Abbreviations
- CCI
Charlson Comorbidity Index
- COVID‐19
coronavirus disease 2019
- ER
emergency room
- HCC
hepatocellular carcinoma
- HE
hepatic encephalopathy
- MELD‐Na
Model for End‐Stage Liver Disease–sodium
TO THE EDITOR:
The short‐term prognosis of coronavirus disease 2019 (COVID‐19) is impacted by underlying cirrhosis and other comorbidities.( 1 ) However, as shown in our multicenter study, inpatient mortality was similar in patients with cirrhosis + COVID‐19 and cirrhosis without COVID‐19, but was higher than a contemporaneous cohort of hospitalized patients with COVID‐19 without cirrhosis.( 2 ) We herein describe the 90‐day postdischarge outcomes in that age‐matched and sex‐matched cohort of patients admitted with cirrhosis + COVID‐19, cirrhosis only, and COVID‐19 only.
Patients and Methods
The inception cohort consisted of nonelective hospitalizations with age‐matched and sex‐matched patients with cirrhosis + COVID‐19 (n = 37), cirrhosis only (n = 127), and COVID‐19 only (n = 108) from 7 North American centers between March 2020 and May 2020.( 2 ) Cirrhosis was diagnosed by liver biopsy or clinical/imaging features. COVID‐19 was diagnosed using polymerase chain reaction tests. We excluded patients with organ transplant, human immunodeficiency virus, and unclear cirrhosis/COVID‐19 diagnoses. Data recorded were demographics, cirrhosis, and inpatient course details and the Charlson Comorbidity Index (CCI) score. Nonelective readmissions and clinical engagement over 90 days were analyzed between groups. Finally, the determinants of readmission with logistic regression using age, sex, ethnicity, race, postdischarge engagement with medical teams (phone/video encounters), COVID‐19 status, cirrhosis status, and CCI score were performed.
Results
Figure 1A shows the patient flow. Final 90‐day analysis included 92 patients with cirrhosis only, 29 patients with cirrhosis + COVID‐19, and 93 patients with COVID‐19 only. Age, sex, ethnicity, and insurance rates were similar across groups. However, patients in the cirrhosis groups regardless of COVID‐19 status had higher CCI scores than the patients with COVID‐19 only, and patients with cirrhosis were more likely to be Caucasian (Table 1). In the group of patients with cirrhosis, the Model for End‐Stage Liver Disease–sodium (MELD‐Na) score and alcohol etiology of cirrhosis were similar.
FIG. 1.
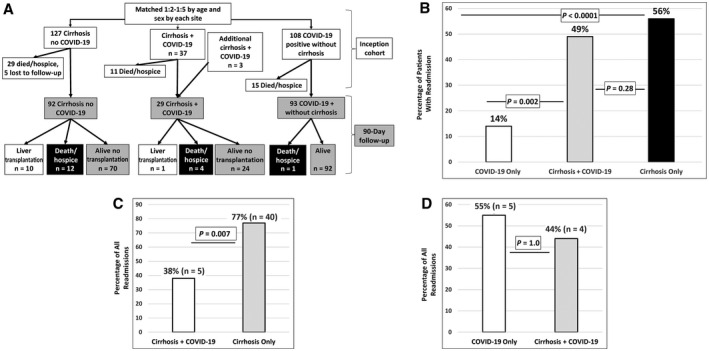
(A) Flowchart of patients from the inception to follow‐up. (B) All‐cause readmission rates between groups showed similar rates within the cirrhosis groups, which was significantly higher than the patients with COVID‐19 only (comparison using chi‐square test). (C) In the patients with cirrhosis who experienced a readmission, the proportion who were readmitted as a result of liver‐related conditions was higher in the cirrhosis‐only group versus the cirrhosis + COVID‐19 group (comparison using chi‐square test). (D) In the patients with COVID‐19 who experienced a readmission, the proportion who were readmitted as a result of COVID‐19–related conditions was statistically similar (comparison using Fisher’s exact test).
TABLE 1.
Comparison and Outcomes Between Groups
| Variable | COVID‐19 Only (n = 93) | Cirrhosis + COVID‐19 (n = 29) | Cirrhosis Only (n = 92) | P Value for All Groups |
|---|---|---|---|---|
| Age, years | 60.6 ± 10.8 | 61.2 ± 10.6 | 59.1 ± 11.2 | 0.54 |
| Men | 37 (40) | 9 (36) | 31 (33) | 0.81 |
| Caucasian race | 46 (49) | 10 (40) | 60 (67) | 0.008 |
| Hispanic ethnicity | 8 (9) | 2 (8) | 3 (3) | 0.34 |
| Uninsured | 23 (25) | 7 (28) | 15 () | 0.23 |
| CCI score | 2.78 ± 2.0 | 6.84 ± 3.0 | 5.98 ± 2.5 | <0.001 |
| MELD‐Na score | — | 18.2 ± 8.0 | 19.5 ± 7.5 | 0.44 |
| Alcohol‐related etiology | — | 10 (34) | 45 (49) | 0.38 |
| Reasons for index admission | ||||
| HE | — | 2 (8) | 24 (26) | |
| Gastrointestinal bleeding | — | 1 (3) | 10 (11) | |
| Ascites, anasarca, renal | — | — | 24 (26) | |
| Other liver‐related reasons | — | — | 11 (12) | |
| Non–COVID‐19 infections | — | 2 (8) | 9 (10) | |
| COVID‐19 related | 93 (100) | 24 (83) | — | |
| Other | — | — | 14 (15) | |
| 90‐day outcomes | ||||
| Total with nonelective readmissions | 13 (14) | 13 (45) | 52 (57) | <0.001 |
| Number of readmissions | 1.3 ± 0.59 (0‐2) | 1.6 ± 0.75 (0‐2) | 1.8 ± 1.0 (0‐3) | 0.16 |
| Death/hospice | 1 (1) | 3 (12) | 11 (12) | 0.009 |
| Liver transplantation | — | 1 (4) | 10 (11) | 0.45 |
| 90‐day engagement | ||||
| Times seen in clinic | 0.98 ± 1.56 | 1.68 ± 2.06 | 1.45 ± 1.79 | 0.26 |
| Phone visits with registered nurse | 1.45 ± 1.98 | 1.83 ± 2.34 | 1.42 ± 2.04 | 0.59 |
| Phone visits with doctor | 1.45 ± 1.79 | 1.30 ± 2.01 | 1.54 ± 2.04 | 0.86 |
| Electronic communications | 0.59 ± 1.46 | 0.39 ± 1.3 | 0.91 ± 2.07 | 0.22 |
| Video visits | 1.03 ± 1.50 | 2.13 ± 2.40 | 1.58 ± 2.24 | 0.02 |
| ER visits | 0.30 ± 0.69 | 1.09 ± 1.70 | 1.14 ± 1.66 | 0.001 |
| ER discharge without admission | 0.13 ± 0.42 | 0.60 ± 1.55 | 0.52 ± 1.33 | 0.02 |
Data are provided as n (%), mean ± standard deviation, or mean ± standard deviation and (range).
P < 0.05 between the groups with COVID‐19 only and cirrhosis + COVID‐19.
P < 0.05 between the groups with COVID‐19 only and cirrhosis only.
Death and Hospice
Of the patients in the group with COVID‐19 only, 1 died (respiratory failure) compared with 4 patients in the cirrhosis + COVID‐19 group (hepatocellular carcinoma [HCC] and advanced cirrhosis and multisystem failure) and 12 patients in the cirrhosis‐only group (HCC and advanced cirrhosis, n = 3; multisystem failure, n = 5; fall, n = 1; metastatic lung cancer, n = 1; variceal bleeding, n = 1; and 1 posttransplant as a result of procedural complications). There was a significantly higher death rate in the patients with cirrhosis regardless of COVID‐19 status versus COVID‐19 only (P = 0.01, cirrhosis + COVID‐19 versus COVID‐19 only; P = 0.75 between cirrhosis groups; Table 1).
Liver Transplantation
A total of 11 patients underwent liver transplantation. Of these patients, 1 with cirrhosis + COVID‐19 cleared the infection at the time of transplant, whereas the remaining patients were in the cirrhosis‐only group. In the cirrhosis‐only group, 1 patient died of posttransplant complications.
Readmissions
A significantly higher proportion of discharged patients with cirrhosis regardless of COVID‐19 required readmissions compared with the patients with COVID‐19 only (Fig. 1B). However, the number of readmissions per patient was statistically similar (Table 1). Major reasons for readmission in the cirrhosis‐only group were infections (n = 11), hepatic encephalopathy (HE; n = 8), gastrointestinal bleeding (n = 6), urgent liver transplantation (n = 6), anasarca (n = 4), renal/metabolic (n = 2), and liver unrelated (n = 12). None of the patients in the group with cirrhosis only developed COVID‐19 during the follow‐up. The patients with cirrhosis + COVID‐19 were mostly readmitted for liver‐unrelated reasons. Of these patients, 4 were hospitalized for COVID‐related symptoms, 1 each for aspiration pneumonia, atrial fibrillation, hemoptysis, and generalized weakness. The only liver‐related reasons for readmission were for HE (n = 3) and HCC (n = 2).
Of the 13 patients with COVID‐19 only who were readmitted, 5 were for COVID‐19 symptoms, 2 were for renal/metabolic reasons, and 1 was for a urinary tract infection, whereas the remaining 5 were admitted for miscellaneous reasons.
Ultimately, the proportion of liver‐related readmissions were higher in the cirrhosis‐only group versus the cirrhosis + COVID‐19 group (77% versus 38%; P < 0.007; Fig. 1C). However, the proportion of COVID‐19–related readmissions was similar across both COVID‐19 groups (55% versus 44%; P = 1.0; Fig. 1D).
Engagement With Medical Teams
The number of clinic visits, phone visits, and electronic communications were similar between groups (Table 1). The patients with cirrhosis + COVID‐19 required a higher number of video visits than the other groups. More patients with cirrhosis with/without COVID‐19 were seen in the emergency room (ER), regardless of whether they were subsequently admitted or discharged from there, compared with the patients with COVID‐19 only.
Regression analysis showed that cirrhosis was the only variable associated with readmission (odds ratio, 6.45; 95% confidence interval, 3.39‐12.26; P < 0.01).
Discussion
Our current results demonstrate that cirrhosis exerts an adverse influence on death and readmissions within 90 days of discharge, which is greater than that of COVID‐19 only. With the pandemic, especially in the earlier stages in North America when the cohort was conceived, cirrhosis care was probably affected.( 3 ) It is likely that patients who were hospitalized had more advanced disease with higher in‐hospital death/hospice rates.( 2 , 4 ) In patients with cirrhosis hospitalized for COVID‐19, readmission was more likely liver unrelated. Liver‐related readmissions were higher in patients with cirrhosis only versus patients with cirrhosis + COVID‐19, but COVID‐19–related readmissions were similar across both COVID‐19 subgroups. This reflects the fact that the inception cohort of patients with cirrhosis + COVID‐19 was COVID‐19 and CDC the patients with advanced COVID‐19 patients, whereas the patients with cirrhosis only followed the usual cirrhosis patterns.( 2 ) Ultimately, the death and readmission rates between the cirrhosis groups were similar. A higher symptom and complex illness burden in cirrhosis was also reflected in greater video encounters and ER visits after discharge, which could have contributed toward higher readmission rates. Therefore, closer proactive follow‐up of patients with cirrhosis may be needed to anticipate or potentially prevent these unscheduled ER visits or readmissions in future studies.
We found continued transplant activity with 12% of patients with cirrhosis only undergoing transplantation. These encouraging findings demonstrate that the procedures in place for patient approval for listing, donor and recipient matching, and peritransplant and posttransplant management are largely robust despite the pandemic, and life‐saving liver transplantation remains a viable approach that should be implemented in eligible patients.( 5 )
We are limited by the small sample size, observational nature, potential variability in practices, and access to therapies across centers. However, this multicenter experience provides granular follow‐up of contemporaneous cohorts of patients with cirrhosis and COVID‐19.
We conclude that during the early period of the pandemic, patients with cirrhosis, regardless of COVID‐19 status, remained at higher risk of death and readmissions compared with age‐matched and sex‐matched patients with COVID‐19 only. The rates of liver transplantation and posttransplant outcomes in the cirrhosis group remain acceptable, and transplantation should be pursued in eligible patients. Given the continued higher rate of poor outcomes in patients with cirrhosis, every effort to minimize and anticipate readmissions in the outpatient setting are needed to reduce this burden.
K. Rajender Reddy advises and has received grants from Gilead and Mallinckrodt; has received grants from Bristol Myers Squibb, Intecept, Grifols, Merck, Sequana, and Exact Sciences; and has other interests in Novartis.
References
- 1. COVID‐19 and CDC . https://www.cdc.gov/coronavirus/2019‐ncov/need‐extra‐precautions/people‐with‐medical‐conditions.html. Accessed December 21, 2020.
- 2. Bajaj JS, Garcia‐Tsao G, Biggins S, Kamath PS, Wong F, McGeorge S, et al. Comparison of mortality risk in patients with cirrhosis and COVID‐19 compared with patients with cirrhosis alone and COVID‐19 alone: multicentre matched cohort. Gut 2020. 10.1136/gutjnl-2020-322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mahmud N, Hubbard RA, Kaplan DE, Serper M. Declining cirrhosis hospitalizations in the wake of the COVID‐19 pandemic: a national cohort study. Gastroenterology 2020;159:1134‐1136.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O'Leary JG, Reddy KR, Garcia‐Tsao G, Biggins SW, Wong F, Fallon MB, et al. NACSELD Acute‐on‐Chronic Liver Failure (NACSELD‐ACLF) score predicts 30‐day survival in hospitalized patients with cirrhosis. Hepatology 2018;67:2367‐2374. [DOI] [PubMed] [Google Scholar]
- 5. AASLD Guidance on COVID‐19 . https://www.aasld.org/sites/default/files/2020‐06/AASLD‐COVID19‐ExpertPanelConsensusStatement‐June252020‐v2‐FINAL.pdf. Accessed December 21, 2020.


