Abstract
It remains uncertain whether immunocompromised patients including solid organ transplant (SOT) recipients will have a robust antibody response to SARS-CoV-2 infection. We enrolled all adult SOT recipients at our center with confirmed SARS-CoV-2 infection who underwent antibody testing with a single commercially available anti-nucleocapsid antibody test at least 7 days after diagnosis in a retrospective cohort. Seventy SOT recipients were studied (56% kidney, 19% lung, 14% liver ± kidney, and 11% heart ± kidney recipients). Thirty-six (51%) had positive anti-nucleocapsid antibody testing, and 34 (49%) were negative. Recipients of a kidney allograft were less likely to have positive antibody testing compared to those who did not receive a kidney (p = .04). In the final multivariable model, the years from transplant to diagnosis (OR 1.26, p = .002) and baseline immunosuppression with more than two agents (OR 0.26, p = .03) were significantly associated with the antibody test result, controlling for kidney transplantation. In conclusion, among SOT recipients with confirmed infection, only 51% of patients had detectable anti-nucleocapsid antibodies, and transplant-related variables including the level and nature of immunosuppression were important predictors. These findings raise the concern that SOT recipients with COVID-19 may be less likely to form SARS-CoV-2 antibodies.
KEYWORDS: antibodies, COVID-19, serology, transplant
Abbreviations: ACR, acute cellular rejection; BiPAP, bilevel positive airway pressure; CKD, chronic kidney disease; ELISA, enzyme-linked immunosorbent assay; EUA, emergency use authorized; HFNC, high-flow nasal cannula; ICU, intensive care unit; NRB, non-rebreather mask; SOT, solid organ transplant
1. INTRODUCTION
With near 20 million confirmed cases of COVID-19 and at least 345,000 associated deaths as of January 1, 2021, the United States remains a major epicenter of SARS-CoV-2 infection.1 This pandemic has significantly impacted all aspects of solid organ transplantation (SOT), including decreased organ procurement, decreased transplant rates in many centers, and thousands of infected SOT recipients across the country. Although there was an initial concern that SOT recipients with COVID-19 would be at risk of poor outcomes, subsequent controlled studies have not confirmed this.2, 3, 4, 5, 6 Nevertheless much uncertainty remains regarding the effect of chronic immunosuppression and optimal therapies for COVID-19 among SOT recipients.7 Organ injury associated with COVID-19 has been linked to an inflammatory response driven by innate responses. While transplant immunosuppression is largely aimed at cognate immunity, transplant recipients on stable immunosuppression may have diminished innate responses, perhaps contributing to their better than expected outcomes with COVID-19.
For SOT patients who recover from COVID-19, as in the general population, the incidence, persistence, and significance of anti-SARS-CoV-2 antibody formation remains unclear. While approximately 78–100% of individuals in the population develop an IgG antibody response to the nucelocaspid protein within 10–21 days following infection, similar data among SOT recipients are limited.8, 9, 10, 11 In one case series, seven SOT recipients hospitalized with COVID-19 were tested for SARS-CoV-2 IgG between 4 and 38 days after symptom onset and all were positive, though larger cohorts are needed.12 Herein we present the clinical characteristics and SARS-CoV-2 antibody response via a commercially available anti-nucelocapsid assay in a large cohort of SOT recipients with COVID-19.
2. MATERIALS AND METHODS
2.1. Patients
Adult (age >18 years) SOT recipients from Columbia University Irving Medical Center with a positive SARS-CoV-2 PCR test between March 13, 2020 and June 23, 2020, and with SARS-CoV-2 antibody testing performed at our institution at least 7 days after the initial positive PCR were included. The SARS-CoV-2 PCR tests used reverse-transcriptase PCR on nasopharyngeal swab specimens via the Roche 6800 platform to diagnose COVID-19.
2.2. Anti-SARS-CoV-2 antibody assay
The commercially available and Emergency Use Authorized (EUA) Roche Elecsys® Anti-SARS-CoV-2 serology test was adopted for all COVID antibody testing at our institution after May 22. This assay tests for anti-nucleocapsid antigen high-affinity antibodies (including IgM, IgG, and IgA) and has a stated sensitivity and specificity of 100% and >99.8%, respectively, when used 7 days from the PCR confirmation of SARS-CoV-2.13 Given the known variability in sensitivity, specificity of the commercially available serologic assays, and the different antibody targets of each, we chose to analyze results with this single assay. For patients who had serologic testing with different assays prior to this date, stored sera were utilized and retested using this specific commercial assay.
The clinical protocol at our center recommended that SOT recipients obtain COVID-19 serology testing after a minimum of 7 days from confirmed SARS-CoV-2 infection by PCR. Additional serology testing beyond the initial test was performed based on test availability, in-person visits to our transplant center and at clinicians’ discretion. Both time from COVID-19 diagnosis by PCR to serology testing date and total number of serology tests performed per patient were collected.
2.3. COVID-19 treatment strategy
Antiviral therapies used against SARS-CoV-2 varied according to severity of disease and available scientific evidence. Initial management for outpatients with mild disease generally consisted of supportive care. Many hospitalized with moderate or severe disease may have received hydroxychloroquine and/or azithromycin based on evidence available at the time of treatment. Inflammatory syndromes and severe disease were managed with either off label or experimental protocols using tocilizumab, high-dose corticosteroids, convalescent plasma, or remdesivir.8 , 12
Regarding immunosuppressive therapy, the general approach at our center was to moderately reduce the overall amount of immunosuppression with a particular emphasis on antimetabolites such as mycophenolate and/or azathioprine. This approach was based on expert opinion developed in conjunction with various organ transplant groups and transplant infectious diseases.
2.4. Analytic approach
Patient characteristics, comorbidities, time from transplant to COVID-19 diagnosis, COVID-19 therapy received, baseline immunosuppression and changes in immunosuppression during COVID-19 treatment, oxygen requirement and clinical outcomes were compared between patients with and without positive antibody testing using ranksum and chi square tests. In addition, logistic regression was utilized to identify independent predictors of antibody detection. All covariates with p-value <0.20 in univariable analyses or with strong clinical importance were included and the final model was built with backwards stepwise progression. A p-value <.05 was considered statistically significant for all analyses. All analyses were performed using STATA 12.1 (College Station, TX). This work was approved by the Columbia University institutional review board.
3. RESULTS
3.1. Patient characteristics and COVID-19 disease severity
Seventy SOT recipients underwent anti-SARS-CoV-2 antibody testing after confirmed symptomatic infection. The median age of the cohort was 57 years, 63% were male, 46% were white, and 39% were of Hispanic ethnicity ( Table 1). There were 39 (56%) kidney transplant recipients (including two kidney-pancreas), 13 (19%) lung transplant recipients, 10 (14%) liver transplant recipients (including one liver-kidney), and 8 (11%) heart transplant recipients (including three heart-kidney). The median time from transplant to COVID-19 diagnosis was 3.21 years. Three (4%) patients were diagnosed with COVID-19 in the first month following transplant, and 18 (26%) patients with COVID-19 were within the first year following transplant. Comorbidities were frequent, with hypertension (73%), diabetes mellitus (42%), and chronic kidney disease (CKD, 76%) being the most common. Forty-two (60%) patients were on >2 immunosuppressive agents at time of diagnosis, and 10 (14%) were treated for acute cellular rejection (ACR) in the 3 months preceding the COVID-19 diagnosis.
TABLE 1.
Baseline demographics among confirmed cases of COVID-19
| All (n = 70) | Positive Ab (n = 36) | Negative Ab (n = 34) | p-value | |
|---|---|---|---|---|
| Days from diagnosis to first serology test, median (IQR) | 47.50 (28.75–68.75) | 52 (31.75–67.75) | 42 (25.75–68.75) | .57 |
| Number of serology tests performed, median (IQR) | 1 (1–2) | 1 (1–2) | 1 (1–2) | .78 |
| Age in years, median n (IQR) | 57 (45–66) | 57 (43.5–68) | 56 (48–65) | .97 |
| Male sex, n (%) | 44 (63) | 27 (75) | 17 (50) | .03 |
| Race, n (%) | .9 | |||
| White | 32 (46) | 15 (42) | 17 (50) | |
| Black | 19 (27) | 11 (31) | 8 (24) | |
| Asian | 2 (3) | 1 (3) | 1 (3) | |
| Other, multiple, or declined | 17 (24) | 9 (25) | 8 (24) | |
| Hispanic Ethnicity, n (%) | 27 (39) | 12 (33) | 15 (44) | .35 |
| Organ Transplant, n (%) | .15 | |||
| Kidney (± pancreas) | 39 (56) | 16 (44) | 23 (68) | |
| Lung | 13 (19) | 7 (19) | 6 (18) | |
| Liver (± kidney) | 10 (14) | 8 (22) | 2 (6) | |
| Heart (± kidney) | 8 (11) | 5 (14) | 3 (9) | |
| Received kidney allograft | 43 (61) | 18 (50) | 25 (74) | .04 |
| Years from transplant to diagnosis, median n (IQR) | 3.21 (0.98–8.57) | 6.08 (2.53–11.61) | 1.51 (0.32–3.74) | <.001 |
| Within 1 month, n (%) | 3 (4) | 0 (0) | 3 (9) | .07 |
| Within 1 year, n (%) | 18 (26) | 3 (8) | 15 (44) | .001 |
| Comorbidities, n (%) | ||||
| HTN | 51 (73) | 24 (67) | 27 (79) | .23 |
| DM | 30 (43) | 14 (39) | 16 (47) | .49 |
| CKD | 53 (76) | 23 (64) | 30 (88) | .02 |
| Chronic lung disease | 15 (21) | 7 (19) | 8 (24) | .68 |
| HIV | 2 (3) | 1 (3) | 1 (3) | .97 |
| BMI >40 Kg/m2 | 5 (7) | 3 (8) | 2 (6) | .69 |
| Baseline immunosuppression, n (%) | ||||
| CNI | 52 (75) | 24 (67) | 28 (82) | .13 |
| Mycophenolate | 57 (81) | 27 (75) | 30 (88) | .16 |
| Steroids | 42 (60) | 19 (53) | 23 (68) | .2 |
| Belatacept | 15 (21) | 5 (14) | 10 (29) | .11 |
| IVIG ± Pheresis | 2 (3) | 0 (0) | 2 (6) | .14 |
| mTOR | 2 (3) | 2 (6) | 0 (0) | .16 |
| Thymoglobulin <3 mo | 10 (14) | 2 (6) | 8 (24) | .03 |
| >2 IS agents | 42 (60) | 17 (47) | 25 (74) | .03 |
| Treated ACR in 3 months prior to diagnosis, n (%) | 10 (14) | 2 (6) | 8 (24) | .03 |
| Immunoglobulin level (IgG) in 3 months prior to diagnosis, median n* (IQR) | 951 (804–1222) | 867 (796–1141) | 959 (818–1222) | .4 |
| Changes in Immunosuppression, n (%) | ||||
| Decrease or hold antimetabolite | 46 (66) | 22 (61) | 24 (71) | .4 |
| Decrease or hold CNI | 8 (11) | 3 (8) | 5 (15) | .4 |
| Therapy, n (%) | ||||
| Hydroxychloroquine | 39 (56) | 22 (61) | 17 (50) | .35 |
| Azithromycin | 25 (36) | 14 (39) | 11 (32) | .57 |
| Remdesivir | 6 (9) | 2 (5.6) | 4 (12) | .35 |
| High dose corticosteroids | 14 (20) | 6 (17) | 8 (24) | .47 |
| Tocilizumab | 6 (9) | 2 (6) | 4 (12) | .35 |
| Convalescent plasma trial | 4 (6) | 1 (3) | 3 (9) | .28 |
| Highest Level of Respiratory Support, n (%) | .77 | |||
| Room air | 41 (59) | 23 (64) | 18 (53) | |
| Nasal Cannula | 21 (30) | 10 (28) | 11 (32) | |
| NRB/high flow/BIPAP | 5 (7) | 2 (6) | 3 (9) | |
| Mechanical ventilation | 3 (4) | 1 (3) | 2 (6) | |
| Highest Level of Medical Support, n (%) | .62 | |||
| Outpatient | 27 (39) | 15 (42) | 12 (35) | |
| Medical Ward | 37 (53) | 19 (53) | 18 (53) | |
| ICU Admission | 6 (9) | 2 (6) | 4 (12) |
Immunoglobulin levels were available in 39 of 70 pts.
Overall, 27 (39%) were treated as outpatient, 37 (53%) were admitted to a general medical floor, and 6 (9%) required intensive care unit (ICU)-level care. In terms of supplemental oxygen requirement, 41 (59%) remained on ambient air, 21 (30%) required supplemental oxygen by nasal cannula, 5 (7%) required either supplemental oxygen by non-rebreather mask, high-flow nasal cannula (HFNC), or bilevel positive airway pressure (BiPAP), and 3 (4%) were mechanically ventillated.
3.2. COVID-19 treatment approach
Thirty-nine (56%) were treated with hydroxychloroquine, 25 (36%) with azithromycin, and 6 (9%) with remdesivir. Immunomodulatory therapies including short course of high-dose corticosteroids and tocilizumab were administered in 14 (20%) and 6 (9%) patients, respectively. Four (6%) patients participated in a randomized controlled trial involving convalescent versus non-convalescent plasma (treatment arm assignment unknown). During treatment, 46 (66%) patients had their antimetabolite decreased or held, and 8 (11%) patients had their calcineurin inhibitor decreased or held.
3.3. Prevalence of SARS-CoV-2 antibodies
Among the 70 patients in the cohort, 36 (51.43%) had at least one positive SARS-CoV-2 antibody test and 34 (48.57%) tested negative (Table 1). The proportion with positive antibody varied by organ: 41% of kidney ± pancreas transplant recipients, 54% of lung transplant recipients, 80% of liver ± kidney transplant recipients, and 63% of heart ± kidney transplant recipients ( Figure 1). Seropositivity was significantly lower in kidney transplant recipients compared to patients who did not receive a kidney transplant (42% vs 65%, p = .04).
FIGURE 1.
Antibody test results by organ transplant type
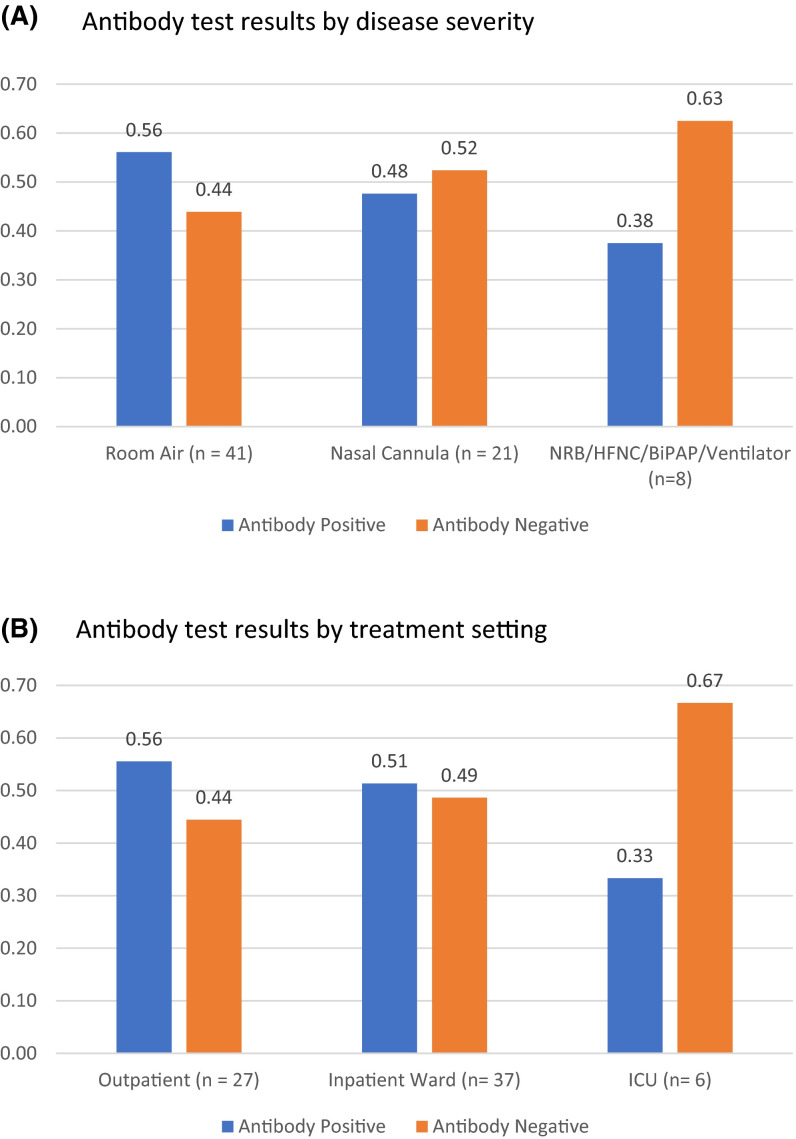
With regard to disease severity, 23 (56%) patients treated without supplemental oxygen, 10 (48%) who received oxygen by nasal cannula, and 3 (38%) who required more intense forms of oxygen support (non-rebreather mask, high-flow nasal cannula, BiPAP, mechanical ventilation) were seropositive ( Figure 2A, p = .77). In terms of treatment setting, 15 (56%) patients treated outpatient, 19 (51%) treated on a general inpatient medical floor, and 2 (33%) treated in the intensive care unit were seropositive (Figure 2B, p = .62).
FIGURE 2.
(A) Antibody test results by disease severity. (B) Antibody test results by treatment setting
Time from transplant to diagnosis of COVID-19 was significantly shorter among seronegative patients as compared to seropositive patients (1.51 vs 6.08 years respectively, p < .001, Table 1). In addition, a greater proportion of patients treated for ACR in the 3 months prior to diagnosis were seronegative (24% vs 6%, p = .03, Table 1).
3.4. Prevalence of SARS-CoV-2 antibodies over time after diagnosis and patients tested multiple times
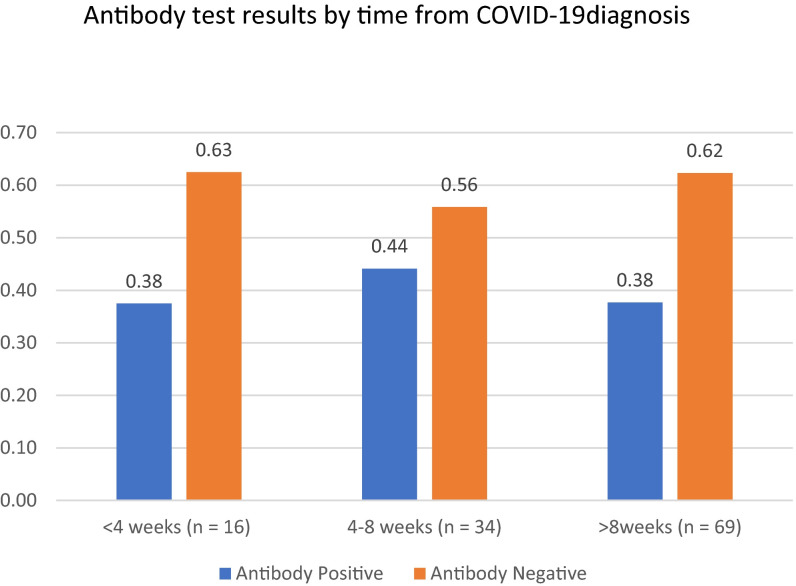
A total of 119 serology tests were performed in 70 patients. Patients were tested at a median of 47.5 days after confirmed diagnosis of COVID-19, with 16 (13%) patients tested within 4 weeks, 34 (29%) tested from 4 to 8 weeks, and 69 (58%) tested more than 8 weeks from original diagnosis ( Figure 3).
FIGURE 3.
Antibody test results by time from COVID-19 diagnosis
A total of 30 (43%) patients were tested more than once. Of these, 24 had consistently positive (n = 10) or negative (n = 14) tests. Among the six patients with discordant results, five had an initial positive test but subsequently tested negative. One patient initially had a negative test, performed 7 days after confirmed COVID-19 infection, followed by a positive test 124 days later.
3.5. Multivariable analysis for predictors of SARS-CoV-2 antibody positivity
In univariable logstic regression, male sex (OR 3.00, p = .03) and years from transplant to COVID-19 diagnosis (OR 1.24, p = .002) were significantly associated with positive antibody testing, while receipt of a kidney allograft (OR 0.36, p = .046), the presence of CKD (OR 0.24, p = .02), use of >2 baseline immunosuppressive agents (OR 0.32, p = .03), and ACR in the last 3 months (OR 0.19, p = .047) were predictive of a negative antibody test result ( Table 2).
TABLE 2.
Multivariable model to predict positive antibody test.
| Covariate | Univariate |
Multivariable |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age (years) | 0.99 | 0.97–1.03 | .89 | |||
| Male sex | 3.00 | 1.09–8.24 | .03 | |||
| Years from transplant to diagnosis | 1.24 | 1.08–1.41 | .002 | 1.26 | 1.09–1.46 | .002 |
| Recipient of a kidney allograft | 0.36 | 0.13–0.98 | .046 | 0.33 | 0.09–1.09 | .07 |
| HTN | 0.52 | 0.18–1.53 | .23 | |||
| DM | 0.72 | 0.28–1.85 | .49 | |||
| CKD | 0.24 | 0.07–0.82 | .02 | |||
| >2 baseline IS agents | 0.32 | 0.12–0.88 | .03 | 0.26 | 0.08–0.86 | .03 |
| ACR in last 3 months | 0.19 | 0.04–0.98 | .047 | |||
| Mechanical ventilation | 0.46 | 0.04–5.29 | .53 | |||
| ICU admission | 0.44 | 0.08–2.58 | .36 | |||
In the final multivariable model, years from transplant to COVID-19 diagnosis (OR 1.26, p = .002), baseline immunosuppression with >2 agents (OR 0.26, p = .03) were significantly associated with antibody test result, while being a recipient of a kidney transplant was no longer significant (OR 0.33, p = .07).
4. DISCUSSION
In this cohort of 70 SOT recipients, only 51% of patients were found to ever have a positive test for anti-nucleocapsid antibodies at a median of 47.5 days following a confirmed diagnosis of COVID-19. This low seroconversion rate stands in sharp contrast to the immunocompetent population, for which data suggest that the large majority of patients (78–100%) develop IgM and IgG antibodies within 10–21 days after infection with SARS-CoV-2.8, 9, 10 , 14 , 15 These results are nevertheless consistent with prior studies showing decreased antibody formation among SOT recipients in the setting of either natural infection or vaccination. Among SOT recipients with natural influenza infection, for example, the seroconversion rate of approximately 65% at four weeks is significantly lower than the 82–95% observed in immunocompetent individuals.16 Similar findings have also been described after vaccination. In a recent study of 161 SOT recipients receiving either the standard or high-dose influenza vaccine, only 56% to 79% of patients seroconverted, rates significantly lower than those in the general population.17 Similar differences in antibody response have also been reported for both hepatitis B and pneumococcal vaccines.18 , 19
Similarly, there also was a significant association between the intensitiy of immunosuppression and lack of anti-nucleocapsid antibody formation in the present study. When serology results were stratified by time from transplant to diagnosis of COVID-19, patients temporally closer to transplant were less likely to be seropositive. This trend was especially pronounced in those diagnosed with COVID-19 within a year of transplantation, with only 3 of 18 patients (16.7%) found to be seropositive. Although likely multifactorial, this temporal association potentially reflects the impact of more intensified initial immunosuppression. Similarly, SOT recipients who were treated for ACR in the 3 months prior to diagnosis or were maintained on >2 immunosuppressive agents at time of diagnosis exhibited lower prevalence of seropositivity. Overall, the above findings suggest a strong correlation between immunosuppression and seroconversion among SOT recipients with COVID-19.
This study also suggests that recipients of kidney transplantation as well as those with CKD are particularly less likely to form antibodies after COVID-19. The diminished antibody response among kidney transplant recipients is likely to be multifactorial and possibly resulting from the use of T cell–depleting induction agents, limited baseline kidney function and a high prevalence of comorbidities such as diabetes and hypertension. Meanwhile, CKD has been shown to result in both diminished B cell (reduced number, increased apoptosis) and T cell (impaired activation/proliferation, reduced expression of costimulatory molecules) function, thereby lowering overall immune response.20 In one study of 165 patients, stage of chronic kidney disease was an independent predictor of seroconversion after hepatitis B immunization, with rates of 40–50% in latter stages of CKD as compared to >95% in those with normal glomerular filtration rates.21 This effect has been replicated in response to vaccination against influenza (30–40% response rates compared to 70% in healthy adults) and pneumococcus (lower anti-pneumococcal anti-IgG titers and increased rates of pneumococcal infections when compared to healthy individuals).22 , 23 Further, patients with CKD have been shown to undergo a more rapid decay of protective antibodies secondary to impaired immune responses, particularly after vaccination.24
The lower seropositivity among the SOT recipients with more severe disease, either due to more intense oxygen requirement or treatment setting, is somewhat surprising. Several studies so far have suggested a positive correlation between disease severity and antibody detection, whether anti-spike or nucleocapsid antibodies are measured. One study of 74 asymptomatic versus symptomatic patients infected with SARS-CoV-2 demonstrated that 40% of asymptomatic patients compared to 12.9% of the symptomatic group became negative for IgG in the early convalescent phase.9 This was further substantiated in a recent correspondence sampling of 34 patients with mild SARS-CoV-2 infection, in which anti-SARS-CoV-2 spike receptor-binding domain IgG levels fell rapidly, with an estimated half-life of 36 days over the observation period.25 Finally, a study of 254 individuals with SARS-CoV-2 infection also showed higher antibody levels among those with more severe disease.26 It is possible that as the antibody testing done in our cohort was per clinical protocol in the outpatient setting, those with critical illness who did not survive the hospitalization were not included in this analysis, leading to bias in this assessment regarding disease severity.
Antibody testing for SARS-CoV-2 has so far been a complex aspect of the COVID-19 pandemic, and the clinical role of current commercially available assays remains uncertain.11 There are a number of FDA emergency use authorized serology tests avaiable, with the majority measuring antibodies to a component of either the spike or the nucleocapsid protein. Diagnostically, some studies suggest that seropositivity to the nucelocaspid protein is more sensitive than spike protein antibody (100% vs. 91%) for detecting early infection although others suggest an equal sensitivity.14 , 27 Meanwhile, the recent tantalizing results in vaccine and monoclonal antibody studies have highlighted the potentially central role of tagerting the spike protein for both therapy and immunogenicity. The FDA emergency use authorized monoclonal antibodies balamnivimab and the combined infusion of casirivimab and imdevimab are major examples of the potential therapeutic impact of targeting the spike protein.28 , 29 More importantly, the striking preliminary results in the phase 3 vaccine trials, including both mRNA and adenovirus vectored platforms uniformly targetting the the spike protein, are strong indicators of the potential protective effects of anti-spike immunoglobulins.30 , 31 Although a recent study among healthcare workers showing that those with either anti-spike or anti-nucelocapsid antibodies were unlikely to be reinfected in the ensuing 6 months, interpreting any serological results beyond simply establishing recent infection remains uncertain at this time.32
Given that SOT recipients were not included the initial phase 3 COVID-19 vaccine trials, there is some uncertainty regarding the efficacy of these vaccines in this immunocompromised population. Although the present study did not measure anti-spike antibodies, the results nevertheless raise important concerns about whether SOT recipients will mount a robust reponse to SARS-CoV-2 vaccination. Further studies on the rates and significance of both B and T cell responses to SARS-CoV-2 are needed to make assertions regarding possible protective immunity to COVID-19.33
There are several limitations to this study. The commercial assay initially adopted in our institution measure anti-nucleocapsid protein antibodies only and thus no assessment can be made of either anti-spike protein antibody formation nor cellular immunity, which recent literature suggests is pervasive, potentially more long-lasting, and present in individuals with no reported history of SARS-CoV-2 infection or known contacts with infected patients.34 There is also a potential survival bias in our cohort, as patients had to be alive for a sufficient time after diagnosis to be tested for antibodies (at time of writing, only 1 patient of the original cohort of 70 is deceased). As such, this cohort included fewer patients with severe illness (9% of patients as compared to 39% in previous work assessing all solid organ transplant patients infected with SARS-CoV-2) which could thereby affect rates of seropositivity.6 However, it is clear that those who survive are the group that will be the population of interest in the study of long-term consequences of antibody formation. Finally, the variable timing of serological testing in this cohort raises the possibility that overall seroconversion may be underestimated, especially among those patients tested late after infection. This is of particular concern because of recent studies suggesting that the mean estimated half life of anti-nucleocapsid IgG is approximately 52 days/7.4 weeks.35 Nevertheless, there was no significant difference in seroversion rates when results were stratified by time periods (<4 weeks, 4–8 weeks and >8 weeks) post diagnosis in this study. Further, among those patients with multiple tests, there was a low rate of discordant results when the time difference between test spanned 52 days after infection.
In summary, over a 3-month period at a large multi-organ transplant center, 70 SOT recipients infected with SARS-CoV-2 underwent serology testing, of whom 36 (51%) were antibody positive. In this population, there was an association between the detection of antibodies and time from transplant, the level of immunosuppression, the organ transplanted as well as CKD. These findings raise the concern that SOT recipients with COVID-19 may be less likely to form SARS-CoV-2 antibodies, neutralizing or otherwise, and there is a suggestion that these antbodies may wane over time. These findings may have potentially major implications for either natural or vaccine related immunity to SARS-COV-2 in this population.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
Data will be available if needed.
Footnotes
Daniel Burack and Marcus R. Pereira are co-first authors.
REFERENCES
- 1.WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/. Accessed 9/10/2020.
- 2.Husain SA, Dube G, Morris H, et al. Early outcomes of outpatient management of kidney transplant recipients with coronavirus disease 2019. Clin J Am Soc Nephrol. 2020;15(8):1174–1178. doi: 10.2215/CJN.05170420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaudhry ZS, Williams JD, Vahia A, et al. Clinical characteristics and outcomes of COVID-19 in solid organ transplant recipients: a case-control study. Am J Transplant. 2020. [DOI] [PMC free article] [PubMed]
- 4.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382(25):2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97(6):1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kates OS, Haydel BM, Florman SS, et al. COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis. 2020.
- 8.Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin Infect Dis. 2020;71(15):778–785. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 11.Deeks JJ, Dinnes J, Takwoingi Y, et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;6:CD013652. doi: 10.1002/14651858.CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fung M, Babik JM. COVID-19 in immunocompromised hosts: what we know so far. Clin Infect Dis. 2021;72(2):340–350. doi: 10.1093/cid/ciaa863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caillard S, Anglicheau D, Matignon M, et al. An initial report from the French SOT COVID Registry suggests high mortality due to Covid-19 in recipients of kidney transplants. Kidney Int. 2020;98(6):1549–1558. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burbelo PD, Riedo FX, Morishima C, et al. Sensitivity in detection of antibodies to nucleocapsid and spike proteins of severe acute respiratory syndrome coronavirus 2 in patients with coronavirus disease 2019. J Infect Dis. 2020;222(2):206–213. doi: 10.1093/infdis/jiaa273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du Z, Zhu F, Guo F, Yang B, Wang T. Detection of antibodies against SARS-CoV-2 in patients with COVID-19. J Med Virol. 2020;92(10):1735–1738. doi: 10.1002/jmv.25820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirzel C, Ferreira VH, L’Huillier AG, et al. Humoral response to natural influenza infection in solid organ transplant recipients. Am J Transplant. 2019;19(8):2318–2328. doi: 10.1111/ajt.15296. [DOI] [PubMed] [Google Scholar]
- 17.Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis. 2018;66(11):1698–1704. doi: 10.1093/cid/cix1082. [DOI] [PubMed] [Google Scholar]
- 18.Kumar D, Rotstein C, Miyata G, Arlen D, Humar A. Randomized, double-blind, controlled trial of pneumococcal vaccination in renal transplant recipients. J Infect Dis. 2003;187(10):1639–1645. doi: 10.1086/374784. [DOI] [PubMed] [Google Scholar]
- 19.Loinaz C, de Juanes JR, Gonzalez EM, et al. Hepatitis B vaccination results in 140 liver transplant recipients. Hepatogastroenterology. 1997;44(13):235–238. [PubMed] [Google Scholar]
- 20.Ma BM, Yap DYH, Yip TPS, Hung IFN, Tang SCW, Chan TM. Vaccination in patients with chronic kidney disease-Review of current recommendations and recent advances. Nephrology. 2021;26(1):5–11. doi: 10.1111/nep.13741. [DOI] [PubMed] [Google Scholar]
- 21.DaRoza G, Loewen A, Djurdjev O, et al. Stage of chronic kidney disease predicts seroconversion after hepatitis B immunization: earlier is better. Am J Kidney Dis. 2003;42(6):1184–1192. doi: 10.1053/j.ajkd.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Eiselt J, Kielberger L, Rajdl D, Racek J, Pazdiora P, Malanova L. Previous vaccination and age are more important predictors of immune response to influenza vaccine than inflammation and iron status in dialysis patients. Kidney Blood Press Res. 2016;41(2):139–147. doi: 10.1159/000443416. [DOI] [PubMed] [Google Scholar]
- 23.Mahmoodi M, Aghamohammadi A, Rezaei N, et al. Antibody response to pneumococcal capsular polysaccharide vaccination in patients with chronic kidney disease. Eur Cytokine Netw. 2009;20(2):69–74. doi: 10.1684/ecn.2009.0153. [DOI] [PubMed] [Google Scholar]
- 24.Kato S, Chmielewski M, Honda H, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3(5):1526–1533. doi: 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild covid-19. N Engl J Med. 2020;383(11):1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roltgen K, Powell AE, Wirz OF, et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol. 2020;5(54) doi: 10.1126/sciimmunol.abe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lumley SF, O’Donnell D, Stoesser NE, et al. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N Engl J Med. 2021;384(6):533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melgaco JG, Azamor T, Ano Bom APD. Protective immunity after COVID-19 has been questioned: What can we do without SARS-CoV-2-IgG detection? Cell Immunol. 2020;353:104114. doi: 10.1016/j.cellimm.2020.104114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 35.Grandjean L, Saso A, Torres A, et al. Humoral response dynamics following infection with SARS-CoV-2. medRxiv. 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available if needed.