Abstract
The purpose of the present study was to explore the relationship between nuclear factor erythroid 2-related factor 2 (Nrf2)/BTB-CNC allogeneic 1 (Bach1)/γ-glutamic acid cysteine synthase (γ-GCS) and chronic obstructive pulmonary disease (COPD). The expression of Nrf2, Bach1, γ-GCS mRNA and protein in the peripheral blood mononuclear cells (PBMCs) of 80 COPD patients and 40 healthy volunteers were studied. Then, the correlation between Nrf2, Bach1, γ-GCS and lung function, inflammation and oxidative stress indicators was analyzed. Compared with healthy controls, Nrf2, Bach1 mRNA and protein levels were significantly increased in the PBMCs of COPD patients, while γ-GCS mRNA and protein levels were significantly decreased. Nrf2 and Bach1 protein levels in the nucleus were significantly elevated in acute exacerbation COPD patients compared with patients with a stable stage of COPD, while γ-GCS mRNA levels were significantly reduced. In addition, it was found that Nrf2 nuclear protein levels were significantly reduced in COPD patients compared with the control group, while Bach1 nuclear protein levels were significantly increased. Correlation analysis in COPD group demonstrated that γ-GCS mRNA was positively correlated with Nrf2 nuclear protein level, but negatively correlated with Bach1 nuclear protein level. Further analysis demonstrated that γ-GCS mRNA and Nrf2 protein in the nucleus was positively correlated with forced expiratory volume in one second (FEV1)/forced vital capacity (FVC)% and FEV1% predicted, and Bach1 protein in the nucleus was negatively correlated with FEV1/FVC% and FEV1% predicted. Additionally, the expression levels of Nrf2, Bach1 and γ-GCS were also associated with smoking. The expression of Nrf2, Bach1 and γ-GCS in peripheral blood mononuclear cells of patients with COPD was dysregulated and related to lung function, which provides a new basis for exploring further the pathogenesis of COPD.
Keywords: chronic obstructive pulmonary disease, peripheral blood mononuclear cells, oxidative stress, nuclear factor erythroid 2-related factor 2, BTB-CNC allogeneic 1, γ-glutamic acid cysteine synthase
Introduction
Chronic obstructive pulmonary disease (COPD) is a common chronic and frequently occurring disease in the respiratory system, and its prevalence and mortality are expected to increase in the coming decades (1). The World Health Organization estimated that COPD is to become the third most prevalent cause of worldwide mortality by 2020(2). The pathogenesis of COPD is complicated, its pathogenesis has not yet been fully elucidated and no breakthrough has been made in clinical treatment. Current research suggests that oxidative stress is one of the important mechanisms leading to the development of COPD (3,4).
Glutathione (GSH) is an important antioxidant in the body and is the main substance in the lung combating the continued damage of endogenous and exogenous oxidants. γ-glutamic acid cysteine synthase (γ-GCS) is the rate-limiting enzyme for the synthesis of GSH and serves an important role in the COPD oxidation/antioxidant system (5). Increased expression and activity of γ-GCS gene, which in turn increases GSH synthesis, may be one of the main mechanisms of COPD antioxidant activity (6). The expression of γ-GCS is mainly concentrated at the transcription level (7). Findings have shown that under oxidative stress (for instance, smog), protein kinase C, PI3K, ERK and other signaling pathways activate γ-GCS mRNA transcription by activating nuclear factor erythroid 2-related factor 2 (Nrf2), activator protein 1 (AP-1) and NF-κB, catalyzing increased GSH synthesis and participating in the antioxidant processes (7). The regulation of γ-GCS expression at the transcriptional level to increase the synthesis of GSH and enhance the body's antioxidant capacity are important for the treatment of many diseases, including COPD as well as certain tumors such as prostate cancer and colon cancer (8-10).
Recently, it was identified that Nrf2 is at the core of oxidative stress that serves an important role in the pathogenesis of COPD (11). Nrf2 belongs to the family of Cap‘n’collar (CNC) transcription factors and binds to the antioxidant response element (ARE) in the upstream promoter region of antioxidant enzymes, which can maintain the oxidation/antioxidant balance state of the cells by regulating the expression of various antioxidant genes such as γ-GCS, nicotinamide adenine dinucleotide phosphate hydrogen, quinone oxidoreductase 1 and heme oxygenase-1 (12,13). Animal studies have identified that the basal and inducible expression of the antioxidant gene in NRF2-deficient mice results in increased sensitivity to oxidative stress, suggesting that the Nrf2/ARE pathway serves a key role in regulating intracellular redox status (14,15). BTB-CNC allogeneic 1 (Bach1), is also a member of the CNC transcription factor family. Bach1 shuttles in the cytoplasm and nucleus to regulate the dynamic balance of oxidation/antioxidation (16,17). Unlike Nrf2, Bach1 can block ARE-mediated transcription of antioxidant genes (18). It has been shown that Nrf2 and Bach1 may serve a competitive regulatory role in the expression of γ-GCS in the pathogenesis of COPD in rats (19). Reichard et al (20) identified that Bach1 competes with Nrf2 for the binding site of the antioxidant reaction, which acts on the antioxidant response element and regulates the expression of downstream antioxidant genes. Wang et al (19) demonstrated that Bach1 and Nrf2 may compete for the reverse regulation of the expression of the antioxidant gene γ-GCS during the development of COPD in rats.
As previous studies have remained at the level of animal experiments, the changes and correlations of Nrf2, Bach1, γ-GCS expression levels in peripheral blood mononuclear cells (PBMCs) of COPD patients was investigated to explore their role in the pathogenesis of COPD, and analyze the role of Nrf2 and Bach1 in the regulation of γ-GCS expression, thus opening up new directions for the clinical treatment of COPD.
Materials and methods
Research objects
A total of 80 patients with COPD (COPD group) who were admitted to the First Affiliated Hospital of Soochow University between June 2016 and March 2018 were enrolled, including 36 patients with acute exacerbation (AE) COPD and 44 patients in stable stage of COPD (stable COPD). The diagnosis and classification of COPD were in accordance with the criteria for the diagnosis and treatment of COPD (21). Patients had clear consciousness, speech communication and behavioral coordination. They had difficulty breathing, cough or chronic cough, and had a history of exposure to risk factors; the first second forced expiratory volume occupancy lung capacity percentage [forced expiratory volume in one second (FEV1)/forced vital capacity (FVC)%] was <70%. Other diseases were excluded; none of the patients were associated with other diseases that could cause asthma or difficulty in breathing, such as bronchiectasis, bronchial asthma or lung cancer. Another 40 healthy subjects were selected as the control group. All healthy controls had a FEV1/FVC% >70% and no respiratory or other diseases. The study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University, and all subjects signed informed consent.
Pulmonary function test
The lung function test was performed according to the instructions of the lung function tester (Sensor Medics Ltd.) under the guidance of a lung function test professional technician. The main observation indexes were FEV1/FVC% and forced expiratory volume in the first second as a percentage of predicted value (FEV1% predicted).
Determination of serum oxidative stress index
As previously described (22,23), the content of malondialdehyde (MDA) was detected by thiobarbituric acid colorimetry; the content of GSH and the activity of oxide dismutase (SOD) were determined by chemical methods. The kits for MDA (cat. no. A003-1-2), GSH (cat. no. A005-1-2) and SOD (cat. no. A001-3-2) were purchased from Nanjing Jiancheng Bioengineering Research Institute and the procedures were carried out in strict accordance with the operating instructions.
Specimen collection and separation of PBMCs
All the blood samples were taken from the cubital vein of the subjects, and the COPD group specimens were collected on the day of hospitalization. EDTA-K2 anticoagulated whole blood (10 ml) was added to an equal volume of Hank's solution, and gently poured into the centrifuge tube pre-filled with 10 ml of Ficoll-Pague lymphocyte separation solution (Thermo Fisher Scientific, Inc.) along the tube wall. Following centrifugation at 1,200 x g for 15 min at 18˚C, the mononuclear cell layer between the plasma layer and the Ficoll-Pague layer were collected and placed in a de-enzyme Eppendorf tube. PBS (0.01 M) was added following centrifugation, the supernatant was discarded and the cell pellet (PBMCs) was collected following washing. Then, 1 ml of TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) was added and the mixture was thoroughly mixed before being stored at -80˚C.
RNA extraction and reverse transcription-quantitative (RT-q) PCR
Total RNA in PBMCs was extracted using TRIzol® (cat. no. R0016; Beyotime Institute of Biotechnology) according to the manufacturer's protocols. The nucleic acid protein analyzer measured absorbance at 260 and 280 nm and the total RNA concentration. The RT-qPCR reaction was carried out using a SYBR Premix Ex Taq kit (Takara Bio, Inc.) according to the manufacturer's protocols, and the reaction system was 20 µl. The reaction conditions were: Pre-denaturation at 94˚C for 4 min into the main cycle, wherein pre-denaturation at 94˚C for 30 sec, denaturation at 56˚C for 60 sec and extension at 72˚C for 40 sec after 40 cycles. The amount of mRNA expression was calculated using the 2-ΔΔCq method (24). β-actin as considered an internal reference. Primer sequences used were: γ-GCS sense: 5'-ATGATAGAACACGGGAGG-3', antisense: 5'-CAAATACCACATAGGCAG-3'; Nrf2 sense: 5'-CCATTTACGGAGACCCAC-3', antisense: 5'-GGATTCACGCATAGGAGC-3'; Bach1 sense: 5'-GTCAGGGCAATGTAAGAGC-3', antisense: 5'-CGTGAGGTCCAGCAGAAT-3'; β-actin sense: 5'-CCTAAGGCCAACCGTGAA-3', antisense: 5'-CTAGGAGCCAGGGCAGTAATC-3'.
Protein extraction and western blot assay
Cytoplasmic and nuclear extraction was performed using the cytoplasmic/nuclear isolation kit (BioVision, Inc.) according to the manufacturer's protocols. La min B (Santa Cruz, USA) was used to detect the purity of the separation. A total protein extraction kit (Tiangen Biotechnology, China) was used to extract total cellular proteins. Following protein denaturation, the volume of each well was 20 µg protein/lane was separated via SDS-PAGE on a 8% gel. The separated proteins were subsequently transferred onto PVDF membranes on ice and blocked with 5% skimmed milk at room temperature for 1 h. The primary antibodies (Abcam) targeting Nrf2 (1:5,000; cat. no. ab76026; Abcam), Bach1 (1:400; cat. no. ab49657; Abcam) and γ-GCS (1:500; cat. no. AF7037; Beyotime Institute of Biotechnology) were added and incubated overnight at 4˚C with GAPDH (1:5,000) as an internal reference. Following incubation with secondary antibody (1:1,000; cat. no. A21020; Abbkine Scientific Co., Ltd.) for 1 h at room temperature. ECL was performed and the film scanned using ImageScanner, the image strip area and gray value were analyzed using FluorChem 8900 software (version 9.5.3; Alpha Innotech Corporation).
Statistical analysis
Data were statistically analyzed using SPSS 20.0 (IBM Corp.). The measurement data were expressed as mean ± standard deviation, and the LSD-t test was performed between the two groups in accordance with the normal distribution, and the one-way analysis of variance followed by Tukey analysis of variance was performed among then ever smoking-healthy (NS-H), smoking-healthy (including former smokers; S-H), never smokers with chronic obstructive pulmonary disease (NS-COPD) and smokers with chronic obstructive pulmonary disease (including former smokers; S-COPD) S-groups. The Mann-Whitney U test was used for comparison between the two groups when the normal distribution was not met, and the Kruskal-Wallis H test was used between the two groups. Count data was analyzed using χ2 tests or Fisher's exact tests. Pearson's or Spearman's correlation was used to analyze the correlation between variables. P<0.05 was considered to indicate a statistically significant difference.
Results
Characteristics of subjects
No differences in general data such as sex, age, and BMI was observed between the two groups. FEV1% predicted and FEV1/FVC% in the COPD group were significantly lower than those in the control group (P<0.05). The level of MDA in plasma of COPD patients increased significantly, while the levels of GSH and SOD decreased significantly. In addition, the level of tumor necrosis factor (TNF)-α in patients with COPD was significantly higher compared with the control group (Table I).
Table I.
Characteristics of patients with COPD and healthy subjects.
| Parameters | Healthy controls (n=40) | COPD (n=80) | P-value |
|---|---|---|---|
| Sex (male/female) | 26/14 | 48/32 | 0.595 |
| Age (years) | 62.33±6.66 | 61.73±7.29 | 0.663 |
| BMI | 22.93±1.52 | 23.31±1.48 | 0.191 |
| Smoking index | 10.25±15.65 | 15.16±16.69 | 0.124 |
| SO2 | 97.05±1.40 | 96.89±1.42 | 0.560 |
| TG (mmol/l) | 1.59±0.72 | 1.40±0.67 | 0.156 |
| TC (mmol/l) | 4.58±0.72 | 4.70±0.90 | 0.456 |
| HDL (mmol/l) | 1.36±0.35 | 1.40±0.48 | 0.641 |
| LDL (mmol/l) | 2.65±0.86 | 2.49±0.76 | 0.300 |
| FEV1% predicted | 106.29±15.84 | 82.01±16.49 | <0.001 |
| FEV1/FVC% | 79.58±6.56 | 59.11±9.70 | <0.001 |
| TNF-α (pg/ml) | 1.04±0.78 | 2.60±1.95 | <0.001 |
| CRP (µg/ml) | 19.60±15.01 | 23.31±16.40 | 0.232 |
| MDA (µmol/l) | 2.94±1.75 | 4.39±1.96 | <0.001 |
| SOD (U/ml) | 123.00±1.75 | 79.82±8.16 | <0.001 |
| GSH (mg/l) | 424.66±7.98 | 290.85±35.38 | <0.001 |
COPD, chronic obstructive pulmonary disease; BMI, body mass index; SO2, oxygen saturation; TG, triglyceride; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; TNF, tumor necrosis factor; CRP, C-reactive protein; MDA, malondialdehyde; SOD, superoxide dismutase; GSH, glutathione.
Expression levels of Nrf2, Bach1 and γ-GCS mRNA and protein in PBMCs
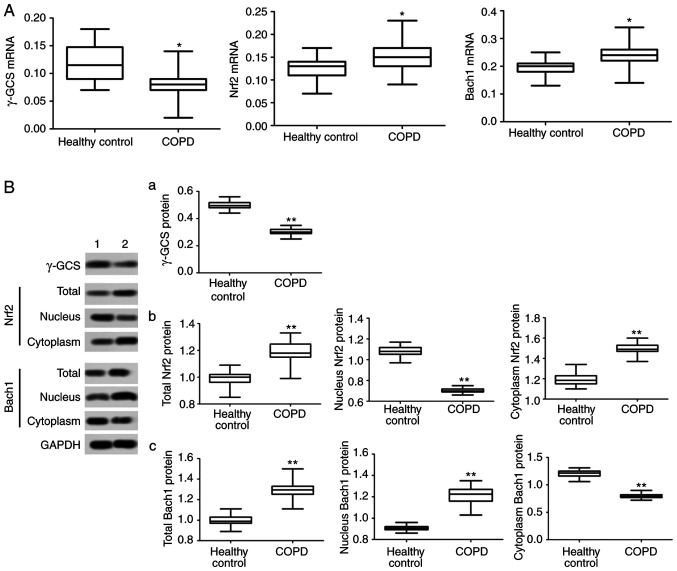
To investigate the causes of systemic oxidation/antioxidant imbalance in COPD patients, the transcription and translation of γ-GCS in PBMCs was analyzed. The results demonstrated that the expression levels of γ-GCS mRNA and protein in PBMCs of COPD patients were significantly decreased (P<0.05, Fig. 1A and B), indicating that the antioxidant capacity of COPD patients decreased. It was also identified that the total protein levels of Nrf2 and Bach1 in PBMCs of COPD patients were significantly higher compared with those in the control group (P<0.05; Fig. 1B).
Figure 1.
mRNA and protein expression of Nrf2, Bach1 and γ-GCS in PBMCs. (A) mRNA expression of γ-GCS,Nrf2 and Bach1 in PBMCs. (B) Protein expression of (a) γ-GCS, (b) Nrf2and (c) Bach1in PBMCs.*P<0.05 and **P<0.01 vs. healthy control. Nrf2, nuclear factor erythroid 2-related factor 2; Bach1, BTB-CNC allogeneic 1; and γ-GCS, γ-glutamic acid cysteine synthase; PBMCs, peripheral blood mononuclear cells.
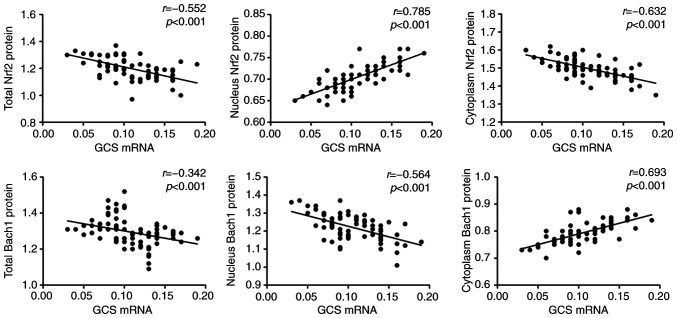
The intranuclear and intracytoplasmic protein expression was analyzed and it was identified that Nrf2 protein levels in the nucleus were significantly reduced in COPD patients compared with the control group, and Bach1 protein levels in the nucleus were significantly increased (P<0.05; Fig. 1B). Correlation analysis in the COPD group demonstrated that γ-GCS mRNA expression level was positively correlated with Nrf2 nuclear protein level (P<0.05; Fig. 2), and negatively correlated with Bach1 nuclear protein level (P<0.05; Fig. 2).
Figure 2.
Relationship among Nrf2, Bach1 and γ-GCS in patients with COPD. Nrf2, nuclear factor erythroid 2-related factor 2; Bach1, BTB-CNC allogeneic 1; γ-GCS, γ-glutamic acid cysteine synthase; COPD, chronic obstructive pulmonary disease.
Relationship between Nrf2, Bach1 and γ-GCS expression and severity of COPD patients
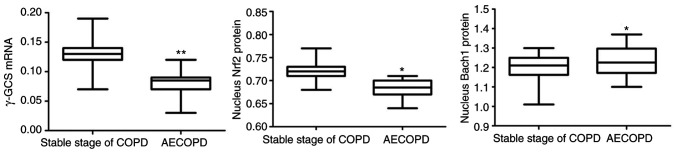
Compared with patients with the stable stage of COPD, FEV1% predicted and FEV1/FVC% were significantly lower in AECOPD patients (P<0.05; Table II), and GSH level was significantly different between the two groups (P<0.05; Table II). The data demonstrated that Nerf2 and Bach1 protein levels in the nucleus were significantly increased in AECOPD patients, while γ-GCS mRNA levels were significantly decreased (P<0.05; Fig. 3).
Table II.
Comparison of clinical data of patients with different levels of COPD.
| Parameters | Stable stage of COPD (n=44) | AECOPD (n=36) | P-value |
|---|---|---|---|
| Sex (male/female) | 23/21 | 25/11 | 0.119 |
| Age (years) | 61.32±7.25 | 62.22±7.30 | 0.583 |
| BMI | 23.42±1.41 | 23.17±1.56 | 0.454 |
| Smoking index | 14.69±15.86 | 15.73±17.63 | 0.782 |
| SO2 | 96.80±1.47 | 97.01±1.35 | 0.512 |
| TG (mmol/l) | 1.41±0.72 | 1.16±0.57 | 0.094 |
| TC (mmol/l) | 4.60±0.91 | 4.81±0.81 | 0.284 |
| HDL (mmol/l) | 1.44±0.46 | 1.35±0.52 | 0.414 |
| LDL (mmol/l) | 2.61±0.70 | 2.35±0.81 | 0.128 |
| FEV1% predicted | 86.54±17.46 | 78.13±15.04 | 0.025 |
| FEV1/FVC% | 61.63±9.58 | 56.03±8.93 | 0.009 |
| TNF-α (pg/ml) | 2.94±2.01 | 2.20±1.80 | 0.090 |
| CRP (µg/ml) | 22.56±16.86 | 24.24±15.77 | 0.649 |
| MDA (µmol/l) | 4.11±1.90 | 4.73±1.98 | 0.158 |
| SOD (U/ml) | 78.97±7.48 | 80.87±8.80 | 0.300 |
| GSH (mg/l) | 304.43±31.96 | 276.47±38.71 | 0.001 |
COPD, chronic obstructive pulmonary disease; AE, acute exacerbation; BMI, body mass index; SO2, oxygen saturation; TG, triglyceride; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low density lipoprotein; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; TNF, tumor necrosis factor; CRP, C-reactive protein; MDA, malondialdehyde; SOD, superoxide dismutase; GSH, glutathione.
Figure 3.
γ-GCS mRNA and Nrf2 and Bach1 protein in the nucleus in patients with stable stage COPD and AECOPE. *P<0.05 and **P<0.01 vs. stable stage of COPD. Nrf2, nuclear factor erythroid 2-related factor 2; Bach1, BTB-CNC allogeneic 1; γ-GCS, γ-glutamic acid cysteine synthase; COPD, chronic obstructive pulmonary disease; AE, acute exacerbation.
Analysis of Nrf2, Bach1 protein and γ-GCS mRNA expression in peripheral blood of smoking and non-smoking subgroups
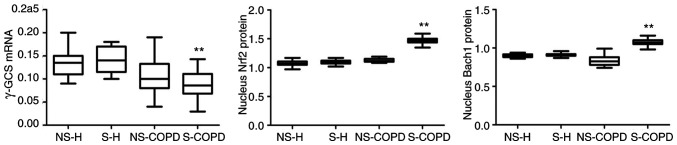
All subjects were subgrouped according to whether they smoked. As shown in Table II, compared with NS-COPD, S-H and NS-H (P<0.05; Fig. 4), Nerf2 and Bach1 protein levels in the nucleus were significantly increased in S-COPD patients, while γ-GCS mRNA levels were significantly decreased (P<0.05; Fig. 4). Similar results were identified in GSH (Table III).
Figure 4.
Nucleus Nrf2, Bach1 protein and γ-GCS mRNA expression in peripheral blood of smoking and non-smoking subgroups. **P<0.01 vs. NS-COPD. The one-way analysis followed by Tukey analysis of variance was performed among the NS-H, S-H, NS-COPD and S-COPD groups. Nrf2, nuclear factor erythroid 2-related factor 2; Bach1, BTB-CNC allogeneic 1; γ-GCS, γ-glutamic acid cysteine synthase; NS-H, never smoking-healthy; S-H: smoking-healthy (including former smokers); NS-COPD, never smokers with chronic obstructive pulmonary disease; S-COPD, smokers with chronic obstructive pulmonary disease (including former smokers).
Table III.
Comparison of clinical data between subgroups of smokers and non-smokers.
| Parameters | NS-H (n=24) | S-H (n=16) | NS-COPD (n=34) | S-COPD (n=46) | P-value |
|---|---|---|---|---|---|
| Sex (male/female) | 8/16 | 14/2 | 11/23 | 44/2 | <0.001 |
| Age (years) | 62.33±7.21 | 62.31±5.73 | 61.91±6.59 | 61.58±7.76 | 0.972 |
| BMI | 22.63±1.64 | 23.37±1.21 | 23.05±1.30 | 23.49±1.58 | 0.122 |
| Smoking index | 0 | 25.62±14.78 | 0 | 26.35±13.75 | <0.001 |
| SO2 | 97.38±1.29 | 96.57±1.43 | 97.13±1.23 | 96.72±1.52 | 0.154 |
| TG (mmol/l) | 1.68±0.62 | 1.48±0.74 | 1.43±0.76 | 1.19±0.58 | 0.032 |
| TC (mmol/l) | 4.48±0.67 | 4.73±0.74 | 4.69±0.81 | 4.69±0.92 | 0.714 |
| HDL (mmol/l) | 1.37±0.35 | 1.35±0.35 | 1.42±0.37 | 1.39±0.56 | 0.955 |
| LDL (mmol/l) | 2.69±0.93 | 2.58±0.73 | 2.39±0.67 | 2.57±0.82 | 0.538 |
| FEV1% predicted | 106.37±17.14 | 104.18±13.66 | 89.05±16.32 | 76.80±14.59 | <0.001 |
| FEV1/FVC% | 82.54±6.30 | 75.14±3.90 | 64.02±8.04 | 55.49±9.22 | <0.001 |
| TNF-α (pg/ml) | 1.01±0.87 | 1.07±0.63 | 1.99±1.44 | 3.05±2.16 | <0.001 |
| CRP (µg/ml) | 18.98±15.98 | 20.53±13.37 | 21.61±11.02 | 24.57±19.34 | 0.531 |
| MDA (µmol/l) | 2.63±1.72 | 3.41±1.67 | 4.31±1.98 | 4.44±1.94 | 0.001 |
| SOD (U/ml) | 123.36±4.94 | 122.45±6.23 | 76.02±7.38 | 82.63±7.54 | <0.001 |
| GSH (mg/l) | 425.70±6.40 | 423.10±9.68 | 307.02±36.42 | 278.89±29.33 | <0.001 |
The one-way analysis of variance was performed among the four groups, and the P-values were obtained by comparing four groups. NS-H, never smoking-healthy; S-H, smoking-healthy (including former smokers); NS-COPD, never smokers with chronic obstructive pulmonary disease; S-COPD, smokers with chronic obstructive pulmonary disease (including former smokers); BMI, body mass index; SO2, oxygen saturation; TG, triglyceride; TC, total cholesterol; HDL, high density lipoprotein; LDL, low density lipoprotein; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; TNF, tumor necrosis factor; CRP, C-reactive protein; MDA, malondialdehyde; SOD, superoxide dismutase; GSH, glutathione.
Relationship between Nrf2, Bach1 protein and γ-GCS mRNA expression and clinicopathological data of patients with COPD
As shown in Table IV, γ-GCS mRNA and Nrf2 nuclear protein level were positively correlated with FEV1/FVC% and FEV1% predicted. By contrast, Bach1 nuclear protein level was negatively correlated with FEV1/FVC% and FEV1% predicted (P<0.05; Table IV). In addition, Nrf2, Bach1 and γ-GCS were also associated with GSH (P<0.05; Table IV).
Table IV.
Correlations between Nrf2, Bach1 and γ-GCS mRNA and other parameters.
| Parameters | γ-GCS mRNA | Nucleus Nrf2 protein | Nucleus Bach1 protein |
|---|---|---|---|
| FEV1% predicted | r=0.357, P=0.001 | r=0.203, P<0.001 | r=0.391, P<0.001 |
| FEV1/FVC% | r=0.444, P<0.001 | r=0.394, P<0.001 | r=0.345, P=0.002 |
| CRP | r=0.259, P=0.020 | r=0.662, P=0.062 | r=0.170, P=0.131 |
| TNF-α | r=0.182, P=0.109 | r=0.049, P=0.667 | r=0.045, P=0.680 |
| MDA | r=0.134, P=0.237 | r=0.145, P=0.204 | r=0.055, P=0.631 |
| SOD | r=0.197, P=0.080 | r=0.105, P=0.364 | r=0.205, P=0.069 |
| GSH | r=0.395, P<0.001 | r=0.345, P=0.002 | r=0.498, P<0.001 |
Pearson's or Spearman's was used to analyze the correlation between variables. Nrf2, nuclear factor erythroid 2-related factor 2; Bach1, BTB-CNC allogeneic 1; γ-GCS, γ-glutamic acid cysteine synthase; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; TNF, tumor necrosis factor; CRP, C-reactive protein; MDA, malondialdehyde; SOD, superoxide dismutase; GSH, glutathione.
Discussion
The present study analyzed the expression levels of Nrf2, Bach1 and γ-GCS in peripheral blood PBMCs from 80 COPD cases and 40 control subjects. A significant increase was observed in Nrf2 and Bach1 expression levels in PBMC in patients with COPD, while γ-GCS expression level was significantly reduced. Further analysis demonstrated that the expression levels of Nrf2, Bach1 and γ-GCS were significantly correlated with lung function decline index (FEV1 % predicted, FEV1/FVC%) and oxidative stress index (GSH), and were associated with smoking history in patients with COPD.
There are significant oxidative and antioxidant imbalances in patients with COPD (25). The anti-oxidative mechanism in the body helps the host to alleviate oxidative stress damage (26). GSH is an indispensable antioxidant for key functions and metabolism in cells which serves an important role in maintaining the normal function of cells (27). γ-GCS is a major antioxidant gene in the body and serves an important role in the antioxidant mechanism of COPD by catalyzing the synthesis of GSH (28). The transcription of γ-GCS is closely related to endogenous GSH level, reflecting the body's antioxidant capacity (29). The present study demonstrated that γ-GCS mRNA and protein levels were significantly reduced in PBMCs in patients with COPD, and correlation analysis demonstrated that γ-GCS was positively correlated with GSH, FVE1% predicted and FEV1/FVC in patients with COPD. It was suggested that the transcription and expression levels of γ-GCS in the cell during the development of COPD are decreased, which led to a decrease in the body's antioxidant capacity and caused deterioration of lung function in patients. The current regulation mechanism for γ-GCS has yet to be elucidated.
Nrf2 and Bach1 are widely present in the body and belong to members of the CNC transcription factor family, all of which are involved in oxidative stress responses (30). Previous studies have shown that Bach1 and Nrf2 are in an unbalanced state in COPD rats (31) and lung tissue of patients with emphysema (32), and elevated levels of Bach1 are identified in emphysema patients (32). The elevated expression of Nrf2 and Bach1 protein was also identified in PBMCs of COPD patients, which suggested that Nrf2 and Bach1 imbalance in COPD patients may be an important mechanism for the development of COPD.
Wang et al (19) demonstrated that Bach1 and Nrf2 may compete for the reverse regulation of γ-GCS expression during the development of COPD in rats. Sykiotis and Bohmann (33) identify that Bach1 serves a negative regulatory role in the anti-oxidation of the body and can compete with Nrf2 for binding to ARE. Gao and Dai (31) also identify that Bach1 and Nrf2 proteins are positively and negatively correlated with γ-GCS mRNA, respectively. The present study demonstrated that Nrf2 protein was positively correlated with γ-GCS mRNA expression, while Bach1 protein was negatively correlated with γ-GCS mRNA. Using transfection technology to alter Bach1 and Nrf2 protein levels in the nucleus, Sykiotis and Bohmann (33) demonstrated that Bach1 nuclear accumulation downregulates ARE-mediated gene expression, resulting in decreased antioxidant enzyme production, and the dynamic equilibrium relationship between Bach1 and Nrf2 in the nucleus affects ARE-mediated gene transcription. The results of the present study demonstrated that Nrf2 and Bach1 nuclear translocation are associated with γ-GCS expression. It was also identified that Nrf2 and Bach1 protein expression levels in the nucleus were correlated with FVE1% predicted and FEV1/FVC%. Therefore, it was hypothesized that oxidative stress stimulated the movement of Nrf2 and Bach1 in the cytoplasm and nucleus during the pathogenesis of COPD: Nrf2 enters the nucleus, Bach1 exits the nucleus and the levels of them in the nucleus participate in the regulation of γ-GCS transcription. Enhancing the expression of the downstream antioxidant gene γ-GCS by promoting Nrf2 nuclear translocation and inhibiting Bach1 nuclear translocation may enhance the host's antioxidant capacity which could be of significance for the treatment of patients with COPD.
Altuntaş et al (34) identified that COPD patients and healthy smokers have higher systemic oxidative stress levels compared with those who do not smoke, indicating that smoking causes an increase in systemic oxidative stress. The present study also identified that Nrf2/Bach1/γ-GCS-mediated oxidation/antioxidant imbalance was associated with smoking history. Due to the limited subjects within this study, it was impossible to make a final conclusion as to whether this imbalance could be attributed to smoking status and gender.
In summary, the present study identified that Nrf2 and Bach1 expression were elevated in PBMCs of patients with COPD, while γ-GCS expression was decreased, and the levels of all three were correlated with lung function, confirming that Nrf2/Bach1/γ-GCS-mediated oxidation/antioxidant imbalance is associated with COPD development. The present study provided new information for further understanding the pathogenesis and development of COPD.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
DL performed most of the experiments and wrote the manuscript, DS performed experiments and analyzed the data, and YZ designed the study and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University, and all subjects signed informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Croft JB, Wheaton AG, Liu Y, Xu F, Lu H, Matthews KA, Cunningham TJ, Wang Y, Holt JB. Urban-rural county and State differences in chronic obstructive pulmonary Disease-United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67:205–211. doi: 10.15585/mmwr.mm6707a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009;4:435–459. doi: 10.1146/annurev.pathol.4.110807.092145. [DOI] [PubMed] [Google Scholar]
- 3.Singh S, Verma SK, Kumar S, Ahmad MK, Nischal A, Singh SK, Dixit RK. Evaluation of oxidative stress and antioxidant status in chronic obstructive pulmonary disease. Scand J Immunol. 2017;85:130–137. doi: 10.1111/sji.12498. [DOI] [PubMed] [Google Scholar]
- 4.Boukhenouna S, Wilson MA, Bahmed K, Kosmider B. Reactive oxygen species in chronic obstructive pulmonary disease. Oxid Med Cell Longev. 2018;2018(5730395) doi: 10.1155/2018/5730395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ray S, Watkins DN, Misso NL, Thompson PJ. Oxidant stress induces gamma-glutamylcysteine synthetase and glutathione synthesis in human bronchial epithelial NCI-H292 cells. Clin Exp Allergy. 2002;32:571–577. doi: 10.1046/j.0954-7894.2002.01294.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Dai AG, Hu RC. aPKC and ERK regulate NRF2-γ-GCS in rat lung with chronic obstructive pulmonary disease. Chin J Pathophysiol. 2007;23:2239–2243. [Google Scholar]
- 7.Lin SD, Dai AG, Xu P. Changes of the actiity and expression of gamma-glutamylcysteine synthetase in patients with chronic obstructie pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi. 2005;28:97–101. (In Chinese) [PubMed] [Google Scholar]
- 8.Lin SD, Zhou QL, Dai AG. Synthetic mechanism and effect of glutathione in lung of rats with chronic obstructive pulmonary disease. Chin Tropical Med. 2007;7:1071–1072. [Google Scholar]
- 9.Kinnula VL, Fattman CL, Tan RJ, Oury T. Oxidative stress in pulmonary fibrosis: A possible role for redox modulatory therapy. Am J Respir Care Med. 2005;172:417–422. doi: 10.1164/rccm.200501-017PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malfa GA, Tomasello B, Acquaviva R, Genovese C, La Mantia A, Cammarata FP, Ragusa M, Renis M, Di Giacomo C. Betula etnensis Raf. (betulaceae) extract induced HO-1 expression and ferroptosis cell death in human colon cancer cells. Int J Mol Sci. 2019;20(pii: E2723) doi: 10.3390/ijms20112723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui W, Zhang Z, Zhang P, Qu J, Zheng C, Mo X, Zhou W, Xu L, Yao H, Gao J. Nrf2 attenuates inflammatory response in COPD/emphysema: Crosstalk with Wnt3a/β-catenin and AMPK pathways. J Cell Mol Med. 2018;22:3514–3525. doi: 10.1111/jcmm.13628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy NM, Kleeberger SR, Yamamoto M, Kensler TW, Scollick C, Biswal S, Reddy SP. Genetic dissection of the Nrf2-dependent redox signaling-regulated transcriptional programs of cell proliferation and cytoprotection. Physiol Genomics. 2007;32:74–81. doi: 10.1152/physiolgenomics.00126.2007. [DOI] [PubMed] [Google Scholar]
- 13.Jin Y, Huang ZL, Li L, Yang Y, Wang CH, Wang ZT, Ji LL. Quercetin attenuates toosendanin-induced hepatotoxicity through inducing the Nrf2/GCL/GSH antioxidant signaling pathway. Acta Pharmacol Sin. 2019;40:75–85. doi: 10.1038/s41401-018-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gbotosho OT, Kapetanaki MG, Ross M, Ghosh S, Weidert F, Bullock GC, Watkins S, Ofori-Acquah SF, Kato GJ. doi: 10.1016/j.exphem.2020.02.005. Nrf2 deficiency in mice attenuates erythropoietic stress-related macrophage hypercellularity. Exp Hematol: Mar 6 2020 doi: 10.1016/j.exphem.2020.02.005 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK. Bach1 Competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H: quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J Biol Chem. 2005;280:16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 17.Su C, Shi Q, Song X, Fu J, Liu Z, Wang Y, Wang Y, Xia X, Song E, Song Y. Tetrachlorobenzoquinone induces Nrf2 activation via rapid Bach1 nuclear export/ubiquitination and JNK-P62 signaling. Toxicology. 2016;363-364:48–57. doi: 10.1016/j.tox.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Yang S, Zhou B, Wei X, Xue F, Nisar MF, Bian C, Huang X, Yang L, Zhang Y, Bartsch JW, Zhong JL. Nrf2- and Bach1 may play a role in the modulation of ultraviolet A-induced oxidative stress by Acetyl-11-Keto-β-boswellic acid in skin keratinocytes. Skin Pharmacol Physiol. 2017;30:13–23. doi: 10.1159/000452744. [DOI] [PubMed] [Google Scholar]
- 19.Wang MF, Dai AG, Hu RC, Gao JL, Zhu LM. Regulation of Nrf2/Bach1 on γ-glutamylcysteine synthetase in the lung tissue of chronic obstructive pulmonary disease and the effect of mitogen-activated protein kinase in rats. Chin J Pathophysiol. 2009;25:959–964. [Google Scholar]
- 20.Reichard JF, Motz GT, Puga A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acids Res. 2007;35:7074–7086. doi: 10.1093/nar/gkm638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabbri L, Pauwels RA, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary updated 2003. COPD. 2004;1:103–141. doi: 10.1081/COPD-120030163. GOLD Scientific Committee. [DOI] [PubMed] [Google Scholar]
- 22.Sharma A, Bist R. Alteration in MDA, GSH level and hematological changes due to thiamine deficiency in Mus musculus. Interdiscip Toxicol. 2018;11:321–325. doi: 10.2478/intox-2018-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sehitoglu MH, Karaboga I, Kiraz A, Kiraz HA. The hepatoprotective effect of Aloe vera on ischemia-reperfusion injury in rats. North Clin Istanb. 2019;6:203–209. doi: 10.14744/nci.2018.82957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Cristóvão C, Cristóvão L, Nogueira F, Bicho M. Evaluation of the oxidant and antioxidant balance in the pathogenesis of chronic obstructive pulmonary disease. Rev Port Pneumol. 2013;19:70–75. doi: 10.1016/j.rppneu.2012.09.002. (In English, Portuguese) [DOI] [PubMed] [Google Scholar]
- 26.Foyer CH, Noctor G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environment. 2010;28:1056–1071. [Google Scholar]
- 27.Venè R, Castellani P, Delfino L, Lucibello M, Ciriolo MR, Rubartelli A. The cystine/cysteine cycle and GSH are independent and crucial antioxidant systems in malignant melanoma cells and represent druggable targets. Antioxid Redox Signal. 2016;15:2439–2453. doi: 10.1089/ars.2010.3830. [DOI] [PubMed] [Google Scholar]
- 28.Gonnelli S, Caffarelli C, Maggi S, Rossi S, Siviero P, Crepaldi G, Nuti R. Utility of QUS assessment in COPD patients treated with GCS: The EOLO study. Bone. 2009;45 (Suppl):S144–S145. [Google Scholar]
- 29.Jin X, Song L, Li Z, Newton IP, Zhao M, Liu W. Dichlorodiphenyldichloroethylene exposure reduces r-GCS via suppressed Nrf2 in HepG2 cells. Environ Toxicol. 2016;31:350–359. doi: 10.1002/tox.22049. [DOI] [PubMed] [Google Scholar]
- 30.El-Deek HEM, Ahmed AM, Mohammed RAA. Aberration of Nrf2-Bach1 pathway in colorectal carcinoma; role in carcinogenesis and tumor progression. Ann Diagn Pathol. 2019;38:138–144. doi: 10.1016/j.anndiagpath.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Gao JL, Dai AG. Bach1 and Nrf2 Regulate γ-Glutamylcysteine synthetase in chronic obstructive pulmonary disease in rats. Chin J Biochemistry Mol Biol. 2008;24:649–655. [Google Scholar]
- 32.Goven D, Boutten A, Leçon-Malas V, Marchal-Sommé J, Amara N, Crestani B, Fournier M, Lesèche G, Soler P, Boczkowski J, Bonay M. Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax. 2008;63:916–994. doi: 10.1136/thx.2007.091181. [DOI] [PubMed] [Google Scholar]
- 33.Sykiotis GP, Bohmann D. Stress-activated Cap‘n’collar transcription factors in aging and human disease. Sci Signal. 2010;3(re3) doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altuntaş E, Turgut T, Ilhan N, Deveci F, Muz HM, Celik I. The levels of oxidant and antioxidant in patients with COPD. Tuberk Toraks. 2003;51:373–379. (In Turkish) [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.