Abstract
Background
Polypharmacy is common and closely linked to drug interactions. The impact of polypharmacy has not been previously quantified in survivors of critical illness who have reduced resilience to stressors. Our aim was to identify factors associated with preadmission polypharmacy and ascertain whether polypharmacy is an independent risk factor for emergency readmission to hospital after discharge from a critical illness.
Methods
A population-wide cohort study consisting of patients admitted to all Scottish general ICUs between January 1, 2011 and December 31, 2013, whom survived their ICU stay. Patients were stratified by presence of preadmission polypharmacy, defined as being prescribed five or more regular medications. The primary outcome was emergency hospital readmission within 1 yr of discharge from index hospital stay.
Results
Of 23 844 ICU patients, 29.9% were identified with polypharmacy (n=7138). Factors associated with polypharmacy included female sex, increasing age, and social deprivation. Emergency 1-yr hospital readmission was significantly higher in the polypharmacy cohort (51.8% vs 35.8%, P<0.001). After confounder adjustment, patients with polypharmacy had a 22% higher hazard of emergency 1-yr readmission (adjusted hazard ratio 1.22, 95% confidence interval 1.16–1.28, P<0.001). On a linear scale of polypharmacy each additional prescription conferred a 3% increase in hazard of emergency readmission by 1 yr (adjusted hazard ratio 1.03, 95% confidence interval 1.02–1.03, P<0.001).
Conclusions
This national cohort study of ICU survivors demonstrates that preadmission polypharmacy is an independent risk factor for emergency readmission. In an ever-growing era of polypharmacy, this risk factor may represent a substantial burden in the at-risk post-intensive care population.
Keywords: critical illness, drug interactions, emergency readmission, hospital readmission, intensive care, outcome, polypharmacy
Editor's key points.
-
•
Polypharmacy is an indicator of comorbidity but also increases risk of drug interactions.
-
•
This study found that one in two patients with preadmission polypharmacy had an emergency readmission to hospital within 1 yr after ICU discharge.
-
•
Polypharmacy patients also experienced longer ICU stays and higher mortality rates.
-
•
There is a likely need for medication reconciliation during or after ICU discharge.
Polypharmacy is an ever-perpetuating global phenomenon fuelled by our aging population and rising tide of multi-morbidity. It occurs throughout all aspects of healthcare, making the application of single disease-focused evidence-based practice more challenging. Between 1995 and 2010 the mean number of dispensed drugs increased from 3.3 to 4.4 and the percentage of patients prescribed five or more medications doubled from 11% to 22%.1 Polypharmacy is strongly associated with potentially serious drug–drug interactions; a prevalence of which also doubled to 13% between 1995 and 2010.1 Primary care analyses demonstrate that for each additional drug prescribed, the odds of a prescription or monitoring error increase by 16%.2 Potential interactions occur at a frequency of around 50% when five or more medications are prescribed and increase to 80% and 100% when 10 and 20 drugs are prescribed, respectively.3 Adverse drug reactions account for 6.5% of hospital admissions (with a 2.2% mortality rate) and an estimated annual cost of £466 million.4 Some 72% of these adverse drug events are avoidable.4
Polypharmacy prevalence is significantly greater in the ICU setting compared with general wards, with between 54% and 64% of patients experiencing a potential drug–drug interaction.5,6 The well described ‘post-intensive care syndrome’ results in patients with ongoing issues related to their acute admission and a reduced resilience to stressors.7 Consequently, these patients are likely at increased risk of adverse medication prescriptions and side-effects. The combination of the ageing population (proportion of over 65s is predicted to increase from 18% in 2016 to 24% in 2036),8 increasing multi-morbidity, and an evolving intensive care population (favouring increases in age) is culminating in a growing polypharmacy intensive care population.
A report into the burden of polypharmacy in 2013 highlighted that the evidence base for multiple interventions in the multi-morbid patient is currently poor.9 There is limited research to date on preadmission polypharmacy in the intensive care setting and its effects on emergency readmission and mortality. We investigated the relationship between preadmission polypharmacy and emergency readmission in patients from a Scottish national ICU registry. We hypothesised that, in intensive care survivors, preadmission polypharmacy would be associated with increased emergency readmission and mortality at 1 yr.
Methods
We used a cohort study design. Data sources were linked registries collated for the PROFILE (PReventing early unplanned hOspital readmission aFter critical ILlnEss) study10: Scottish Intensive Care Society Audit Group (SICSAG),11 Scottish Morbidity Record of acute hospital admissions (SMR01), Scottish death records, acute psychiatric hospital admissions (SMR04), and Prescribing Information System (PIS). The SICSAG registry is subject to regular validation assessments and includes Scottish intensive care activity and derives a population from 24 adult ICUs which serve a population of 5.1 million.12 The PIS records all prescriptions dispensed in Scotland since 2009, with high levels of linkage accuracy (95% by 2014) and completeness.13
All data were anonymised before release and analysed in a safe-haven environment. The study formed a secondary analysis of a subset of the population included in the PROFILE study10 which gained approval from the Privacy Advisory Committee of NHS National Services Scotland (Reference-PAC12/14) and The Research Ethics Committee granted a waiver of consent (Reference-NR/1403AB5).
Participants
The cohort comprised adult patients (aged ≥16 yr) admitted to a Scottish ICU and who were subsequently discharged alive from their index hospital stay between January 1, 2011 and December 31, 2013. These participants were a subset of the original PROFILE dataset and this timeframe chosen as full required prescribing data were available.
Exposure
Preadmission polypharmacy was defined as five or more monthly prescribed drugs, a threshold suggested by NHS Scotland guidance and the most prevalent numerical definition used in current literature.14,15 We derived this by calculating the total number of dispensed items to each patient over a 1 yr period before admission and then divided this by 12 to create a monthly mean number of prescriptions. The origin of prescribing data is available in the Supplementary material. The primary exposure variable was represented as a binary variable (presence vs absence) of preadmission polypharmacy. Acknowledging that a binary cut-off for polypharmacy may be viewed as arbitrary, as a secondary exposure we modelled the monthly mean number of prescriptions as a continuous variable. In sensitivity analyses, we modelled an additional continuous measure of polypharmacy using the number of distinct drugs prescribed over the preadmission 1-yr period (tertiary exposure).
Outcomes
The primary outcome was emergency hospital readmission within 1 yr of discharge from index hospital stay. Emergency vs elective admission type was coded in SMR01 with a validated accuracy of >93%.16 The secondary outcome was mortality within 1 yr of index admission discharge. Complete follow-up was assumed. Emigration from Scotland was unrecorded in the database, however it is known to be low (<0.8% of residents aged ≥16 yr annually in 2013).17
Confounders
The following confounders were used to adjust in multivariable models: number of comorbidities, sex, age, Scottish Index of Multiple Deprivation (SIMD),18 remoteness of residence,19 Acute Physiology Score, number of previous emergency/elective hospital admissions, outpatient attendances, psychiatric admissions, ICU admission type (elective/emergency), ICU admission diagnosis, mechanical ventilation/renal replacement therapy/cardiovascular support/tracheostomy use (binary), and length of stay pre/during/post-ICU. See Supplementary material for details relating to confounders.
Statistical analysis
Baseline characteristics and cohort analyses
Data analyses were undertaken using Stata/IC.V.14 (StataCorp, Texas 77845, USA). Baseline characteristics were compared between polypharmacy and non-polypharmacy groups using χ2 and Wilcoxon rank-sum. We evaluated the relationship between mean monthly dispensed drugs and both age and social deprivation graphically.
Primary analysis
The primary analysis was to investigate the association between preadmission polypharmacy on the primary outcome of 1-yr emergency readmission to hospital. Fine and Gray competing risk regression analysis was used allowing for the competing risk of death. We took a sequential approach to model building. Firstly, we performed a univariable analysis between polypharmacy and 1-yr emergency readmission. The second model was a multivariable model adjusting for confounders including comorbidities listed above. This model is presented as the primary multivariable model. However, because the number of comorbidities and polypharmacy is correlated (Spearman's rho 0.37, P<0.001) and comorbidity count is potentially causally related to polypharmacy, we followed with a third multivariable model adjusting for confounders but excluding the number of comorbidities.
We calculated cumulative risk data for emergency 1-yr readmission to hospital using the Stata cumulative incidence function ‘stcrreg’ followed by ‘stcurve’. In order to visualise the results, we used logistic regression to calculate predicted probabilities for emergency readmission and then graphed using a margins plot. The logistic regression model included all confounders.
To increase robustness, we first analysed using a binary cut-off for polypharmacy (primary exposure) and then progressed (using the same models) to considering polypharmacy as a continuous scale entered as a linear term (secondary exposure).
Secondary analysis
The secondary analysis investigated the association between polypharmacy on the secondary outcome of 1-yr mortality. Cox regression analysis was used in the same sequential approach to the primary analysis. Further details are available in the Supplementary material.
Sensitivity analysis
For the primary and secondary analyses, polypharmacy was calculated using the monthly mean number of prescriptions. To evaluate robustness of the main analysis, we used the number of distinct drugs prescribed over the preceding year to derive the exposure. We used the same sequential Fine and Gray model approach as in the primary/secondary analysis. We also evaluated age/polypharmacy and comorbidity/polypharmacy interactions. In addition, we undertook a propensity score matched analysis in an attempt to further control for confounding. Further detail is provided in the Supplementary material.
Drug category analysis
We investigated which drug categories contributed most towards readmission risk. A Fine and Gray fully adjusted multivariable model was run for each of the 44 drug chapter codes for the primary outcome of 1-yr emergency readmission. Chapter codes were sorted into associated categories. Chapter codes with n<50 or no associated category were placed in ‘Other’ (Supplementary Table S1).
Results
Baseline characteristics
A total of 23 844 patients, aged ≥16 yr, were admitted to a Scottish ICU and subsequently discharged from hospital alive between January 1, 2011 and December 31, 2013. Median age was 62 (inter-quartile range [IQR] 47–72) yr, 22% had at least one emergency inpatient admission in the preceding year, 58.1% had at least one comorbidity, and 11.4% had three or more comorbidities. Median length of ICU stay was 1 (IQR 0–4) day with a median post-ICU stay of 8 (IQR 4–18) days. Emergency 1-yr readmission occurred in 40.6% and 1-yr mortality in 8.2%. Median monthly dispensed medications were three (IQR 1–5) with a median of nine distinct prescriptions in the past 12 months (IQR 5–15) (Table 1). Further baseline characteristics are available in the Supplementary material.
Table 1.
Baseline characteristics of the whole intensive care cohort, non-polypharmacy cohort, and polypharmacy cohort. See Supplementary Table S2 in the Supplementary material for more detailed characteristics of the full, non-polypharmacy, and polypharmacy cohorts. IQR, inter-quartile range.
| Whole cohort |
No polypharmacy cohort |
Polypharmacy cohort |
P-value | |
|---|---|---|---|---|
| (n=23 844) | (n=16 706) | (n=7138) | ||
| Patient characteristics | ||||
| Female, n (%) | 10 166 (42.6) | 6590 (39.4) | 3576 (50.1) | <0.001 |
| Age at admission to ICU, yr, median (IQR) | 62 (47–72) | 59 (44–70) | 66 (55–75) | <0.001 |
| Scottish Index of Multiple Deprivation, n (%) | <0.001 | |||
| First quartile (most deprived) | 6174 (25.9) | 4127 (24.7) | 2047 (28.7) | |
| Second quartile | 5402 (22.7) | 3591 (21.5) | 1811 (25.4) | |
| Third quartile | 4741 (19.9) | 3259 (19.5) | 1482 (20.8) | |
| Fourth quartile | 4194 (17.6) | 3099 (18.6) | 1095 (15.3) | |
| Fifth quartile (least deprived) | 3311 (13.9) | 2616 (15.7) | 695 (9.7) | |
| Remoteness of residence, n (%) | 0.073 | |||
| Urban area | 15 944 (66.9) | 11 202 (67.1) | 4742 (66.4) | |
| Accessible | 5456 (22.9) | 3813 (22.8) | 1643 (23.0) | |
| Remote | 1001 (4.2) | 698 (4.2) | 303 (4.2) | |
| Very remote | 855 (3.6) | 557 (3.3) | 298 (4.2) | |
| Indices of pre-existing patient health | ||||
| Unplanned inpatient admissions in the year before index stay, n (%) | <0.001 | |||
| 0 | 16 206 (68.0) | 12,299 (73.6) | 3907 (54.7) | |
| 1 | 4705 (19.7) | 2967 (17.8) | 1738 (24.3) | |
| 2 or more | 2933 (12.3) | 1440 (8.6) | 1493 (20.9) | |
| Count of Charlson comorbidities, n (%) | <0.001 | |||
| 0 | 9983 (41.9) | 8183 (49.0) | 1800 (25.2) | |
| 1 | 7468 (31.3) | 5336 (31.9) | 2132 (29.9) | |
| 2 | 3673 (15.4) | 2109 (12.6) | 1564 (21.9) | |
| 3 or more | 2720 (11.4) | 1078 (6.5) | 1642 (23.0) | |
| Indices of critical illness severity | ||||
| Type of admission to ICU | 0.009 | |||
| Elective surgery | 8630 (36.2) | 6124 (36.7) | 2506 (35.1) | |
| Emergency surgery | 4807 (20.2) | 3394 (20.3) | 1413 (19.8) | |
| Non-operative | 10 222 (42.9) | 7055 (42.2) | 3167 (44.4) | |
| APACHE II score at admission to ICU, median (IQR) | 15 (11–19) | 14 (10–19) | 16 (12–21) | <0.001 |
| Acute physiology score at admission to ICU, median (IQR | 10 (7–15) | 10 (7–15) | 11 (8–16) | <0.001 |
| Outcomes | ||||
| Length of ICU stay, days, median (IQR) | 1 (0–4) | 1 (0–4) | 2 (1–4) | <0.001 |
| Length of index hospital stay, days, median (IQR) | 13 (7–27) | 12 (7–25) | 15 (8–29) | <0.001 |
| Readmission outcome at 1 yr | <0.001 | |||
| Emergency readmission | 9685 (40.6) | 5985 (35.8) | 3700 (51.8) | |
| Alive with no readmission | 13 687 (57.4) | 10 444 (62.5) | 3243 (45.4) | |
| Died with no readmission | 472 (2.0) | 277 (1.7) | 195 (2.7) | |
| Mortality at 1 yr | 1963 (8.2) | 1140 (6.8) | 823 (11.5) | <0.001 |
| Time until death (days, median+quartiles) | 153 (69, 248) | 158 (75, 252) | 144 (61, 244) | 0.024 |
| Prescribing | ||||
| Average monthly dispensed items (median+quartiles) | 3 (1, 5) | 1 (0, 3) | 7 (6, 10) | <0.001 |
| Total distinct items in past 12 months (median+quartiles) | 9 (5, 15) | 7 (3, 10) | 17 (13, 22) | <0.001 |
Cohort analyses
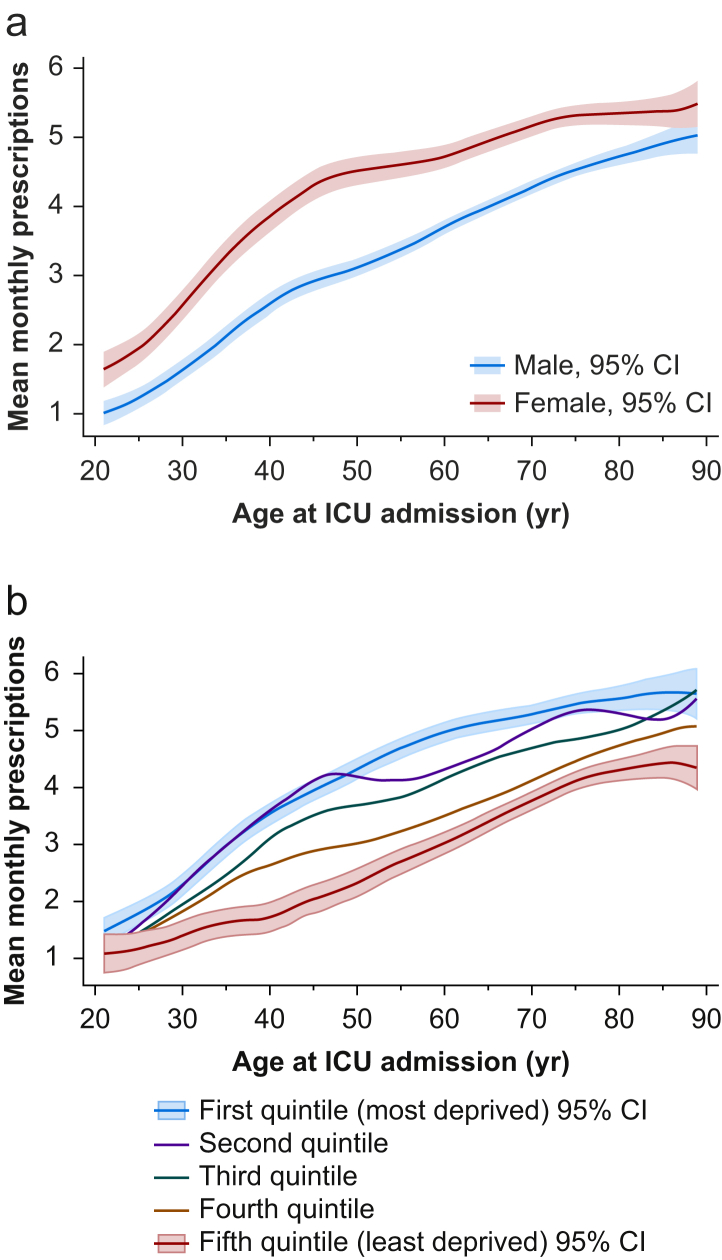
The prevalence of preadmission polypharmacy (five or more medications) was 29.9% (n=7138). Polypharmacy patients were more likely to be female (50.1% vs 39.4%, P<0.001), older (median 66 [IQR 55–75] vs 59 IQR [44–70] yr, P<0.001) and living in more socially deprived areas (P<0.001). Polypharmacy patients had greater APACHE II scores on admission (median 16 [IQR 12–21] vs median 14 [IQR 10–19], P<0.001), required greater cardiovascular system support (44.1% vs 40.2%, P<0.001), less mechanical ventilation (56.9% vs 62.0%, P<0.001), and longer ICU stay (median 2 [IQR 1–4] vs 1 IQR [0–4] days, P<0.001) (Table 1). The full version of baseline characteristics table including individual comorbidities prevalence is available in the Supplementary material (Supplementary Table S2). Throughout the age range, women were prescribed a greater number of mean monthly dispensed drugs than men (Fig. 1a) and social deprivation was also associated with higher mean monthly dispensed drugs (Fig. 1b).
Fig 1.
Mean number of monthly prescriptions in the year preceding critical care index admission by age at index admission stratified by (a) gender or (b) social deprivation as determined from Scottish Index of Multiple Deprivation (SIMD, version 2009). First and fifth quintile 95% confidence intervals for social deprivation are only shown for clarity. CI, confidence interval.
Primary analysis
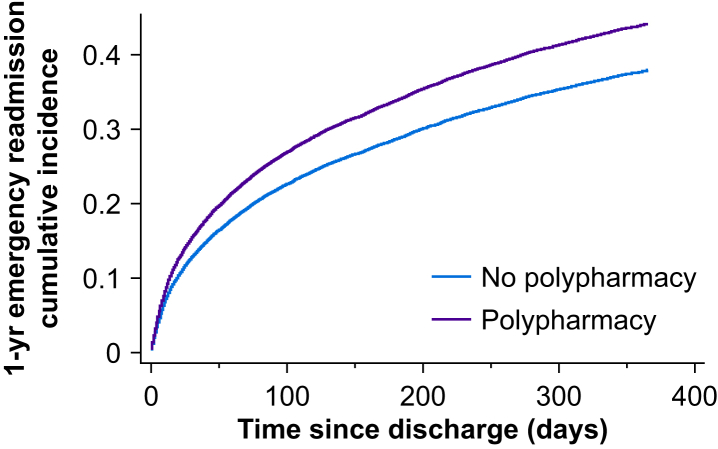
Emergency 1-yr hospital readmission was significantly higher in the polypharmacy cohort (51.8% vs 35.8%, P<0.001). Univariable analysis demonstrated a 61% increase in hazard of 1-yr emergency readmission {hazard ratio (HR) 1.61 (95% confidence interval [CI] 1.54–1.67), P<0.001}. After adjustment for confounders (including comorbidities), there was a 22% relative increase in hazard (adjusted hazard ratio [adjHR] 1.22 [95% CI 1.16–1.28], P<0.001, Fig. 2). Multivariable analysis adjusted for confounders, after excluding number of comorbidities, yielded a 30% relative increase in hazard for emergency 1-yr admission (adjHR 1.30 [95% CI 1.24–1.37], P<0.001).
Fig 2.
Cumulative incidence plot for 1-yr emergency readmission by time since discharge from hospital after index critical care stay stratified by presence of polypharmacy.
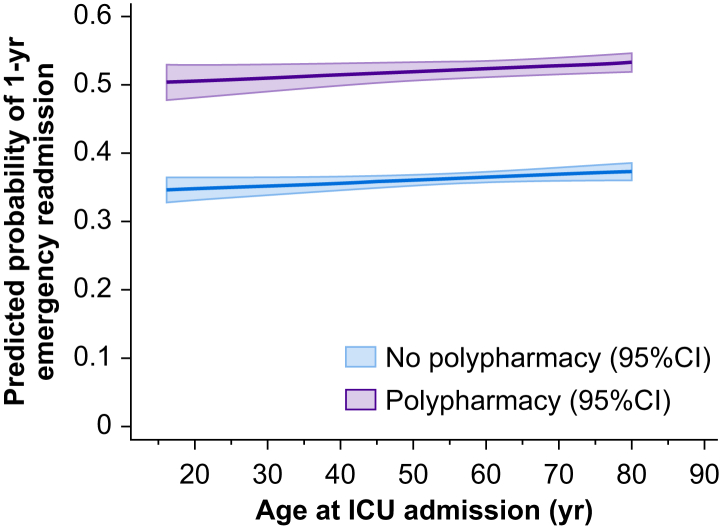
For a ‘typical’ patient (median 62 yr) the predicted adjusted probability of 1-yr emergency readmission was 37% in the non-polypharmacy cohort and 53% in the polypharmacy cohort. At all ages, preadmission polypharmacy markedly increased the probability of emergency readmission by 1-yr post-hospital discharge (Fig. 3). In those patients experiencing emergency readmission by 1 yr after discharge, the median time to readmission was sooner in the polypharmacy cohort at 55 (IQR 13–159) vs 59 (IQR 17–162) days.
Fig 3.
Predicted probability of 1-yr emergency readmission after discharge from hospital containing index critical care stay according to age, stratified by presence of polypharmacy. CI, confidence interval.
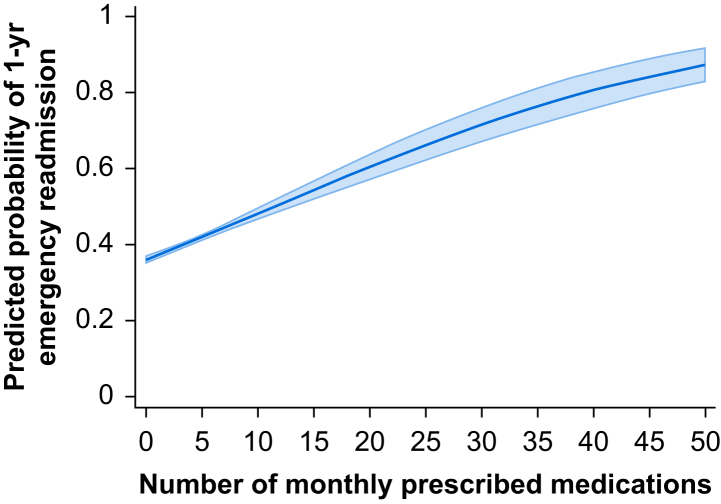
Analysing the mean number of dispensed items per month as a continuous variable rather than binary, the risk of 1-yr emergency hospital readmission was 6% higher for each additional dispensed medication per month (HR 1.06 [95% CI 1.06–1.07], P<0.001) in the univariable model. With adjustment for confounders, the additional risk for each additional prescription was 3% (adjHR 1.03 [95% CI 1.02–1.03], P<0.001). Multivariable model excluding number of comorbidities yielded the additional risk at 4% per medication (adjHR 1.04 [95% CI 1.03–1.04], P<0.001). Predicted probabilities demonstrated a linear relationship between mean prescriptions per month and probability of emergency hospital readmission (Fig. 4).
Fig 4.
Predicted probability of 1-yr emergency readmission after discharge from hospital containing index critical care stay on a linear scale of polypharmacy. Shaded area represents 95% confidence interval.
Secondary analysis
Preadmission polypharmacy was associated with higher 1-yr mortality (11.5% vs 6.8%, P<0.001). A univariable model showed polypharmacy to incur a 9% increase risk in hazard for 1-yr mortality (HR 1.09 [95% CI 1.00–1.19], P=0.06). A fully adjusted multivariable model showed a non-significant increase in risk in hazard (adjHR 1.06 [95% CI 0.95–1.18], P=0.29) which remained non-significant upon the withdrawal of comorbidities from the model (adjHR 1.05 [95% CI 0.95–1.17], P=0.34).
Sensitivity analysis
Using the tertiary exposure of polypharmacy, calculated via the number of distinct prescriptions over a 12-month period, the median number of dispensed items was nine with IQR of 5–15. The univariable model showed a 4% increase in readmission risk for each additional dispensed medication (HR 1.04 [95% CI 1.04–1.04], P<0.001). With adjustment for confounders, the additional risk for each additional prescription was 2% (adjHR 1.02 [95% CI 1.02–1.02], P<0.001). Multivariable model excluding number of comorbidities yielded a similar result (adjHR 1.02 [95% CI 1.02–1.03], P<0.001). The association between polypharmacy and emergency hospital readmission did not vary by age (P-value for interaction=0.08) (Supplementary Table S3). However, polypharmacy was associated with a higher risk of readmission in patients without comorbidity compared with those with comorbidity (adjHR 1.36 vs 1.24, P-value for interaction=0.002). The matched propensity score analysis used two matched cohorts with a total of 9712 patients. The propensity matched approach found a comparable effect on the hazard for emergency readmission (adjHR 1.21 [95% CI 1.14–1.29], P<0.001) compared with the primary analysis. There are further details in the Supplementary material (Supplementary Tables S4 and S5, Fig. S1).
Drug category analysis
After an adjusted multivariable model, the drug chapters which conferred the greatest increase in risk were: oxygen (adjHR 1.32), anti-parkinsonian agents (adjHR 1.29) and anti-migraine medications (adjHR 1.28) (Supplementary Fig. S2). Full results available in Supplementary material.
Discussion
In this complete, large national database cohort study focusing on ICU survivors, patients with preadmission polypharmacy experienced higher emergency readmission rates. Within a year of post-hospital discharge, one in two patients with preadmission polypharmacy experienced emergency readmission to hospital. Polypharmacy patients also experienced longer ICU stays and higher mortality rates. Patient factors associated with polypharmacy included female sex, greater age, and social deprivation.
The prevalence of preadmission polypharmacy in our study was 30%. It is difficult to directly compare this with wider literature for a multitude of reasons. Firstly, the definition of polypharmacy is variable,15 secondly, the prevalence of polypharmacy is increasing over time,1 thirdly, prevalence varies markedly on the age of the cohort population,1,20,21 and finally, the prevalence varies geographically worldwide.21, 22, 23, 24 Emergency readmission rates to hospital after a critical care index stay were similar in our study (cohort emergency readmission 15% at 30 days and 41% at 1 yr) when comparing with other large cohort studies of general ICU discharged patients (16% at 30 days).25
Whilst much literature exists showing the detrimental effects of inappropriate polypharmacy, it is largely restricted to geriatric services,26, 27, 28, 29 with some evidence in the postoperative period.30,31 Polypharmacy has been included in risk scores for readmission for general medical patients.32, 33, 34 Our results demonstrated that each additional medication attributed a 3–4% increased risk of 1-yr emergency readmission, which is comparable to a study in a non-ICU survivor cohort.26 There are no previous comparable studies which have stratified emergency readmission by presence of preadmission polypharmacy in ICU survivors. Our results, unique in current literature and reporting a large cohort, highlight the importance of preadmission polypharmacy in relation to healthcare burden after critical illness.
Factors associated with ICU survivor readmission have been well documented and are divided into system, clinical, and patient factors.10 ‘Post-intensive care syndrome’ results in patients with reduced resilience to stressors who are subsequently at increased readmission risk.7 It is credible that these patients would be at particular risk for emergency readmission when subjected to known medication side-effects, drug–drug interactions, and potentially inappropriately prescribed medications—all known to directly correlate with number of prescriptions.3,22
Our study has a number of strengths. We used a large cohort of data covering a whole population over a 3-yr time period. Our coverage was complete for ICU admissions throughout Scotland and the quality and robustness of the data were high. We had the ability to capture nationwide hospital readmission, rather than a single site. Caution must be taken when extrapolating the results to other countries as our study was solely based in Scotland and clinical practice and organisation vary considerably between healthcare systems. Our dataset contained multiple factors relating to the ICU and hospital admission resulting in a diverse range of data and the ability to adjust for potential confounders. Our primary endpoint was at 1 yr rather than the commonly reported 30 or 90 days. The strength of this prolonged follow-up is related to capturing a longer period of post-ICU discharge risk which is known to extend well beyond 90 days.35 Our visual data enable readers to draw conclusions on a linear scale for both time after discharge and number of regular medications, rather than being limited to binary endpoints.
Limitations within our study include variation in the literature surrounding the definition of polypharmacy. Our binary definition was five or more regular medications, as this threshold has been suggested for use within the health system in which the cohort was derived, and is also the most common literature threshold.14,15 In sensitivity analyses, we progressed from a binary cut-off to a linear scale of prescribed medications. There are discussions that polypharmacy should focus less on the number of medications prescribed and rather focus on the appropriateness of the individual prescribed items. This has led to the terms ‘appropriate’ and ‘inappropriate’ polypharmacy.9 Our study did not have access to the information required to determine polypharmacy appropriateness. However, we were able to evaluate the effect of certain drug classes and also used two separate measures of polypharmacy. We were unable to evaluate polypharmacy after ICU discharge, which is of concern to clinicians managing post-ICU services.
We opted to report the multivariable model including comorbidities as a confounder as the primary analysis. However, a further potential limitation is that polypharmacy correlates with the number of comorbidities which, depending on inclusion in a statistical model, may confound or ‘over-adjust’ an association between polypharmacy and readmission. To evaluate this effect, we developed statistical models both with and without adjustment for the number of comorbidities. We demonstrated that polypharmacy remained associated with readmission even after adjustment for comorbidities. This builds evidence for the biological plausibility that polypharmacy has its own causative effect via drug–drug interactions and side-effects in a susceptible and physiologically frail population following on from ICU discharge. However, we were also unable to investigate this putative causal mechanism because of limitations in the datasets.
Our study has implications for clinical practice. The importance of polypharmacy is recognised to have implications for policy and practice in general,9 confirmed in our study by the impact of polypharmacy on patient outcomes and subsequent healthcare resource use. The ICU itself, the downstream hospital ward, the community pharmacy, or a post-ICU discharge clinic may be the ideal clinical setting to perform medication reconciliation and identify interactions to minimise ongoing risk. It could also act as a time for patients and families to receive medication-related education, especially focusing on long-term conditions known to have high readmission risks. Studies in other clinical settings have shown that medication reconciliation combined with education, support, and pharmaceutical care-plans resulted in fewer adverse drug events and lower hospital utilisation compared with usual care.36,37
Further studies are required to add to the body of evidence regarding the mechanism through which polypharmacy contributes to emergency readmission and the potential effectiveness of a dedicated medication reconciliation for patients after a critical care admission.
Conclusions
Preadmission polypharmacy is prevalent in the intensive care setting. Our study highlights polypharmacy as an independent risk factor for emergency readmission after critical illness. We have demonstrated that one in two patients with preadmission polypharmacy experience emergency readmission to hospital within 1 yr from discharge after a critical illness. Further understanding of the mechanisms leading to readmission and possible interventions could improve clinical care and reduce the post-ICU financial burden.
Authors' contributions
Had full access to all the data in the study and take responsibility for the integrity of the data and the data analysis: AT, NL
Contributed to the conception and design of the study: all authors
Drafted the manuscript: AT, NL
Revised it critically for important intellectual content: all authors
Gave final approval of the version to be published: all authors
Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: all authors
Acknowledgements
We thank all the clinicians in the Scottish ICUs who contributed data to the SICSAG registry, the SICSAG team for undertaking data extraction, and the electronic Data Research and Innovation Service for undertaking linkage.
Handling editor: Paul Myles
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.09.035.
Declarations of interest
The authors declare that they have no conflicts of interest
Funding
Chief Scientist Office for Scotland (reference CZH/401026).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Guthrie B., Makubate B., Hernandez-Santiago V. The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995-2010. BMC Med. 2015;13:74. doi: 10.1186/s12916-015-0322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avery A.J., Ghaleb M., Barber N. The prevalence and nature of prescribing and monitoring errors in English general practice: a retrospective case note review. Br J Gen Pract. 2013;63:e543–e553. doi: 10.3399/bjgp13X670679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doan J., Zakrzewski-Jakubiak H., Roy J. Prevalence and risk of potential cytochrome P450-mediated drug-drug interactions in older hospitalized patients with polypharmacy. Ann Pharmacother. 2013;47:324–332. doi: 10.1345/aph.1R621. [DOI] [PubMed] [Google Scholar]
- 4.Pirmohamed M., James S., Meakin S. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uijtendaal E.V., van Harssel L.L.M., Hugenholtz G.W.K. Analysis of potential drug-drug interactions in medical intensive care unit patients. Pharmacotherapy. 2014;34:213–219. doi: 10.1002/phar.1395. [DOI] [PubMed] [Google Scholar]
- 6.Ray S., Bhattacharyya M., Pramanik J. Drug-drug interactions in the ICU. Crit Care. 2009;13:P495. [Google Scholar]
- 7.Lone N.I., Lee R., Salisbury L. Predicting risk of unplanned hospital readmission in survivors of critical illness: a population-level cohort study. Thorax. 2019;74:1046–1054. doi: 10.1136/thoraxjnl-2017-210822. [DOI] [PubMed] [Google Scholar]
- 8.Office for National Statistics . 2017 July. Overview of the UK population.https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/articles/overviewoftheukpopulation/july2017 Available from: [Google Scholar]
- 9.Duerden M., Avery T., Payne R. The Kings Fund; London: 2013. Polypharmacy and medicines optimisation. Making it safe and sound. [Google Scholar]
- 10.Walsh T.S., Salisbury L., Donaghy E. PReventing early unplanned hOspital readmission aFter critical ILlnEss (PROFILE): protocol and analysis framework for a mixed methods study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-012590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scottish Intensive Care Society Audit Group . ISD Scotland Publications; Edinburgh: 2016. Scottish intensive care society audit group Annual report: audit of intensive care units in Scotland 2016 reporting on 2015. [Google Scholar]
- 12.SICSAG . 2017. Scottish intensive care society audit group annual report: data quality.http://www.sicsag.scot.nhs.uk/quality/data.html Available from: [Google Scholar]
- 13.Alvarez-Madrazo S., McTaggart S., Nangle C. Data resource profile: the scottish national prescribing information system (PIS) Int J Epidemiol. 2016;45 doi: 10.1093/ije/dyw060. 714–5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scottish Government Model of Care Polypharmacy Working Group . 2nd Edn. Scottish Government; Edinburgh: 2015. Polypharmacy guidance. [Google Scholar]
- 15.Masnoon N., Shakib S., Kalisch-Ellett L. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230. doi: 10.1186/s12877-017-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NSS . 2015. Assessment of SMR01 data Scotland 2014-2015.http://www.isdscotland.org/Health-topics/Hospital-care/Publications/2012-05-08/assessment-of-SMr01Data-2010-2011Scotlandreport.pdf Available from: [Google Scholar]
- 17.Scotland NRo . 2017. Migration between Scotland and overseas.http://www.nrscotland.gov.uk/statistics-and-data/statistics/statistics-by-theme/migration/migration-statistics/migration-between-scotland-and-overseas Available from: [Google Scholar]
- 18.Scottish Government . 2009. SIMD scottish index of multiple deprivation 2009 general report.http://www.gov.scot/resource/doc/289599/0088642.pdf Available from: [Google Scholar]
- 19.Scottish Government . 2014. Scottish government urban rural classification 2013-2014.http://www.gov.scot/Publications/2014/11/2763/0 Available from: [Google Scholar]
- 20.Payne R.A. The epidemiology of polypharmacy. Clin Med. 2016;16:465–469. doi: 10.7861/clinmedicine.16-5-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hovstadius B., Hovstadius K., Astrand B. Increasing polypharmacy - an individual-based study of the Swedish population 2005-2008. BMC Clin Pharmacol. 2010;10:16. doi: 10.1186/1472-6904-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallagher P., Lang P.O., Cherubini A. Prevalence of potentially inappropriate prescribing in an acutely ill population of older patients admitted to six European hospitals. Eur J Clin Pharmacol. 2011;67:1175. doi: 10.1007/s00228-011-1061-0. [DOI] [PubMed] [Google Scholar]
- 23.Kim H.-A., Shin J.-Y., Kim M.-H. Prevalence and predictors of polypharmacy among Korean elderly. PLoS One. 2014;9 doi: 10.1371/journal.pone.0098043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onoue H., Koyama T., Zamami Y. Trends in polypharmacy in Japan: a nationwide retrospective study. J Am Geriatr Soc. 2018;66:2267–2273. doi: 10.1111/jgs.15569. [DOI] [PubMed] [Google Scholar]
- 25.Hua M., Gong M.N., Brady J. Early and late unplanned rehospitalizations for survivors of critical illness. Crit Care Med. 2015;43:430–438. doi: 10.1097/CCM.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basnet S., Zhang M., Lesser M. Thirty-day hospital readmission rate amongst older adults correlates with an increased number of medications, but not with Beers medications. Geriatr Gerontol Int. 2018;18:1513–1518. doi: 10.1111/ggi.13518. [DOI] [PubMed] [Google Scholar]
- 27.Fabbietti P., Di Stefano G., Moresi R. Impact of potentially inappropriate medications and polypharmacy on 3-month readmission among older patients discharged from acute care hospital: a prospective study. Aging Clin Exp Res. 2018;30:977–984. doi: 10.1007/s40520-017-0856-y. [DOI] [PubMed] [Google Scholar]
- 28.Rosted E., Schultz M., Sanders S. Frailty and polypharmacy in elderly patients are associated with a high readmission risk. Dan Med J. 2016;63:A5274. [PubMed] [Google Scholar]
- 29.Campbell S.E., Seymour D.G., Primrose W.R. A systematic literature review of factors affecting outcome in older medical patients admitted to hospital. Age Ageing. 2004;33:110–115. doi: 10.1093/ageing/afh036. [DOI] [PubMed] [Google Scholar]
- 30.McIsaac D.I., Wong C.A., Bryson G.L. Association of polypharmacy with survival, complications, and healthcare resource use after elective noncardiac surgery: a population-based cohort study. Anesthesiology. 2018;128:1140–1150. doi: 10.1097/ALN.0000000000002124. [DOI] [PubMed] [Google Scholar]
- 31.Härstedt M., Rogmark C., Sutton R. Polypharmacy and adverse outcomes after hip fracture surgery. J Orthop Surg. 2016;11:151. doi: 10.1186/s13018-016-0486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taha M., Pal A., Mahnken J.D. Derivation and validation of a formula to estimate risk for 30-day readmission in medical patients. Int J Qual Health Care. 2014;26:271–277. doi: 10.1093/intqhc/mzu038. [DOI] [PubMed] [Google Scholar]
- 33.Dorajoo S.R., See V., Chan C.T. Identifying potentially avoidable readmissions: a medication-based 15-day readmission risk stratification algorithm. Pharmacotherapy. 2017;37:268–277. doi: 10.1002/phar.1896. [DOI] [PubMed] [Google Scholar]
- 34.Logue E., Smucker W., Regan C. Admission data predict high hospital readmission risk. J Am Board Fam Med. 2016;29:50–59. doi: 10.3122/jabfm.2016.01.150127. [DOI] [PubMed] [Google Scholar]
- 35.Lone N.I., Gillies M.A., Haddow C. Five-year mortality and hospital costs associated with surviving intensive care. Am J Respir Crit Care Med. 2016;194:198–208. doi: 10.1164/rccm.201511-2234OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phatak A., Prusi R., Ward B. Impact of pharmacist involvement in the transitional care of high-risk patients through medication reconciliation, medication education, and postdischarge call-backs (IPITCH Study) J Hosp Med. 2016;11:39–44. doi: 10.1002/jhm.2493. [DOI] [PubMed] [Google Scholar]
- 37.Jack B.W., Chetty V.K., Anthony D. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178–187. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.