD, et al. Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID‐19 in association with glucose‐lowering drugs: a nationwide cohort study. BMC Med. 2020;18(1):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen Y, Yang D, Cheng B, et al. Clinical characteristics and outcomes of patients with diabetes and COVID‐19 in association with glucose‐lowering medication. Diabetes Care. 2020;43(7):1399‐1407. [DOI] [PubMed] [Google Scholar]
- 16. Kosiborod M, Berwanger O, Koch GG, et al. Effects of dapagliflozin on prevention of major clinical events and recovery in patients with respiratory failure due to COVID‐19: the design and rationale for the DARE‐19 study [published online ahead of print, December 15, 2020]. Diabetes Obes Metab. 2021. 10.1111/dom.14296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grenet G, Mekhaldi S, Mainbourg S, et al. DPP‐4 Inhibitors and Respiratory Infection: A Systematic Review and Meta‐analysis of the Cardiovascular Outcomes Trials. Diabetes Care. 2021;44(3):e36‐e37. https://doi.org/10.2337/dc20‐2018. [DOI] [PubMed] [Google Scholar]
- 18. Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analyzing data and undertaking meta‐analyses. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. Chichester, UK: John Wiley & Sons Ltd; 2011. [Google Scholar]
- 19. Review Manager (RevMan) [Computer program] Version [5.3]. Copenhagen, Denmark: The Nordic Cochrane Centre TCC; 2014. [Google Scholar]
- 20. Higgins JPT, Sterne JAC, Savović J, et al. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev. 2016;10:29‐31. [Google Scholar]
- 21. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 22. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 23. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 24. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995‐2008. [DOI] [PubMed] [Google Scholar]
- 25. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295‐2306. [DOI] [PubMed] [Google Scholar]
- 26. Heerspink HJL, Stefánsson BV, Correa‐Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436‐1446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effect of canagliflozin compared to placebo on the risk for a) lower respiratory tract infection and b) influenza.
Table S1. Summary of participants baseline characteristics of special interest.
Table S2. Risk of bias across the included studies.
Table S3. Subgroup analyses for safety outcomes of interest according to SGLT‐2 inhibitor treatment. Data are presented as risk ratio (95% confidence interval)
Table S4. Relative frequency of safety events of interest across the selected trials.
Data Availability Statement
Data available on request from the authors.


