Abstract
Background
There are limited data on surgical complications for patients that have delayed surgery after severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. We aimed to analyze the surgical outcomes of patients submitted to surgery after recovery from SARS‐CoV‐2 infection.
Methods
Asymptomatic patients that had surgery delayed after preoperative reverse‐transcription polymerase chain reaction (RT‐PCR) for SARS‐CoV‐2 were matched in a 1:2 ratio for age, type of surgery and American Society of Anesthesiologists to patients with negative RT‐PCR for SARS‐CoV‐2.
Results
About 1253 patients underwent surgical procedures and were subjected to screening for SARS‐CoV‐2. Forty‐nine cases with a delayed surgery were included in the coronavirus disease (COVID) recovery (COVID‐rec) group and were matched to 98 patients included in the COVID negative (COVID‐neg) group. Overall, 22 (15%) patients had 30‐days postoperative complications, but there was no statistically difference between groups –16.3% for COVID‐rec and 14.3% for COVID‐neg, respectively (odds ratio [OR] 1.17:95% confidence interval [CI] 0.45–3.0; p = .74). Moreover, we did not find difference regarding grades more than or equal to 3 complication rates – 8.2% for COVID‐rec and 6.1% for COVID‐neg (OR 1.36:95%CI 0.36‐5.0; p = .64). There were no pulmonary complications or SARS‐CoV‐2 related infection and no deaths within the 30‐days after surgery.
Conclusions
Our study suggests that patients with delayed elective surgeries due to asymptomatic preoperative positive SARS‐CoV‐2 test are not at higher risk of postoperative complications.
Keywords: SARS‐CoV‐2, surgical complications, surgical oncology
1. INTRODUCTION
The new coronavirus (SARS‐CoV‐2) pandemic has been impairing the diagnosis and treatment of chronic diseases with major impact on public health, such as cardiovascular disease and cancer. This could be justified by the concern among oncological patients after several reports pointed to the worst outcomes for SARS‐CoV‐2 disease during cancer treatment. 1 , 2 However, the delay in diagnosis and treatment of cancer has a negative impact on prognosis. Recently, a model for predicting the effect of coronavirus disease 2019 (COVID‐19) on cancer screening and treatment in the United States estimated an increase of almost 10,000 excess deaths to the next decade, including just breast and colorectal cancer. 3
Moreover, recent data from COVIDSurg collaborative 4 reported a 30‐day mortality rate of 23.8% in a series of patients with perioperative SARS‐CoV‐2 infection, with overall pulmonary complication rates of 51.2%. In addition, a matched cohort study that included 41 cases with SARS‐CoV‐2 positive patients reinforced a higher 30‐day mortality and complication rates for the SARS‐CoV‐2 positive patients compared with controls. 5
In this setting, actions for protecting the access to health services have been proposed and are under practice during the pandemic. Despite the weakness of evidence, preoperative screening for SARS‐CoV‐19 has been proposed for elective cancer surgeries in Europe 6 and North America, 7 and also became a recommendation in Brazil since April 2020. 8 Therefore, we have implemented universal screening for SARS‐CoV‐2 with reverse‐transcription polymerase chain reaction (RT‐PCR) nasopharyngeal swabs for all surgical procedures in our institution since late April 2020. Notably, we found a preoperative positivity rate of 7.6% among asymptomatic patients scheduled for elective surgeries. 9 These patients had their surgeries postponed, and the next raised question is about the safer strategy for re‐scheduling.
Although it has been suggested a significant increase in morbidity and mortality rates for perioperative SARS‐CoV‐2 positive patients, it is not clear if these patients still have an increased risk of surgical complications in a delayed surgery after complete recovery from SARS‐CoV‐2 infection. Our aim was to evaluate the surgical morbidity and mortality among patients with delayed surgery due to asymptomatic positive SARS‐CoV‐2 at a tertiary comprehensive cancer center.
2. METHODS
2.1. Patients
Since April 22, 2020, all patients scheduled for surgical procedures at AC Camargo Cancer Center were subjected to preoperative RT‐PCR test for SARS‐CoV‐2. The preoperative screening protocol included: (1) All patients with scheduled elective surgery were contacted for performing SARS‐CoV‐2 test, 2–3 days before surgical admission; (2) patients underwent epidemiological survey about flu symptoms or contact with infected relatives 5 days before surgery; (3) patients were tested with RT‐PCR for SARS‐CoV‐2 from nasopharyngeal swabs; (4) before and after surgery, all patients were oriented to remain in social isolation; and (5) patients with positive results had the admission canceled, a new SARS‐CoV‐2 test was collected after 14 days, and the surgery was re‐scheduled only after a negative test. There were no additional costs for the patients with respect to screening and the study had the Institutional Review Board approval (#4.072.209).
From April 22 to July 2, 2020, a total of 1253 patients underwent surgical procedures at AC Camargo Cancer Center and were subjected to screening for SARS‐CoV‐2 by nasopharyngeal swabs. Eighty‐five (6.8%) tests were positive for SARS‐CoV‐2% and 17.6% (15/85) positive cases had emergency procedures. All elective surgeries with positive SARS‐CoV‐2 had admission canceled and surgery postponed (n = 70). Until the end of July 2, 49 cases have already been operated after a subsequent negative test and were included in the COVID recovery (COVID‐rec) group. Figure 1 depicts the patient's flow chart.
Figure 1.
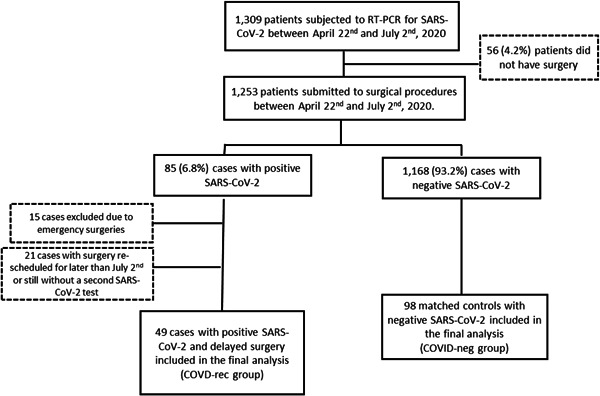
Flow‐chart of the 147 patients included in the study. COVID, coronavirus disease; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
Patients with delayed surgery due to SARS‐CoV‐2 positive (COVID‐rec) were matched in a 1:2 ratio to those with primary SARS‐CoV‐2 negative (COVID‐neg) that had the surgery performed according to the first schedule. Patients underwent the surgical procedures by the same surgical teams and during the same period of time. Moreover, patients with SARS‐CoV‐2 positive test were oriented for social distance and their symptoms development were followed. Oncological surgeries were considered as resections performed with curative intent and nononcological surgeries were those performed in patients without curative intent or related to the oncological management (e.g., ureteral catheter in a pelvic tumor recurrence or hysteroscopy in a patient previously treated for breast cancer).
For both groups we analyzed demographic and clinical variables such as: age, gender, body mass index, American Society of Anesthesiologists (ASA) Physical Status Classification System, 10 surgical procedure length, intensive care unit (ICU) admission, hospital stay length, type of surgery (oncological surgeries or non‐oncological surgeries), and Eastern Cooperative Oncology Group (ECOG) Performance Status. 11 Complications were recorded according to Clavien‐Dindo classification. 12
2.2. Statistical analysis
The patients in the COVID‐rec group (n = 49) were matched in a 1:2 ratio for age, type of surgery (oncological surgeries or non‐oncological surgeries) and ASA (1 and 2 vs. 3 and 4) to those in the COVID‐neg group (n = 98). We calculated the propensity score using a logistic regression model including type of surgery, ASA and age to balance these variables between the studied groups. A database was constructed using SPSS, version 20.0 for Mac (SPSS; Inc.). Descriptive statistics were described for both groups. The χ 2, Fisher's exact test were used to analyze the correlations between categorical variables and Mann–Whitney for continuous variables. Odds ratios (ORs) were assessed with logistic regression. For all tests, p < .05 was considered to be significant.
3. RESULTS
Forty‐nine cases had elective surgery delayed due to asymptomatic positive RT‐PCR for SARS‐CoV‐2 (COVID‐rec) and 98 controls with preoperative negative RT‐PCR for SARS‐CoV‐2 (COVID‐neg) were included in the study. The median time between the positive SARS‐CoV‐2 and definitive surgery was 25 days (range, 12–84). Interestingly, 3 (6.1%) cases had the second positive test, and only had a negative test after 20, 35, and 82 days.
Data on symptoms after positive RT‐PCR for SARS‐CoV‐2 were retrieved from 48 (98%) cases, and notably only 9 (22.9%) cases had symptoms related to SARS‐CoV‐2 infection. All cases that developed symptoms had a mild presentation such as coryza, myalgia and anosmia, and any patient required hospital admission. Of the three cases with a second positive test, two developed symptoms but any of them had surgical complications.
There were no statistically differences between groups regarding age, body mass index, gender, performance status, surgical time length, and hospital stay length. For the COVID‐rec group, 25 (51%) cases had oncological surgeries and 24 (49%) nononcological surgeries. In addition, 2 (4.1%) cases of COVID‐rec groups had emergency surgeries due to complications during the delaying period. For COVID‐neg group, 6 (6.1%) cases with emergency surgeries were included (p = .71). Table 1 describes the surgical procedures.
Table 1.
Description of the 147 cases included in the study
| Case | SARS‐CoV‐2 status | Age | ASAa | ECOGb | Oncological surgery | Surgical procedure | Clavien–Dindoc |
|---|---|---|---|---|---|---|---|
| 1 | Positive | 48 | 2 | 1 | No | Biliary drainage | IIIb |
| 2 | Positive | 68 | 3 | 0 | Yes | Pulmonary lobectomy MISd | IIIa |
| 3 | Positive | 76 | 2 | 0 | Yes | Skin resection | IIIa |
| 4 | Positive | 57 | 3 | 1 | No | Splenic embolization | IIIa |
| 5 | Positive | 57 | 2 | 0 | Yes | Cytoreductive surgery | II |
| 6 | Positive | 48 | 2 | 0 | No | Implantable venous catheter | II |
| 7 | Positive | 64 | 2 | 0 | No | Renal arteriography | I |
| 8 | Positive | 60 | 2 | 0 | Yes | Rectosigmoidectomy MIS | I |
| 9 | Positive | 62 | 2 | 1 | No | Implantable venous catheter | None |
| 10 | Positive | 19 | 2 | 0 | No | Hemangioma embolization | None |
| 11 | Positive | 49 | 2 | 0 | Yes | Total thyroidectomy | None |
| 12 | Positive | 60 | 2 | 0 | No | Implantable venous catheter | None |
| 13 | Positive | 51 | 2 | 0 | Yes | Brain tumor resection | None |
| 14 | Positive | 46 | 2 | 0 | No | Oophorectomy | None |
| 15 | Positive | 72 | 3 | 1 | No | Ureteral stent implant | None |
| 16 | Positive | 13 | 1 | 0 | Yes | Skin resection | None |
| 17 | Positive | 55 | 2 | 0 | Yes | Total hysterectomy | None |
| 18 | Positive | 26 | 1 | 0 | No | Hysteroscopy | None |
| 19 | Positive | 55 | 2 | 1 | No | Celiac plexus block | None |
| 20 | Positive | 34 | 2 | 0 | Yes | Axillary lymphadenectomy | None |
| 21 | Positive | 62 | 2 | 0 | Yes | Partial breast resection | None |
| 22 | Positive | 69 | 3 | 0 | No | Implantable venous catheter | None |
| 23 | Positive | 31 | 1 | 0 | Yes | Transurethral bladder resection | None |
| 24 | Positive | 40 | 1 | 0 | No | Total hysterectomy | None |
| 25 | Positive | 38 | 2 | 0 | Yes | Simple mastectomy | None |
| 26 | Positive | 56 | 2 | 0 | No | Skin resection | None |
| 27 | Positive | 58 | 2 | 0 | Yes | Total gastrectomy | None |
| 28 | Positive | 52 | 2 | 0 | No | Lymph node biopsy | None |
| 29 | Positive | 38 | 1 | 0 | No | Cervical conization | None |
| 30 | Positive | 52 | 2 | 0 | No | Implantable venous catheter | None |
| 31 | Positive | 38 | 2 | 0 | Yes | Simple mastectomy | None |
| 32 | Positive | 68 | 2 | 0 | No | Total thyroidectomy | None |
| 33 | Positive | 59 | 2 | 0 | Yes | Radical prostatectomy MISd | None |
| 34 | Positive | 48 | 2 | 0 | Yes | Skin resection | None |
| 35 | Positive | 48 | 2 | 0 | Yes | Skin resection | None |
| 36 | Positive | 62 | 2 | 0 | No | Total hysterectomy | None |
| 37 | Positive | 28 | 1 | 0 | Yes | Radical orchiectomy | None |
| 38 | Positive | 51 | 2 | 0 | Yes | Partial penectomy | None |
| 39 | Positive | 46 | 1 | 0 | Yes | Total thyroidectomy | None |
| 40 | Positive | 53 | 1 | 0 | Yes | Radical prostatectomy MISd | None |
| 41 | Positive | 55 | 2 | 0 | No | Salpingectomy MISd | None |
| 42 | Positive | 45 | 1 | 0 | Yes | Skin resection | None |
| 43 | Positive | 35 | 1 | 0 | No | Cervical conization | None |
| 44 | Positive | 39 | 2 | 0 | Yes | Partial parotidectomy | None |
| 45 | Positive | 49 | 2 | 0 | Yes | Axillary lymphadenectomy | None |
| 46 | Positive | 17 | 2 | 0 | No | Skin resection | None |
| 47 | Positive | 81 | 2 | 1 | No | Implantable venous catheter | None |
| 48 | Positive | 45 | 3 | 1 | No | Ureteral stent implant | None |
| 49 | Positive | 55 | 2 | 0 | Yes | Simple mastectomy | None |
| 50 | Negative | 55 | 3 | 0 | No | Implantable venous catheter | IVb |
| 51 | Negative | 55 | 3 | 0 | No | Ileostomy closure | IVa |
| 52 | Negative | 48 | 2 | 0 | Yes | Total gastrectomy MISd | IIIb |
| 53 | Negative | 48 | 3 | 1 | No | Biliary drainage | IIIa |
| 54 | Negative | 52 | 2 | 0 | No | Total hysterectomy MISd | IIIa |
| 55 | Negative | 49 | 2 | 0 | Yes | Rectal amputation | IIIa |
| 56 | Negative | 69 | 2 | 1 | No | Ureteral stent implant | II |
| 57 | Negative | 51 | 3 | 1 | Yes | Simple mastectomy | II |
| 58 | Negative | 61 | 2 | 0 | No | Small bowel resection | II |
| 59 | Negative | 38 | 2 | 0 | Yes | Radical mastectomy | II |
| 60 | Negative | 46 | 2 | 0 | Yes | Simple mastectomy | II |
| 61 | Negative | 60 | 2 | 0 | Yes | Axillary lymphadenectomy | II |
| 62 | Negative | 59 | 2 | 1 | Yes | Pulmonary lobectomy MISd | II |
| 63 | Negative | 77 | 3 | 0 | Yes | Skin resection | I |
| 64 | Negative | 68 | 4 | 1 | No | Implantable venous catheter | None |
| 65 | Negative | 62 | 2 | 0 | Yes | Skin resection | None |
| 66 | Negative | 68 | 2 | 1 | No | Endoscopic gastrostomy | None |
| 67 | Negative | 68 | 3 | 0 | Yes | Maxillectomy | None |
| 68 | Negative | 53 | 2 | 0 | Yes | Skin resection | None |
| 69 | Negative | 66 | 2 | 0 | No | Tracheoplasty | None |
| 70 | Negative | 45 | 2 | 0 | Yes | Skin resection | None |
| 71 | Negative | 49 | 2 | 0 | Yes | Simple mastectomy | None |
| 72 | Negative | 48 | 2 | 0 | Yes | Simple mastectomy | None |
| 73 | Negative | 72 | 2 | 2 | No | Ureteral stent implant | None |
| 74 | Negative | 61 | 2 | 0 | No | Ureteral stent implant | None |
| 75 | Negative | 45 | 2 | 0 | No | Hysteroscopy | None |
| 76 | Negative | 39 | 2 | 0 | No | Craniotomy | None |
| 77 | Negative | 57 | 2 | 0 | Yes | Skin resection | None |
| 78 | Negative | 46 | 2 | 0 | No | Breast plastic | None |
| 79 | Negative | 55 | 2 | 0 | Yes | Skin resection | None |
| 80 | Negative | 55 | 3 | 1 | No | Bowel bleeding angiography | None |
| 81 | Negative | 16 | 2 | 0 | No | Ileostomy closure | None |
| 82 | Negative | 48 | 2 | 0 | No | Eye brachytherapy implant | None |
| 83 | Negative | 57 | 2 | 0 | No | Cystoscopy | None |
| 84 | Negative | 39 | 2 | 0 | Yes | Axillary lymphadenectomy | None |
| 85 | Negative | 62 | 3 | 1 | No | Esophageal prosthesis | None |
| 86 | Negative | 46 | 2 | 0 | No | Hysteroscopy | None |
| 87 | Negative | 55 | 2 | 0 | No | Laryngeal biopsy | None |
| 88 | Negative | 45 | 2 | 0 | No | Hysteroscopy | None |
| 89 | Negative | 52 | 2 | 0 | No | Total hysterectomy MISd | None |
| 90 | Negative | 64 | 3 | 1 | No | Prostate endoscopic resection | None |
| 91 | Negative | 39 | 2 | 0 | Yes | Radical mastectomy | None |
| 92 | Negative | 57 | 3 | 1 | No | Choledocoplasty | None |
| 93 | Negative | 55 | 2 | 0 | Yes | Partial breast resection | None |
| 94 | Negative | 51 | 2 | 0 | Yes | Pulmonary resection MIS | None |
| 95 | Negative | 52 | 2 | 0 | No | Biliary drainage | None |
| 96 | Negative | 66 | 3 | 1 | No | Biliary drainage | None |
| 97 | Negative | 51 | 2 | 0 | Yes | Eye enucleation | None |
| 98 | Negative | 49 | 2 | 0 | Yes | Radical mastectomy | None |
| 99 | Negative | 40 | 2 | 0 | No | Total hysterectomy MISd | None |
| 100 | Negative | 38 | 2 | 0 | Yes | Skin resection | None |
| 101 | Negative | 45 | 2 | 0 | Yes | Simple mastectomy | None |
| 102 | Negative | 24 | 1 | 0 | Yes | Partial parotidectomy | None |
| 103 | Negative | 38 | 2 | 0 | Yes | Axillary lymphadenectomy | None |
| 104 | Negative | 58 | 2 | 0 | Yes | Liver resection | None |
| 105 | Negative | 39 | 1 | 0 | No | Anal fistulectomy | None |
| 106 | Negative | 25 | 2 | 0 | No | Oophoroplasty MISd | None |
| 107 | Negative | 81 | 4 | 0 | No | Eye brachytherapy implant | None |
| 108 | Negative | 45 | 1 | 0 | Yes | Partial breast resection | None |
| 109 | Negative | 64 | 2 | 0 | Yes | Paraortic lymphadenectomy | None |
| 110 | Negative | 59 | 3 | 0 | Yes | Partial breast resection | None |
| 111 | Negative | 52 | 2 | 0 | No | Partial thyroidectomy | None |
| 112 | Negative | 38 | 1 | 0 | Yes | Total thyroidectomy | None |
| 113 | Negative | 51 | 1 | 0 | Yes | Partial breast resection | None |
| 114 | Negative | 19 | 2 | 0 | No | Implantable venous catheter | None |
| 115 | Negative | 58 | 2 | 0 | Yes | Skin resection | None |
| 116 | Negative | 46 | 2 | 0 | Yes | Simple mastectomy | None |
| 117 | Negative | 58 | 2 | 0 | Yes | Axillary lymphadenectomy | None |
| 118 | Negative | 34 | 1 | 0 | Yes | Total thyroidectomy | None |
| 119 | Negative | 48 | 2 | 0 | Yes | Partial breast resection | None |
| 120 | Negative | 40 | 2 | 0 | No | Paravertebral tumor biopsy | None |
| 121 | Negative | 57 | 2 | 0 | Yes | Simple mastectomy | None |
| 122 | Negative | 53 | 1 | 0 | Yes | Radical prostatectomy MISd | None |
| 123 | Negative | 25 | 2 | 0 | No | Cervical conization | None |
| 124 | Negative | 20 | 1 | 0 | No | Anal fistulectomy | None |
| 125 | Negative | 62 | 2 | 0 | Yes | Skin resection | None |
| 126 | Negative | 31 | 1 | 0 | Yes | Total thyroidectomy | None |
| 127 | Negative | 55 | 2 | 0 | Yes | Total thyroidectomy | None |
| 128 | Negative | 26 | 2 | 0 | No | Cervical conization | None |
| 129 | Negative | 49 | 2 | 0 | Yes | Hysteroscopy | None |
| 130 | Negative | 35 | 2 | 0 | No | Hepatic angiography | None |
| 131 | Negative | 31 | 2 | 0 | Yes | Partial nephrectomy MIS | None |
| 132 | Negative | 68 | 2 | 0 | Yes | Radical prostatectomy MIS | None |
| 133 | Negative | 51 | 2 | 0 | Yes | Skin resection | None |
| 134 | Negative | 27 | 2 | 0 | Yes | Oropharyngeal biopsy | None |
| 135 | Negative | 72 | 2 | 2 | No | Eye brachytherapy implant | None |
| 136 | Negative | 16 | 2 | 0 | No | Biliary drainage | None |
| 137 | Negative | 24 | 1 | 0 | No | Skin resection | None |
| 138 | Negative | 55 | 2 | 0 | Yes | Simple mastectomy | None |
| 139 | Negative | 56 | 2 | 0 | No | Hysteroscopy | None |
| 140 | Negative | 34 | 1 | 0 | Yes | Total thyroidectomy | None |
| 141 | Negative | 48 | 2 | 0 | Yes | Radical mastectomy | None |
| 142 | Negative | 70 | 2 | 0 | Yes | Transurethral bladder resection | None |
| 143 | Negative | 75 | 2 | 0 | Yes | Radical nephrectomy MISd | None |
| 144 | Negative | 60 | 3 | 0 | Yes | Partial breast resection | None |
| 145 | Negative | 60 | 2 | 1 | No | Hysteroscopy | None |
| 146 | Negative | 60 | 3 | 1 | No | Transurethral bladder resection | None |
| 147 | Negative | 38 | 1 | 0 | No | Cervical conization | None |
ASA: American Society of Anesthesiologists risk classification. 10
ECOG: Eastern Cooperative Oncology Group Performance Status.
Clavien–Dindo: Clavien–Dindo classification of surgical complications. 11
MIS: Minimally Invasive Surgery.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Overall, 22 (15%) patients had 30‐days postoperative complications, but there was no statistically difference between groups – 16.3% for COVID‐rec and 14.3% for COVID‐neg, respectively (OR 1.17: 95% confidence interval [CI] 0.45–3.0; p = .74). Moreover, we did not find difference regarding Grades ≥ 3 complication rates – 8.2% for COVID‐rec and 6.1% for COVID‐neg (OR 1.36: 95%CI 0.36–5.0; p = .64). Yet, we had no pulmonary complications or SARS‐CoV‐2 related infection during the hospital stay length or during the 30‐days after surgery for both groups. Table 2 summarizes the clinical and demographic data between groups and Table 3 describes the surgical complications of Grades ≥ 3.
Table 2.
Clinical and demographic characteristics of the 147 patients submitted to surgical procedures from April 22 to July 2, 2020
| Variable | COVID‐nega group | COVID‐recb group | Total | ||
|---|---|---|---|---|---|
| n = 98 (%) | n = 49 (%) | p value | 147 (%) | ||
| Age, mean; median (range) year | 49.8; 51 (16–81) | 50.1; 52 (13–81) | .86 | 49.9; 51 (13–81) | |
| Body mass index, mean; median (range) kg/m2 | 26.8; 25.9 (16.9–53.9) | 27.6; 27.5 (18.8–43) | .33 | 27.1; 26.6 (16.9–53.9) | |
| Surgical time length, mean; median (range) (min) | 119.0; 100 (10–670) | 110.2; 79 (10–362) | .54 | 116.1; 93 (10–670) | |
| Hospital stay length, mean; median (range) (days) | 3.48; 1.0 (0–62) | 3.08; 1.0 (0–47) | .28 | 3.35; 1.0 (0–62) | |
| Gender | Male | 40 (40.8) | 16 (33.3) | .38 | 56 (38.4) |
| Female | 58 (59.2) | 32 (66.7) | 90 (61.6) | ||
| ASAc | 1 and 2 | 82 (83.7) | 44 (89.8) | .31 | 126 (85.7) |
| 3 and 4 | 16 (16.3) | 5 (10.2) | 21 (14.3) | ||
| ECOGd | 0 and 1 | 83 (84.7) | 42 (85.7) | .87 | 125 (85.0) |
| 2 and 3 | 15 (15.3) | 7 (14.3) | 22 (15.0) | ||
| Surgical type | Oncological | 53 (54.1) | 25 (51.0) | .72 | 78 (53.1) |
| Nononcological | 45 (45.9) | 24 (49.0) | 69 (46.9) | ||
| Surgical Department | Gastrointestinal | 17 (17.3) | 10 (20.4) | .73 | 27 (18.4) |
| Gynecology | 16 (16.3) | 10 (20.4) | 26 (17.7) | ||
| Breast | 21 (23.5) | 5 (14.3) | 26 (17.7) | ||
| Skin Cancer | 14 (14.3) | 5 (10.2) | 19 (12.9) | ||
| Urology | 12 (12.2) | 7 (14.3) | 19 (12.9) | ||
| Head and Neck | 11 (11.2) | 7 (14.3) | 18 (12.2) | ||
| Otherse | 8 (8.2) | 4 (8.2) | 12 (8.2) | ||
| Intensive care unit | No | 92 (93.9) | 41 (85.4) | .12 | 133 (91.1) |
| Yes | 6 (6.1) | 7 (14.6) | 13 (8.9) | ||
| Morbidity (Clavien–Dindof) | none | 84 (85.7) | 41 (83.7) | .74 | 125 (85.0) |
| I | 1 (1.0) | 2 (4.1) | 3 (2.0) | ||
| II | 7 (7.1) | 2 (4.1) | 9 (6.1) | ||
| IIIa | 3 (3.1) | 3 (6.1) | 6 (4.1) | ||
| IIIb | 1 (1.0) | 1 (2.0) | 2 (1.4) | ||
| IVa | 1 (1.0) | 0 (0) | 1 (0.7) | ||
| IVb | 1 (1.0) | 0 (0) | 1 (0.7) | ||
COVID‐neg: patients that had surgeries after a negative RT‐PCR test for SARS‐CoV‐2.
COVID‐rec: asymptomatic patients that had surgeries delayed due to positive RT‐PCR test for SARS‐CoV‐2.
ASA: American Society of Anesthesiologists risk classification. 10
ECOG: Eastern Cooperative Oncology Group Performance Status.
Others: Vascular surgery, Intervention Radiology, Neurosurgery and Reconstructive Surgery.
Clavien–Dindo: Clavien–Dindo classification of surgical complications. 11
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 3.
Characteristics of the 10 patients with Clavien–Dindoa Grades III and IV submitted to surgical procedures from April 22 to July 2, 2020
| Group | Age (year) | ASAb | Oncological surgery | Surgical procedure | Clavien–Dindoa | Complication type | Treatment | ICUc |
|---|---|---|---|---|---|---|---|---|
| COVID‐rec | 57 | 3 | No | Splenic embolization | IIIa | Abdominal abscess | Guide drainaged | No |
| COVID‐rec | 76 | 2 | Yes | Skin resection | IIIa | SSe infection | Local suture | No |
| COVID‐rec | 68 | 3 | Yes | Pulmonary lobectomy | IIIa | Pleural effusion | Pleural drainage | No |
| COVID‐rec | 61 | 2 | No | Biliary drainage | IIIb | Biliary leakage | Re‐drainage | No |
| COVID‐neg | 49 | 2 | Yes | Rectal amputation | IIIa | Abdominal abscess | Guide drainage | No |
| COVID‐neg | 52 | 2 | No | Hysterectomy | IIIa | Abdominal abscess | Guide drainage | No |
| COVID‐neg | 48 | 3 | No | Biliary drainage | IIIa | SS bleeding | Local suture | No |
| COVID‐neg | 48 | 2 | Yes | Total gastrectomy | IIIb | Small bowel obstruction | Laparotomy | No |
| COVID‐neg | 55 | 3 | No | Ileostomy closure | IVa | Anastomotic leakage | Laparotomy | Yes |
| COVID‐neg | 55 | 3 | No | Implantable venous catheter | IVb | Catheter infection | Catheter removal | Yes |
Clavien–Dindo: Clavien–Dindo classification of surgical complications. 11
ASA: American Society of Anesthesiologists risk classification. 10
ICU: Intensive Care Unit admission after complication.
Guided drainage: Image guided procedure.
SS: Surgical Site.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
For the COVID‐rec group, 18.2% of patients that developed symptoms after suspended surgery had any type of complications (Grades ≥ 1) compared with 16.2% for those who did not have any symptom (p = 1.0). Moreover, Grades ≥ 3 complications were found in COVID‐rec and COVID‐neg groups in 9.1% and 8.1% of cases, respectively (p = 1.0). Interestingly, delaying time length for surgery, analyzed as a continuous variable, was not related to a higher risk of complications (p = .18).
We found no deaths within the 30‐days after surgery. However, after a longer follow‐up, we observed five deaths. All deaths occurred after 45 days of follow‐up: three for the COVID‐rec group and two for the COVID‐neg. No death occurred directly after SARS‐CoV‐2 infection. For the COVID‐rec group, one patient died after bone marrow transplant and catheter infection; one related to empyema after a pulmonary segmentectomy; and one related to hepatic progression of colon cancer after biliary drainage. For the two COVID‐neg group cases, one died due to congestive heart failure and one after ovarian cancer progression.
4. DISCUSSION
According to the World Health Organization, Brazil is the second country in the number of cases and deaths by COVID‐19 disease. 13 After considering the implications in delaying oncologic care, and the availability of ward and ICU beds, our institution opted to resume elective surgeries and implemented the strategy of universal preoperative testing. In our preliminary experience including 540 patients, the positivity rate was 7.6% among asymptomatic preoperative patients, allowing us to perform 84.1% of the surgeries electively scheduled. 9
Recently, the published data from the COVIDSurg collaborative 4 included 1115 patients with perioperative positive SARS‐CoV‐2 (835 with emergency surgeries and 280 with elective surgeries). SARS‐CoV‐2 infection was confirmed preoperatively in 294 (26.1%) patients. The overall 30‐day mortality in this study was 23.8%, with all‐cause mortality rates of 18.9% in elective patients and 25.6% in emergency patients (hazard ratio [HR] 1.67, 1.06–2.63; p = .026). Moreover, the mortality rates for minor and major surgeries were 16.3% and 26·9%, respectively (HR 1.52, 1.01–2.31; p = .047); for cancer surgery and benign cases of 27.6% and 22.1%, respectively (HR 1.55, 1.01–2.39; p = .046); and for ASA 3‐5 and 1‐2 were 32.2% and 12.1%, respectively (2.35, 1.57–3.53; p < .0001). Mortality in patients with SARS‐CoV‐2 occurred mainly in those who had postoperative pulmonary complications, which was about 50% of patients.
In addition, Doglietto et al. 5 reported a matched cohort study that included 41 cases with SARS‐CoV‐2 positive and compared with 82 negative cases. The 30‐day mortality (19.5% vs. 2.4%: OR 9.5; 95%CI 1.77–96.5) and any complication rates (85.3% vs. 53.6%: OR 4.98; 95%CI 1.81–16) were significantly higher for the SARS‐CoV‐2 positive cases. In contrast form our study, only seven cases of elective SARS‐CoV‐2 positive cases were included and only 13.4% (11/82) controls were treated during the same period of time.
Due to the devastating impact on morbidity and mortality in SARS‐CoV‐2 positive patients submitted to surgical procedures even for minor procedures, consideration should be given for delaying nonemergency procedures and promoting alternative nonoperative treatments for surgery delay. Extrapolating the data from COVIDSurg Collaborative, 4 for the 272 elective cases, we estimate that up to 53 deaths could have been potentially avoided after applying a preoperative SARS‐CoV‐2 test and subsequent surgery delay.
Universal preoperative screening is now crucial, mainly in places with a high burden of SARS‐CoV‐2 positive cases. As stated, robust data suggest a highly unacceptable complication and mortality rates even for elective surgeries in SARS‐CoV‐2 positive patients, and these surgeries should be delayed. However, to date the only study that addressed the complication rates for patients that had delayed surgeries after a positive SARS‐CoV‐2 was recently published by COVIDSurg Collaborative. 14 They reported in a series of 112 patients that time from positive SARS‐CoV‐2 and surgery correlated to pulmonary complications and mortality. The authors found no pulmonary complications or deaths when the surgery was performed after 4 weeks of the positive test, suggesting that a 4‐week interval between the positive test and surgery may be a safe parameter.
As far as we know, we present the second series that evaluated this topic and we found no difference in complication rates between patients with previous positive SARS‐CoV‐2 compared with matched controls. Moreover, all cases were operated only after a negative SARS‐CoV‐2 test, and no case developed pulmonary disease or SARS‐CoV‐2 infection during the 30‐days after surgery. These findings suggest that it may be safe to postpone and operate patients after a negative control SARS‐CoV‐2 test. Notably, due to positive SARS‐CoV‐2, 2 elective surgeries were delayed and after oncological complications these cases had emergency procedures, however, with negative SARS‐CoV‐2 at this time and no deaths even after emergency procedures.
Our strategy was based on a negative control test, despite the interval between tests. The patients were planned to be re‐tested after 2 weeks from the first positive test and surgery were only performed after a negative test. Although our data suggest that this parameter is safe for re‐scheduling, we still need to determine if it is safe to operate after 3–4 weeks from the first positive test, even after a second (control) positive test or if a subsequent third test is necessary in an asymptomatic patient.
Although being an institution dedicated to cancer treatment, we expanded the analysis for nononcological surgeries and the COVID‐rec cases were matched for cases treated during the same period of time and by the same surgical teams. Despite the relatively low number of patients with SARS‐CoV‐2 positive with delayed surgeries, it is the first matched control study that evaluated this population, and our findings may contribute with valuable data for literature on this topic. However, we should point out the weaknesses of a retrospective single center study.
In conclusion, patients with delayed elective surgeries due to asymptomatic preoperative positive SARS‐CoV‐2 test are not at higher risk of postoperative complications after having a negative test before surgery. If ward and ICU beds are available, elective surgeries can be scheduled safely with preoperative screening for SARS‐CoV‐2 based on systematic RT‐PCR SARS‐CoV‐2 testing.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
SYNOPSIS
Delayed elective surgeries due to asymptomatic preoperative positive SARS‐CoV‐2 test are not at higher risk of postoperative complications. Elective surgeries can be scheduled safely with preoperative screening with RT‐PCR SARS‐CoV‐2 testing.
ACKNOWLEDGMENT
The study did not receive funding.
Baiocchi G, Aguiar S, Duprat JP, et al. Early postoperative outcomes among patients with delayed surgeries after preoperative positive test for SARS‐CoV‐2: A case‐control study from a single institution. J Surg Oncol. 2021;123:823–833. 10.1002/jso.26377
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Miyashita H, Mikami T, Chopra N, et al. Do patients with cancer have a poorer prognosis of COVID‐19? An experience in New York City. Ann Oncol. 2020;31(8):1088‐1089. 10.1016/j.annonc.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liang W, Guan W, Chen R, et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335‐337. 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharpless NE. COVID‐19 and cancer. Science. 2020;368(6497):1290. 10.1126/science.abd3377 [DOI] [PubMed] [Google Scholar]
- 4. Nepogodiev D, Bhangu A, Glasbey JC, et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS‐CoV‐2 infection: an international cohort study. Lancet. 2020;396(10243):27‐38. 10.1016/S0140-6736(20)31182-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doglietto F, Vezzoli M, Gheza F, et al. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID‐19) in Italy. JAMA Surg. 2020;155(8):691. 10.1001/jamasurg.2020.2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bogani G, Signorelli M, Ditto A, Raspagliesi F. Surgical oncology at the time of COVID‐19 outbreak. J Surg Oncol. 2020;122(2):115‐116. 10.1002/jso.25975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu AC, Schmiesing CA, Mahoney M, et al. COVID‐19 Preoperative assessment and testing: from surge to recovery. Ann Surg. 2020;272(3):e230‐e235. 10.1097/SLA.0000000000004124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pinheiro RN, Coimbra FJF, Costa‐Jr WLDA, et al. Surgical cancer care in the COVID‐19 era: front line views and consensus. Rev Col Bras Cir. 2020:47. 10.1590/0100-6991e-20202601 [DOI] [PubMed] [Google Scholar]
- 9. Aguiar S, Baiocchi G, Duprat JP, et al. Value of preoperative testing for SARS‐CoV‐2 for elective surgeries in a cancer center during the peak of pandemic in Brazil. J Surg Oncol. 2020;122:1293‐1295. 10.1002/jso.26146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mayhew D, Mendonca V, Murthy BVS. A review of ASA physical status ‐ historical perspectives and modern developments. Anaesthesia. 2019;74(3):373‐379. 10.1111/anae.14569 [DOI] [PubMed] [Google Scholar]
- 11. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649‐655. http://www.ncbi.nlm.nih.gov/pubmed/7165009 [PubMed] [Google Scholar]
- 12. Dindo D, Demartines N, Clavien P‐A. Classification of surgical complications. Ann Surg. 2004;240(2):205‐213. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. WHO Coronavirus Disease (COVID‐19) Dashboard . Situation by country, territory or area. https://covid19.who.int. Accessed August 20, 2020.
- 14. COVIDSurg Collaborative . Delaying surgery for patients with a previous SARS CoV‐2 infection [published online ahead of print September 25, 2020]. Br J Surg. 2020. 10.1002/bjs.12050 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


