Abstract
To evaluate the efficacy of corticosteroids on coronavirus disease 2019 (COVID‐19) patients with different levels of disease severity. In our multicenter study, 543 patients with confirmed COVID‐19 were classified as non‐severe group and severe group, and then were compared respectively for all‐cause mortality and length of hospital stay between those who received corticosteroids and not. By searching in PubMed, Web of Science, Embase, and CNKI, we identified 13 retrospective studies and 6 random control trials eligible for criteria of inclusion, and conducted comprehensive meta‐analyses assessing the impacts of corticosteroids on mortality, length of stay, duration of RNA clearance and duration of fever. Our multicenter study demonstrated that low‐dose corticosteroids can reduce mortality in the multivariable Cox regression analysis for severe patients (p = .03), while presented no influence in univariable analysis for non‐severe patients (p = .14). From multivariable analyses, patients with corticosteroids in non‐severe group had longer duration of hospitalization (p = .003), but did not in severe group (p = .18). Moreover, for severe patients, corticosteroids can evidently shorten duration of fever. The same results were summarized in the meta‐analyses supplemented with the result that corticosteroids delayed viral clearing in non‐severe patients. Corticosteroids should be considered based on patient's condition. For patients with non‐severe COVID‐19, corticosteroid was not recommended as a routine therapeutic initiative as that presented prolonged duration of hospitalization and delayed viral clearing, as well as no positive impact on prognosis. While low‐dose corticosteroids may benefit patients with severe COVID‐19 for it can manifestly lower risk of death and improve the clinical status to some extent.
Keywords: corticosteroid, COVID‐19, meta‐analysis, multi‐center retrospective study
Highlights
-
1.
The role of corticosteroid in COVID‐19 patients was comprehensively summarized by combining the multicenter study and meta‐analysis.
-
2.
Corticosteroid was recommended for severe COVID‐19 patients as that can manifestly lower risk of death and improve the clinical status to some extent, while not for non‐severe patients.
1. INTRODUCTION
In December 2019, the first pneumonia case caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), now known as coronavirus disease 2019 (COVID‐19), was identified in Wuhan, Hubei Province, China. 1 SARS‐CoV‐2 infection was then quickly spread globally. 2 , 3 , 4 Evidence shows that the person‐to‐person transmission is the main cause of the large‐scale outbreak of COVID‐19. 5 As of September 20, 2020, there has been more than 30 million confirmed cases worldwide and nearly one million deaths.
Previous studies had reported various therapeutic initiatives and effect assessment related to COVID‐19, including antiviral, immunotherapy, corticosteroid therapy, and other symptomatic supportive treatments, but there are still no specific drugs for treatment and control. Thereinto, corticosteroid was widely used in patients with COVID‐19 pneumonia, while its efficacy remains controversial. Given the experience and lessons of severe acute respiratory syndrome (SARS) treatment, improper use of systemic corticosteroids can increase the risk of secondary infection, osteonecrosis of the femoral head, hypokalemia, etc. 6 , 7 , 8 Meanwhile, several observational studies in the early stage of COVID‐19 outbreak suggested that corticosteroid treatment may increase the mortality to some extent. 9 , 10 Tang et al. 11 called for caution in the use of corticosteroids for COVID‐19 and did not recommend this as a routine treatment.
To date, three meta‐analysis articles summarized the effects of corticosteroid therapy mainly including studies on SARS and Middle East respiratory syndrome (MERS), as well as several early‐stage observational studies of COVID‐19, and suggested that employment of corticosteroids cannot improve survival or reduce length of hospital stay, but spark some adverse effects. 12 , 13 , 14 Due to the heterogeneity of disease among SARS, MERS, and COVID‐19, and non‐specificity of the study cohorts for the literatures of COVID‐19 included in the meta‐analysis, the reliability of the results needs further verification. Afterwards, multiple cohort studies and randomized controlled trials (RCTs) primarily to compare the mortality of COVID patients who did receive or not receive corticosteroid emerged within a short period. In early September, a prospective meta‐analysis of clinical trials of critically ill patients with COVID‐19 reported that administration of systematic corticosteroid was associated with lower 28‐day all‐cause mortality, which clearly demonstrated the benefit of corticosteroid therapy. 15 However, there is currently no high‐quality meta‐analysis to systematically compare the effects of corticosteroid therapy in patients with different severity levels and the impact on other outcome indicators, such as length of hospital stay, RNA clearance time, and duration of fever.
Thus, our study aimed at two domains: (1) to evaluate the efficacy of corticosteroid on mortality, length of hospital stay and changes in body temperatures between severe and non‐severe patients with COVID‐19 from our multi‐center study; (2) to review and summarize high‐quality evidence for efficacy of corticosteroid on COVID‐19 from online databases providing a comprehensive understanding to facilitate clinical practice.
2. METHODS
2.1. Multi‐center study
2.1.1. Study design
This multi‐center retrospective study included 543 adult (≥18 years old) patients confirmed with COVID‐19 between December 29, 2019 and February 17, 2020 from 14 hospitals in Hubei Province of China. The 14 hospitals involved in the study are government‐designated hospitals to receive and treat the patients with COVID‐19, and seven of them were from Wuhan, the outbreak center of COVID‐19 then. According to World Health Organization interim guidance (https://www.who.int/) and Chinese new coronavirus infected pneumonia diagnosis and treatment plan (http://www.nhc.gov.cn/), all patients in this study were classified into the non‐severe and the severe. Of them, patients who used corticosteroid were assigned to corticosteroid cohorts respectively, while those who did not were assigned to no corticosteroid cohorts. The ethics committee reviewed and approved this study (Ethics committee, Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China, IRB ID:TJ‐C20200144). The written informed patient consent was waived owing to the emerging infectious disease.
2.1.2. Data collection
All data collected from the hospital's electronic medical record system of every hospital were as follow: demographic characteristics (age and gender), medical history of chronic disease (hypertension, diabetes, chronic respiratory, liver, and renal disease), sign and symptoms (fever, cough, shortness of breath, temperature, respiratory rate, and heart rate), laboratory findings on admission (PaO2, WBC, C‐reactive protein (CRP), alanine aminotransferase, bilirubin, alkaline phosphatase, creatinine and lactate dehydrogenase) and treatment information (antiviral, antibacterial, immunotherapy, oxygen delivery, and corticosteroid therapy), as well as clinical outcomes (mortality and length of hospital stay). These data were then collated, analyzed and interpreted by researchers from the Department of Respiratory and Critical Care Medicine, Tongji Hospital, Huazhong University of Science and Technology.
2.1.3. Exposure, outcome and definition
The main exposure was the employment of corticosteroid therapy between severe/corticosteroid cohort (SC) and non‐severe/corticosteroid cohort (NC). We explicitly recorded the type of corticosteroid, daily dosage and duration of treatment. The primary outcomes included mortality and length of hospital stay. Fever was defined as axillary temperature exceeding 37.3°C. The date of disease onset was marked by the first appearance of fever (either reported by the patient or confirmed by measurement), cough, or other related clinical symptoms with or without abnormal imaging results.
2.1.4. Statistical analysis
Continuous variables were expressed as median (interquartile range) as they were not distributed normally, and categorical variables were expressed as number (%). The Mann–Whitney U test was employed for comparison of continuous data. χ 2 test or the Fisher exact test was used to compare the categorical data as appropriate. To explore the efficacy of corticosteroid therapy on patients' clinical outcome (mortality or length of hospital stay), univariable and multivariable Cox regression were processed. Statistical analyses were performed using SPSS (Version 26.0), and figures were plotted using software package R (version 3.6.3). p < .05 (two‐sided) was considered statistically significant.
2.2. Meta‐analysis
2.2.1. Search strategy
Our meta‐analysis mainly focused on the efficacy of corticosteroid therapy on COVID‐19 patients. We searched electronic databases including PubMed, Web of Science, Embase, and CNKI (China National Knowledge Infrastructure) up to September 20, 2020 with language restriction of English and Chinese. The searching strategy were as follow: (“COVID‐19” OR “SARS‐CoV‐2” OR “2019 novel coronavirus” OR “2019‐nCoV” OR "Coronavirus disease 2019") AND ("corticosteroid" OR “glucocorticoid” OR “methylprednisolone” OR “dexamethasone” OR “prednisone” OR “hydrocortisone”). The decision on literature inclusion was reached by two authors (Y Zhan and Q Huang) after detailed screening and resolution of disagreement. The included literatures covered retrospective cohort studies and RCTs, where included patients were confirmed with COVID‐19 and the study grouping was based on the use of corticosteroid. The exclusion process and criteria of ineligible studies was shown in Fig S1.
2.2.2. Data extraction and quality assessment
Two authors (Y Zhan and Y Gu) independently browsed included literatures for full text and extracted the original data using the predefined form: (1) basic information (first author, research year, study design and research country); (2) patient characteristics (total number of participants, severity of disease, gender and age); (3) exposure or intervention details (type of corticosteroid, dosage and duration of therapy); and (4) outcomes (mortality, length of hospital stay, duration of RNA clearance and duration of fever). To assess the risk of bias, two authors (Y Zhan and Y Gu) used the Newcastle–Ottawa scale (NOS) for the retrospective studies 16 and Jadad score for the RCTs. 17 The NOS scores were achieved by evaluating selection, comparability and outcome categories with a maximum of 9. While the Jadad scores were obtained by evaluating three domains of randomization, blinding, and dropouts/withdrawals with a maximum of 5. We considered the study with a NOS score ≥6 or Jadad score ≥3 as high quality.
2.2.3. Statistical analysis
The mete‐analysis was conducted using Review Manager 5.3 (The Cochrane Collaboration). We pooled eligible data and described the continuous data by mean difference (MD) with 95% confidence interval (CI), while the dichotomous data by risk ratio (RR) with 95% CI. Studies with an I² statistic less than 50% were considered to have no heterogeneity, where the fixed‐effects model was chosen. While those with an I² statistic ≥50% are considered to have heterogeneity, where the random‐effects model was chosen. A sensitivity analysis was conducted by detecting the stability for the composite outcome of the remaining studies when excluding one study at a time. The funnel plots were used to assess the potential publication bias. p < .05 (two‐sided) was considered statistically significant.
3. RESULTS
3.1. Multi‐center study
3.1.1. Basic characteristics
A total of 543 patients with confirmed COVID‐19 were included in this multi‐center retrospective study, 79 in the NC, 196 in the non‐severe/no corticosteroid cohort (NN), 178 in the SC and 90 in the severe/no corticosteroid cohort (SN). Overall, almost 6%(31/543) of patients had died, of which there were two deaths in NC and 1 death in NN with no significant difference (p = .14), as well as 13 deaths in SC and 15 deaths in SN with significant difference (p = .02). The baseline characteristics among these four cohorts were displayed in Table 1.
Table 1.
Baseline characteristics of COVID‐19 patients treated with or without corticosteroid in non‐severe and severe group
| Non‐severe group | Severe group | |||||
|---|---|---|---|---|---|---|
| Corticosteroid | No corticosteroid | p Value | Corticosteroid | No corticosteroid | p Value | |
| Number of patients | 79 | 196 | 178 | 90 | ||
| Demographic information | ||||||
| Age, years | 48 (32–58) | 44 (32–56) | 0.27 | 55 (45–64) | 56 (40–67) | .95 |
| Gender | ||||||
| Male | 30 (39%) | 79 (40%) | .84 | 89 (50%) | 46 (51%) | .86 |
| Female | 49 (61%) | 117 (60%) | 89 (50%) | 44 (49%) | ||
| Comorbidity | 31 (39%) | 55 (28%) | .07 | 109 (61%) | 55 (61%) | .98 |
| Hypertension | 12 (15%) | 22 (11%) | .37 | 46 (26%) | 23 (26%) | .96 |
| Diabetes | 2 (3%) | 7 (4%) | .66 | 32 (18%) | 14 (16%) | .62 |
| Chronic respiratory disease | 3 (4%) | 7 (4%) | .93 | 13 (7%) | 3 (3%) | .28 |
| Liver disease | 3 (4%) | 4 (2%) | .40 | 9 (5%) | 2 (2%) | .27 |
| Renal disease | 0 | 2 (1%) | .37 | 5 (3%) | 1 (1%) | .37 |
| Days from the onset to admission | 6 (4–9) | 6 (4–8) | .99 | 8 (6–10) | 9 (5–11) | .99 |
| Signs and symptoms | ||||||
| Fever | 70 (89%) | 160 (82%) | .16 | 162 (91%) | 79 (88%) | .41 |
| Cough | 56 (71%) | 131 (67%) | .51 | 117 (66%) | 51 (57%) | .15 |
| Shortness of breath | 14 (18%) | 31 (16%) | .70 | 79 (44%) | 33 (37%) | .23 |
| RR, bpm | 20 (20–20) | 20 (20–21) | .49 | 20 (20–21) | 20 (20–20) | .005 |
| HR, bpm | 85 (80–102) | 86 (78–96) | .26 | 88 (80–100) | 84 (77–98) | .08 |
| Laboratory findings | ||||||
| PaO2, mmHg | 89 (74–111) | 98 (83–104) | .24 | 75 (62–96) | 86 (76–105) | .007 |
| White blood cell count, ×109/L | 4.5 (3.2–5.8) | 4.5 (3.6–5.7) | .69 | 4.9 (3.8–6.3) | 4.6 (3.6–5.9) | .45 |
| Neutrophil count, ×109/L | 3.1 (1.8–4.7) | 2.7 (1.9–3.6) | .14 | 3.7 (2.4–5.1) | 3.2 (2.2–4.5) | .22 |
| Lymphocyte count, ×109/L | 1.0 (0.7–1.4) | 1.3 (0.9–1.7) | .0002 | 0.9 (0.6–1.2) | 1.0 (0.8–1.4) | .003 |
| CRP, mg/L | 19.0 (9.4–41.9) | 13.3 (5.4–28.6) | .03 | 32.7 (15.3–61.2) | 23.1 (8.5–51.1) | .01 |
| Alanine aminotransferase, U/L | 17 (13–27) | 20 (14–30) | .19 | 21 (15–36) | 20 (13–28) | .10 |
| Total bilirubin, umol/L | 9.7 (7.2–12.4) | 8.9 (6.8–11.5) | .34 | 8.4 (6.1–10.9) | 8.3 (5.8–10.6) | .59 |
| Alkaline phosphatase, U/L | 56 (49–70) | 58 (49–71) | .80 | 59 (48–70) | 59 (47–76) | .77 |
| Creatinine, umol/L | 67 (54–83) | 61 (51–76) | .07 | 69 (55–85) | 66 (54–83) | .77 |
| Lactate dehydrogenase, U/L | 226 (176–258) | 201 (171–244) | .08 | 278 (222–361) | 246 (198–299) | .007 |
| Treatments | ||||||
| Antiviral | 76 (96%) | 174 (89%) | .06 | 167 (94%) | 83 (92%) | .62 |
| Antibacterial | 77 (97%) | 104 (53%) | <.0001 | 168 (94%) | 75 (83%) | .003 |
| Immunotherapy | 30 (38%) | 26 (13%) | <.0001 | 77 (43%) | 20 (22%) | .0007 |
| Oxygen delivery | 1 (1%) | 0 | .29 | 162 (91%) | 86 (96%) | .22 |
| Outcomes | ||||||
| Deaths | 2 (3%) | 1 (1%) | .14 | 13 (7%) | 15 (17%) | .02 |
| Length of hospital stay | 13 (11–18) | 10 (7–13) | <.0001 | 14 (10–18) | 10 (8–13) | <.0001 |
Note: Data are expressed as n (%), median (IQR).
Abbreviations: COVID‐19, coronavirus disease 2019; CRP, C‐reactive protein; HR, heart rate; IQR, interquartile range; PaO2, arterial partial pressure of oxygen; RR, respiratory rate.
Administration of corticosteroids were delivered in patients of NC and SC cohorts within 3 days after admission. Three types of drugs were mainly given orally or intravenously, namely methylprednisolone, hydrocortisone and dexamethasone. The corticosteroid values recorded in the study were all calculated and analyzed after conversion to methylprednisolone according to the formula: 0.3 mg dexamethasone = 8 mg methylprednisolone = 1.6 mg methylprednisolone (Table 2). The values of daily dosage were not different significantly (p = .52) in patients who received corticosteroid between NC and SC cohorts, and both values of median dosage were 40 regarded as a low‐dose administration. While the median treatment period in NC was 6 days (4–9) shorter evidently than that in SC (8 days [5–10]) with p = .003.
Table 2.
Detail information of corticosteroid therapy
| Non‐severe group | Severe group | p Value | |
|---|---|---|---|
| Number of patients | 79 | 178 | |
| Dosage, mg/d | 40.0 (33.3–40.0) | 40.0 (31.4–52.1) | .52 |
| Treatment period, days | 6 (4–9) | 8 (5–10) | .003 |
Note: Data are expressed as median (IQR). The doses of all‐type corticosteroids were uniformly converted into methylprednisolone (dexamethasone 0.3 mg = hydrocortisone 8 mg = methylprednisolone 1.6 mg).
Abbreviations: COVID‐19, coronavirus disease 2019; IQR, interquartile range.
3.1.2. Mortality and length of hospital stay
For mortality, there was no difference between NC and NN as was shown in Table 1, while it's different significantly between SC and SN (p = .02). To explore the efficacy of corticosteroid therapy on mortality for severe group, some variables different significantly in Table 1 (p < .05) were included into univariable Cox regression to screen for potential confounding factors, including RR, arterial partial pressure of oxygen (PaO2), lymphocyte count, CRP, lactate dehydrogenase, antibacterial and immunotherapy. We chose as the final inclusion into multivariable Cox regression the factors that was significantly different in univariable Cox regression analysis and potentially affected the prognosis, namely CRP, lactate dehydrogenase, immunotherapy, and corticosteroid therapy. Result of multivariable Cox regression analysis suggested that administration of corticosteroids could clearly reduce the mortality for patients in severe group (Figure 1A).
Figure 1.
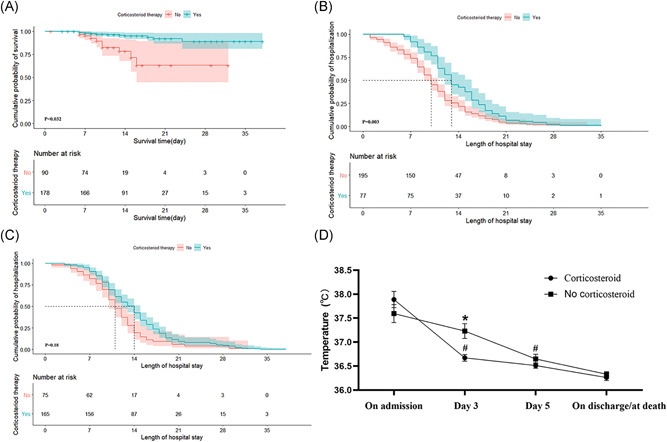
Efficacy of corticosteroids on patients with coronavirus disease 2019 (COVID‐19) in multi‐center study. Shown are the multivariable Cox regression curves for (A) the influence of corticosteroid therapy on mortality in patients with severe COVID‐19, (B) the impact of corticosteroid therapy on length of hospital stay in patients with non‐severe COVID‐19 and (C) the impact of corticosteroid therapy on length of hospital stay in patients with severe COVID‐19. The changes of temperatures in patients with severe COVID‐19 are displayed (D). *p < .05 versus the temperatures of patients who received corticosteroids. # p < .05 versus the temperatures of patients who were measured on admission
In the same way above, we performed Cox regression analyses regarding the influence of corticosteroids on the length of hospital stay for non‐severe patients and severe patients respectively. Eventually, the variables included in the multivariable Cox regression analysis for non‐severe group were antibacterial and corticosteroid therapy, while CRP, PaO2, lactate dehydrogenase, immunotherapy, antibacterial and corticosteroid therapy for severe group. Surprisingly, corticosteroid therapy can obviously prolong the length of hospital stay for non‐severe group (Figure 1B), but not for severe group (Figure 1C).
3.1.3. Changes in temperature
Combined with the result of meta‐analysis (Figure 2), we purposefully collected the temperature values of patients in severe group on admission, on Day 3, on Day 5 and on discharge/death. As indicated in Figure 1D, THE mean temperature on admission in SC cohort was 37.9°C slightly higher than that of 37.6°C in SN cohort without any difference(p = .26). But on Day 3, patients who received corticosteroids restored rapidly to a mean temperature of 36.7°C, and there was significant difference either compared with temperature on admission (p < .0001) or compared with the mean value on Day 3 (37.2°C) in SN cohort (p = .003). While the temperature in SN cohort restored gradually to normal range on Day 5. We can easily conclude that corticosteroids may shorten the duration of fever.
Figure 2.
Effect of corticosteroids on duration of fever in patients with COVID‐19. CI, confidence interval; COVID‐19, coronavirus disease 2019
3.2. Meta‐analysis
3.2.1. Basic characteristics of included literatures
We ultimately included 19 literatures with a total of 8867 subjects after rapid and detailed screening, of which 13 were retrospective studies and 6 were RCTs. 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 The search and selection process were shown in FigS1 The majority of studies were conducted in various provinces of China, and the remaining were from multiple countries globally. Non‐severe patients with COVID‐19 were investigated in 10 retrospective studies, while severe patients in 5 retrospective studies. Subjects of RCTs were mainly severe patients. All studies were considered as high‐quality with the NOS scores for retrospective studies higher than 6 and the Jadad scores for RCTs higher than 3. The basic information for each study included was introduced in Table S1.
3.2.2. Mortality
To explore the efficacy of corticosteroids on mortality, 11 studies with 8302 subjects were analyzed, of which 5 were retrospective studies and 6 were RCTs (Figure 3). On the whole, the use of corticosteroids can significantly reduce the mortality in patients with COVID‐19 (RR = 0.87; 95% CI: 0.80–0.94; I 2 = 26%), and there was no evident heterogeneity. For subgroup analyses of retrospective studies, corticosteroids cannot reduce the mortality either in non‐severe patients (RR = 0.64; 95% CI: 0.38–1.06; I 2 = 0%) or in severe patients (RR = 0.72; 95% CI: 0.49–1.08; I 2 = 0%). While the subgroup analysis for RCTs indicated that administration of corticosteroids was associated with lower mortality (RR = 0.88; 95% CI: 0.81–0.95; I 2 = 49%) and the sensitivity analysis was stable.
Figure 3.
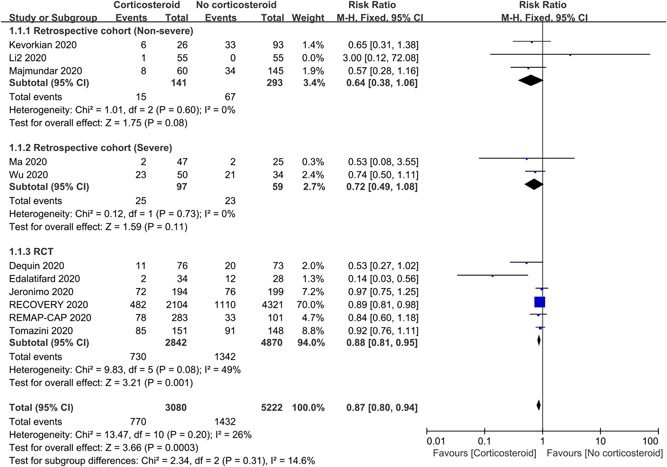
Efficacy of corticosteroids on mortality in patients with COVID‐19. CI, confidence interval; COVID‐19, coronavirus disease 2019
3.2.3. Length of hospital stay
To assess the influence of corticosteroids on length of hospital stay, 12 studies with 1300 patients were included in the meta‐analysis, of which 10 were retrospective studies and 2 were RCTs (Figure 4). Overall, corticosteroids may prolong the duration of hospitalization (MD = 2.48 days; 95% CI: 0.51–4.45; I 2 = 82%). Subgroup analyses of retrospective studies suggested that corticosteroids can significantly increase the length of stay in non‐severe patients (MD = 4.61 days; 95% CI: 2.75–6.46; I 2 = 63%) and sensitivity analysis was stable, but not in severe patients (MD = −1.39 days; 95% CI: −4.38 to 1.60; I 2 = 0%). There was also no impact for subgroup analysis of RCTs (MD = −2.36 days; 95% CI: −8.90 to 4.18; I 2 = 90%).
Figure 4.
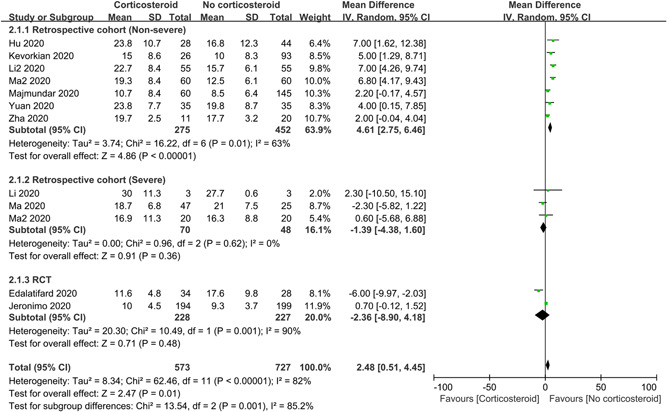
Influence of corticosteroids on length of hospital stay in patients with COVID‐19. CI, confidence interval; COVID‐19, coronavirus disease 2019
3.2.4. Duration of RNA clearance
To investigate the impact of corticosteroids on duration of RNA clearance, only 11 retrospective studies with 627 subjects were analyzed (Figure 5). Pooled data indicated that corticosteroids cannot delay the RNA clearing (MD = 1.56 days; 95% CI: −0.01 to 3.14; I 2 = 56%). But the subgroup analysis of non‐severe patients demonstrated that corticosteroid therapy was associated with prolonged duration of RNA clearance (MD = 2.03 days; 95% CI: 0.30–3.7; I 2 = 57%) and sensitivity analysis was stable.
Figure 5.
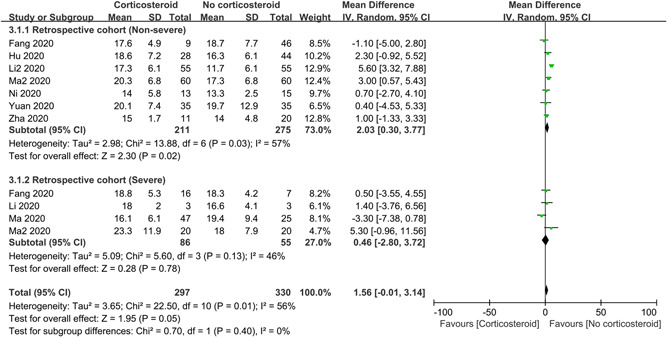
Impact of corticosteroids on duration of viral RNA clearance in patients with COVID‐19. CI, confidence interval; COVID‐19, coronavirus disease 2019
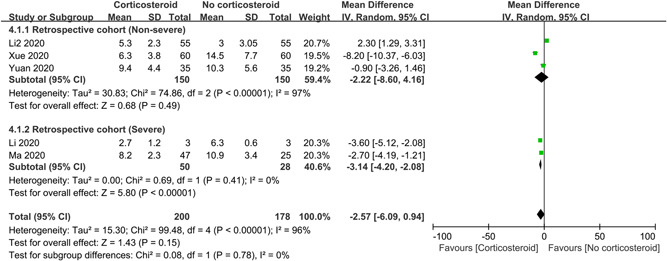
3.2.5. Duration of fever
To evaluate the influence of corticosteroids on duration of fever, 5 retrospective studies with 378 patients were included in the meta‐analysis, where there were 300 non‐severe patients and 78 severe patients (Figure 2). The overall result showed corticosteroid therapy had no distinct impact on duration of fever (MD = −2.57 days; 95% CI: −6.09 to 0.94; I 2 = 96%). Subgroup analyses suggested that corticosteroids can shorten the duration of fever in severe patients (MD = −3.14 days; 95% CI: −4.20 to −2.08; I 2 = 0%), but not in non‐severe patients.
3.2.6. Publication bias
We produced four funnel plots for these four endpoints respectively to assess the publication bias. As shown in Figure S2, there was no obvious publication bias in the studies included in our meta‐analysis.
4. DISCUSSION
This is the first study to explore the effectiveness of corticosteroid therapy for patients with COVID‐19 by combining clinical research and meta‐analysis. The multicenter retrospective study demonstrated that low‐dose corticosteroids can significantly lower the risk of death and shorten duration of fever among patients in severe group, while prolong duration of hospitalization in among patients in non‐severe group. The meta‐analysis confirmed the same results and found that corticosteroids may delay viral clearing in non‐severe patients. Therefore, corticosteroids exhibited different therapeutic efficacy in patients with non‐severe or severe COVID‐19.
Consistent with many previously published studies, 1 , 37 compared with non‐severe patients, severe patients have worse baseline characteristics and higher therapeutic requirements in our multicenter study, such as older age, more comorbidities, and worse biochemical indicators. Ye et al. suggested that patients with COVID‐19 should be treated differently according to the severity of the disease. 38 To date, corticosteroid therapy remains as one of the pivotal adjuvant initiatives for COVID‐19, the role of which should be assessed respectively between non‐severe patients and severe patients to facilitate clinical decision‐making.
Hasan et al. classified the disease process of COVID‐19 as three stages, namely early infection, lung progression and hyperinflammation phase. 39 In the stage of early infection, patients often presented with some mild or nonspecific symptoms, such as fever and cough. He proposed that treatment at this stage was primarily targeted toward symptomatic relief and avoided the use of corticosteroids. Surprisingly, what resembled to Hasan's proposal was that corticosteroids were not recommended for patients with non‐severe COVID‐19 as that cannot significantly reduce mortality or duration of fever, and even may increase the duration of hospital stay and delay viral RNA clearing, which were concluded from our meta‐analysis and further validated in our multi‐centered study. A preliminary clinical trial 31 reported that the use of dexamethasone was not associated with 28‐day mortality in patients who received no oxygen therapy, which provided a robust evidence demonstrating the unnecessity of corticosteroids among non‐severe COVID‐19 patients. In addition, consistent with studies on SARS and MERS, 40 , 41 systematic corticosteroids in COVID‐19 may also lead a delayed viral RNA clearing and a prolonged length of hospital stay. As indicated by Hasan, 39 stages of early infection and lung phase were considered as viral response phase where virus performed rapid replication, while early initiation of corticosteroids at this phase resulted in delayed viral clearance (thus a higher subsequent plasma viral load). 40 Although it's reported in early‐stage observational studies that the prolonged duration of hospitalization was primarily associated with the more severe baseline characteristics in patients who received corticosteroid therapy, we considered the delayed role of corticosteroids on viral clearing as main cause for prolonged duration of hospitalization on account of the relatively balanced baseline characteristics between NC and NN cohorts in our multi‐centered study. Consequently, for patients with non‐severe COVID‐19, corticosteroid therapy presented no manifest improvement in survival, and delayed viral clearing and increase length of hospital stay instead.
In severe COVID‐19 pneumonia, patients' symptoms worsen rapidly and were considered in hyperinflammation phase where proper corticosteroid was recommended to suppress the cytokine storm. 39 , 42 Corticosteroid is a classical immunosuppressive drug that helps in delaying or halting the progress of pneumonia and has been effective for the treatment of ARDS, 43 , 44 and meanwhile serves as an anti‐inflammatory agent that reduces systemic inflammation, decreases exudation into the lung tissue, promotes the absorption of inflammation, and prevents alveolar damage. 45 These effects of corticosteroids may improve the prognosis of severe COVID‐19 patient to some extent. In the meta‐analysis of RCTs regarding mortality, the RR was 0.88 with 95% CI from 0.81 to 0.95 in favor of corticosteroid therapy. Given that subjects in RCTs substantially consisted of patients with severe COVID‐19, the administration of corticosteroids in severe‐type patients should be adopted properly, which was also suggested in a prospective meta‐analysis of clinical trials. 15 Moreover, we also conducted Cox regression analysis to further confirm the beneficial role of corticosteroids in survival of severe patients. As indicated, due to the host' susceptibility to hyperinflammation in severe‐type patients, corticosteroids functioned as the anti‐inflammatory agent with the capability to suppress inflammation and lower body temperature rapidly in patients at this stage. 39 This effect was also demonstrated in the subgroup analysis of retrospective studies (severe) and multicenter study. In addition to a shorter duration of fever, a faster improvement of SpO2 was found in cases of severe SARS‐CoV‐2 pneumonia treated with low‐dose, short‐term methylprednisolone, which was possibly associated with the effectiveness of corticosteroid on hypoxemia. 44 , 46 To summarize briefly, corticosteroid therapy can help in either reducing the mortality or improving the clinical status in patients with severe COVID‐19. Furthermore, previous meta‐analysis demonstrated that corticosteroids may increase length of hospital stay. 12 , 13 In contrast, a recently published clinical trial 32 suggested that corticosteroids decreased length of stay evidently. While the length of stay was not significantly prolonged in severe‐type patients who received low‐dose corticosteroids either in multivariable Cox regression or in our meta‐analysis. Interestingly, although the duration of RNA clearing was not collected in our multicentered study, the result of subgroup meta‐analysis suggested that corticosteroids had no significant impact on duration of RNA clearance in patients with severe COVID‐19, which may be associated with patients at this stage primarily characterized with host hyperinflammation activity and having completed the stage of virus replication. It can be speculated that the clearing of viral RNA may be closely related to the length of the patient's hospital stay. Besides, we also considered the severity of disease and some unknown reasons possibly masking the role of corticosteroids. More large‐scale and rigorously designed random control trials are required to further elucidate the specific impact of corticosteroid on length of hospital stay and virus clearing in the future.
There remain some limitations in this study. First of all, the exact time of viral RNA shedding was untested timely and recorded owing to the overwhelming medical pressure and restrained medical resources then. What's more, the dynamic change of temperatures for non‐severe patients was not collected with the values lacking too much to statistically analyze. Finally, the literature search was not performed in each database on account of the inaccessibility.
In conclusion, the therapeutic response to corticosteroids differed in patients with different severity of disease. For patients with non‐severe COVID‐19, corticosteroids were not recommended as a routine therapeutic initiative as that presented the prolonged duration of hospitalization and delayed viral RNA clearing, as well as no impact on mortality and duration of fever. While low‐dose administration of corticosteroids may benefit the patients with severe COVID‐19 for it can manifestly lower risk of death and shorten duration of fever without significant influence for length of hospital stay. It can be evidently concluded that corticosteroids may be more suitable for severe patients relative to non‐severe patients. Therefore, corticosteroids should be considered or not prudently based on patient's condition, which was expected to help guiding physicians in the corticosteroid management for COVID‐19 in clinical practice to some extent.
CONFLICT OF INTERESTS
All authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Jungang Xie participated in study design. Yuan Zhan and Jin Shang collected the epidemiological and clinical data, performed the statistical analysis, and drafted the manuscript. Qian Huang and Yiya Gu Conducted the literature search and data extraction. Jungang Xie revised the final manuscript. All authors reviewed and approved the final version of the manuscript.
Supporting information
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
The authors would like to thank all cooperation unit for their support. They also acknowledge all health‐care workers involved in the prevention and control of the epidemic situation. The author would like to thank them for their contributions to the diagnosis and treatment of patients and the staff who record and collect the clinical data carefully. This study was supported by the National Natural Science Foundation of China (No. 81973986, 81570033), the National key basic research and development program (973 Program, No. 20l5CB553403), and the National Key R&D Program of China (2016YFC1304500, 2018YFC1311900).
Zhan Y, Shang J, Gu Y, Huang Q, Xie J Efficacy of corticosteroid in patients with COVID‐19: a multi‐center retrospective study and meta‐analysis. J Med Virol. 2021;93:4292‐4302. 10.1002/jmv.26914
Yuan Zhan and Jin Shang contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470‐473. 10.1016/S0140-6736(20)30185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong JEL, Leo YS, Tan CC. COVID‐19 in Singapore‐current experience: critical global issues that require attention and action. JAMA. 2020;323:1243‐1244. 10.1001/jama.2020.2467 [DOI] [PubMed] [Google Scholar]
- 4. Van Cuong L, Giang HTN, Linh LK, et al. The first Vietnamese case of COVID‐19 acquired from China. Lancet Infect Dis. 2020;20:408‐409. 10.1016/S1473-3099(20)30111-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019‐nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970‐971. 10.1056/NEJMc2001468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin YC, Yen LL, Chen SY, et al. A preliminary study on the treatment of severe acute respiratory syndrome with glucocorticoid. Chinese J Prev Med. 2003;37:233‐235. [Google Scholar]
- 7. He R, Liu Z, Duan X. Adverse effects associated with corticosteroids therapy in 57 SARS cases. Adverse Drug Reactions J. 2003;5:374‐377. [Google Scholar]
- 8. Shen J, et al. Investigation of proximal femoral marrow with magnetic resonance imaging in recovered patients with severe acute respiratory syndrome. Zhonghua Jie He He Hu Xi Za Zhi. 2006;29:189‐193. [PubMed] [Google Scholar]
- 9. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang C, Wang Y, Lv H, Guan Z, Gu J. Caution against corticosteroid‐based COVID‐19 treatment. Lancet. 2020;395:1759‐1760. 10.1016/S0140-6736(20)30749-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu S, et al. Effectiveness and safety of glucocorticoids to treat COVID‐19: a rapid review and meta‐analysis. Ann Transl Med. 2020;8:627. 10.21037/atm-20-3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li H, Chen C, Hu F, et al. Impact of corticosteroid therapy on outcomes of persons with SARS‐CoV‐2, SARS‐CoV, or MERS‐CoV infection: a systematic review and meta‐analysis. Leukemia. 2020;34:1503‐1511. 10.1038/s41375-020-0848-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Ao G, Qi X, Zeng J. The influence of corticosteroid on patients with COVID‐19 infection: a meta‐analysis. Am J Emerg Med. 2020. 10.1016/j.ajem.2020.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID‐19: a meta‐analysis. JAMA. 2020;324:1330. 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25:603‐605. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 17. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1‐12. 10.1016/0197-2456(95)00134-4 [DOI] [PubMed] [Google Scholar]
- 18. Zha L, Li S, Pan L, et al. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID‐19). Med J Aust. 2020;212:416‐420. 10.5694/mja2.50577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yuan M, Xu X, Xia D, et al. Effects of corticosteroid treatment for non‐severe COVID‐19 pneumonia: a propensity score based analysis. Shock. 2020;54:638‐643. 10.1097/SHK.0000000000001574 [DOI] [PubMed] [Google Scholar]
- 20. Fang X, Mei Q, Yang T, et al. Low‐dose corticosteroid therapy does not delay viral clearance in patients with COVID‐19. J Infect. 2020;81:147‐178. 10.1016/j.jinf.2020.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu Z, Lv Y, Xu C, et al. Clinical use of short‐course and low‐dose corticosteroids in patients with non‐severe COVID‐19 during pneumonia progression. Front Public Health. 2020;8:355. 10.3389/fpubh.2020.00355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li XG, et al Clinical research of glucocorticoid treatment in severe cases of COVID‐19. J Capital Med Univ. 2020;41(3):1006‐7795. [Google Scholar]
- 23. Ni Q, et al. Effect of low‐to‐moderate dose glucocorticoids on viral clearance in COVID‐19: a restrospective study. Chin J Clin Infect Dis. 2020;13(1):1674‐2397. [Google Scholar]
- 24. Xue P, et al. Clinical observation of short‐term and low‐dose glucocorticosteroid in treatment of moderate COVID‐19 in elderly patients. Shandong medicine. 2020. 10.3969/j.issn [DOI] [Google Scholar]
- 25. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934‐943. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma Q, et al Corticosteroid therapy for patients with severe novel coronavirus disease 2019. Eur Rev Med Pharmacol Sci. 2020;24:8194‐8201. 10.26355/eurrev_202008_22508 [DOI] [PubMed] [Google Scholar]
- 27. Li Q, Li W, Jin Y, et al. Efficacy evaluation of early, Low‐lose, short‐term corticosteroids in adults hospitalized with nonsevere COVID‐19 pneumonia: a retrospective cohort study. Infect Dis Ther. 2020;9:823‐836. 10.1007/s40121-020-00332-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kevorkian JP, Riveline JP, Vandiedonck C, et al. Early short‐course corticosteroids and furosemide combination to treat non‐critically ill COVID‐19 patients: an observational cohort study. J Infect. 2020;4825:e22‐e24. 10.1016/j.jinf.2020.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Majmundar M, Kansara T, Lenik JM, et al. Efficacy of corticosteroids in non‐intensive care unit patients with COVID‐19 pneumonia from the New York Metropolitan region. PLOS One. 2020;15:e0238827. 10.1371/journal.pone.0238827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma Y, Zeng H, Zhan Z, et al. Corticosteroid use in the treatment of COVID‐19: a multicenter retrospective study in Hunan, China. Front Pharmacol. 2020;11:1198. 10.3389/fphar.2020.01198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Group RC, et al Dexamethasone in hospitalized patients with COVID19—preliminary report. N Engl J Med. 2020;384:693‐704. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Edalatifard M, Akhtari M, Salehi M, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID‐19 patients: results from a randomised controlled clinical trial. Eur Respir J. 2020;56:2002808. 10.1183/13993003.02808-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jeronimo CMP, Farias MEL, Val FFA, et al. Methylprednisolone as adjunctivev therapy for patients hospitalized with COVID‐19 (Metcovid): a randomised, double‐blind, phase IIb, placebo‐controlled Trial. Clin Infect Dis. 2020. 10.1093/cid/ciaa1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dequin PF, Heming N, Meziani F, et al. Effect of Hydrocortisone on 21‐day mortality or respiratory support among critically ill patients with COVID‐19: a randomized clinical trial. JAMA. 2020;324:1298. 10.1001/jama.2020.16761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Angus DC, Derde L, Al‐Beidh F, et al. Effect of hydrocortisone on Mortality and organ support in patients with severe COVID‐19: the REMAP‐CAP COVID‐19 corticosteroid domain randomized clinical trial. JAMA. 2020;324:1317. 10.1001/jama.2020.17022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator‐free in patients with moderate or severe acute respiratory distress syndrome and COVID‐19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307. 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ye Z, Rochwerg B, Wang Y, et al. Treatment of patients with nonsevere and severe coronavirus disease 2019: an evidence‐based guideline. CMAJ. 2020;192:E536‐E545. 10.1503/cmaj.200648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Siddiqi HK, Mehra MR. COVID‐19 illness in native and immunosuppressed states: a clinical‐therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405‐407. 10.1016/j.healun.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS‐associated coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304‐309. 10.1016/j.jcv.2004.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arabi YM, Mandourah Y, Al‐Hameed F, et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757‐767. 10.1164/rccm.201706-1172OC [DOI] [PubMed] [Google Scholar]
- 42. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thompson BT. Glucocorticoids and acute lung injury. Crit Care Med. 2003;31:S253‐S257. 10.1097/01.CCM.0000057900.19201.55 [DOI] [PubMed] [Google Scholar]
- 44. Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17:233‐247. 10.1038/nri.2017.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rhen T, Cidlowski JA. Anti‐inflammatory action of glucocorticoids—New mechanisms for old drugs. N Engl J Med. 2005;353:1711‐1723. 10.1056/NEJMra050541 [DOI] [PubMed] [Google Scholar]
- 46. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID‐19 pneumonia. Signal Transduct Target Ther. 2020;5:57. 10.1038/s41392-020-0158-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


