Abstract
We describe a case of proven transmission of SARS-CoV-2 from lung donor to recipient. The donor had no clinical history or findings suggestive of infection with SARS-CoV-2 and tested negative by reverse transcriptase polymerase chain reaction (RT-PCR) on a nasopharyngeal (NP) swab obtained within 48 h of procurement. Lower respiratory tract testing was not performed. The recipient developed fever, hypotension, and pulmonary infiltrates on posttransplant day (PTD) 3, and RT-PCR testing for SARS-CoV-2 on an NP swab specimen was non-reactive, but positive on bronchoalveolar lavage (BAL) fluid. One thoracic surgeon present during the transplantation procedure developed COVID-19. Sequence analysis of isolates from donor BAL fluid (obtained at procurement), the recipient, and the infected thoracic surgeon proved donor origin of recipient and health-care worker (HCW) infection. No other organs were procured from this donor. Transplant centers and organ procurement organizations should perform SARS-CoV-2 testing of lower respiratory tract specimens from potential lung donors, and consider enhanced personal protective equipment for HCWs involved in lung procurement and transplantation.
KEYWORDS: clinical research/practice, donors and donation: donor-derived infections, infection and infectious agents – viral, infectious disease
Abbreviations: BAL, bronchoalveolar lavage; CT, computed tomography; Ct, cycle threshold; DDD, donor-derived disease; HCW, health-care worker; ICU, intensive care unit; NP, nasopharyngeal; OPO, organ procurement organization; RT-PCR, reverse transcriptase polymerase chain reaction; SOT, solid organ transplant
1. INTRODUCTION
Unexpected transmission of infection from donor to recipient is uncommon, occurring in fewer than 1% of transplant recipients.1 Nonetheless, donor-derived disease (DDD) is associated with poor outcomes including graft loss or death noted in about one-third of affected recipients.1 Emerging pathogens create particular challenges in assessment of disease transmission risk, and recent infectious agents of concern have included H1N1 pandemic influenza (2009), West Nile virus, Ebola virus, and Zika virus.2, 3, 4
The disruption in solid organ transplantation (SOT) resulting from the COVID-19 epidemic has, however, been unprecedented. Particularly in hard-hit areas early in the pandemic, resource limitations led to a drastic decline in SOTs.5 Transplant centers and organ procurement organizations (OPOs) have been forced to grapple with many unknowns regarding the most effective donor clinical assessment and testing processes to reduce the risk of donor to recipient disease transmission. Further, OPOs and transplant centers have to consider how to protect their staff from exposure to potential donors infected with SARS-CoV-2.
To our knowledge, in the United States, no proven case of donor to recipient transmission of SARS-CoV-2 has been reported. We report a case of proven donor to recipient transmission of SARS-CoV-2 despite negative pre-procurement donor nasopharyngeal (NP) SARS-CoV-2 testing. Lower respiratory tract testing was not performed. In addition, we describe transmission to a health-care worker (HCW) associated with the transplant procedure.
2. CASE REPORT AND EPIDEMIOLOGIC INVESTIGATION
2.1. Epidemiologic review
Laboratory studies and recipient medical records were reviewed. Donor medical records from DonorNet were also reviewed. At the transplant center (and for transplant center employees participating in procurement), per institutional policy, HCWs were considered to have a high-risk exposure if prolonged contact occurred while not wearing appropriate personal protective equipment (PPE) (mask and eyewear protection for routine exposures, and N95/PAPR for aerosol generating procedures). Exposed employees were notified and those with high-risk exposures were instructed to undergo SARS-CoV-2 testing 5 days from the last exposure. All employees with low risk exposures were provided with a list of symptoms consistent with COVID-19 and instructed to contact occupational health for testing should symptoms develop.
2.2. Organ donor
The organ donor was a woman from the upper Midwest who suffered severe brain injury in an automobile accident and quickly progressed to brain death during a 2-day hospital admission. Computed tomography (CT) of the chest performed on the day of admission showed areas of consolidation within the posterior right lower lobe. The radiologist interpretation was that these areas represented atelectasis and pulmonary contusion. The left lung was clear aside from mild atelectasis at the base. A bronchoscopy performed 1 day after admission showed inflammation in the trachea and minimal thick white secretions, which were not persistent, and submucosal erythema and punctate hemorrhage in the distal trachea and mainstem bronchi felt to be secondary to contusion. SARS-CoV-2 RT-PCR testing was performed within 48 h of procurement on a nasopharyngeal swab and was resulted as not detected (Roche Cobas SARS-CoV-2, Roche Molecular Systems). History obtained from family revealed no history of travel or any recent fever, cough, headache, or diarrhea. It is unknown if the donor had any recent exposures to persons known or suspected to be infected with SARS-CoV-2.
2.3. Lung recipient
The bilateral lung recipient had chronic obstructive lung disease. A rapid SARS-CoV-2 RT-PCR performed on an NP swab (DiaSorin Molecular) was negative 12 h before transplant. The transplant procedure was uncomplicated, and the patient arrived in the intensive care unit (ICU) mechanically ventilated with low oxygen requirements and minimal vasoactive support. Her initial immunosuppression included methylprednisolone 1 mg/kg every 12 h for six doses followed by a taper, tacrolimus, and mycophenolate mofetil without any induction immunosuppression.
A persistently low cardiac index was noted on posttransplant day (PTD) 2, and a transthoracic echocardiogram revealed acute right ventricular dysfunction. On PTD 3, the patient developed worsening fever, hypotension, and ventilator requirements. CT imaging of the chest showed multifocal consolidations. Due to the sudden worsening in respiratory status and evolving lung infiltrates, a bronchoscopy was performed and BAL samples were collected from both lungs. HCWs present during the bronchoscopy all wore full PPE. Quantitative culture and Gram stain of the BAL fluid revealed a few polymorphonuclear leukocytes and no significant bacterial growth. Due to the atypical presentation of septic shock with cardiomyopathy, the BAL fluid was sent for SARS-CoV-2 PCR testing along with a second NP swab. The NP swab was not detected but the BAL sample was positive for SARS-CoV-2 with a low cycle threshold (Ct) value. Repeat testing the following day revealed positive testing of both tracheal aspirate and NP swab. Due to concern for donor origin of the SARS-CoV-2, BAL fluid obtained from the donor at the time of procurement was tested at the transplant center and was positive with a low Ct value ( Table 1). The patient’s ongoing posttransplant course in the ICU was complicated by multisystem organ failure requiring prolonged mechanical ventilation and circulatory support. Specific treatment for COVID-19 has included remdesivir (two 5-day courses) and convalescent plasma on two occasions. Tacrolimus was continued and mycophenolate mofetil was held; her corticosteroid dosing was maintained at methylprednisolone 30 mg daily then tapered. She developed worsening respiratory distress and required veno-venous extracorporeal membrane oxygenation. Her overall clinical status continued to decline and she was not considered a candidate for re-transplantation. Support was withdrawn and she died on PTD 61. Her SARS-CoV-2 PCR remained positive on PTD 60 with a Ct value of 29.3.
TABLE 1.
SARS-CoV-2 test results
| Day –2 | Day 0 | Day +3 | Day +4 | Day +27 | Day +60 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | Donor | Donor | Recipient | Recipient | Surgeon | Recipient | Recipient | Recipient | ||
| Specimen | NP swab | BALa,b | NP swab | BAL | NP swab | NP swab | NP swab | Tracheal aspirate | NP swab | NP swab |
| Result | Not detected | Positive Ct = 8.5/9.5 | Not detected | Positive Ct = 8.1/9.2 | Not detected | Positive Ct = 23.6 | Positive Ct = 35.7 | Positive Ct = 9.8/10.4 | Positive Ct = 13.6/13.9 | Positive Ct = 29.3 |
Abbreviations: BAL, bronchoalveolar lavage; Ct, cycle threshold; NP, nasopharyngeal.
BAL obtained by procurement team and tested at transplant center after recipient tested positive.
Single Ct values are representative of SARS-CoV-2 RdRp and N genes, shared fluorophore one target (Alinity System); two Ct values are representative of SARS-CoV-2 two targets and ORF1ab genes, separate fluorophores (DiaSorin).
2.3.1. Epidemiologic investigation
Testing for SARS-CoV-2 was performed 5 or more days postexposure for high-risk exposed HCWs. In addition, some HCW with low-risk exposures chose to be tested. While one thoracic surgeon tested positive for SARS-CoV-2 on PTD 4 (Table 1), a surgical trainee, two anesthesia faculty, two anesthesia and one surgery critical care trainee, two nurses, the procuring surgeon, and one perfusionist tested negative. No member of the procurement team was infected. The surgeon was not present at the time of procurement, but did prepare the lungs for implantation and performed the transplant procedure. He did describe potential exposure to mucous and sputum when cutting off the bronchial staple line resulting in deflation. No other exposed HCW was diagnosed with SARS-CoV-2 linked to this event.
2.3.2. Laboratory methods
All experiments using SARS-CoV-2 were performed at the University of Michigan in compliance with containment procedures in laboratories approved for use by the University of Michigan Institutional Biosafety Committee and Environment, Health & Safety. The use of residual specimens from hospitalized patients with COVID-19 has been approved by the University of Michigan Institutional Review Board.
All RT-PCR testing and associated cycle threshold values were based on assays performed in the Michigan Medicine Clinical Microbiology Laboratory including the Simplexa™ COVID-19 Direct (DiaSorin) and Alinity m SARS-CoV-2 assay (Abbott Molecular) with limits of detection of 500 and 100 SARS-CoV-2 genomic RNA copies/ml, respectively.
Viral genomic RNA was extracted from the original specimens using PureLink Viral RNA Kits (Invitrogen). Complementary DNA corresponding to the genome was amplified by RT-PCR in two multiplex reactions using the ARTIC network V3 primer set. Sequencing libraries were prepared using the NEBNext Ultra II kit and sequenced on an Illumina MiSeq with 2 × 250 bp reads, V2 chemistry. Reads were aligned to the Wuhan-1 reference (GenBank: MN908947.3) with BWA-MEM version 0.7.15. Sequencing adaptors were removed and the ARTIC primer was trimmed with iVar version 1.2.1. Consensus sequences were determined with samtools (version 1.5) mpileup and iVar version 1.2.1, placing an N at reference positions with fewer than 10 reads.
Whole genome consensus sequences were aligned to the Wuhan-Hu-1 reference with MAFFT (version 7.467)6 as implemented in the augur pipeline.7 We constructed the phylogenetic tree with IQ-TREE using a GTR model and 1000 ultrafast bootstrap replicates and performed ancestral reconstruction for node sequences with TreeTime (version 0.7.6).8
All analysis code, patient sequences, and metadata are available https://github.com/lauringlab/DonorDerivedInfection. Original sequence reads are available at www.ncbi.nlm.nih.gov/sra under BioProject.
2.3.3. Laboratory results
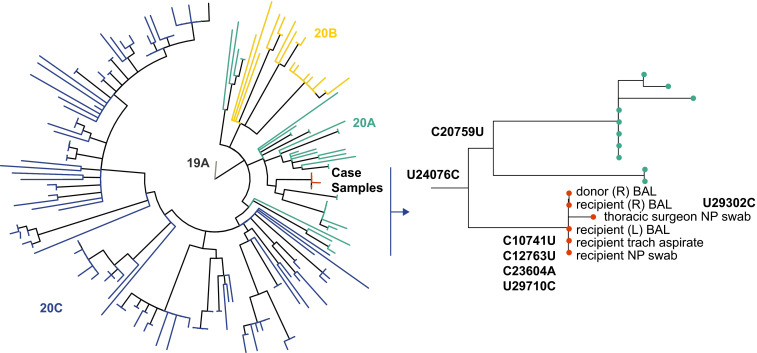
Five of the six sequences were identical at the consensus level: donor right BAL (PTD 0), recipient left BAL (PTD 3), recipient right BAL (PTD 3), recipient tracheal aspirate (PTD 4), and recipient nasopharyngeal swab (PTD 4). The virus from the surgeon differed at just one position relative to the other samples ( Figure 1). We performed a whole genome phylogenetic analysis with these sequences—126 SARS-CoV-2 sequences from the same hospital, and an additional 59 sequences from across the state of Michigan. The six sequences associated with this case formed a cluster within clade 20A, which was distinct from other lineages and circulating in Michigan (Figure 1). These data demonstrate that both the transplant recipient and the surgeon acquired SARS-CoV-2 from the donor lungs.
FIGURE 1.
Phylogenetic analysis of the six sequences associated with this case [Color figure can be viewed at wileyonlinelibrary.com]
3. DISCUSSION
We describe a case of donor-derived infection with SARS-CoV-2 with secondary transmission to a HCW. Sequencing proved donor origin of the infection. Transmission occurred despite RT-PCR testing on a donor NP sample obtained within 48 h of procurement and no clinical or radiological evidence suggestive of donor infection with SARS-CoV-2; lower respiratory tract testing was not performed. Donor-derived infection with SARS-CoV-2 has significant implications for the health of the recipient, but also for HCWs who may be exposed prior to recipient diagnosis.
Donor-derived infection with SARS-CoV-2 in a lung recipient is not surprising as donor-derived infection with viruses that infect the respiratory tract has been well described.1 While SARS-CoV-2 infection has been detected in kidneys, liver, heart, and blood,9 , 10 no other organs were donated in this case and thus this report provides no information on the risk of transmission to non-lung recipients. Of note, transmission of respiratory viruses (including H1N1 2009 pandemic influenza) has been almost exclusively detected in lung recipients.1 A recent case series of suspected transmissions based on early posttransplant diagnosis of COVID-19 revealed no proven donor origin of infection, but high mortality for early posttransplant COVID-19.11
While one base pair difference was noted in the transplant surgeon, an alternative source of infection is highly unlikely, given that the lineage associated with the donor, recipient, and surgeon sequences is quite distinct from other viral sequences circulating in our community at that time. While it is impossible to completely rule out alternate infection sources based on genome sequencing, transmission can lead to fixation of new viral mutations, consistent with the additional polymorphism in the surgeon.
This report does raise questions regarding the appropriate SARS-CoV-2 screening for potential donors. While Organ Procurement and Transplantation Network policies do not require SARS-CoV-2 donor testing, optional fields were added to DonorNet to indicate test results.12 The American Society of Transplantation recommends a combination of clinical and laboratory donor screening. Laboratory testing for non-lung donors should include at least one sample from the respiratory tract, and consideration for a second sample obtained within 24–48 h of procurement. For thoracic organ donors, SARS-CoV-2 testing of the lower respiratory tract is recommended by both AST and the International Society for Heart and Lung Transplantation (ISHLT), if safe and feasible.13 , 14 In the reported case, clinical and laboratory screening was not suggestive of infection with SARS-CoV-2, as family reported no suggestive symptoms and pulmonary imaging showed non-specific findings consistent with atelectasis combined with pulmonary contusion (consistent with her motor vehicle accident), and laboratory screening with RT-PCR performed on an NP swab within 48 h of procurement was negative.
While testing of BAL fluid for SARS-CoV-2 would have diagnosed donor infection in this case, very limited data exist on the incremental sensitivity obtained by SARS-CoV-2 testing on BAL fluid or other lower respiratory tract (LRT) specimens as compared to NP swabs. Case reports do, however, describe patients with negative SARS-CoV-2 testing in NP swabs but positive tests on BAL specimens and testing on BAL fluid may remain positive after testing on upper respiratory specimens becomes negative.15, 16, 17 Barriers to testing BAL specimens on potential lung donors include lack of validation of testing of LRT specimens—although some assays do have FDA emergency use authorization on BAL specimens18—as well as possible difficulty obtaining the LRT specimens in a timely fashion resulting in delays in procurement.
Since both the donor and recipient had negative SARS-CoV-2 pre-procedure testing, by institutional protocol, HCWs were not required to wear N95 masks and eye protection. During the double lung transplant procedure, HCWs are exposed to material expelled from the donor lung and one HCW was proven to be infected, likely during the transplant. Thus, transplant centers should consider the possible benefit of N95 masks and eye wear protection during lung transplantation even with negative donor testing as recommended by the ISHLT, while levels of community infection remain high.14
We describe a case of proven donor-derived infection with SARS-CoV-2 in a lung transplant recipient despite negative clinical and laboratory screening of the donor complicated by secondary transmission to a HCW. While barriers to implementation do exist, OPOs and transplant centers—particularly in areas of high SARS-CoV-2 transmission in the community—should perform SARS-CoV-2 testing of LRT specimens on potential lung transplant donors.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1.Kaul DR, Vece G, Blumberg E, et al. Ten years of donor-derived disease: a report of the disease transmission advisory committee. Am J Transplant. 2020;21(2):689–702. doi: 10.1111/ajt.16178. [DOI] [PubMed] [Google Scholar]
- 2.Organ Procurement and Transplantation Network. Identifying Risk Factors for West Nile Virus (WNV). During evaluation of potential living donors. https://optn.transplant.hrsa.gov/resources/guidance/identifying-risk-factors-for-west-nile-virus-wnv-during-evaluation-of-potential-living-donors/. Published 2013. Accessed November 30, 2020.
- 3.Organ Procurement and Transplantation Network. Guidance on zika virus. https://optn.transplant.hrsa.gov/news/guidance-on-zika-virus/. Published 2016. Accessed November 30, 2020.
- 4.Kaul DR, Mehta AK, Wolfe CR, Blumberg E, Green M. Ebola virus disease: implications for solid organ transplantation. Am J Transplant. 2015;15(1):5–6. doi: 10.1111/ajt.13093. [DOI] [PubMed] [Google Scholar]
- 5.Loupy A, Aubert O, Reese PP, Bastien O, Bayer F, Jacquelinet C. Organ procurement and transplantation during the COVID-19 pandemic. Lancet. 2020;395(10237):e95–e96. doi: 10.1016/S0140-6736(20)31040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadfield J, Megill C, Bell SM, et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018;34(23):4121–4123. doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sagulenko P, Puller V, Neher RA. TreeTime: maximum-likelihood phylodynamic analysis. Virus Evol. 2018;4(1):vex042. doi: 10.1093/ve/vex042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braun F, Lütgehetmann M, Pfefferle S, et al. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet. 2020;396(10251):597–598. doi: 10.1016/S0140-6736(20)31759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puelles VG, Lutgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones JM, Kracalik I, Rana MM, et al. SARS-CoV-2 infections among recent organ recipients, March-May 2020, United States. Emerg Infect Dis. 2020;27(2):552–555. doi: 10.3201/eid2702.204046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Organ Procurement and Transplantation Network. COVID-19. https://optn.transplant.hrsa.gov/covid-19/. Published 2020. Accessed November 25, 2020.
- 13.American Society of Transplantation. SARS-CoV-2 (Coronavirus, 2019-nCoV): recommendations and guidance for organ donor testing. https://www.myast.org/sites/default/files/Donor%20Testing_100520_revised_ReadyToPostUpdated10-12.pdf. Published 2020. Accessed November 25, 2020.
- 14.Guidance from the International Society of Heart and Lung Transplantation. Regarding the SARS CoV-2 pandemic. https://ishlt.org/ishlt/media/documents/SARS-CoV-2_Guidance-for-Cardiothoracic-Transplant-and-VAD-center.pdf. Published 2020. Accessed January 13, 2021.
- 15.Gualano G, Musso M, Mosti S, et al. Usefulness of bronchoalveolar lavage in the management of patients presenting with lung infiltrates and suspect COVID-19-associated pneumonia: a case report. Int J Infect Dis. 2020;97:174–176. doi: 10.1016/j.ijid.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez RM. Clinical samples for SARS-CoV-2 detection: review of the early literature. Clin Microbiol Newsl. 2020;42(15):121–127. doi: 10.1016/j.clinmicnews.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simplex COVID-19 Direct, Package Insert, DiaSorin Molecular. 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.