Abstract
Vitamin D, a key nutrient/prohormone classically associated with skeletal health, is also an important immunomodulator, with pleotropic effects on innate and adaptive immune cells. Outcomes of several chronic, autoimmune, and infectious diseases are linked to vitamin D. Emergent correlations of vitamin D insufficiency with coronavirus-induced disease 2019 (COVID-19) severity, alongside empirical and clinical evidence of immunoregulation by vitamin D in other pulmonary diseases, have prompted proposals of vitamin D supplementation to curb the COVID-19 public health toll. In this review paper, we engage an immunological lens to discuss potential mechanisms by which vitamin D signals might regulate respiratory disease severity in severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) infections, vis a vis other pulmonary infections. It is proposed that vitamin D signals temper lung inflammatory cascades during SARS-CoV2 infection, and insufficiency of vitamin D causes increased inflammatory cytokine storm, thus leading to exacerbated respiratory disease. Additionally, analogous to studies of reduced cancer incidence, the dosage of vitamin D compounds administered to patients near the upper limit of safety may serve to maximize immune health benefits and mitigate inflammation and disease severity in SARS-CoV2 infections. We further deliberate on the importance of statistically powered clinical correlative and interventional studies, and the need for in-depth basic research into vitamin D-dependent host determinants of respiratory disease severity.
Keywords: immunoregulation, vitamin D
Graphical Abstract
Review on Vitamin D-dependent immunoregulatory mechanisms underlying respiratory disease severity in SARS-CoV2 infections.
Graphical Abstract.
INTRODUCTION
Respiratory viruses such as coronavirus and influenza pose a major public health threat due to their fast-spreading nature, epidemic/pandemic potential, and high morbidity/mortality through rapid induction of respiratory disease. In this regard, the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), has proven particularly challenging, with relatively short latency and prolonged infectious periods,1 case fatality rates ranging from 2 to 10% in the most affected countries (see mortality analysis at https://coronavirus.jhu.edu/data/mortality), and confoundingly disproportionate effects on certain vulnerable populations such as the elderly, the overweight, African Americans, and patients with preexisting chronic disease conditions.2–4 Given the scale of the SARS-CoV2-induced coronavirus disease 2019 (COVID-19) pandemic—with over 59,100,000 confirmed cases and over 1,400,000 deaths in 216 countries worldwide (as of November 24, 2020; World Health Organization)—there is an urgent need to define the host determinants of disease susceptibility. In this review paper, we delve into the clinical and empirical evidence supporting vitamin D regulation of host immunity in the context of COVID-19 and other diseases, and speculate on possible immunologic mechanisms by which vitamin D might dictate COVID-19 patient outcomes. It is proposed that vitamin D signals temper lung inflammatory cascades during SARS-CoV2 infection, and insufficiency of vitamin D causes increased inflammatory cytokine storm, thus leading to exacerbated respiratory disease.
VITAMIN D METABOLISM IN MAMMALS
Vitamin D3 (cholecalciferol) is produced in the skin from 7-dehydrocholesterol through a 2-step process by UVB radiation from the sun, to first form pre-D3, which then isomerizes to vitamin D3 in a nonenzymatic process. The rate of vitamin D3 production in the skin is regulated by multiple factors, such as melanin pigmentation level and UVB intensity (as determined by seasonal angle of the sun, latitude, clothing, and sunscreen usage). Vitamin D may also be procured from dietary sources in the form of vitamin D3 or cholecalciferol (from animal sources such as fatty fish), and in the form of vitamin D2 or ergocalciferol (from plant sources such as mushrooms). The cytochrome P450 mixed-function oxidase (CYP) family of enzymes in the endoplasmic reticulum and mitochondria, next catalyze the conversion of vitamin D3 into 25-hydroxy vitamin D (25OHD, also known as calcidiol), largely in the liver. The CYP27B1 hydroxylase next converts 25OHD into the bioactive form 1,25-dihydroxy vitamin D [1,25(OH)2D, also known as calcitriol], mainly in the kidneys. 1,25(OH)2D is implicated in a variety of physiologic effects such as bone and calcium homeostasis, as well as transcriptional regulation. Gene regulatory effects of 1,25(OH)2D are largely mediated through the vitamin D receptor (VDR) transcription factor of the steroid hormone nuclear receptor family, by binding to the vitamin D response elements in transcriptional regulatory genomic regions. The genomic effects of VDR are exerted in the context of a heterodimeric partner, RXR, and may vary from one cell-type to another depending on distinct binding partners (see previous review papers on the topic).5–8 In this review paper, we use vitamin D to broadly include the bioactive 1,25(OH)2D and the 25OHD precursor forms, both of which together typically define the vitamin D status of an individual.
Based on the 2020 Consensus statement from the 2nd International Conference on Controversies in Vitamin D 25(OH)D, the serum concentrations between 50 nmol/L (20 ng/ml) and 125 nmol/L (50 ng/ml) appear to be safe and sufficient in the general population for skeletal health.9 However, it has been shown in numerous studies that higher 25-hydroxyvitamin D serum concentrations are associated with a reduction of diseases other than rickets and osteomalacia, for instance, a lower cancer incidence and cancer mortality.10 The dose of vitamin D required to increase serum 25OHD in people to a minimum serum 25OHD of 20 ng/ml (50 nmol/L) is approximately 800 IU daily, whereas increasing to a minimum level of 30 ng/ml (75 nmol/L) requires approximately 4,000 IU daily.9 Potential risk of vitamin D toxicity is typically defined as a 25(OH)D level > 100 ng/ml (>250 nmol/ml) in adults, thus offering a substantial range of vitamin D dose for supplementation efforts. Of note, in various disease states, Endocrine Society recommended doses of 10,000 IU/day (250 μg/day), and in some studies even higher doses have been used.11,12 Hence, short-term treatment with high doses of vitamin D can be safely used in emergency situations, such as COVID-19 patients requiring supplemental oxygenation. In the future, to maximize the beneficial outcomes of supplementation in the context of immune health, an in-depth, systematic dose-dependent risk versus benefit analyses of vitamin D supplementation in its relatively newer role of immune regulation and disease mitigation beyond skeletal health are critically needed.
VITAMIN D AND COVID-19
In general, age, certain genetic or ethnic backgrounds, and preexistent chronic disease conditions are linked to disproportionately exacerbated respiratory disease severity in COVID-19.13–16 Intriguingly, vitamin D insufficiency is also pronounced in the elderly and African Americans owing to inefficiencies in vitamin D uptake, metabolism, and/or signaling.5,17–20 Likewise, chronic disease conditions such as heart disease and diabetes are also linked to inadequate vitamin D levels, thus supporting a possible indirect link between vitamin D insufficiency and COVID-19 severity.8 Promisingly, preliminary retrospective analyses of serum vitamin D levels in South Asian and Swiss COVID-19 patients reveal a strong correlation between vitamin D insufficiency and respiratory disease severity.21,22 A similar indirect correlation between vitamin D and COVID-19 severity is implicated by recent observations of increased mortality in the higher latitude northern US states that receive lower UVB dose compared with the southern states.15,16 Likewise, cursory cross-sectional global analyses have uncovered higher COVID-19-related mortality in countries with lower average vitamin D status.14,23
Linkage between vitamin D insufficiency and COVID-19 severity is consistent with several historic, anecdotal, and clinical studies linking inadequate vitamin D levels to a variety of infectious, chronic, and autoimmune diseases such as respiratory syncytial virus (RSV), tuberculosis (TB), HIV, HBV, HSV, Dengue virus, malaria, leprosy, cancer, multiple sclerosis (MS) and inflammatory bowel disease (IBD).5,6,24–40 Likewise, strong association between vitamin D status and seasonal influenza infections incidence is well known.41–44 Hepatitis C virus infection also shows an inverse relationship between vitamin D levels and viral load, liver fibrosis, and treatment outcomes.45 Direct studies using mouse models of mycobacterial and listeria infections demonstrate that disease progression is severely aggravated when mice are rendered vitamin D deficient.46–49 Recent data from our studies demonstrate that vitamin D deficiency also underlies increased susceptibility to chronic lymphocytic choriomeningitis virus (LCMV) infection in mice (unpublished observations). Collectively, the above-referenced clinical correlations and meta-level pathogen clearance/disease outcome analyses linking vitamin D and disease severity underscore the importance of investigations into vitamin D-dependent immunological mechanisms of disease control or susceptibility.
IMMUNE REGULATION BY VITAMIN D
Cells of both innate and adaptive immune systems work in concert to clear pathogens. In addition to mediating direct antipathogen functions, innate immune responses also serve as beacons and catalysts for adaptive immune responses to effectively cull the pathogen. Notably, vitamin D influences cells of the both innate and adaptive immune systems.17,50,51 Macrophages,37,46,47 dendritic cells (DCs),52 neutrophils,53 NK cells, NK-T cells, effector cluster of differentiation (CD)4 and CD8 T cells,54 regulatory T cells (Tregs),55–60 and B cells,60,61 all express the VDR (a zinc-finger nuclear transcription factor that exerts direct gene regulation and cell signaling effects) or CYP27B1 enzyme (required for formation of bioactive vitamin D for intracrine action), or both. The expression of VDR changes in a cell-type specific manner with their developmental state.62,63 For example, macrophages and DCs down-regulate VDR following maturation, whereas T cells tend to up-regulate VDR expression following activation. Types of infections also differentially regulate VDR expression through distinct mechanisms. For instance macrophages/monocytes up-regulate the expression of VDR and CYP27B1 during mycobacterial infections through TLR-triggering, thus enabling increased responsiveness to paracrine and intracrine vitamin D signals.64 Other innate cells such as neutrophils are also functionally activated by vitamin D signals.65 In fact, immunologic role of vitamin D was first identified in mycobacterial infections, where it promotes host defense by augmenting chemotaxis, phagocytosis, and production of antimicrobial peptides by macrophages and monocytes.46,66–68
Mouse and human CD8 and CD4 T cells express VDR and the CYP27B1 enzyme (required for formation of bioactive vitamin D), and up-regulate VDR expression upon polyclonal in vitro stimulation.17,37,54,69,70 We have recently shown that antigen-specific CD8 T cells also up-regulate VDR following in vivo activation during viral infection.71 Intriguingly, contrary to immunostimulatory effects of vitamin D on monocytes/macrophages, vitamin D signals are largely inhibitory in adaptive immune cells such as T cells and B cells.5,65 Exposure of CD4, CD8 T cells, and B cells to calcitriol in vitro suppresses proliferation and effector differentiation.17,72 Our data show reduced expression of effector molecule, granzyme B, in VDR-deficient LCMV-specific effector CD8 T cells.71 Likewise, B cells produce reduced IgM and IgG effector antibodies in the absence of vitamin D signals.72 Differentiation of TH1 and TH17 effector subsets is also suppressed by vitamin D, while the effects of vitamin D on TH2 cells are seemingly stimulatory, albeit this needs to be further clarified due to conflicting reports.73–75 Development and function of iNKT cells is also regulated by vitamin D.76–79 In contrast, vitamin D promotes the development and the suppressive function of Tregs—the prototypic suppressive cells of the adaptive immune system, critical for inhibition of innate and effector responses.80–91 Treg-enhancing effects of vitamin D are mediated either through direct signaling in Treg cells, or indirectly through induction of tolerogenic DCs.52,92–94
As a critical link between innate and adaptive immune systems, DCs serve as an important target for vitamin D-mediated immunoregulation.52,93,94 Vitamin D signals lead to impaired expression of key functional markers in DCs including: (i) MHC-II and costimulatory molecules (such as CD80. CD86, CD40), (ii) inflammatory cytokines and chemokines (such as IL-12 and IL-23, which drive inflammatory TH1 and TH17 responses), and (iii) also promote the expression of immunosuppressive cytokines and chemokines (such as IL-10 and CCL-22, which support immunosuppressive Treg cell function and migration).52,94–98 The migratory properties and tissue tropism of T cells are also modulated by DCs in a vitamin D-dependent manner through regulation of chemokine receptor expression. For example, in the in vitro studies, vitamin D promotes CCR10 expression and skin homing of T cells in response to CCL27, but suppresses the expression of gut-homing receptors CCR9 and α4β7.55 Similar to DCs,99 vitamin D also suppresses the ability of macrophages to activate T cells by reducing expression of MHC-II, costimulatory molecules, and inflammatory cytokines, despite activating their phagocytic and chemotactic activity.100–104 Vitamin D also suppresses the production of other inflammatory or prodifferentiation cytokines such as IFN-γ, IL-2, IL-17, and IL-21 by CD4 T cells.87 The antipathogen effects of vitamin D possibly extend to nonimmune cells as well, as indicated by increased intestinal epithelial barrier function in case of autoimmune intestinal disorders.105–107 Therefore, vitamin D regulates several immune cells including macrophages, DCs, NK cells, B cells, and T cells by largely inhibiting inflammation and proliferation while also enhancing antipathogen innate immunity at the same time.52,108–110
DISEASE CONTEXT-DEPENDENT REGULATION OF HOST IMMUNITY BY VITAMIN D
Given the multitude of immune cells regulated by the bioactive form of vitamin D [1,25(OH)2D], it is proposed that protective effects of vitamin D are mediated by unique immunoregulatory mechanisms in a disease-specific manner. Although the precise vitamin D-regulated immune mediators may vary in each disease, in general vitamin D promotes immunoprotection by maintaining a healthy balance of inflammation and effector responses.111 For instance, in mycobacterial infection, vitamin D inhibits pathologic inflammatory mediators and matrix degrading enzymes, while enhancing the IFN-γ-induced antimicrobial peptides to block mycobacterial replication.26 In the mouse model of MS, vitamin D signaling in TH1 cells regulates their migration in response to inflammatory CXCR3 ligands,112 and also suppresses pathogenic B cell responses.60,61,72 In the case of HSV-induced Behcet's inflammatory disease, vitamin D mediates protective, anti-inflammatory effects through down-regulation of TLRs.113,114 Anti-inflammatory effects of vitamin D are also implicated in successful organ transplantation.73,115–118 In endotoxin-induced acute respiratory distress syndrome (ARDS), inadequate vitamin D level is associated with increased levels of inflammatory cytokines such as IL-1β and TNF-α.119,120 In contrast to largely beneficial effects of vitamin D, certain infections (e.g., citrobacter and leishmania) were confusingly enhanced or unaffected by vitamin D. Subsequent categoric immunologic studies revealed inhibitory effects of vitamin D on inflammatory TH1 effector subset necessary for pathogen control.38,121,122 These instances of disease context-dependent immune modulation by vitamin D underscore the importance of studying vitamin D regulation of SARS-CoV2 immune responses for the design of novel immunotherapies and exploring timely vitamin D supplementation in vulnerable populations.
VITAMIN D AND RESPIRATORY DISEASE SEVERITY
Strong host inflammation is a lead cause of exacerbated lung disease in TB and influenza.123,124 Although rapid induction of proinflammatory cytokines and chemokines is a key part of early immune anti-viral defense, and is also critical for recruitment of additional innate cells (such as monocytes and NK cells) as well as adaptive immune cells to sites of viral replication in the lung, overexuberant and/or prolonged inflammation in the lung causes significant lung damage, ARDS and mortality.111 In the highly pathogenic 1918 Influenza A, pulmonary pathology and fatal outcome were associated with accelerated activation of proinflammatory genes that remained unabated until death.125–128 Hence, therapies aimed at reducing the cytokine storm are proposed to be beneficial for severe cases of influenza.129
As in pathogenic influenza, SARS-CoV2 infection in humans is associated with replication of the virus in the lower respiratory tract, which is accompanied by severe inflammation owing to increased localization of inflammatory macrophages to lung airways.13,123,125, 130–135 Profound impact of vitamin D on immunity to a variety of respiratory/mucosal diseases characterized by inflammation (influenza, TB, MS)13,42,44,125 supports vitamin D-dependent regulation of innate and adaptive inflammatory mediators/regulators of pulmonary immunopathology and ARDS during COVID-19 as well (Fig. 1). Increased inflammation is implicated as the prime cause for morbidity/mortality in vulnerable patients, with more severe COVID-19 cases associated with greater inflammatory cytokine induction and vitamin D insufficiency.14–16,125,130,134–138 Atypical innate immune responses comprising reduced type-I IFN production and increased inflammatory cytokines such as IL-6 have been recently identified in COVID-19132,139–145 (Fig. 1). 1,25(OH)2D exerts suppressive effects on the production of IL-6 by innate monocytes,146 albeit its effect on type-I IFNs in the context of viral infections remains largely unexplored. Additional potential vitamin D-regulated innate immune cell targets include neutrophils, inflammatory M1 macrophages and plasmacytoid DCs. Other immune cells implicated in protecting against severe lung injury and pulmonary fibrosis in respiratory infections such as influenza, SARS, MERS, RSV include innate lymphoid cells 2 ILC2, invariant NKT iNKT cells, M2 macrophages, myeloid-derived suppressor cells MDSC, and γδT cells.123 Hence, the role of these cell-types in regulating COVID-19 lung immunopathology in a vitamin D-dependent manner needs to be rigorously evaluated in the future.
FIGURE 1.
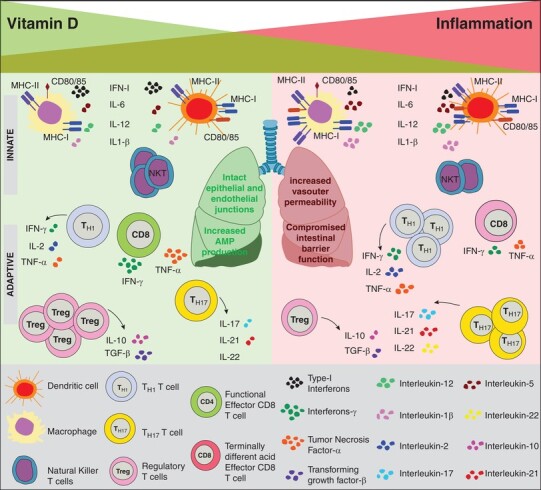
Model of immunomodulation by vitamin D in COVID-19. The decreasing green shaded triangle indicates decreasing vitamin D status. Increasing intensity of red shade indicates increasing inflammation with decreasing vitamin D levels. Infection in vitamin D sufficient hosts (green half of the figure, corresponding to serum levels of 25(OH)D > 25–30 ng/ml, defined as sufficient) is expected to induce optimal activation of innate immune cells such as macrophages (with robust antimicrobial peptide, AMP, production) and DCs with robust up-regulation of MHC and costimulatory molecules, and regulated production of proinflammatory cytokines. Balanced differentiation of effector CD8 and CD4 T cell subsets under conditions of vitamin D sufficiency is also expected to promote robust antiviral responses, with regulated production of inflammatory cytokines. Vitamin D sufficiency is also predicted to promote the development of immunoprotective NK-T cells, and maintain epithelial junctional integrity and endothelial vascular permeability, thus minimizing pulmonary damage. Contrarily, host vitamin D insufficiency or deficiency (red half of the figure, corresponding to serum levels of 25(OH)D ≤ 10 ng/ml (25 nM) defined as deficient, and 10–20 ng/ml defined as insufficient) is expected to lead to aberrant activation of innate inflammatory mediators such as macrophages and DCs, leading to exacerbated inflammation, more pronounced expansion and terminal differentiation of effector CD8 and inflammatory CD4 T cell subsets, and diminished Treg induction and NK-T cell development. Likewise, the junctional integrity of lung epithelial and vascular endothelial cells is also predicted to be impaired, thus leading to pulmonary edema, lung injury and functional impairment and ARDS. Conditions of vitamin D hyper-supplementation are not depicted in this model. A better understanding of disease-context-dependent immunomodulatory effects of vitamin D in SARS-CoV2 infections at pulmonary sites of viral growth and secondary lymphoid sites of immune activation will illuminate potential beneficial effects of normalizing vitamin D levels to sufficient or hyper-supplemented levels
The adaptive immune components, CD8 and CD4 T cells might also serve to exacerbate inflammation at lung sites as “latecomer” inflammatory mediators attracted by the initial innate chemoattractants at lung sites (Fig. 1). Indeed, vitamin D-dependent regulation of chemokine receptor expression on effector T cells and Tregs, the anti-proliferative effects of vitamin D on effector T cells, and the anti-inflammatory effects of vitamin D likely modulate the overall expansion, migration, differentiation status, and function of T cells in lung sites of SARS-CoV2 infection. In addition to immune mediators of inflammation, it is important to consider epithelial/endothelial cells as candidate vitamin D-regulated mediators of lung damage and ARDS in COVID-19110,129 (Fig. 1). Airway epithelial and endothelial cells secrete a variety of immune mediators such as antimicrobial peptides, cytokines, and chemokines, such as IFN-I, IL-6, G-CSF, and GM-CSF. Potential vitamin D-dependent innate and adaptive immune regulators of lung inflammation and COVID-19 severity are summarized in Fig. 1.
VITAMIN D SUPPLEMENTATION IN SARS-COV2 INFECTIONS
According to the National Health and Nutrition Examination Survey (NHANES), while massive public health efforts of milk fortification have effectively controlled severe rachitic deficiency, vitamin D insufficiency is widespread in the US as well as Europe.147–151 Notable vitamin D insufficiency in the elderly, African Americans, and patients with chronic disease conditions (diabetes and heart disease) and their increased susceptibility to COVID-19 has prompted proposals for vitamin D supplementation.
Potential beneficial effects of vitamin D supplementation are supported by vitamin D restoration of antimycobacterial immune defects in sera from TB-susceptible African Americans with vitamin D insufficiency46,67,68 (also see selected studies highlighted in Table 1). Furthermore, Dr. Niels Finsen's Nobel Prize winning cure of epidermal TB through sunlight lends historic support.152 Metadata analysis of studies involving a large cohort of participants (>11,000) also promisingly found a protective effect of higher vitamin D levels in acute respiratory infections153 (Table 1). Even in bacterial sepsis, AIDS, and parasitic infections, meta data analysis of controlled human trials have shown improved clinical outcomes following supplementation (Table 1).29,31,154 These findings have reinvigorated vitamin D supplementation trials in influenza vaccines after unclear results in the past due to confounding factors such as lack of pre- and posttreatment vitamin D measurements, high baseline vitamin D, and use of low vitamin D doses.44,155 Random controlled trials showing effective augmentation of bioavailable vitamin D and modulation of CD4 T cells, inflammation, and Treg cells support the proposal that protective effects of vitamin D might be mediated through immune-regulation.156,157 Likewise, induction of Treg cells and concomitant suppression of inflammation in organ transplant recipients through vitamin D supplementation further bolsters the inflammation regulatory role of vitamin D.83 Moreover, recent amelioration of MS, diabetes and cardiac disease (with inflammation as a key underlying factor) through vitamin D supplementation bolsters the proposal for vitamin D supplementation in mitigating respiratory disease in SARS-CoV2 infections as well28,30,58,112 (Table 1). It is predicted that augmenting the overall vitamin D levels in the population to “sufficient” levels will mitigate disease severity in the general population, and will be especially beneficial in curbing the disproportionate COVID-19 morbidity and mortality in vulnerable subsets through direct regulation of immune cell activation, proliferation, and inflammatory responses. Several vitamin D-regulated immune target genes (such as MHC-I CCR10, FBP, CAMP, RANKL, IL-6, IL-1β, NFKBIA, CCL2, TNF-α, and cell cycle progression genes ATM p53, CDK6, CDKN2A) have been identified in primary white blood cells as well as in the monocytic cell line THP-1.158–164 Previous studies supporting beneficial effects of vitamin D supplementation in a variety of infectious diseases and other chronic diseases with underlying inflammatory issues are summarized in Table 1. Rigorous correlations of host vitamin D status with molecular and cellular mediators of inflammation and respiratory disease severity in COVID-19 in large-sized diverse patient cohorts, as well as in preclinical murine models of SARS-CoV2 infection will help advance vitamin D supplementation efforts on a solid footing. Currently there are over 16 clinical trials listed on clinicaltrials.gov aimed at correlating patient vitamin D status with disease severity and evaluating the dose-dependent beneficial effects of vitamin D supplementation.
TABLE 1.
Clinical studies showing beneficial effects of vitamin D supplementation
| Disease | References |
|---|---|
| Tuberculosis |
Martineau et al.153
Nursyam et al.175 Morcos et al.176 Wejse et al.177 |
| Influenza | Aloia and Li-Ng,43 |
| AIDS |
Arpadi et al.178
Reviewed in Alvarez et al., 2019 and Teixeira et al.29,31 |
| Schistosomiasis | Snyman et al.179 |
| Sepsis |
Amrein et al.180
Leaf et al.181 Quraishi et al.182 Miroliaee et al.183,184 Ginde et al.151 |
| Coronary disease |
Sokol et al.185
Farrokhian et al.186 Bahrami et al.187,188 Manson et al.28 |
| Diabetes | Pittas et al.30 |
| Multiple sclerosis |
Kouchaki et al.34
Berezowska et al.35 |
| Dengue |
Ahmed et al.32
Martinez-Moreno et al.33 |
Selected clinical studies of vitamin D supplementation and meta-analysis of multiple disease-relevant clinical trials showing infection or disease protective effects are presented.
VALUE OF PRECLINICAL STUDIES
Given that inflammatory responses are (i) rapidly induced after infection, (ii) undergo dynamic changes with disease progression, and (iii) likely show tissue-specific dichotomy in local lung tissues of viral replication compared with the secondary lymphoid sites of immune activation, murine SARS-CoV2 infection model is indispensable for better understanding of when and how vitamin D regulates COVID-19. The hACE2-Tg mouse model of SARS-CoV2, expressing the human ACE2 receptor under the control of the human cytokeratin 18 promoter in airway and gut epithelial cells, closely simulates the human viral replication patterns and COVID-19 disease pathology—with interstitial pneumonia, lymphocyte, and monocyte infiltration in alveolar interstitium and macrophage infiltration in alveolar cavities—thus, offering high translational value for SARS-CoV2 pathogenesis and immunologic studies.165–167 Importantly, the full range of vitamin D disparity and supplementation modalities observed in the clinic are easily recapitulated in mouse models through controlled diets with well-defined levels of bioactive vitamin D, thus resulting in serum calcidiol levels that aptly mimic clinically defined states of vitamin D deficiency, insufficiency, sufficiency, and supplementation. Likewise, ready use in the clinic of low calcemic vitamin D analogs such as paricalcitol offers rapid translational potential for the preclinical supplementation studies, where equivalency of dose based on body weight and route of administration are easily achieved due to similarities in vitamin D source, absorption, metabolism, and signaling.170,172
Powerful genetic models of vitamin D deficiency (VDR−/−), supplementation (CYP27B1−/−), and conditional ablation (VDRfl/fl) exist to query systemic and cell-type specific vitamin D function. Mice harboring VDR deficiency168 are unable to transmit vitamin D signals and exhibit clinical manifestations of severe vitamin D deficiency (i.e., rickets, osteomalacia, hypocalcemia, and hyperparathyroidism). The CYP27B1−/− mice are unable to convert inactive calcidiol into its bioactive calcitriol form, and develop rickets, osteomalacia, and so on. This is a powerful reagent to tightly control the dose and duration of vitamin D signals by rapidly elevating serum levels via administration of bioactive calcitriol (half-life < 12 h), but not calcidiol.169,170 Additionally, conditional VDR knockout mice (VDRfl/fl) have been developed for controlling vitamin D signals in specildiscfic cell types using the classic immunology tool of bone marrow chimerism.171 Thus, the field is uniquely poised to conduct rigorous and systematic inquisitions into when and how vitamin D signals regulate COVID-19, as a springboard for further clinical studies and future supplementation trials, to commandeer immune responses based on clinical needs.
SUMMARY
Several respiratory diseases are strongly linked to vitamin D inadequacy, and potential beneficial effects of vitamin D supplementation are indicated in multiple disease conditions with underlying immune alterations. Similar albeit limited preliminary indications of association between vitamin D insufficiency and COVID-19 severity exist. Given the magnitude of the pandemic, larger-scale clinical studies will provide more robust correlations between vitamin D status, inflammation, respiratory disease severity, and mortality from COVID-19. In addition, immune system-independent effects of vitamin D on SARS-COV2 replication and disease progression also need to be considered through possible modulation of ACE2 and the renin–angiotesin system. Similarly, indirect regulation of immune responses to SARS-CoV2 by vitamin D-dependent regulation of mucosal microbiota is also plausible, as shown in the case of cystic fibrosis.173,174 Promisingly, the ready availability of highly translational murine models of vitamin D deficiency, insufficiency, and supplementation are expected to catalyze the critical cause–effect type studies in the tractable murine models of SARS-CoV2 infection. Methodical stepwise evaluation of how vitamin D regulates lung immunopathology during early and late stages of SARS-Cov2 infection will illuminate the immunologic underpinnings of ARDS in COVID-19, and provide mechanistic insights into vitamin D-dependent regulation of inflammation and ARDS. This will also offer evidence-based support for vitamin D supplementation as a safe, inexpensive, and readily available option to improve COVID-19 outcomes in vulnerable people (e.g., elderly and African Americans who generally have lower amounts of vitamin D), and help identify potential new therapeutic targets to temper inflammation and ARDS in SARS-CoV2 and other respiratory infections of future pandemic potential (such as novel coronaviruses and influenza viruses).
AUTHORSHIP
V.K. and S.S. contributed equally to this work. All authors contributed to the manuscript preparation.
ACKNOWLEDGMENTS
This work was supported by ACS (S.S.) and NIH (S.S. and V.K.). The authors would like to thank Ms. Meenakshi Dunga for help with Figure preparation and Ms. Heather Maylor-Hagen for assistance with Bibliography.
Abbreviations
- 1,25(OH)2D
1,25-dihydroxy vitamin D
- 25OHD
25-hydroxy vitamin D
- ARDS
acute respiratory distress syndrome
- CD
cluster of differentiation
- COVID-19
coronavirus-induced disease 2019
- CYP
cytochrome P450 mixed-function oxidase
- DC
dendritic cells
- LCMV
lymphocytic choriomeningitis virus
- RSV
respiratory syncytial virus
- IBD
inflammatory bowel disease
- MS
multiple sclerosis
- SARS-CoV2
severe acute respiratory syndrome coronavirus 2
- TB
tuberculosis
- Treg
regulatory T cells
- VDR
vitamin D receptor
Contributor Information
Vandana Kalia, Department of Pediatrics, Division of Hematology and Oncology, University of Washington School of Medicine, Seattle, Washington, USA; Ben Towne Center for Childhood Cancer Research, Seattle Children's Research Institute, Seattle, Washington, USA.
George P Studzinski, Department of Pathology, New Jersey Medical School, Rutgers University, Newark, New Jersey, USA.
Surojit Sarkar, Department of Pediatrics, Division of Hematology and Oncology, University of Washington School of Medicine, Seattle, Washington, USA; Ben Towne Center for Childhood Cancer Research, Seattle Children's Research Institute, Seattle, Washington, USA; Department of Pathology, University of Washington School of Medicine, Seattle, Washington, USA.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1. Bar-On YM, Flamholz A, Phillips R, Milo R. SARS-CoV-2 (COVID-19) by the numbers. Elife. 2020;9:e57309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382:2534–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Du RH, Liang LR, Yang CQ et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55:2000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamidian Jahromi A, Hamidianjahromi A. Why African Americans are a potential target for COVID-19 infection in the United States (USA). J Med Internet Res. 2020;22:e19934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sarkar S, Hewison M, Studzinski GP, Li YC, Kalia V. Role of vitamin D in cytotoxic T lymphocyte immunity to pathogens and cancer. Crit Rev Clin Lab Sci. 2015:1–14. [DOI] [PubMed] [Google Scholar]
- 6. Studzinski GP, Harrison JS, Wang X, Sarkar S, Kalia V, Danilenko M. Vitamin D control of hematopoietic cell differentiation and leukemia. J Cell Biochem. 2015;116:1500–1512. [DOI] [PubMed] [Google Scholar]
- 7. Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Christakos S, Hewison M, Gardner DG et al. Vitamin D: beyond bone. Ann N Y Acad Sci. 2013;1287:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giustina A, Adler RA, Binkley N et al. Consensus statement from 2(nd) International Conference on Controversies in Vitamin D. Rev Endocr Metab Disord. 2020;21:89–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han J, Guo X, Yu X et al. 25-Hydroxyvitamin D and total cancer incidence and mortality: a meta-analysis of prospective cohort studies. Nutrients. 2019;11:2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vieth R. The mechanisms of vitamin D toxicity. Bone Miner. 1990;11:267–272. [DOI] [PubMed] [Google Scholar]
- 12. Jacobus CH, Holick MF, Shao Q et al. Hypervitaminosis D associated with drinking milk. N Engl J Med. 1992;326:1173–1177. [DOI] [PubMed] [Google Scholar]
- 13. Grant WB, Lahore H, McDonnell SL et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;32:1195–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marik PE, Kory P, Varon J. Does vitamin D status impact mortality from SARS-CoV-2 infection. Med Drug Discov. 2020;6:100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rhodes JM, Subramanian S, Laird E, Kenny RA. Editorial: low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment Pharmacol Ther. 2020;51:1434–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–496. [DOI] [PubMed] [Google Scholar]
- 18. Namgung R, Mimouni F, Campaigne BN, Ho ML, Tsang RC. Low bone mineral content in summer-born compared with winter-born infants. J Pediatr Gastroenterol Nutr. 1992;15:285–288. [DOI] [PubMed] [Google Scholar]
- 19. Namgung R, Tsang RC, Specker BL, Sierra RI, Ho ML. Low bone mineral content and high serum osteocalcin and 1,25-dihydroxyvitamin D in summer- versus winter-born newborn infants: an early fetal effect?. J Pediatr Gastroenterol Nutr. 1994;19:220–227. [DOI] [PubMed] [Google Scholar]
- 20. Holick MF, Binkley NC, Bischoff-Ferrari HA et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. [DOI] [PubMed] [Google Scholar]
- 21. Khalil I, Barma P. Sub-continental atmosphere and inherent immune system may have impact on novel corona virus’ 2019 (nCovid-19) prevalence in South East Asia. Mymensingh Med J. 2020;29:473–480. [PubMed] [Google Scholar]
- 22. D'Avolio A, Avataneo V, Manca A et al. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12:1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benskin LL. A basic review of the preliminary evidence that COVID-19 risk and severity is increased in vitamin D deficiency. Front Public Health. 2020;8:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Srinivasan A, Syal K, Banerjee D et al. Low plasma levels of cholecalciferol and 13-cis-retinoic acid in tuberculosis: implications in host-based chemotherapy. Nutrition. 2013;29:1245–1251. [DOI] [PubMed] [Google Scholar]
- 25. Bearden A, Abad C, Gangnon R, Sosman JM, Binkley N, Safdar N. Cross-sectional study of vitamin D levels, immunologic and virologic outcomes in HIV-infected adults. J Clin Endocrinol Metab. 2013;98:1726–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Teles RM, Graeber TG, Krutzik SR et al. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science. 2013;339:1448–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goulart LR, Ferreira FR, Goulart IM. Interaction of TaqI polymorphism at exon 9 of the vitamin D receptor gene with the negative lepromin response may favor the occurrence of leprosy. FEMS Immunol Med Microbiol. 2006;48:91–98. [DOI] [PubMed] [Google Scholar]
- 28. Manson JE, Cook NR, Lee IM et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alvarez N, Aguilar-Jimenez W, Rugeles MT. The potential protective role of vitamin D supplementation on HIV-1 infection. Front Immunol. 2019;10:2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pittas AG, Dawson-Hughes B, Sheehan P et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Teixeira N, Pereira BM, Oliveira IKF et al. Effect of vitamin D3 supplementation on HIV-infected adults: a systematic review. Nutr Hosp. 2019;36:1205–1212. [DOI] [PubMed] [Google Scholar]
- 32. Ahmed S, Finkelstein JL, Stewart AM et al. Micronutrients and dengue. Am J Trop Med Hyg. 2014;91:1049–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martinez-Moreno J, Hernandez JC, Urcuqui-Inchima S. Effect of high doses of vitamin D supplementation on dengue virus replication, Toll-like receptor expression, and cytokine profiles on dendritic cells. Mol Cell Biochem. 2020;464:169–180. [DOI] [PubMed] [Google Scholar]
- 34. Kouchaki E, Afarini M, Abolhassani J et al. High-dose omega-3 fatty acid plus vitamin D3 supplementation affects clinical symptoms and metabolic status of patients with multiple sclerosis: a randomized controlled clinical trial. J Nutr. 2018;148:1380–1386. [DOI] [PubMed] [Google Scholar]
- 35. Berezowska M, Coe S, Dawes H. Effectiveness of vitamin D supplementation in the management of multiple sclerosis: a systematic review. Int J Mol Sci. 2019;20:1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bruce D, Yu S, Ooi JH, Cantorna MT. Converging pathways lead to overproduction of IL-17 in the absence of vitamin D signaling. Int Immunol. 2011;23:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ooi JH, McDaniel KL, Weaver V, Cantorna MT. Murine CD8+ T cells but not macrophages express the vitamin D 1alpha-hydroxylase. J Nutr Biochem. 2014;25:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ryz NR, Patterson SJ, Zhang Y et al. Active vitamin D (1,25-dihydroxyvitamin D3) increases host susceptibility to Citrobacter rodentium by suppressing mucosal Th17 responses. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1299–G1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garland CF, Comstock GW, Garland FC, Helsing KJ, Shaw EK, Gorham ED. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;2:1176–1178. [DOI] [PubMed] [Google Scholar]
- 40. Mullin GE, Dobs A. Vitamin d and its role in cancer and immunity: a prescription for sunlight. Nutr Clin Pract. 2007;22:305–322. [DOI] [PubMed] [Google Scholar]
- 41. Grant WB, Garland CF. The role of vitamin D3 in preventing infections. Age Ageing. 2008;37:121–122. [DOI] [PubMed] [Google Scholar]
- 42. Cannell JJ, Vieth R, Umhau JC et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aloia JF, Li-Ng M. Re: epidemic influenza and vitamin D. Epidemiol Infect. 2007;135:1095–1096.author reply 1097–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goldstein MR, Mascitelli L, Pezzetta F. Pandemic influenza A (H1N1): mandatory vitamin D supplementation?. Med Hypotheses. 2010;74:756. [DOI] [PubMed] [Google Scholar]
- 45. Hoan NX, Tong HV, Song LH, Meyer CG, Velavan TP. Vitamin D deficiency and hepatitis viruses-associated liver diseases: a literature review. World J Gastroenterol. 2018;24:445–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu PT, Stenger S, Li H et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. [DOI] [PubMed] [Google Scholar]
- 47. Selvaraj P. Vitamin d, vitamin d receptor, and cathelicidin in the treatment of tuberculosis. Vitam Horm. 2011;86:307–325. [DOI] [PubMed] [Google Scholar]
- 48. Waters WR, Palmer MV, Nonnecke BJ, Whipple DL, Horst RL. Mycobacterium bovis infection of vitamin D-deficient NOS2-/- mice. Microb Pathog. 2004;36:11–17. [DOI] [PubMed] [Google Scholar]
- 49. Bruce D, Whitcomb JP, August A, McDowell MA, Cantorna MT. Elevated non-specific immunity and normal Listeria clearance in young and old vitamin D receptor knockout mice. Int Immunol. 2009;21:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–1183. [DOI] [PubMed] [Google Scholar]
- 51. Bikle DD. Vitamin D and immune function: understanding common pathways. Curr Osteoporos Rep. 2009;7:58–63. [DOI] [PubMed] [Google Scholar]
- 52. Penna G, Amuchastegui S, Giarratana N et al. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol. 2007;178:145–153. [DOI] [PubMed] [Google Scholar]
- 53. Takahashi K, Nakayama Y, Horiuchi H et al. Human neutrophils express messenger RNA of vitamin D receptor and respond to 1alpha,25-dihydroxyvitamin D3. Immunopharmacol Immunotoxicol. 2002;24:335–347. [DOI] [PubMed] [Google Scholar]
- 54. Baeke F, Korf H, Overbergh L et al. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J Steroid Biochem Mol Biol. 2010;121:221–227. [DOI] [PubMed] [Google Scholar]
- 55. Sigmundsdottir H, Pan J, Debes GF et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. [DOI] [PubMed] [Google Scholar]
- 56. Shirakawa AK, Nagakubo D, Hieshima K, Nakayama T, Jin Z, Yoshie O. 1,25-dihydroxyvitamin D3 induces CCR10 expression in terminally differentiating human B cells. J Immunol. 2008;180:2786–2795. [DOI] [PubMed] [Google Scholar]
- 57. Agmon-Levin N, Theodor E, Segal RM, Shoenfeld Y. Vitamin D in systemic and organ-specific autoimmune diseases. Clin Rev Allergy Immunol. 2013;45:256–266. [DOI] [PubMed] [Google Scholar]
- 58. Nashold FE, Nelson CD, Brown LM, Hayes CE. One calcitriol dose transiently increases Helios+ FoxP3+ T cells and ameliorates autoimmune demyelinating disease. J Neuroimmunol. 2013;263:64–74. [DOI] [PubMed] [Google Scholar]
- 59. Prietl B, Treiber G, Mader JK et al. High-dose cholecalciferol supplementation significantly increases peripheral CD4 Tregs in healthy adults without negatively affecting the frequency of other immune cells. Eur J Nutr. 2013;53:751–759. [DOI] [PubMed] [Google Scholar]
- 60. Terrier B, Derian N, Schoindre Y et al. Restoration of regulatory and effector T cell balance and B cell homeostasis in systemic lupus erythematosus patients through vitamin D supplementation. Arthritis Res Ther. 2012;14:R221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hartmann B, Heine G, Babina M et al. Targeting the vitamin D receptor inhibits the B cell-dependent allergic immune response. Allergy. 2011;66:540–548. [DOI] [PubMed] [Google Scholar]
- 62. Kreutz M, Andreesen R, Krause SW, Szabo A, Ritz E, Reichel H. 1,25-dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood. 1993;82:1300–1307. [PubMed] [Google Scholar]
- 63. Hewison M, Freeman L, Hughes SV et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170:5382–5390. [DOI] [PubMed] [Google Scholar]
- 64. Krutzik SR, Hewison M, Liu PT et al. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181:7115–7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vanherwegen AS, Gysemans C, Mathieu C. Regulation of immune function by vitamin d and its use in diseases of immunity. Endocrinol Metab Clin North Am. 2017;46:1061–1094. [DOI] [PubMed] [Google Scholar]
- 66. Chun RF, Adams JS, Hewison M. Immunomodulation by vitamin D: implications for TB. Expert Rev Clin Pharmacol. 2011;4:583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chung C, Silwal P, Kim I, Modlin RL, Jo EK. Vitamin D-cathelicidin axis: at the crossroads between protective immunity and pathological inflammation during infection. Immune Netw. 2020;20:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fabri M, Stenger S, Shin DM et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3:104ra102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010;11:344–349. [DOI] [PubMed] [Google Scholar]
- 70. Cantorna MT. Why do T cells express the vitamin D receptor. Ann N Y Acad Sci. 2011;1217:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yuzefpolskiy Y, Baumann FM, Penny LA, Studzinski GP, Kalia V, Sarkar S. Vitamin D receptor signals regulate effector and memory CD8 T cell responses to infections in mice. J Nutr. 2014;144:2073–2082. [DOI] [PubMed] [Google Scholar]
- 72. Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–1647. [DOI] [PubMed] [Google Scholar]
- 73. Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–4980. [DOI] [PubMed] [Google Scholar]
- 74. Palmer MT, Lee YK, Maynard CL et al. Lineage-specific effects of 1,25-dihydroxyvitamin D(3) on the development of effector CD4 T cells. J Biol Chem. 2011;286:997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tang J, Zhou R, Luger D et al. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J Immunol. 2009;182:4624–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Waddell A, Zhao J, Cantorna MT. NKT cells can help mediate the protective effects of 1,25-dihydroxyvitamin D3 in experimental autoimmune encephalomyelitis in mice. Int Immunol. 2015;27:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yu S, Zhao J, Cantorna MT. Invariant NKT cell defects in vitamin D receptor knockout mice prevents experimental lung inflammation. J Immunol. 2011;187:4907–4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yu S, Cantorna MT. Epigenetic reduction in invariant NKT cells following in utero vitamin D deficiency in mice. J Immunol. 2011;186:1384–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yu S, Cantorna MT. The vitamin D receptor is required for iNKT cell development. Proc Natl Acad Sci USA. 2008;105:5207–5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Korf H, Wenes M, Stijlemans B et al. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology. 2012;217:1292–1300. [DOI] [PubMed] [Google Scholar]
- 81. Penna G, Roncari A, Amuchastegui S et al. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106:3490–3497. [DOI] [PubMed] [Google Scholar]
- 82. Gorman S, Kuritzky LA, Judge MA et al. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. J Immunol. 2007;179:6273–6283. [DOI] [PubMed] [Google Scholar]
- 83. Zhou Q, Qin S, Zhang J, Zhon L, Pen Z, Xing T. 1, 25(OH)2D3 induces regulatory T cell differentiation by influencing the VDR/PLC-gamma1/TGF-beta1/pathway. Mol Immunol. 2017;91:156–164. [DOI] [PubMed] [Google Scholar]
- 84. Kalia V, Ahmed R, Sarkar S. CD8 T cell memory to pathogens. Encyclopedia of Immunobiology. 1st ed.. Michale Ratcliffe Elsevier; 2016:300–317.Editors: Dr. [Google Scholar]
- 85. Kalia V, Penny LA, Yuzefpolskiy Y, Baumann FM, Sarkar S. Quiescence of memory CD8(+) T cells is mediated by regulatory t cells through inhibitory receptor CTLA-4. Immunity. 2015;42:1116–1129. [DOI] [PubMed] [Google Scholar]
- 86. Laidlaw BJ, Cui W, Amezquita RA et al. Production of IL-10 by CD4(+) regulatory T cells during the resolution of infection promotes the maturation of memory CD8(+) T cells. Nat Immunol. 2015;16:871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jeffery LE, Burke F, Mura M et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rouse BT, Sarangi PP, Suvas S. Regulatory T cells in virus infections. Immunol Rev. 2006;212:272–286. [DOI] [PubMed] [Google Scholar]
- 89. Mempel TR, Pittet MJ, Khazaie K et al. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–141. [DOI] [PubMed] [Google Scholar]
- 90. Dittmer U, He H, Messer RJ et al. Functional impairment of CD8(+) T cells by regulatory T cells during persistent retroviral infection. Immunity. 2004;20:293–303. [DOI] [PubMed] [Google Scholar]
- 91. Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ghoreishi M, Bach P, Obst J, Komba M, Fleet JC, Dutz JP. Expansion of antigen-specific regulatory T cells with the topical vitamin d analog calcipotriol. J Immunol. 2009;182:6071–6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Szeles L, Keresztes G, Torocsik D et al. 1,25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J Immunol. 2009;182:2074–2083. [DOI] [PubMed] [Google Scholar]
- 94. Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. [DOI] [PubMed] [Google Scholar]
- 95. Griffin MD, Kumar R. Effects of 1alpha,25(OH)2D3 and its analogs on dendritic cell function. J Cell Biochem. 2003;88:323–326. [DOI] [PubMed] [Google Scholar]
- 96. Pedersen AW, Holmstrom K, Jensen SS et al. Phenotypic and functional markers for 1alpha,25-dihydroxyvitamin D(3)-modified regulatory dendritic cells. Clin Exp Immunol. 2009;157:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Piemonti L, Monti P, Sironi M et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–4451. [DOI] [PubMed] [Google Scholar]
- 98. Gauzzi MC, Purificato C, Donato K et al. Suppressive effect of 1alpha,25-dihydroxyvitamin D3 on type I IFN-mediated monocyte differentiation into dendritic cells: impairment of functional activities and chemotaxis. J Immunol. 2005;174:270–276. [DOI] [PubMed] [Google Scholar]
- 99. Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr. 2003;23:117–145. [DOI] [PubMed] [Google Scholar]
- 100. Xu H, Soruri A, Gieseler RK, Peters JH. 1,25-Dihydroxyvitamin D3 exerts opposing effects to IL-4 on MHC class-II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand J Immunol. 1993;38:535–540. [DOI] [PubMed] [Google Scholar]
- 101. Almerighi C, Sinistro A, Cavazza A, Ciaprini C, Rocchi G, Bergamini A. 1Alpha,25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine. 2009;45:190–197. [DOI] [PubMed] [Google Scholar]
- 102. D'Ambrosio D, Cippitelli M, Cocciolo MG et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Stoffels K, Overbergh L, Bouillon R, Mathieu C. Immune regulation of 1alpha-hydroxylase in murine peritoneal macrophages: unravelling the IFNgamma pathway. J Steroid Biochem Mol Biol. 2007;103:567–571. [DOI] [PubMed] [Google Scholar]
- 104. Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res Clin Pract. 2007;77:47–57. [DOI] [PubMed] [Google Scholar]
- 105. Palmer MT, Weaver CT. Linking vitamin d deficiency to inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2245–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Liu W, Chen Y, Golan MA et al. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J Clin Invest. 2013;123:3983–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Du J, Chen Y, Shi Y et al. 1,25-Dihydroxyvitamin D protects intestinal epithelial barrier by regulating the myosin light chain kinase signaling pathway. Inflamm Bowel Dis. 2015;21:2495–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hewison M. Vitamin D and innate and adaptive immunity. Vitam Horm. 2011;86:23–62. [DOI] [PubMed] [Google Scholar]
- 109. Hewison M. Vitamin D and immune function: an overview. Proc Nutr Soc. 2012;71:50–61. [DOI] [PubMed] [Google Scholar]
- 110. Kong J, Grando SA, Li YC. Regulation of IL-1 family cytokines IL-1alpha, IL-1 receptor antagonist, and IL-18 by 1,25-dihydroxyvitamin D3 in primary keratinocytes. J Immunol. 2006;176:3780–3787. [DOI] [PubMed] [Google Scholar]
- 111. Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7:4240–4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Grishkan IV, Fairchild AN, Calabresi PA, Gocke AR. 1,25-Dihydroxyvitamin D3 selectively and reversibly impairs T helper-cell CNS localization. Proc Natl Acad Sci USA. 2013;110:21101–21106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gungor S, Gokdemir G, Cicek YG, Topal IO, Canat D. The effect of 25(OH)D on endothelial and immunological markers in Behcet's disease. J Dermatolog Treat. 2016;27:254–259. [DOI] [PubMed] [Google Scholar]
- 114. Do JE, Kwon SY, Park S, Lee ES. Effects of vitamin D on expression of Toll-like receptors of monocytes from patients with Behcet's disease. Rheumatology (Oxford). 2008;47:840–848. [DOI] [PubMed] [Google Scholar]
- 115. Infante M, Ricordi C, Padilla N et al. The role of vitamin D and omega-3 PUFAs in islet transplantation. Nutrients. 2019;11:2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ferrandiz-Pulido C, Torres IB, Juarez-Dobjanschi C et al. Vitamin D deficiency in solid-organ transplant recipients from a Spanish Mediterranean population. Clin Exp Dermatol. 2019;44:e103–e109. [DOI] [PubMed] [Google Scholar]
- 117. Obi Y, Hamano T, Ichimaru N et al. Vitamin D deficiency predicts decline in kidney allograft function: a prospective cohort study. J Clin Endocrinol Metab. 2014;99:527–535. [DOI] [PubMed] [Google Scholar]
- 118. Bitetto D, Fabris C, Falleti E et al. Vitamin D and the risk of acute allograft rejection following human liver transplantation. Liver Int. 2010;30:417–444. [DOI] [PubMed] [Google Scholar]
- 119. Li YC, Chen Y, Liu W, Thadhani R. MicroRNA-mediated mechanism of vitamin D regulation of innate immune response. J Steroid Biochem Mol Biol. 2014;144(Pt A):81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Chen Y, Liu W, Sun T et al. 1,25-Dihydroxyvitamin D promotes negative feedback regulation of TLR signaling via targeting microRNA-155-SOCS1 in macrophages. J Immunol. 2013;190:3687–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bezerra I, Oliveira-Silva G, Braga D et al. Dietary vitamin D3 deficiency increases resistance to Leishmania (Leishmania) amazonensis infection in mice. Front Cell Infect Microbiol. 2019;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ehrchen J, Helming L, Varga G et al. Vitamin D receptor signaling contributes to susceptibility to infection with Leishmania major. FASEB J. 2007;21:3208–3218. [DOI] [PubMed] [Google Scholar]
- 123. Newton AH, Cardani A, Braciale TJ. The host immune response in respiratory virus infection: balancing virus clearance and immunopathology. Semin Immunopathol. 2016;38:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Biondo C, Lentini G, Beninati C, Teti G. The dual role of innate immunity during influenza. Biomed J. 2019;42:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Perrone LA, Plowden JK, Garcia-Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008;4:e1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cilloniz C, Shinya K, Peng X et al. Lethal influenza virus infection in macaques is associated with early dysregulation of inflammatory related genes. PLoS Pathog. 2009;5:e1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kobasa D, Jones SM, Shinya K et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. [DOI] [PubMed] [Google Scholar]
- 129. Walsh KB, Teijaro JR, Wilker PR et al. Suppression of cytokine storm with a sphingosine analog provides protection against pathogenic influenza virus. Proc Natl Acad Sci USA. 2011;108:12018–12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. 2020;80:607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Vardhana SA, Wolchok JD. The many faces of the anti-COVID immune response. J Exp Med. 2020;217:e20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jamilloux Y, Henry T, Belot A et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020;19:102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Felsenstein S, Herbert JA, McNamara PS, Hedrich CM. COVID-19: immunology and treatment options. Clin Immunol. 2020;215:108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Bindoli S, Felicetti M, Sfriso P, Doria A. The amount of cytokine-release defines different shades of Sars-Cov2 infection. Exp Biol Med (Maywood). 2020;245:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Daneshkhah Ali, Agrawal Vasundhara, Eshein Adam, Subramanian Hariharan, Roy Hemant Kumar, Backman Vadim. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients. Aging Clinical and Experimental Research. 2020;32 (10):2141–2158. 10.1007/s40520-020-01677-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lau FH, Majumder R, Torabi R, Saeg F, Hoffman R, Cirillo JD, Greiffenstein P. Vitamin D insufficiency is prevalent in severe COVID-19. medRxiv. 2020. doi: 10.1101/2020.04.24.20075838. [DOI] [Google Scholar]
- 138. De Smet D, De Smet K, Herroelen P, Gryspeerdt S, Martens GA, Vitamin D deficiency as risk factor for severe COVID-19: a convergence of two pandemics. medRxiv. 2020. 10.1101/2020.05.01.20079376 [DOI] [Google Scholar]
- 139. Trouillet-Assant S, Viel S, Gaymard A et al. Type I IFN immunoprofiling in COVID-19 patients. J Allergy Clin Immunol. 2020;146:206–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Major J, Crotta S, Llorian M et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science. 2020;369:712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Magrone T, Magrone M, Jirillo E. Focus on receptors for coronaviruses with special reference to angiotensin-converting enzyme 2 as a potential drug target - a perspective. Endocr Metab Immune Disord Drug Targets. 2020;20:807–811. [DOI] [PubMed] [Google Scholar]
- 142. Hadjadj J, Yatim N, Barnabei L et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Broggi A, Ghosh S, Sposito B et al. Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science. 2020;369:706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Blanco-Melo D, Nilsson-Payant BE, Liu WC et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Arunachalam PS, Wimmers F, Mok CKP et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science. 2020;369:1210–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Dickie LJ, Church LD, Coulthard LR, Mathews RJ, Emery P, McDermott MF. Vitamin D3 down-regulates intracellular Toll-like receptor 9 expression and Toll-like receptor 9-induced IL-6 production in human monocytes. Rheumatology (Oxford). 2010;49:1466–1471. [DOI] [PubMed] [Google Scholar]
- 147. Herrick KA, Storandt RJ, Afful J et al. Vitamin D status in the United States, 2011–2014. Am J Clin Nutr. 2019;110:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Cashman KD, Dowling KG, Skrabakova Z et al. Vitamin D deficiency in Europe: pandemic?. Am J Clin Nutr. 2016;103:1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Cashman KD, Kiely M. Tackling inadequate vitamin D intakes within the population: fortification of dairy products with vitamin D may not be enough. Endocrine. 2016;51:38–46. [DOI] [PubMed] [Google Scholar]
- 150. Ginde AA, Liu MC. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169:626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ginde AA, Mansbach JM. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kock W. The first Nobel Prize for medicine to the Nordic countries (Niels Ryberg Finsen 1903). Hist Sci Med. 1982;17:144–147. [PubMed] [Google Scholar]
- 153. Martineau AR, Jolliffe DA, Hooper RL et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Yamshchikov AV, Oladele A, Leonard MK Jr, Blumberg HM, Ziegler TR, Tangpricha V. Vitamin D as adjunctive therapy in refractory pulmonary tuberculosis: a case report. South Med J. 2009;102:649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kriesel JD, Spruance J. Calcitriol (1,25-dihydroxy-vitamin D3) coadministered with influenza vaccine does not enhance humoral immunity in human volunteers. Vaccine. 1999;17:1883–1888. [DOI] [PubMed] [Google Scholar]
- 156. Konijeti GG, Arora P, Boylan MR et al. Vitamin D supplementation modulates T cell-mediated immunity in humans: results from a randomized control trial. J Clin Endocrinol Metab. 2016;101:533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zerofsky MS, Jacoby BN, Pedersen TL, Stephensen CB. Daily cholecalciferol supplementation during pregnancy alters markers of regulatory immunity, inflammation, and clinical outcomes in a randomized controlled trial. J Nutr. 2016;146:2388–2397. [DOI] [PubMed] [Google Scholar]
- 158. Carlberg C, Neme A. Machine learning approaches infer vitamin D signaling: critical impact of vitamin D receptor binding within topologically associated domains. J Steroid Biochem Mol Biol. 2019;185:103–109. [DOI] [PubMed] [Google Scholar]
- 159. Neme A, Seuter S, Malinen M et al. In vivo transcriptome changes of human white blood cells in response to vitamin D. J Steroid Biochem Mol Biol. 2019;188:71–76. [DOI] [PubMed] [Google Scholar]
- 160. Seuter S, Neme A, Carlberg C. Epigenome-wide effects of vitamin D and their impact on the transcriptome of human monocytes involve CTCF. Nucleic Acids Res. 2016;44:4090–4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Neme A, Seuter S, Carlberg C. Selective regulation of biological processes by vitamin D based on the spatio-temporal cistrome of its receptor. Biochim Biophys Acta Gene Regul Mech. 2017;1860:952–961. [DOI] [PubMed] [Google Scholar]
- 162. Verway M, Bouttier M, Wang TT et al. Vitamin D induces interleukin-1beta expression: paracrine macrophage epithelial signaling controls M. tuberculosis infection. PLoS Pathog. 2013;9:e1003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Heikkinen S, Vaisanen S, Pehkonen P, Seuter S, Benes V, Carlberg C. Nuclear hormone 1alpha,25-dihydroxyvitamin D3 elicits a genome-wide shift in the locations of VDR chromatin occupancy. Nucleic Acids Res. 2011;39:9181–9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Nurminen V, Seuter S, Carlberg C. Primary vitamin D target genes of human monocytes. Front Physiol. 2019;10:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Shang J, Wan Y, Luo C et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci USA. 2020;117:11727–11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Bao L, Deng W, Huang B et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583:830–833. [DOI] [PubMed] [Google Scholar]
- 167. McCray PB Jr, Pewe L, Wohlford-Lenane C et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. 2007;81:813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Li YC, Pirro AE, Amling M et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA. 1997;94:9831–9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Vanhooke JL, Prahl JM, Kimmel-Jehan C et al. CYP27B1 null mice with LacZreporter gene display no 25-hydroxyvitamin D3-1alpha-hydroxylase promoter activity in the skin. Proc Natl Acad Sci USA. 2006;103:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Rowling MJ, Gliniak C, Welsh J, Fleet JC. High dietary vitamin D prevents hypocalcemia and osteomalacia in CYP27B1 knockout mice. J Nutr. 2007;137:2608–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ni W, Glenn DJ, Gardner DG. Tie-2Cre mediated deletion of the vitamin D receptor gene leads to improved skeletal muscle insulin sensitivity and glucose tolerance. J Steroid Biochem Mol Biol. 2016;164:281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Marcinkowska E. The vitamin D sytem in humans and mice: similar but not the same. Reports - Medical Cases, Images, and Video. 2020;3(1):1. [Google Scholar]
- 173. Kanhere M, Chassaing B, Gewirtz AT, Tangpricha V. Role of vitamin D on gut microbiota in cystic fibrosis. J Steroid Biochem Mol Biol. 2018;175:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Kanhere M, He J, Chassaing B et al. Bolus weekly vitamin D3 supplementation impacts gut and airway microbiota in adults with cystic fibrosis: a double-blind, randomized, placebo-controlled clinical trial. J Clin Endocrinol Metab. 2018;103:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006;38:3–5. [PubMed] [Google Scholar]
- 176. Morcos MM, Gabr AA, Samuel S et al. Vitamin D administration to tuberculous children and its value. Boll Chim Farm. 1998;137:157–164. [PubMed] [Google Scholar]
- 177. Wejse C, Gomes VF, Rabna P et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179:843–850. [DOI] [PubMed] [Google Scholar]
- 178. Arpadi SM, McMahon D, Abrams EJ et al. Effect of bimonthly supplementation with oral cholecalciferol on serum 25-hydroxyvitamin D concentrations in HIV-infected children and adolescents. Pediatrics. 2009;123:e121–e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Snyman JR, de Sommers K, Steinmann MA, Lizamore DJ. Effects of calcitriol on eosinophil activity and antibody responses in patients with schistosomiasis. Eur J Clin Pharmacol. 1997;52:277–280. [DOI] [PubMed] [Google Scholar]
- 180. Amrein K, Sourij H, Wagner G et al. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: a randomized, double-blind, placebo-controlled pilot study. Crit Care. 2011;15:R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Leaf DE, Raed A, Donnino MW, Ginde AA, Waikar SS. Randomized controlled trial of calcitriol in severe sepsis. Am J Respir Crit Care Med. 2014;190:533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Quraishi SA, De Pascale G, Needleman JS et al. Effect of cholecalciferol supplementation on vitamin d status and cathelicidin levels in sepsis: a randomized, placebo-controlled trial. Crit Care Med. 2015;43:1928–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Miroliaee AE, Salamzadeh J, Shokouhi S et al. Effect of vitamin D supplementation on procalcitonin as prognostic biomarker in patients with ventilator associated pneumonia complicated with vitamin D deficiency. Iran J Pharm Res. 2017;16:1254–1263. [PMC free article] [PubMed] [Google Scholar]
- 184. Miroliaee AE, Salamzadeh J, Shokouhi S, Sahraei Z. The study of vitamin D administration effect on CRP and Interleukin-6 as prognostic biomarkers of ventilator associated pneumonia. J Crit Care. 2018;44:300–305. [DOI] [PubMed] [Google Scholar]
- 185. Sokol SI, Srinivas V, Crandall JP et al. The effects of vitamin D repletion on endothelial function and inflammation in patients with coronary artery disease. Vasc Med. 2012;17:394–404. [DOI] [PubMed] [Google Scholar]
- 186. Farrokhian A, Raygan F, Bahmani F et al. Long-term vitamin D supplementation affects metabolic status in vitamin D-deficient type 2 diabetic patients with coronary artery disease. J Nutr. 2017;147:384–389. [DOI] [PubMed] [Google Scholar]
- 187. Bahrami LS, Sezavar Seyedi Jandaghi SH, Janani L et al. Vitamin D supplementation and serum heat shock protein 60 levels in patients with coronary heart disease: a randomized clinical trial. Nutr Metab (Lond). 2018;15:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Bahrami LS, Ranjbar G, Norouzy A, Arabi SM. Vitamin D supplementation effects on the clinical outcomes of patients with coronary artery disease: a systematic review and meta-analysis. Sci Rep. 2020;10:12923. [DOI] [PMC free article] [PubMed] [Google Scholar]


