Abstract
The immune system plays a crucial role in the response against severe acute respiratory syndrome coronavirus 2 with significant differences among patients. The study investigated the relationships between lymphocyte subsets, cytokines, and disease outcomes in patients with coronavirus disease 2019 (COVID‐19). The measurements of peripheral blood lymphocytes subsets and cytokine levels were performed by flow cytometry for 57 COVID‐19 patients. Patients were categorized into two groups according to the severity of the disease (nonsevere vs. severe). Total lymphocytes, T cells, CD4+ T cells, CD8+ T cells, B cells, and natural killer cells were decreased in COVID‐19 patients and statistical differences were found among different severity of illness and survival states (P ˂ 0.01). The levels of IL‐6 and IL‐10 were significantly higher in severe and death groups and negatively correlated with lymphocyte subsets counts. The percentages of Th17 in the peripheral blood of patients were higher than those of healthy controls whereas the percentages of Th2 were lower. For the severe cases, the area under receiver operating characteristic (ROC) curve of IL‐6 was the largest among all the immune parameters (0.964; 95% confidence interval: 0.927–1.000, P < 0.0001). In addition, the preoperative IL‐6 concentration of 77.38 pg/ml was the optimal cutoff value (sensitivity: 84.6%, specificity: 100%). Using multivariate logistic regression analysis and ROC curves, IL‐6 > 106.44 pg/ml and CD8+ T cell counts <150 cells/μl were found to be associated with mortality. Measuring the immune parameters and defining a risk threshold can segregate patients who develop a severe disease from those with a mild pathology. The identification of these parameters may help clinicians to predict the outcome of the patients with high risk of unfavorable progress of the disease.
Keywords: COVID‐19, cytokines, flow cytometry, lymphocytes, prognosis, SARS‐CoV2
Graphical Abstract
COVID‐19 patients with poor prognosis exhibited a significant depletion of lymphocyte‐subset counts, with remarkably increasing concentrations of IL6 and IL10.

Abbreviations
- AUC
Area under the ROC curve
- CD
cluster of differentiation
- COVID‐19
coronavirus disease 2019
- CT
computerized tomography
- DP
double positive T cells for CD4 and CD8
- MERS
Middle East respiratory syndrome
- NK
natural killer
- ROC
receiver operating characteristic
- RTE
recent thymic emigrant
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- Th
T helper lymphocyte
- TCM
central memory T cells
- TEMRA
terminally differentiated T cells
- WHO
World Health Organization
1. INTRODUCTION
In 2019, a new coronavirus was identified as the cause of a disease outbreak that emerged in Wuhan, a city in Hubei's province in China. This novel virus referred to as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) rapidly spread to other provinces of China and to other countries, hence becoming a global health threat and triggering unprecedented measures worldwide. In February 2020, the World Health Organization (WHO) formally named this respiratory infection coronavirus disease 2019 (COVID‐19). A month later, the rapidly escalating health crisis led the WHO to declare the SARS‐COV‐2 outbreak a pandemic. 1 As of December 27, the virus had infected at least 79 million people worldwide resulting in more than 1.7 million deaths. On the same day, 97,857 cases were reported in Algeria with an apparent fatality rate of 2.78%. 2
Although most of SARS‐CoV‐2 infected patients generally display mild‐to‐moderate symptoms and undergo spontaneous regression, approximately 14% of infected subjects experience a severe disease characterized by a significant hypoxia inherent to the respiratory distress syndrome. The remaining COVID‐19 patients, who are mainly individuals over 60 and/or with comorbidities, develop a critical condition, which requires admission to the intensive care unit. 3 It is likely that the variation in the overall immune response to SARS‐CoV‐2 is partly responsible for the clinical heterogeneity. Therefore, the identification of the immune signature of patients with COVID‐19 is crucial for predicting the outcome of infection as well as informing clinical risk‐based stratification, which can help for early intervention and treatment.
It is widely accepted that lymphocytes play a central role in the defense mechanisms against viral respiratory infection. Therefore, a better understanding of the dynamic changes of lymphocyte populations and the cytokine production in COVID‐19 patients is a prerequisite for the development of efficient therapeutics, vaccines, and outcome‐prediction tools. A growing number of investigations have reported phenotypic alterations in lymphocyte subsets and overproduction of the inflammatory cytokines, leading to a cytokine storm, indicating a potential association between this immune dysregulation and viral pathogenesis. 4 , 5 Several meta‐analysis studies have identified the relevant risk factors correlated with the progression of the disease. Among these factors lymphocytes counts and cytokines levels have been of great interest. 6 , 7
The aim of this single‐center study was to characterize the changes of peripheral blood lymphocyte subsets and quantify plasma cytokine levels in an Algerian cohort of SARS‐CoV‐2 infected patients with distinct disease severity. In addition, the study explored the predictive value of these parameters as a prognosis tool to determine the mortality and severity risks in COVID‐19 patients.
2. METHODS
2.1. Study design and participants
This study was conducted at the department of medical immunology at Issaad Hassani University Hospital in Algiers, Algeria. A total of 57 patients were enrolled in the study from March 22 to April 29, 2020. Diagnosis of COVID‐19 was confirmed by the detection of SARS‐COV‐2 nucleic acid in nasopharyngeal swab samples using a real‐time RT‐PCR test. Blood samples were collected at the moment of the first in hospital consultation for COVID‐19 symptoms, before any treatment. The clinical outcomes (survival or death) of all patients were followed up to 28 d after admission in the hospital. All patients received the following treatment: hydroxychloroquine 200 mg + azithromycin 500 mg + vitamin C 250 mg + zinc 10 mg. To assess the impact of infection on the polarization of T helper lymphocytes (Th), 24 age‐ and sex‐matched healthy individuals without SARS‐CoV‐2 infection were recruited as healthy controls.
The study was approved by the institutional ethics committee of Issaad Hassani University Hospital. Informed consent was obtained from all subjects enrolled in the study.
2.2. Clinical classification of the COVID‐19 patients
Disease severity classification of COVID‐19 patients was performed according to the interim guidelines from the WHO and the National Health Commission of China 8 , 9 : (i) The mild disease group was defined as patients displaying mild, clinical symptoms with no pneumonia on computerized tomography (CT) imaging. (ii) Patients with a moderate illness were characterized by fever, respiratory symptoms, and a CT imaging indicating the presence of pneumonia. (iii) Patients belonging to the severe disease group were those who met at least one of the following criteria: shortness of breath and respiratory rate ≥ 30 breaths/min; SpO2 ≤ 93% at a rest state; PaO2/FiO2 ≤ 300 mmHg; and/or lung infiltrates >50% of the lung field within 24–48 h. (iv) Critical patients were defined as those meeting at least one of the following conditions: patients with respiratory failure who were in need of mechanical ventilation; patients displaying signs of cardiovascular shock; and patients with other organ failures, which required monitoring in the intensive care unit.
For the purpose of the following study, the cases were divided into nonsevere (mild to moderate) and severe groups (severe to critical).
2.3. Data collection
Data on demographic characteristics, comorbidities, severity assessment on admission, as well as clinical outcomes were retrieved from a medical record system. All data were reviewed and validated by a team of trained physicians.
2.4. Measurement of cytokine levels
Approximately 4–5 ml of anticoagulant fresh peripheral blood were collected from patients with COVID‐19 and healthy controls. The plasma samples were separated by centrifugation at 2500 rpm for 10 min at 4°C, and immediately stored at −80°C until analysis.
IL‐2, IL‐4, IL‐6, IFNγ, IL‐10, IL‐17A, and TNFα were quantified using BD Cytometric Bead Array Human Th1/Th2/Th17 Cytokine Kit (BD Biosciences, San Jose, CA, USA) following manufacturer's instructions. Briefly, 50 μl of mixed capture beads were incubated with 50 μl of cytokines standards or plasma samples along with 50 μl of phycoerythrin detection reagent at room temperature for 3 h. The captures beads were washed and resuspended in 300 μl of wash buffer and were then acquired on the BD FACSLyric flow cytometer (Becton Dickinson, San Jose, CA, USA). The acquired data were subsequently analyzed for individual cytokine concentrations using the FCAP Array Software Version 3.0 (BD Biosciences). The detection limits were, respectively: 2.6 pg/ml for IL‐2; 4.9 pg/ml for IL‐4; 2.4 pg/ml for IL‐6; 4.5 pg/ml for IL‐10 pg/ml; 3.8 pg/ml for TNF‐α; 3.7 pg/ml for IFNγ; and 18.9 pg/ml for IL‐17A.
2.5. Quantification of lymphocyte subsets
For the immune cell phenotyping, 1 ml of peripheral blood (EDTA—anticoagulated whole blood) was used within 8 h from collection. Lymphocytes were stained with the monoclonal antibodies listed in the Supporting Information Table S1. After red blood cell removal using a lysis buffer followed by washing steps, cells were stained and acquired using a BD FACSLyric flow cytometer (Becton Dickinson) and the data were analyzed using BD FACSuite Software (BD Biosciences).
Lymphocyte subsets were defined as follows10: T cells (CD3+), CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), B cells (CD3−CD19+), and natural killer (NK) cells (CD3−CD16+CD56+). In CD4+ T cells compartment, recent thymic emigrant (RTE) were defined as (CD4+CD31+CD45RA+), effector and central memory CD4+ T cells as (CD4+CD45RO+), and for those who remain as (CD4+CD45RA+). CD8+ T cells maturation subsets were identified using CCR7 (CD197) and CD45RA and include naïve (CCR7+CD45RA+), terminally differentiated T cells (TEMRA; CCR7−CD45RA+), central memory (CCR7+CD45RA−), and effector memory (CCR7−CD45RA−).
2.6. T cell activation and intracellular cytokine staining
Heparinized blood samples collected from COVID‐19 patients and healthy controls were cultured at 1 h after collection. Whole blood (500 μl) was diluted 1:1 with RPMI 1640 supplemented with PMA (2.5 ng/ml, Sigma‐Aldrich, St Louis, MO, USA), ionomycin (1 μg/ml, Sigma‐Aldrich), and BD GolgiStop (BD Biosciences). Cells were incubated for 4 h at 37°C in the presence of 5% CO2. A total of 100 μl of the stimulated cells were stained with an antibody cocktail containing anti‐CD3 and anti‐CD4 antibodies (Supporting Information Table S1). The cells were then fixed with 300 μl of BD Cytofix Fixation Buffer (BD Bioscience) and permeabilized with 2 ml of BD Perm/Wash permeabilization buffer (BD Biosciences) following the manufacturer instructions. The cells were washed and incubated with the human Th1/Th2/Th17 phenotyping cocktail (BD Biosciences) and anti‐CD3 for 30 min. For each sample, 20,000 CD4+ cells were acquired on BD FACSLyric flow cytometer (Becton Dickinson) and analyzed with BD FACSuite Software (BD Biosciences). Th1, Th2, and Th17 cells were identified as CD3+CD4+ cells producing IFNγ, IL‐4, and IL‐17, respectively. Results were expressed as a percentage of IL‐17+ (Th17) or IL‐4+ (Th2) or IFNγ+ (Th1) expressing cells from CD3 and CD4+ T cell population.
2.7. Statistical analysis
All statistical analyses were performed by SPSS software (IBM Statistic 20.0) and GraphPad Prism software 6. Shapiro‐Wilk normality test was conducted to estimate the distribution of the data. Categorical variables were expressed as frequency rates or percentages and significance was detected by χ2 or Fisher's exact test. Continuous variables were expressed as mean and sd or medians and interquartile range (IQR) values. For normally distributed continuous variables, differences between groups were compared using independent group t‐test; conversely, the Mann‐Whitney U‐test was used for continuous variables that were not normally distributed. Correlations were determined using the Spearman rank correlation analysis. Receiver operating characteristic (ROC) curve analysis was conducted to evaluate the ability of the immunologic parameters in predicting patient outcomes. The optimal cut‐off points were obtained by calculating Youden's index. Backward stepwise binary logistic regression was performed on the covariates. Survival analysis was performed using Kaplan‐Meier method. For all statistical analysis, P ˂ 0.05 was considered statistically significant.
3. RESULTS
3.1. Demographics and baseline characteristics
A total of 57 patients with SARS‐CoV‐2 infection were included in this study. The baseline characteristics of patients were summarized in Table 1. The mean age of the subjects was 59.72 yr. A total of 40 patients (70.18%) were males and 17 (29.82%) were females. Of the 23 patients with comorbidities (40.35%), hypertension (19.30%), and diabetes (12.18%) were the most common underlying diseases, followed by benign tumors and pulmonary diseases, with almost identical percentages for each condition at around of 7.02%, hypothyroidism 5.26%, and cardiovascular diseases (1.75%).
TABLE 1.
Baseline characteristics of patients
| All patients N = 57 | Nonsevere N = 31 | Severe N = 26 | P‐value | |
|---|---|---|---|---|
| Age (years) | 59.72 (±14.95) | 53.71 (±14.42) | 66.88 (±12.38) | ˂0.001 |
|
Sex Male Female |
40/57 (70.18%) 17/57 (29.82%) |
19/31 (61.29%) 12/31 (38.71%) |
21/26 (80.77%) 5/26 (19.23%) |
0.109 |
|
Underlying diseases Any Hypertention Cardiovascular disease Diabetes Tumors (benign) Pulmonary diseases Hypothyroidism Others |
23/57 (40.35%) 11/57 (19.30%) 1/57 (1.75%) 7/57 (12.28%) 4/57 (7.02%) 4/57 (7.02%) 3/57 (5.26%) 4/57 (7.02%) |
5/31 (16.12%) 2/31 (6.45%) 0/31 (0%) 1/31 (3.23%) 1/31 (3.23%) 0/31 (0%) 2/31 (6.45%) 2/31 (6.45%) |
18/26 (69.23%) 9/26 (34.62%) 1/26 (3.85%) 6/26 (23.08%) 3/26 (11.54%) 4/26 (15.39%) 1/26 (3.85%) 3/26 (11.54%) |
˂0.001 |
|
Clinical outcomes Cured and discharge Death |
42/57 (73.68%) 15/57 (26.32%) |
31/31 (100%) 0/31 (0%) |
11/26 (42.31%) 15/26 (57.69%) |
˂0.001 |
Data are expressed as mean ± sd or n/N (%), where N is the total number of patients with available data. P‐values comparing nonsevere and severe group are from independent group t‐test, χ2, or Fisher's exact test.
According to the guidelines criteria described in Section 2 (Methods), the patients were divided into two groups with respect to the severity of their illness: Among the patients, 31 cases (54.38%) were classified as nonsevere group and 26 (45.62%) categorized as severe group. Compared with the patients in the nonsevere group, patients in the severe group were older (66.88 ± 12.38 yr vs. 53.71 ± 14.42 yr, P < 0.001) and were commonly associated with underlying comorbidities (18/26 [69.23%] vs. 5/31 [16.12%], P < 0.001). In addition, there were no significant gender differences between the two groups. The patients in the severe group showed a higher mortality than that in the nonsevere group (15/26 (57.69%) vs. 0/31 (0%); P < 0.001). Based on the outcome of the disease, 42 patients were ultimately discharged (73.68%) and 15 patients died (26.32%).
3.2. Lymphocyte subsets and cytokine alterations in COVID‐19 patients with different disease severity and outcomes
The quantification of peripheral blood lymphocyte subsets shown in Table 2 and Supporting Information Table S2 were compared with those already established in the literature as a reference 10 for adults with the same age group. Our data showed that lymphocyte absolute counts were below the normal range in 36 (63.15%) patients with a median of lymphocyte counts of 758.0 cells/μl (IQR, 480.5–1288.5). A significant decrease in total lymphocyte counts was found in the severe cases compared to the nonsevere group (513.5 vs. 1199 cells/μl; P ˂ 0.001) (Fig. 1A). Furthermore, this decrease was more pronounced in the fatal cases when compared to survived patients (401 vs. 983.5 cells/μl; P ˂ 0.001) (Fig. 1B). The absolute counts of the main lymphocyte subsets (T cells, B cells, and NK cells) were decreased in more than one‐third of patients with COVID‐19. T cells decreased in 33 (57.89%) patients, B cells decreased in 31 (54.38%) patients, and NK cells decreased in 21 (36.84%) patients. Among patients with nonsevere COVID‐19, the median value of total T cells, B cells, and NK cells counts were 789, 150, and 171 cells/μl, respectively, whereas the median values decreased to 354, 40, and 76 in the severe group. Moreover, this study found that the median values of these subsets were significantly higher in nonsurvivors than in the survivor group (277 vs. 726 cells/μl; P < 0.001 for T cells, 39 vs. 112.5 cells/μl; P = 0.006 for B cells and 58 vs. 157.5 cells/μl; P = 0.003 for NK cells) (Fig. 1B).
TABLE 2.
The laboratory findings of patients (categorized by severity of illness and survival states)
| Nonsevere vs. severe | Survival vs. death | |||||||
|---|---|---|---|---|---|---|---|---|
| Normal range* | All patients (N = 57) | Nonsevere (N = 31) | Severe (N = 26) | P‐value | Survival (N = 42) | Death (N = 15) | P‐value | |
|
Lymphocyte subsets Lymphocytes (T cells + B cells + natural killer [NK] cells)/μl T cells (CD3+)/μl B cells (CD3−CD19+)/μl NK cells (CD3−CD16+CD56+)/μl |
1000–2500 700–1900 100–400 100–400 |
758 (480.5–1288.5) 521 (305.5–859.5) 92(38–175.5) 136 (66–216.5) |
1199 (704–1623) 789 (465–1153) 150 (67–249) 171 (133–292) |
513.5 (271–701) 354 (178–489.50) 40 (26.75–92.25) 76 (49.75–139.50) |
˂0.001 ˂0.001 ˂0.001 ˂0.001 |
983.5 (610.75–1372.5) 726 (391.75–968.75) 112.5 (56–181.5) 157.5 (96.5–250.5) |
401 (243–642) 277 (146–463) 39 (27–92) 58 (39–123) |
˂0.001 ˂0.001 0.006 0.003 |
|
T cells subsets CD4+ T cells/μl CD8+ T cells/μl CD4+/CD8+ DP T cells (CD4+CD8+)/μl CD4+CD45RA+ T cells/CD4+ T cells % RTEs (CD3+CD4+CD45RA+CD31+)/CD4+ T cells % CD4+CD45RO+ T cells/CD4+ T cells% Naïve CD8+ T cells (CD8+CD45RA+CCR7+)/CD8+ T cells % TEMRA CD8+ T cells (CD8+CD45RA+CCR7−)/CD8+ T cells % CM CD8+ T cells (CD8+ CD45RA−CCR7+)/T CD8+ T cells % EM CD8+ T cells (CD8+CD45RA−CCR7−)/CD8+ T cells % Activated CD4+ T cells (CD4+HLADR+CD38+)/CD4+ T cells % a Activated CD8+ T cells (CD8+HLADR+CD38+)/CD8+ T cells % a CD4+ IFNγ+ (Th1)/CD4+ T cells % b CD4+ IL‐4+ (Th2)/CD4+ T cells % b CD4+ IL‐17A+ (Th17)/CD4+ T cells % b |
400–1300 200–700 1.5–2.9 2–88 29.4–55.4 6.4–41.7 44.4–68.9 28.6–64.3 14.4–48.8 6.1–14.3 6.4–16.7 1.2–2.3 2.5–6.7 / / / |
288 (149–440) 208 (119–328) 1.4 (0.965–1.995) 8 (3.5–18.5) 27 (19.5–42.75) 15 (8.5–22.5) 73 (57.25–80.5) 11.3 (5.17–25.17) 46.29 (31.83–60.24) 4.19 (2.965–5.26) 32.56 (19.99–40.45) 3 (1.9–4.85) 6.8 (3.295–14.6) 16.34 (11.48–20.80) 2.53 (1.24–3.97) 1.56 (0.67–3.8) |
395 (264–661) 295 (197–432) 1.46 (0.91–2.05) 8 (4–19) 27 (19–37.50) 15 (10.50–25.50) 73 (62.50–81) 17.21 (7.21–33.83) 46.29 (30.50–58.05) 4.07 (3.14–5.32) 29.85 (22.38–36.69) 2.65 (1.36–5.43) 6.34 (2.79–10.03) 17.25 (12.76–24.29) 2.45 (1.24–4.66) 0.98 (0.53–3.02) |
160 (82.50–250.50) 126.5 (58.25–203.50) 1.35 (1.16–1.94) 6 (2–14) 27.25 (19.62–48.50) 12 (7.25–21.75) 72.75 (51.50–80.38) 7.31 (3.46–15.43) 47.27 (34.80–62.44) 4.415 (2.78–5.20) 34.62 (16.90–52.45) 3 (2.50–4.40) 9 (4–20) 14.17 (9.58–19.19) 2.53 (1.2–3.79) 1.65 (1.36–5.12) |
˂0.001 ˂0.001 0.974 0.275 0.380 0.476 0.380 0.014 0.344 0.898 0.293 0.239 0.234 0.112 0.739 0.105 |
299 (192.25–513) 273 (162.5–374.75) 1.3 (0.85–1.83) 8.5 (4.75–19) 29 (20.38–41.13) 16.75 (10–22.13) 71 (58.88–79.63) 15.17 (6.26–32.79) 46.80 (30.88–60.23) 4.09 (2.54–5.28) 30.28 (19.05–36.83) 2.6 (1.51–4.52) 6.42 (2.77–8.85) 17.25 (12.76–24.29) 2.43 (1.51–3.98) 1.32 (0.63–3.55) |
146 (81–321) 119 (50–163) 1.5 (1.28–2.7) 4 (2–10) 23.5 (18–53) 11 (8–24) 76.5 (47–82) 6.57 (3.08–14.3) 43.55 (34.2–60.3) 4.8 (3.27–5.25) 42.75 (20.48–57.97) 3.4 (2.78–5.6) 9.5 (5.4–23.75) 12.65 (7.42–17.64) 2.63 (0.92–4.19) 1.56 (1.31–5.84) |
0.004 ˂0.001 0.030 0.022 0.928 0.253 0.928 0.016 0.951 0.310 0.052 0.051 0.053 0.042 0.692 0.333 |
|
Cytokines IL‐17A (pg/ml) IFNγ (pg/ml) TNFα (pg/ml) IL‐10 (pg/ml) IL‐6 (pg/ml) IL‐4 (pg/ml) IL‐2 (pg/ml) |
/ / / / / / / |
0 (0–8.18) 0.41 (0–2.32) 0.86 (0.12–2.10) 4.11 (1.45–8.13) 52.25 (13.03–127.08) 0.86 (0.35–1.40) 0 (0–0) |
0 (0–18.19) 0 (0–1.38) 1.04 (0.37–1.99) 2.13 (0.75–4.11) 20.22 (3.38–42.59) 1 (0.56–1.65) 0 (0–0.05) |
0 (0–0.94) 0.71 (0.14–2.95) 0.39 (0–2.36) 7.45 (4.5–11.57) 136.45 (89.12–255.80) 0.79 (0–1.09) 0 (0–0) |
0.240 0.038 0.214 ˂0.001 ˂0.001 0.137 0.407 |
0 (0–11.24) 0.21 (0–2.48) 0.86 (0.17–1.57) 2.85 (0.9–6.51) 24.65 (6.52–71.52) 0.97 (0.53–1.58) 0 (0–0.01) |
0 (0–0) 0.56 (0–1.01) 0.6 (0–3.46) 7.88 (4.33–13.72) 198.27 (138.96–288.02) 0.79 (0–0.97) 0 (0–0) |
0.385 0.708 0.729 0.001 ˂0.001 0.060 0.596 |
*Reference values for the lymphocyte subsets, according to Yi et al. 10
The number of COVID‐19 patients who were tested for activated CD4+ T cells and CD8+ T cells was 22 and 19 in the nonsevere and severe group, 29 and 12 in the survival and death group, respectively.
The number of COVID‐19 patients who were tested for Th1, Th2, and Th17, was 16 and 14 in the nonsevere and severe group, 20 and 10 in the survival and death group, respectively.
Data are presented as medians and interquartile ranges. P‐values comparing nonsevere and severe group, survival and death group are from independent group t‐test, or Mann‐Whitney U‐test.
COVID‐19: coronavirus disease 2019; CD4+ T cells; CD8+ T cells; DP: double positive; RTEs: recent thymic emigrants; TEMRA: terminally differentiated effector memory‐RA; CM: central memory; and EM: effector memory.
FIGURE 1.

Lymphocyte subsets and cytokine levels in coronavirus disease 2019 (COVID‐19) patients. (A) and (B) Lymphocyte subsets in different groups. (C) Proportion of Th1, Th2, and Th17 cells among healthy controls and COVID‐19 patients. (D) and (E) Cytokines levels in different groups
In addition to the changes occurring in lymphocyte subpopulations, the study extended our analysis to the different subsets of T lymphocytes. CD4+ T cells and CD8+ T cells were, respectively, decreased in 42 (73.68%) and 27 (47.36%) COVID‐19 patients. Interestingly our data revealed that CD4+/CD8+ ratio was below the normal range in 33 patients (57.89%). When compared to the nonsevere group, a more substantial decrease in both CD4+ T cells and CD8+ T cells was noticed in the severe group (160 vs. 395 cells/μl; P < 0.001 for CD4+ T cells, 126.5 vs. 295 cells/μl; P < 0.001 for CD8+ T cells) (Fig. 1A). A similar tendency was also observed between fatal cases and survivors (146 vs. 299 cells/μl; P = 0.004 for CD4+ T cells, 119 vs. 273 cells/μl; P < 0.001 for CD8+ T cells) (Fig. 1B). The CD4+/CD8+ ratio showed no difference between severe and nonsevere cases whereas a statistically significant difference was observed between survivors and deceased patients (P = 0.03). The frequencies of naïve CD8+ T cells (CD8+, CD45RA+, CCR7+) decreased in 45 patients (78.94%), which were markedly lower in severe cases compared to nonsevere COVID‐19 patients (7.31% vs. 17.21%; P = 0.014). Lower frequencies were also found in the deceased group compared to patients who were cured and discharged from the hospital (6.57% vs. 15.17%; P = 0.016). The percentages of effector memory CD8+ T cells (CD3+, CD8+, CD45RA−, CCR7−) increased in 47(82.45%) patients. Interestingly, there was no statistical difference between severe and nonsevere cases (P = 0.293), but a clear tendency to significance (P = 0.052) was found between dead patients and survivors. Quantification of (CD4+, CD45RA+) T cells, RTE (CD4+, CD45RA+, CD31+), effector, and central memory CD4+ T cells (CD4+, CD45RO+), TEMRA CD8+ T cells (CD3+, CD8+, CD45RA+, CCR7−), and central memory CD8+ T cells (CD3+, CD8+, CD45RA−, CCR7+) did not show any significant difference regardless of the disease severity and the survival. However, the study observed that more than half of the patients 30 (52.63%) had decreased percentages of (CD4+, CD45RA+) T cells without a perturbation in thymic output of RTE in 48(84.21%) patients. Furthermore, this study noticed an increase of (CD4+, CD45RO+) T cells in 31(54.38%) patients, whereas only 25 (43.85%) patients had an increase of their peripheral TEMRA CD8+ T cells. Moreover, and most of the patients had low frequencies of central memory CD8+ T cells 45 (78.94%).
In parallel to the quantification of T cell subsets upon SARS‐COV2 infection, the study also used HLA‐DR and CD38 to determine the activation status of T cells. 11 , 12 , 13 , 14 This study observed that both median frequencies of CD38+HLA‐DR+ CD4+T cells (3%, IQR 1.9–4.85) and CD38+HLA‐DR+ CD8+ T cells (6.8%, 3.295–14.6) were above the upper reference limits in COVID‐19 patients. Interestingly, increased activated CD4+T cells and CD8+ T cells was observed in patients with a fatal outcome compared to those who survived (P = 0.051 and P = 0.053).
In order to characterize the nature of CD4 T helper response to SARS‐CoV‐2 infection, the percentages of CD4+IFNγ+ (Th1), CD4+IL‐4+ (Th2), and CD4+IL‐17A+ (Th17) cells were assessed by flow cytometry. As shown in Figure 1C and Table 2, COVID‐19 patients exhibited a significant reduction in the proportion of IL‐4 producing CD4+ T cells and a significant increase of Th17 frequency when compared to healthy subjects (P < 0.0001 and P = 0.009). On the other hand, only a significant decrease of Th1 cells was observed in the patients who died compared to those who recovered (P = 0.042). Interestingly, no differences were found between nonsevere and severe cases in terms Th frequencies. Besides the analysis of Th populations, the study also assessed plasma cytokine levels in these patients (Table 2). IL‐6 and IL‐10 concentrations were markedly higher in deceased cases compared to the survivors (P < 0.001 and P = 0.001) (Fig. 1E). The patients who had a severe clinical disease showed a significant increase of IL‐6, IL‐10, and IFNγ (P < 0.001, P = 0.001 and P = 0.038) (Fig. 1D).
3.3. Correlation between lymphocyte subsets and cytokines
In order to gain a broader understanding of the immune response triggered by SARS‐COV2 infection, the study examined the correlation between the different lymphocyte subsets and the cytokine levels (IL‐2, IL‐4, IL‐6, IL‐10, TNFα, IFNγ, and IL‐17A) (Table 3, Fig. 2). The analysis revealed a strong positive correlation between IL‐6 and IL‐10 (r = 0.783, P < 0.0001). The total lymphocyte T cell, CD4+ T cell, CD8+ T cell, B cell, NK cell, and, double positive (DP) cell numbers as well as the frequencies of naïve CD8+ T cells were negatively correlated with IL‐6 and IL‐10 levels (P < 0.01). In contrast, IL‐6 and IL‐10 concentrations were positively correlated with the frequencies of terminally differentiated TEMRA CD8+ T cells (P < 0.05, P < 0.01). Furthermore, the study found that the levels of IL‐4 and TNFα were positively correlated with total lymphocyte, T cell, NK cell, CD4+T cell, CD8+ T cell, and naïve CD8+ T cell counts. No correlation was found between theses lymphocyte subsets and IL‐17A concentrations. Similarly, there was no significant correlation between IL‐17A and Th17 cells (data not shown).
TABLE 3.
Correlations between lymphocytes subsets and cytokines
| Cytokines Lymphocyte subsets | IL‐17 A | IFNγ | TNFα | IL‐10 | IL‐6 | IL‐4 | IL‐2 |
|---|---|---|---|---|---|---|---|
| Lymphocytes | 0.058 | −0.162 | 0.271* | −0.455** | −0.554** | 0.295* | 0.123 |
| T cells (CD3+) | 0.030 | −0.229* | 0.290* | −0.465** | −0.547** | 0.296* | 0.107 |
| B cells (CD3−CD19+) | 0.102 | −0.158 | 0.221* | −0.463** | −0.501** | 0.111 | 0.117 |
| Natural killer (NK) cells (CD3−CD16+CD56+) | 0.154 | ,009 | 0.308** | −0.323** | −0.442** | 0.340** | 0.109 |
| CD4+ T cells | 0.082 | −0.221* | 0.316** | −0.482** | −0.537** | 0.295* | 0.110 |
| CD8+ T cells | −0.015 | −0.162 | 0.237* | −0.370** | −0.469** | 0.237* | 0.056 |
| DP T cells (CD3+CD4+CD8+) | −0.216 | −0.193 | 0.067 | −0.344** | −0.473** | 0.286* | 0.048 |
| CD4+CD45RA+ T cells | 0.174 | 0.016 | 0.031 | −0.124 | −0.061 | 0.108 | −0.117 |
| CD4+CD45RO+ T cells | −0.174 | −0.016 | −0.031 | 0.124 | 0.061 | −0.108 | 0.117 |
| RTEs (CD3+CD4+CD45RA+CD31+) | 0.212 | −0.052 | 0.096 | −0.251* | −0.187 | 0.207 | 0.041 |
| Naive CD8+ T cells (CD8+CD45RA+CCR7+) | 0.036 | −0.283* | 0.258* | −0.468** | −0.415** | 0.297* | 0.249* |
| TEMRA CD8+ T cells (CD8+CD45RA+CCR7−) | 0.063 | 0.351** | −0.071 | 0.364** | 0.234* | −0.199 | −0.258* |
| CM CD8+ T cells (CD8+CD45RA−CCR7+) | −0.164 | −0.274* | 0.085 | −0.205 | −0.097 | 0.299* | 0.154 |
| EM CD8+ T cells (CD8+CD45RA−CCR7−) | 0.000 | −0.084 | −0.152 | 0.036 | 0.114 | −0.090 | −0.024 |
| Activated CD4+ T cells (CD4+HLADR+CD38+) | −0.034 | 0.084 | −0.197 | 0.210 | 0.284* | −0.298* | −0.051 |
| Activated CD8+ T cells (CD8+HLADR+CD38+) | 0.007 | −0.148 | −0.076 | 0.113 | 0.266* | −0.144 | −0.038 |
The correlations were analyzed by the Spearman test. Data expressed as correlation coefficient.
* P < 0.05; ** P < 0.01. DP: double positive; RTEs: recent thymic emigrants; TEMRA: terminally differentiated effector memory‐RA; CM: central memory; and EM: effector memory.
FIGURE 2.
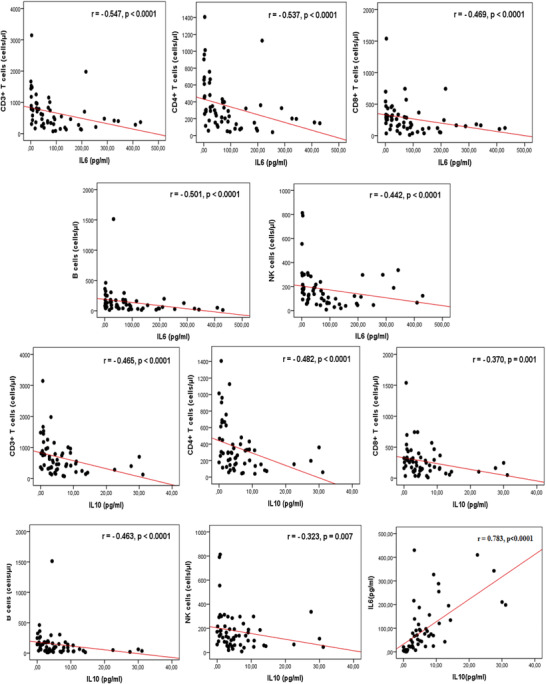
Correlations between lymphocytes subsets and cytokines. Plasma IL‐6 and IL‐10 levels were negatively correlated with total T cells count, CD4+ T cells counts, CD8+ T cell counts, B cell counts, and natural killer (NK) cell counts. A positive correlation was found between IL‐6 and IL‐10
3.4. Potential immunologic markers to identify severe cases among COVID‐19 patients
As previously described, low count of lymphocyte subsets and high levels of cytokines were associated with increased disease severity. ROC curve and area under ROC curve (AUC) were generated to evaluate the potential use of these parameters as diagnosis tool to identify severe cases. As shown in Figure 3 and Supporting Information Table S3, the number of total lymphocytes, T cells, B cells, CD4+T cells, and IL‐6, IL‐10 plasma levels had a good diagnosis efficiency, among which IL‐6 was the best parameter (0.967; 95% confidence interval [CI]: 0.927–1.000, P < 0.0001) for distinguishing severe and nonsevere cases. The preoperative IL‐6 concentration of 77.38 pg/ml was the optimal cutoff value with a sensitivity and specificity of 84.6% and 100%, respectively.
FIGURE 3.
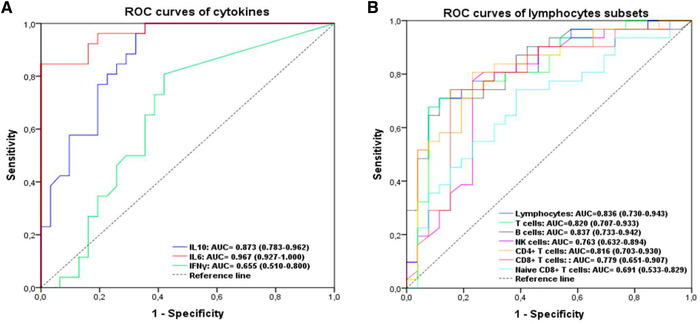
Efficiency of cytokines and lymphocytes subsets in identification of severe coronavirus disease 2019 (COVID‐19) patients. (A) Receiver operating characteristic (ROC) curves of cytokines. (B) ROC curves of lymphocyte subsets
3.5. CD8+ T cells and IL‐6 as potential prognosis factors to predict COVID‐19‐relared mortality
As shown in Supporting Information Table S4, the AUC derived from the ROC curve of CD8+ T cells was larger than that derived from T cells, CD4+T cells, DP cells, B cells, and NK cells (AUC CD8+ T cells = 0.812 [0.675–0.949] vs. AUC T cells = 0.795 [0.652–0.938], AUC CD4+T cells = 0.749 [0.593–0.906], AUC DP = 0.700 [0.542–0.858], AUC B cells = 0.740 [0.599–0.882], AUC NK cells = 0.761 [0.602–0.920]). Furthermore, the AUC of IL‐6 (0.946 [0.885–1.000], P ˂ 0.0001) was much larger than that of IL‐10 (0.786 [0.664–0.907], P = 0.001). Based on Youden's index, the optimal cutoff value was 150 cells/μl for CD8+ T cells (sensitivity: 81%, specificity: 73.3%) and 106.44 pg/ml for IL‐6 (sensitivity: 86.7%, specificity: 92.9%). Consequently, CD8+ T cell counts reduction and IL‐6 elevation are obviously the most predominant predictive factors for the clinical outcome. Hence, after adjusting for confounding factors including age and underlying diseases, the multivariate logistic regression showed that CD8+ T cell counts <150 cells/μl (odds ratio [OR] = 13.08, 95% CI: 1.282–133.505, P = 0.03) and IL‐6 >106.44 pg/ml (OR = 91.46, 95% CI: 8.959–933.640, P ˂ 0.0001) are two independent prognostic factors for death (Table 4). The Nagelkerke R 2 value was 0.741.
TABLE 4.
Univariate and multivariate analysis to identify risk factors related to mortality
| Univariate analysis | Multivariate analysis* | |||||
|---|---|---|---|---|---|---|
| P‐value | Odds ratio [OR] | 95% confidence interval [CI] | P‐value | OR | 95% CI | |
| IL‐6 > 106.44 pg/ml | ˂0.0001 | 84.5 | 12.688–562.761 | ˂0.0001 | 91.46 | 8.959–933.640 |
| CD8+ T cells ˂150 cells/μl | ˂0.0001 | 11.7 | 2.942–46.429 | 0.03 | 13.08 | 1.282–133.505 |
After adjusting for gender and underlying diseases.
In the light of these data, the study assigned patients into four groups based on their IL 6 plasma levels and CD8+ T cell counts: group I: IL‐6 ⩽ 106.44 pg/ml and CD8+ T cells ⩾150 cells/μl, n = 32; group II: IL‐6 > 106.44 pg/ml and CD8+ T cells ⩾150 cells/μl, n = 6; group III: IL‐6 ⩽ 106.44 pg/ml and CD8+ T cells ˂150 cells/μl, n = 9; and group IV: IL‐6 > 106.44 pg/ml and CD8+ T cells ˂150 cells/μl, n = 10. The Kaplan‐Meier survival curves revealed that compared to group I the patients from the other groups had worse survival rates (P ˂ 0.0001). In line with these findings, the study found the survival rates of groups I to IV were, respectively: 100%, 33.33%, 77.8%, and 10% (Fig. 4A). Strikingly, the ROC curve of the model combining CD8+ T cell count reduction and IL‐6 elevation had a larger AUC than each parameter separately (0.960 [0.913–1.000], P ˂ 0.0001) (Fig. 4B).
FIGURE 4.
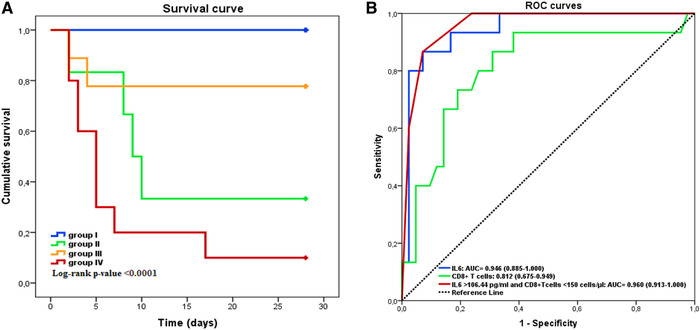
Efficiency of cytokines and lymphocytes subsets in predicting the clinical outcomes of coronavirus disease 2019 (COVID‐19) patients. (A) Kaplan‐Meier survival curves in four groups of COVID‐19 patients. Group I: IL‐6 ⩽ 106.44 pg/ml and CD8+ T cells ⩾150 cells/μl, n = 32. Group II: IL‐6 > 106.44 pg/ml and CD8+ T cells ⩾150 cells/μl, n = 6. Group III: IL‐6 ⩽ 106.44 pg/ml anCD8+ T cells ˂150 cells/μl, n = 9. Group IV: IL‐6 > 106.44 pg/ml and CD8+ T cells ˂150 cells/μl, n = 10. (B) Performance of ROC curves of IL‐6, CD8+ T cells and the combined model in predicting the mortality of patients
4. DISCUSSION
Here, the study presents an immune cell profiling in a cohort of 57 COVID‐19 patients with a particular emphasis on lymphocytes. Our data revealed an intriguing association between the quantitative composition of T cell subsets and cytokine levels with the clinical manifestations of COVID‐19 patients.
In line with previous reports, our data showed that the majority of patients, especially those who developed a severe disease with a fatal outcome, exhibited a significant drop of total lymphocyte counts and increased concentration of IL‐6 and IL‐10. 15 , 16 , 17 , 18 , 19 , 20 These immunologic abnormalities were also found in patients infected by Middle East respiratory syndrome (MERS)‐CoV and SARS‐CoV. 21 , 22 , 23 Our data suggest that the decrease of lymphocyte counts in COVID‐19 patients is mainly due to the reduction of CD4+ T cells and B cells. Surprisingly, NK and CD8+ T cells were below the normal range in only 36.84% and 47.37% of the study subjects. These findings are somehow different from those described earlier where the decline of CD8+ T cells and NK cells was more frequently observed in COVID‐19 patients than a reduction of CD4+ T cells. 24 , 25 These discrepancies might originate from the differences in the therapeutic approaches, the variation in the timing of clinical manifestations as well as the time of sample collections. Indeed, it has been shown that after a declining phase, all the lymphocytes tend to go back to their normal levels after the clearance of the virus.
The reason behind the lymphopenia inherent to SARS‐COV2 infection remains largely unknown. The low expression levels of the virus entry receptor in the immune cell compartment argue against the idea that the lymphopenia results from the direct interaction between the virus and the lymphocytes. 26 This is further supported by the fact that the SARS‐COV2 genetic material is barely detectable in the immune cell compartment. 27 Hence it seems more plausible to hypothesize that the lymphopenia is a direct consequence of the substantial cell migration to the site of infection where the immune response is initiated. Therefore, the proinflammatory environment might also contribute to the observed lymphopenia in COVID‐19 patients. Consistent with this idea, previous reports have shown that the levels of IL‐6 were negatively correlated with the lymphocyte counts in COVID‐19 patients whereas convalescent patients were found to have restored their lymphocyte numbers paired with lower proinflammatory cytokine levels. 28 IL‐6 is a pleiotropic cytokine secreted by a large variety of cells. 29 Recent investigations reported that active pathogenic T cells as well as CD14+CD16+ inflammatory monocytes activated by GM‐CSF secrete IL‐6 upon SARS‐CoV‐2 infection. 30 , 31 Interestingly, the IL‐6 receptor antagonist, tocilizumab, was found to increase the number of circulating lymphocytes in COVID‐19 patients. 32
In accordance with previous studies, our data also revealed that the concentration of IL‐10, an anti‐inflammatory cytokine, is positively correlated with the severity of COVID‐19. 33 The increased levels of IL‐10 in the plasma of COVID‐19 patients probably reflect a host response to prevent the harmful effect of the cytokine storm. However, the induction of IL‐10 expression to dampen the overexaggerated inflammation seems to be inefficient as the fatal outcome is associated with the highest levels of IL‐6 and IL‐10.
It is widely accepted that the composition of lymphocytes is under a strict homeostatic regulation to ensure a proper defense against invading organisms and to prevent the development of detrimental uncontrolled immune responses. Considering the central role of the lymphocyte subsets in the immune response against viral infections, the study sought to determine the changes in T cell composition among COVID‐19 patients. A detailed analysis of T cell compartment revealed a significant immune cell alteration when the clinical manifestations become apparent. These changes are predominantly characterized by increased percentages of activated T lymphocytes, which in turn were correlated with the severity and the outcome of the disease. In line with these findings Mazzoni et al. 34 reported an increased frequency of CM CD4+ T cells with a significant reduction of naïve and CM CD8+ T cells in COVID‐19 patients; however, CD8+ T lymphocytes are skewed toward a terminally differentiated phenotype. Information on the phenotype of SARS‐CoV‐2 specific T cells is scarce. According to the study conducted by Weiskopf et al., 35 virus specific CD4+ and CD8+ T cells were characterized predominantly as either CM CD4+ T cells or Effector Memory (EM) and TEMRA CD8+ T cells. Both of these CD8+ subsets are important for the control of infections. EM and TEMRA CD8+ T cells were shown to display potent cytotoxicity and home efficiently to inflamed peripheral tissues, whereas central memory home to secondary lymphoid organs and display a high proliferative capacity. 36
Antigen recognition leads to the activation of T cells followed by the loss of CD45RA expression and the acquisition of CD45RO isoform. 37 Therefore, early studies considered that the expression of CD45RA in T cells as an indication of a naïve state, whereas CD45RO+ T cells were defined as a pool of antigen‐experienced T cells, mainly composed of recently activated effector and memory T cells. 37 However, subsequent reports have demonstrated that T cells, which encountered an antigen may revert from a primed CD45RO+ to a CD45RA+ phenotype, 38 , 39 entailing that the CD45RA+ T cells may contain a fraction of antigen‐experienced clones. Unfortunately, the surface markers used in the current study were not suitable for the accurate characterization of naïve CD4+ T cell subsets. It is now widely accepted that after the thymic involution the maintenance of T cells is exclusively achieved through a peripheral expansion resulting in different patterns of naïve T cells in the young and elderly. 40 , 41 As a consequence, these events might substantially influence the potential of T cell activation and subsequently T cell differentiation. 42 , 43 Therefore, it is tempting to speculate that the low frequency of a severe COVID‐19 cases in young subjects is at least partly due to a difference in the nature of T cells between young and old patients.
During an infection, the initial antigen priming of naïve CD4 T cells combined to specific cues provided by cytokines signaling lead to their differentiation into at least four distinct subsets including Th1, Th2, Th17, and iTreg. The phenotypic and functional identity of T helper cells is defined by the pattern of the cytokines produced following their differentiation. 44 Our analysis revealed that SARS‐COV2 infection is associated with increased numbers of IL‐17‐producing CD4+ T cells in the peripheral blood compared to healthy controls. This suggests that Th17 inflammatory response could contribute to the immunopathology of the disease. Previous reports have suggested that this Th17 response might play a role in the immunity against the virus and/or the immunopathology caused by the infection. 45 , 46 However, our findings argue against the involvement of Th17 response in the development of COVID‐19 pathology as the severity of the disease did not correlate with the percentage of Th17 cells and the levels of IL‐17A. The study also evaluated the capacity of CD4+ T cells to produce IFNγ, an important cytokine that promotes antiviral immunity. 47 No significant difference has been found between patients and healthy controls; however, the patients who died showed reduced frequency of IFNγ producing CD4+ T cells compared to those who survived. This indicates that a poor outcome is not only associated with a decreased number of lymphocyte counts but also with an impaired CD4 T cell functionality. 48
Effective prediction criteria can allow physicians to provide an appropriate medical care for the patients with severe COVID‐19. A ROC curve analysis showed that low count of lymphocyte subsets and high levels of IL‐6 and IL‐10 have a potential value to segregate patients with a severe disease from those who experienced a mild pathology. In addition, the aforementioned parameters allowed predicting a fatal outcome in our cohort of COVID‐19 patients. Moreover, potential mortality risk factors for COVID‐19 were analyzed by a multivariable binary logistic regression. The results indicated that a CD8+ T cell counts <150 cells/μl and IL‐6 >106.44 pg/ml are two independent prognostic factors for death. Similar results were documented in another study, 49 which showed that CD8+ T cell counts <165 cells/μl and IL‐6 > 20 pg/ml are two reliable prognosis indicators that accurately stratify patients into risk categories and predict COVID‐19 mortality. Furthermore, the ROC curve of the model combining these two parameters was reported to display a better performance than the commonly used CURB‐65 scores (confusion, urea, respiratory rate, blood pressure, and age).
It is worth noting that our study has some limitations. First, this was a pilot single‐center study with a small sample size. Hence, a multicenter study with larger cohort would significantly enhance the strength of our findings. Second, quantification of soluble cytokines was performed only in the patients with COVID‐19. In fact, the recruitment of healthy subjects was extremely difficult during the peak of the pandemic because of the multiple preventive measures to limit the spread of infection. Moreover, some patients with COVID‐19 have developed a bacterial superinfection, which probably influenced their immunologic parameters. Further, 6.9% (95% CI, 4.3–9.5%) of patients with COVID‐19 develop a bacterial infection that contributes to the exacerbation of the disease. 50 Holub et al. reported that the number of lymphocyte subsets depends on the bacterial etiology of sepsis. 51 Indeed, Gram‐positive sepsis causes stronger suppression of peripheral blood lymphocyte subsets in comparison to sepsis due to Gram‐negative pathogens. Consequently, as an indicator of bacterial infection, 52 procalcitonin measurement would be desirable to rule out patients with co‐infection, which probably influenced their immunologic parameters.
The study presents here the first description of immunologic characteristics of a cohort of Algerian patients with COVID‐19 that confirms some data described in previous reports. The study also showed that the quantification IL‐6 and IL‐10 plasma associated with total lymphocyte counts and their subsets can be used as potential immunologic markers for the early diagnosis of severe cases. Moreover, both IL‐6 levels and CD8+ T cell counts may help to predict the outcome of the disease. Nevertheless, further prospective investigations are necessary to define precisely the immunologic profile during the course of the disease in order to improve the clinical and therapeutic management of COVID‐19 patients.
AUTHORSHIP
B.B. and L.L.M. were the major contributors in writing of the manuscript. S.Y.R., A.D., B.M., Z.L., and L.B. participated in the design and coordination of the study and contributed to the drafting of the manuscript. I.B., W.S., F.M., N.Z.L., L.K., and I.A. participated in performing study. A.K., M.O., R.K., D.M., A.K., S.A., R.M.H., F.D., M.G., and R.D. helped to analyze data and to edit the manuscript. All authors read and approved the final manuscript.
B.B. and L.L.M contributed equally to the work.
DISCLOSURES
The authors declare no conflicts of interest.
Supporting information
Table S1: List of monoclonal antibodies used for flow cytometry analysis
Table S2: Characteristics of Peripheral Lymphocyte Subsets in patients with COVID‐19.
Table S3: AUROCs of immune‐inflammatory parameters for the severity of COVID‐19
Table S4: AUROCs of immune‐inflammatory parameters for the mortality of COVID‐19
ACKNOWLEDGMENTS
The authors thank Dr. Mark Bix for his critical reading of the manuscript. They also thank Mohamad Kurdi for the technical support.
Belaid B, Lamara Mahammad L, Mihi B, et al. T cell counts and IL‐6 concentration in blood of North African COVID‐19 patients are two independent prognostic factors for severe disease and death. J Leukoc Biol. 2022;111:269–281. 10.1002/JLB.4COVA1020-703R
REFERENCES
- 1. Ludwig S, Zarbock A. Coronaviruses and SARS‐CoV‐2: a brief overview. Anesth Analg. 2020;131:93‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO . COVID‐19 weekly epidemiological update. 2020;1;4. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-29-december-2020 [Google Scholar]
- 3. Aylward B, Liang W. Report of the WHO‐China Joint Mission on Coronavirus Disease 2019 (COVID‐19). WHO‐China Jt Mission Coronavirus Dis 2019. 2020;2019:16‐24. [Google Scholar]
- 4. Jesenak M, Brndiarova M, Urbancikova I, et al. Immune parameters and COVID‐19 infection—associations with clinical severity and disease prognosis. Front Cell Infect Microbiol. 2020;10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID‐19: current state of the science. Immunity. 2020;52:910‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang ZL, Hou YL, Li DT, et al. Laboratory findings of COVID‐19: a systematic review and meta‐analysis. Scand J Clin Lab Invest. 2020;80:441‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feng X, Li S, Sun Q, et al. Immune‐inflammatory parameters in COVID‐19 cases: a systematic review and meta‐analysis. Front Med;7. Epub ahead of print 2020. 10.3389/fmed.2020.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (2019‐nCoV) infection is suspected: interim guidance 28 January 2020. WHO. 2020;10. [Google Scholar]
- 9. Chinese NHC and state A of T . Diagnosis and treatment protocol for novel coronavirus pneumonia. Chin Med J (Engl). 2020;133:1087‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yi JS, Rosa‐Bray M, Staats J, et al. Establishment of normative ranges of the healthy human immune system with comprehensive polychromatic flow cytometry profiling. PLoS One. 2019;14:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller JD, van der Most RG, Akondy RS, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710‐722. [DOI] [PubMed] [Google Scholar]
- 12. Ndhlovu Z, Kamya P, Mewalal N, et al. Magnitude and kinetics of CD8+ T cell activation during hyperacute HIV infection impacts viral set point. Immune. 2016;43:591‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McElroy AK, Akondy RS, Davis CW, et al. Human Ebola virus infection results in substantial immune activation. Proc Natl Acad Sci U S A. 2015;112:4719‐4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Z, Zhu L, Nguyen THO, et al. Clonally diverse CD38+HLA‐DR+CD8+ T cells persist during fatal H7N9 disease. Nat Commun;9. Epub ahead of print 2018. 10.1038/s41467-018-03243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu B, Fan C, Wang A, et al. Suppressed T cell‐mediated immunity in patients with COVID‐19: a clinical retrospective study in Wuhan, China. J Infect. 2020;81:51‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020;2019:762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID‐19). Front Immunol. 2020;11:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gan J, Li J, Li S, et al. Leucocyte subsets effectively predict the clinical outcome of patients with COVID‐19 pneumonia: a retrospective case‐control study. Front Public Heal. 2020;8:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu R, Wang Y, Li J, et al. Decreased T cell populations contribute to the increased severity of COVID‐19. Clin Chim Acta. 2020;508:110‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID‐19 pneumonia. J Infect Dis. 2020;221:1762‐1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y, Li J, Zhan Y, et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun. 2004;72:4410‐4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim ES, Choe PG, Park WB, et al. Clinical progression and cytokine profiles of Middle East respiratory syndrome coronavirus infection. J Korean Med Sci. 2016;11:1717‐1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. He Z, Zhao C, Dong Q, et al. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int J Infect Dis. 2005;6:323‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jiang Y, Wei X, Guan J, et al. COVID‐19 pneumonia: CD8 + T and NK cells are decreased in number but compensatory increased in cytotoxic potential. Clin Immunol. 2020;218:108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cell Mol Immunol. 2020;17:7‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou P, Yang X, Wang X, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature;581. Epub ahead of print 2020. 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 28. Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine pro fi les in the peripheral blood of SARS‐CoV‐2 infected patients. EBioMedicine;55. Epub ahead of print 2020. 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tanaka T, Narazaki M, Kishimoto T. IL‐6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou Y, Fu B, Zheng X, et al. In severe pulmonary syndrome patients of a new coronavirus. bioRxiv Prepr. Epub ahead of print 2020. 10.1101/2020.02.12.945576. [DOI] [Google Scholar]
- 31. Zhang D, Guo R, Lei L, et al. COVID‐19 infection induces readily detectable morphological and inflammation‐related phenotypic changes in peripheral blood monocytes, the. J Leukoc Biol. 2020;1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Netea MG, Rovina N, Koulouris N, et al. Clinical and Translational Report Complex Immune Dysregulation in COVID‐19 Patients with Severe Respiratory Failure ll Clinical and Translational Report Complex Immune Dysregulation in COVID‐19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020;27:992‐1000.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopatho. 2017;5:517‐528. [DOI] [PubMed] [Google Scholar]
- 34. Mazzoni A, Annunziato F, Cosmi L. Impaired immune cell cytotoxicity in severe COVID‐19 is IL‐6 dependent. J Clin Invest. 2020;130:4694‐4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weiskopf D, Schmitz KS, Raadsen MP, et al. Phenotype and kinetics of SARS‐CoV‐2–specific T cells in COVID‐19 patients with acute respiratory distress syndrome. Sci Immunol. 2020;5:1‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martin MD, Badovinac VP. Defining memory CD8 T cell. Front Immunol. 2018;9:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Merkenschlager M, Terry L, Edwards R, et al. Limiting dilution analysis of proliferative responses in human lymphocyte populations defined by the monoclonal antibody UCHL1: implications for differential CD45 expression in T cell memory formation. Eur J Immunol. 1988;18:1653‐1662. [DOI] [PubMed] [Google Scholar]
- 38. Bell EB, Sparshott SM. Interconversion of CD45R subsets of CD4 T cells in vivo. Nature. 1990;348:163‐166. [DOI] [PubMed] [Google Scholar]
- 39. Michie Colin A., Mclean Angela, Christopher Alcock PCLB. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;359:710‐713. [DOI] [PubMed] [Google Scholar]
- 40. Kohler S, Thiel A. Life after the thymus: CD31+ and CD31 human naive CD4+ T‐cell subsets. Blood. 2009;113:769‐774. [DOI] [PubMed] [Google Scholar]
- 41. den Braber I, Mugwagwa T, Vrisekoop N, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288‐297. [DOI] [PubMed] [Google Scholar]
- 42. Pekalski ML, Ferreira RC, Coulson RMR, et al. Postthymic expansion in human CD4 naive T cells defined by expression of functional high‐affinity IL‐2 receptors. J Immunol. 2013;190:2554‐2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mold JE, Réu P, Olin A, et al. Cell generation dynamics underlying naive T‐cell homeostasis in adult humans. PLoS Biol. 2019;17:1‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Populations CDTC, Zhu J, Yamane H, et al. Differentiation of effector CD4 T cell populations. AnnuRevImmunol. 2010;28:445‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cov S, Sas EM, Cov S, et al. The potential role of Th17 immune responses in coronavirus immunopathology and vaccine‐induced immune enhancement. Microbes Infect. 2020;22:165‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu D, Yang XO. TH17 responses in cytokine storm of COVID‐19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53:368‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kang S, Brown HM. Direct antiviral mechanisms of interferin‐gamma. Immune Netw. 2018;18:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen G, Zhao J, Ning Q, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019 clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Luo M, Liu H, Wei S, et al. IL‐6 and CD8 + T cell counts combined are an early predictor of in‐hospital mortality of patients with COVID‐19. 5. Epub ahead of print 2020. 10.1172/jci.insight.139024. [DOI] [PMC free article] [PubMed]
- 50. Langford BJ, So M, Raybardhan S, et al. Bacterial co‐infection and secondary infection in patients with COVID‐19: a living rapid review and meta‐analysis. Clin Microbiol Infect. Epub ahead of print 2020. 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Holub M, Klučková Z, Helcl M, et al. Lymphocyte subset numbers depend on the bacterial origin of sepsis. Clin Microbiol Infect. 2003;9:202‐211. [DOI] [PubMed] [Google Scholar]
- 52. Cheval C, Timsit JF, Garrouste‐Orgeas M, et al. Procalcitonin (PCT) is useful in predicting the bacterial origin of an acute circulatory failure in critically ill patients. Intensive Care Med Suppl. 2000;26:153‐158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: List of monoclonal antibodies used for flow cytometry analysis
Table S2: Characteristics of Peripheral Lymphocyte Subsets in patients with COVID‐19.
Table S3: AUROCs of immune‐inflammatory parameters for the severity of COVID‐19
Table S4: AUROCs of immune‐inflammatory parameters for the mortality of COVID‐19


