Abstract
Renewable carbon sources are a rapidly growing field of research because of the finite supply of fossil carbon. The lignocellulosic biomass walnut shell (WS) is an attractive renewable feedstock because it has a high lignin content (38–44 wt %) and is an agricultural waste stream. Lignin, a major component of lignocellulosic biomass that is currently a waste stream in pulping processes, has unique potential for chemical upgrading because its subunits are aromatic. In the interest of improving the sustainability and reducing the environmental impact of biomass processing, valorization of agricultural waste streams is important. Herein, three lab-scale, batch organosolv procedures are explored in the interest of optimal isolation of protected WS lignin (WSL). One system uses acetic acid, one MeOH, and the final EtOH as the primary solvent. The optimal condition for protected WSL isolation, which resulted in a 64% yield, was methanol and dilute sulfuric acid with formaldehyde to act as a protecting group at 170 °C. Select samples were upgraded by hydrogenolysis over a nickel catalyst. Protected lignin recovered from the optimal condition showed 77% by weight conversion to monomeric phenols, demonstrating that the protected WSL can selectively afford high value products. One key finding from this study was that MeOH is a superior solvent for isolating WSL versus EtOH because the latter exhibited lignin recondensation. The second was that the Ni/C-catalyzed reductive catalytic fractionation (RCF) directly of WS biomass was not selective relative to RCF of isolated WSL; conversion of raw WS to monomers produced significantly more side products.
1. Introduction
Industrial-scale chemical manufacturing is currently dependent on carbon in the form of coal, natural gas, and liquid oil as feedstock.1 Increasing consumption of single-use plastics and other products made from these non-renewable feedstock is a positive climate forcer. To mitigate global warming and to move toward a more sustainable industrial system for chemical and material production, an alternative carbon feedstock is necessary. Non-food lignocellulosic biomass is a promising alternative; it is renewable and readily available in existing agricultural waste streams.2 The primary components of typical lignocellulosic biomass are acid-soluble lignin (ASL) and acid-insoluble lignin (AIL) (combined 10–25 wt %), cellulose (40–60 wt %), and hemicellulose (20–40 wt %).3 Non-organic or other unquantified material in biomass is categorized as ash, which is subcategorized as acid-soluble ash (ASA) and acid-insoluble ash (AIA).
In recent years, many studies have focused on the chemical valorization of lignin, a structural component of biomass. Lignin is an amorphous, three-dimensional polymer composed primarily of three aromatic monomers (Figure 1a), which vary by species in ratio and linkage frequency. Notably, β-O-4 bonds make up 40–60% of all linkages in lignin and β-5 bonds 6–12% (Figure 1b).4 As an abundant, naturally occurring aromatic polymer, lignin has unique potential to serve as a feedstock for bulk aromatic chemicals if effective and economic depolymerization methods can be realized.5,6
Figure 1.
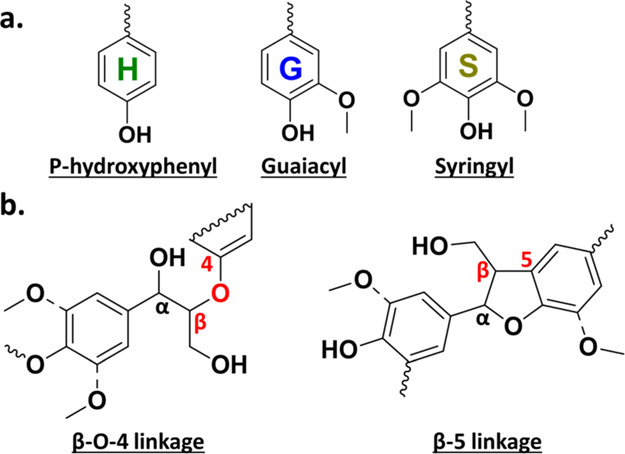
Lignin (a) monomers and (b) predominant linkages.
Currently, the presence of lignin in industry is primarily as a byproduct of pulping processes such as Kraft. The black liquor produced by Kraft pulping, which comprises highly degraded lignin, is commonly burned for heat, while intact cellulose fibers are used to produce valuable products such as paper.7 Delignification pretreatment is vital to cellulose-fed processes because entanglement between the structural lignin network and linear cellulose makes lignocellulosic biomass recalcitrant.8−11 Hence, lignin removal and valorization has become a hot research topic, with notable industrial progress such as the product “liquid wood”, registered as ARBOFORM by TECNARO.12
There is a variety in composition between all the available lignocellulosic species, so certain species will be better suited for production of certain chemical compounds. Woody biomass is between 14 and 25% lignin depending on whether it is soft versus hard wood, and many of these biomass feedstock have been well-studied.13,14 Grass-type biomass ranges from 15 to 20% lignin and has ferulate/diferulate linkages in their cell walls. Under hydrodeoxygenation (HDO) conditions, their isolated monomers have methyl ferulate tails.15−17 Nut shells, which contain 26–45% lignin, fall into neither of the preceding categories.
While they have been studied to some degree, nut shell lignins are less characterized.18,19 Due to large variations in lignin content, ash content, and lignin character, lignocellulosic feedstock must be studied by species to determine if they are amenable to pretreatment and upgrading. Methods that work for one biomass may not necessarily work for another due to the complexity of the feedstock.20
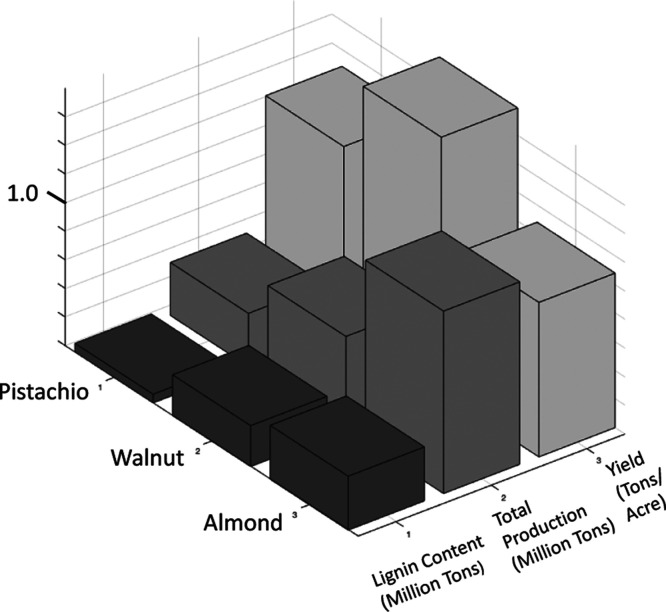
Industrial-scale agriculture uses large amounts of land, fertilizer, pesticides, and water. The implications of these demands are habitat loss, disruption in the water cycle, environmental contamination and injustices, and disruptions in local ecological cycles.21 Therefore, it is sensible to use agricultural waste that is already being generated rather than fueling new agricultural growth. In California alone, walnuts are the sixth most valuable crop, valued at $1.3 billion in 2019.22 The only other nuts in the top 50 highest value crops are pistachio in the fourth place and almonds in the first. Thus, any of these nut-shell waste streams is a potentially untapped resource for non-food lignocellulosic material. In this study, walnut shell (WS) was investigated because it has both the highest lignin content by mass and the highest crop yield by land area (Figure 2).22,23
Figure 2.
Lignin content, crop production, and crop yield comparing pistachio (P), walnut (WS), and almond (A).
Notable past works using WS biomass with a variety of different organosolv systems, lignin valorization methods, and characterization approaches have been reported, although no comprehensive studies were found.24−30 Other examples of low environmental impact and abundant biomasses used in similar studies are plant trimmings such as banana leaf, other nut shells, or invasive species.18,19
A process called reductive catalytic fractionation (RCF) of lignocellulose is an emerging biorefinery method that combines the fractionation of biomass with lignin depolymerization, referred to as “lignin first” strategy.31−35 Currently, the literature for RCF of WS has not been reported. Rather, the main focus of the utilization of WS waste has been through pyrolysis. Naderi and Vesali-Naseh reported the use of water and high temperature (300 °C) to convert WS into hydrochar.36 However, these were performed on 10–30 mg scales of WS and the converted WS overall is turned into carbon-densified hydrochar, which needs further characterization. Kar reported pyrolysis on WS, which yielded 30%, which required harsh conditions in order to convert the WS to hydrochar.37
Organosolv pretreatment was chosen as the delignification method of focus after direct RCF of WS using our selected method was found to give a large mixture of untractable products. Organosolv lignin isolation can be accomplished under relatively mild conditions using an alcoholic medium, which circumvents the need for toxic volatile organic solvents. Organosolv lignin extraction for valorization and pretreatment purposes has been widely reported as an effective, green, and non-destructive delignification method.4,11,28−46 The non-destructive nature of organosolv delignification as a pretreatment is especially attractive because cellulose valorization processes often require harsher conditions, which lead to lignin recondensation. To reduce waste, it is important that lignin fractions are isolated and stabilized while leaving cellulose intact, so that utilization of the whole biomass is possible.47
In this study, three organosolv methods for WSL removal are investigated. The first (System A), a formic acid (FA)/acetic acid (AA)/water system under atmospheric pressure, was found to be the least effective, with lignin yields ranging from 23 to 27%. The second system, refluxing 5–7 wt % hydrochloric acid (HCl) in ethanol (EtOH) or methanol (MeOH) at ambient pressure (System B), was found to give high product recovery, up to 70% yield of isolated lignin, but with severe loss in lignin quality due to recondensation. The third system (System C), organosolv in aqueous sulfuric acid (H2SO4), MeOH, and formaldehyde under 15–20 bar hydrogen (H2) in a Parr reactor, gave 60–64% yield of stabilized lignin. This lignin showed high selectivity and yield for monomeric products when subjected to our chosen method of Ni/C-catalyzed hydrogenolysis. In contrast, the same catalytic hydrogenolysis reaction applied directly to raw WS gave a large mix of products, which cannot be easily upgraded.
Our three systems were selected due to the accessibility of the solvents and reagents as commodity chemicals. EtOH is the major product of biorefining. AA is a commodity chemical that is less hazardous than halide-based acids such as HCl. Finally, formaldehyde was added to the organosolv process because of its recognized protecting group reactivity; it helps prevent carbocation-based recondensation of lignin as has been described by Luterbacher.47
Organosolv lignin products were analyzed by heteronuclear single quantum coherence nuclear magnetic resonance (HSQC-NMR) and gel-permeation chromatography (GPC). WSL, which was deemed intact by these analysis methods, was subjected to hydrogenolysis over a nickel catalyst.
2. Experimental Section
2.1. Materials
Untreated, ground WS was purchased from Agra Grit in a 35/50 particle size. A 10 wt % nickel catalyst on activated carbon support (Ni/C) was synthesized following previously reported procedures.32,48 Formic acid (88 wt % in water) and methanol were purchased from Fischer. Sulfuric acid (95–98 wt %), hydrochloric acid (38 wt %), and acetic acid (glacial, 99 wt %) were purchased from Sigma-Aldrich. Ethanol (200 proof) was purchased from Gold Shield. Commercial chemicals were used as received. Water was filtered to nano-purity at 18 Ω resistance by a Millipore “Q-Guard 2” machine. Reactions which took place under pressure were done in a Parr 5000 multi-reactor instrument.
2.2. WS Composition Analysis
Composition of WS biomass was analyzed following the NREL procedure.49 300 mg of raw WS biomass was combined with 3 Ml of 72 wt % H2SO4 in a dry pressure tube and stirred with a dry glass rod. The tubes, still equipped with stir rods, were placed on a shaker rack immersed in a 35 °C water. Starting at 0 min, the contents of each pressure tube were stirred every 10 min for 1 h while continuously shaking. At the end of the hour, 84 mL of nanopure water was added to each pressure tube. Pressure tubes with the mixtures were then capped, agitated by inversion, and autoclaved on liquids setting; 121 °C for 1 h. The tubes were allowed to cool completely to room temperature before removal of the cap. They were inverted before aliquots were removed to ensure homogeneity.
To measure AIL, solids post-hydrolysis were removed by gravity filtration and allowed to dry for 4–6 h in a 105 °C oven and then weighed to obtain the oven dry weight. In a tared crucible, the dry solid underwent pyrolysis in a furnace on ramp setting with the following program: room temperature to 105 °C, hold at 105 °C for 12 min, ramp to 250 °C at 10 °C/min, hold at 250 °C for 30 min, ramp to 575 °C at 20 °C/min, hold at 575 °C for 180 min, temperature drop to 105 °C, and then hold until a full 24 h from initial ramp had passed. Contents of the crucible after pyrolysis were AIA, the mass of which was subtracted from the oven dry weight taken earlier, to give the mass of AIL. The filtrate from the pressure tubes was diluted by a factor of 10 with water. UV–vis measurement of the resulting solution was used to calculate a range of ASL content assuming that Beer’s Law was obeyed. The known extinction coefficients published in the referenced NREL procedure were used to give upper and lower limits for ASL content.49 Agilent Technologies 1260 Infinity high performance liquid chromatography (HPLC) equipped with UV and refractive index detection was used to quantify xylose, glucose, and fructose against a 10 mM tert-butanol internal standard and the corresponding calibration curves. Composition analysis was replicated five times, and final values were determined by averaging results weighted by starting biomass.
2.3. Biomass Fractionation
Biomass fractionation methods were optimized starting from published procedures.25,50 Organosolv methods differ in solvent systems but generally follow the pathway in Figure 3. Three solvent systems were chosen for this study; formic acid/acetic acid/water (FA/AA/H2O) (System A), EtOH or MeOH and concentrated HCl (System B), and H2SO4 (aq)/MeOH/formaldehyde (System C). For System A; 20/30/50 v/v/v of FA/AA/H2O solution was combined with biomass in a 10 mL/1 g ratio in a round bottom flask. The flask was then heated to 60 °C for 1 h and then 110–135 °C for 3 h with constant stirring at 500 rpm. For System B: 5–10% volume concentrated HCl in EtOH or MeOH was combined with biomass in a 10 mL/1 g ratio in a round bottom flask and then heated to reflux at 80–120 °C for 6 h while stirring at 500 rpm. For System C: in a 75 mL stainless steel Parr vessel, 2 g of biomass was combined with 20 mL of MeOH, 20 mL of 0.045 N H2SO4, and 4 mL of formaldehyde. The reactor was sealed and purged five times with nitrogen (N2) and then heated for 30 min under 15–20 bar N2 while stirring at 700 rpm.
Figure 3.
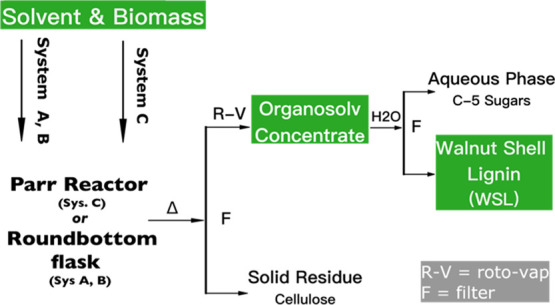
Organosolv process diagram.
The workup for each system was comparable. The organosolv solution was allowed to cool, and then the biomass pulp was removed by filtration and rinsed with EtOH or MeOH depending on the starting solvent or 0.05 M AA in the case of System A. The washes and filtrate were combined and concentrated to approximately 10% original volume by rotary evaporation. For System B, the resulting concentrate was added dropwise to 150 mL water while stirring at 200 rpm. Once the dropwise addition was complete, another 100 mL of water was added. For Systems A and C, water was added to the concentrate to bring the volume up to twice the original. For all three systems, after water was introduced, the solution was placed in a refrigerator overnight and then filtered. Water was used to rinse the recovered precipitated WSL. The WSL was air-dried overnight and then weighed. The organosolv lignin (WSL) was analyzed by GPC and HSQC-NMR. Let it be noted that lignin recovered from Systems A and C are orange-brown in color, while lignins recovered from System B are pale pink-purple. Each organosolv experiment was replicated at least three times. Yields are reported as wt % of theoretical acid soluble lignin (ASL) in the starting dry WS material.
2.4. Lignin Re-precipitation
Isolated organosolv lignin from System B or C was heated while stirring in the alcoholic solvent; it was originally extracted in EtOH or MeOH at a 1 g/100 mL ratio at 70 °C until either complete solvation was achieved or 24 h had elapsed. The liquor was then decanted and concentrated by rotary evaporation. The concentrate was added dropwise to 150 mL of water while stirring. The precipitate was filtered out, dried overnight, weighed, and characterized by GPC and HSQC-NMR. All experiments were replicated at least three times.
2.5. Reductive Catalytic Upgrading of Lignin
Lignin was depolymerized and deoxygenated using a 10 wt % Ni/C catalyst as reported by Luo et al.50,51 In a 75 mL Parr vessel, 50–100 mg of WSL or raw WS was combined with 5–10 mg of 10 wt % Ni/C catalyst and 20 Ml OF MeOH. After purging with H2 five times, the reaction was run under 35 bar H2 at 225 °C for 12 h while stirring at 700 rpm. Once the reaction was cooled, the contents were filtered to remove the solid catalyst, which was rinsed with three 3 mL portions of MeOH. Wash and filtrate were combined and concentrated by rotary evaporation until an oil was achieved. The oil was further dried under vacuum in a tared vial until the mass stabilized. The final product was analyzed by HPLC with a ZORBAX Eclipse XDB-C18 column (250 × 74.6 mm) set at 30 °C. The aromatic biophenol products were compared and quantified versus calibration curves of standards. All experiments were replicated at least three times. Yields are reported as wt % of starting ASL in dry WS material.
3. Results and Discussion
3.1. Composition
Untreated WS was found to be 8% moisture by mass. The composition of dry WS mass was determined by this study as follows: 12 wt % cellulose, 24 wt % hemicellulose, 37 wt % ASL, 7 wt % AIL, and 20 wt % inorganics.52 Our findings are in accordance with reported values.53 The uncertainty in lignin content is due to uncertainty in the extinction coefficient for ASL. The upper and lower limits for ASL composition are determined by the upper and lower molar absorptivity coefficients defined by the NREL method followed.52 Inorganic or un-characterized content in the biomass is classified as ash, which is further categorized as AIA or ASA. The full composition is presented in Table 1. Reported yields are based on dry lignocellulosic biomass, meaning the 8 wt % moisture is excluded. For lignin yields, because the utilized organosolv methods are all acidic, only ASL is considered accessible and the maximum theoretical lignin recovery is 37 wt % of dry WSL mass. Therefore, the yield is the weight percent recovered out of theoretical ASL weight.
Table 1. WS Biomass Composition Obtained Using the Standard NREL Method.
| component | wt % |
|---|---|
| ASL | 37 ± 4 |
| AIL | 7 |
| cellulose | 12 |
| hemicellulose | 24 |
| AIA | 13 |
| ASAa | 7 ± 4 |
To bring mass balance to 100%.
3.2. RCF of WS Biomass over Ni/C
To assess the viability of the “lignin first” strategy with WS biomass, 10 wt % Ni/C catalyst was used in both MeOH and EtOH at 225 °C. These conditions have been shown to achieve 40–50% selective yield of monomeric phenols based on lignin content in a diverse array of woody biomass.32,44,50,52 In the case of WS biomass, 53% of lignin was converted to lignin oil in EtOH solvent. However, analysis showed a large number of products (Figure S5). The same reaction in MeOH led to 100% conversion of lignin-to-lignin oil. However, these reactions also yielded an intractable number of products (Figure S5). The low selectivity shows that WS biomass is not suitable for direct RCF by this catalyst. Lignin extraction, stabilization, isolation, and subsequent upgrading are necessary to access meaningful aromatic/phenolic products from WS lignin. We describe next WSL lignin characterization and hydrogenolysis catalysis over Ni/C from three organosolv lignin extraction systems.
3.3. Solvent, Pretreatment, and the Effect of Pressure
In the organosolv lignin isolation process, acid induces carbocation formation in benzylic positions. The carbocation reacts with other lignin molecules in a condensation reaction or with the solvent.55 As reported by Luterbacher, formaldehyde facilitates production of uncondensed lignin by converting the α- and γ-hydroxyl groups to a stable 1,3-dioxane/acetal blocking the formation of benzylic carbocations.47
It has been previously reported that organosolv extraction is dependent on the solvent to biomass ratio, with higher ratios giving higher yields.56 To test if the solubility of lignin in the organosolv solution is a limiting factor for the extraction of WSL at 1:10 WS/solvent g/mL for System A, an analogous experiment was run with a 1:20 WS/solvent g/mL ratio. Results shown in Table 2 indicate that the solution was not being saturated with lignin at the 1:10 ratio since yields with the 1:20 ratio were comparable. Solvent to biomass ratio experiments were repeated with Systems B and C. For System B, it was found that doubling the solvent to biomass ratio did result in higher yields but the effect was modest.56
Table 2. Effect of Solvent and Soxhlet Extractives on Lignin Extraction Systems A, B, and C.
| entry | system | Soxhleta | biomass/solvent (g/mL) | isolated lignin (% yield)b |
|---|---|---|---|---|
| 1 | A | N | 1/10 | 30 |
| 2 | A | Y | 1/10 | 27 |
| 3 | A | N | 1/20 | 30 |
| 4 | A | Y | 1/20 | 23 |
| 5c | A | N | 1/10 | 27 |
| 6 | Bd | N | 1/10 | 34 |
| 7 | Bd | N | 1/20 | 43 |
| 8 | Be | N | 1/10 | 35 |
| 9 | Be | N | 1/20 | 48 |
| 10 | C,170 °C | N | 1/10 | 67 |
| 11 | C,170 °C | N | 1/20 | 65 |
WS pretreated with Soxhlet extraction in ethanol and water for 24 h each.
Based on theoretical ASL of 37 wt % of dry WS biomass.
Reaction under an N2 atmosphere.
EtOH.
MeOH.
To check if extractives hinder the solvolysis of WSL, System A organosolv was run with WS that had been pretreated by Soxhlet extraction in EtOH and water.55 Only 2% of WS mass was extracted after 24 h of EtOH followed by 24 h of water pretreatment. Furthermore, there was no notable difference in isolated lignin yield or quality from WS that underwent Soxhlet extraction pretreatment versus WS that did not. The small amounts of extractives and negligible effect on the organosolv results led to the conclusion that Soxhlet treatment is unnecessary for WSL extraction, and all subsequent experiments were conducted without Soxhlet extraction of WS biomass. An organosolv experiment in System A was also conducted under an inert N2 atmosphere, as opposed to air, to check the extraction process for air sensitivity. Results indicated no air sensitivity; thus, further experiments were conducted without protection from air. However, System C is conducted under pressure in a Parr reactor so inert atmosphere N2 was used.
Complete results from the aforementioned screening experiments are summarized in Table 2. The higher yields obtained by Systems B and C indicate that WSL is more suited for alcohol solvated extractions. Therefore, B and C were pursued for further optimization.
3.4. Optimization of WSL Isolation
Solvent acidity, reaction time, and temperature were explored as reaction parameters. Optimization results are given in Table 3.
Table 3. Optimization of Organosolv Systems for WSL Isolation.
| entry | temperature (°C) | time (h) | pressurea (bar) | solvent system (system symbol) | solvent ratio (by volume) | biomass recovered (wt %) | lignin yield (wt %)d |
|---|---|---|---|---|---|---|---|
| 1 | 85 | 6 | 1 | HCl/EtOH (B)c | 5/95 | 83 ± 6 | 46 ± 8 |
| 2 | 120 | 6 | 1 | HCl/EtOH (B)c | 7/93 | 71 | 55 ± 16 |
| 3 | 85 | 6 | 1 | HCl/MeOH (B)c | 5/95 | 70 | 35 ± 2 |
| 4 | 150 | 0.5 | 15–18 | H2SO4/MeOH/HCOH (C)b | 5/5/1 | 56 ± 2 | 26 ± 4 |
| 5 | 160 | 0.5 | 15–18 | H2SO4/MeOH/HCOH (C)b | 5/5/1 | 50 | 52 ± 8 |
| 6 | 170 | 0.5 | 15–18 | H2SO4/MeOH/HCOH (C)b | 5/5/1 | 32 ± 8 | 62 ± 2 |
| 7b | 180 | 0.5 | 15–18 | H2SO4/MeOH/HCOH (C)b | 5/5/1 | 41 ± 2 | 88 ± 7 |
| 8e | 190 | 0.5 | 15–18 | H2SO4/MeOH/HCOH (C)b | 5/5/1 | 26 ± 2 | 37 ± 2 |
Nitrogen pressure.
0.045 N aqueous sulfuric acid.
Concentrated HCl.
Theoretical ASL assumed to be 37 wt % of dry lignocellulosic WS.
Product significantly degraded.
Generally, a higher acid concentration and temperature improved lignin yield, a trend which has been previously reported for various other organosolv processes.57 It was observed that WSL yields in System C afforded a smaller range over multiple experiments than did System B. It was also found that temperatures above 180 °C or reaction times longer than 0.5 h for System C resulted in charred material (dark color and coarser) rather than the orange, fine powder collected from WSL isolation at lower temperatures.
Table 4 shows previous reports of WS lignin extraction for comparison to our work. Our optimized conditions offer higher yields and shorter reaction times for WSL isolation.
Table 4. Comparison of Reported Literature Results on the Extraction of Lignin from WS.
3.5. WSL Characterization
GPC in dimethylformamide (DMF) was used to characterize the molecular weight and dispersity of isolated WSL (Figure 4). WSL from Systems A and B with MeOH and C gave by GPC a single distribution with average Mn in the range of 5–7 kg mol–1 and a polymer dispersity index in the range of 4–7, typical of organosolv lignin (Figure 4, Table S1).46,58 However, crude lignin isolated from System B in EtOH organosolv afforded a bimodal GPC distribution. One peak is in the expected range, with an Mn value of 4–5 kg mol–1, while the second larger peak has an Mn over 100 kg mol–1. It should be noted that samples which afforded this bimodal distribution took up to a day to dissolve in DMF, while those which displayed a single distribution dissolved readily in less than 10 min.
Figure 4.
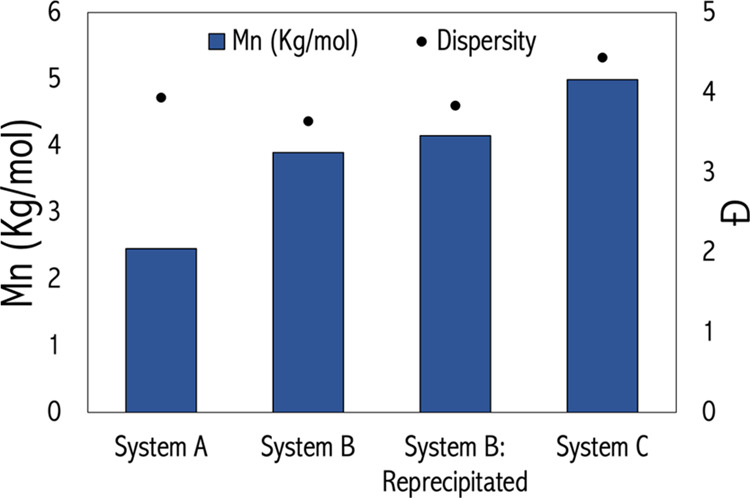
WSL characterization by GPC in DMF is presented above with x-axis referring to lignin samples from different extraction methods, y-axis (left) is the number average molecular weight (Mn), and y-axis (right) is dispersity (Đ). System B data correspond to the low molecular weight fraction and System C for reactions run at 170 °C.
To investigate the difference in GPC results between the MeOH and EtOH System B organosolv lignins, a sample from the EtOH process was reprecipitated. Only 30–40% of the original WSL by weight was recovered after reprecipitation. As seen in Figure 4, GPC on the purified product gave a single peak distribution of lower molecular weight (Mn = 5–7 kg mol–1), which closely resembled those of Systems A and B with MeOH and C. The solubility of the reprecipitated lignin product was also improved; it dissolved readily in DMF in 10 min. The same process on all other samples did not return different GPC results, and no change in solubility was observed.
In summary, EtOH-derived WSL had poor solubility, gave a bimodal distribution by GPC, and a low reprecipitation yield. MeOH, a stronger nucleophile, yielded WSL samples which did not show these characteristics under the same conditions. Organosolv lignin is typically of molecular weight between 2 and 5 kg/mol, and high molecular weight fractions (≫5 kg/mol) are often recondensed material.6,60 Therefore, we attribute these characteristics to inadequate protection of carbocation intermediates by EtOH, which resulted in recondensation.
HSQC-NMR is a well-established method for lignin analysis as the chemical shifts for peaks corresponding to the monomers and monomeric linkages found in lignin are well characterized.59 β-O-4 and β–β linkages and aromatic monomer peaks are assigned in the HSQC spectra, displayed in Figure 5a,b, for System C WSL. Therefore, higher heat must aid the solvation process. Following this trend, System C at 180 °C yielded the highest organosolv product recovery at a theoretical lignin yield of 81–95%. However, HSQC-NMR of the isolated solid, which was darker in color and coarser in texture, revealed that the native lignin structure was not preservedand high molecular weight factions (≫5 kg/mol) are recondensed material.61
Figure 5.
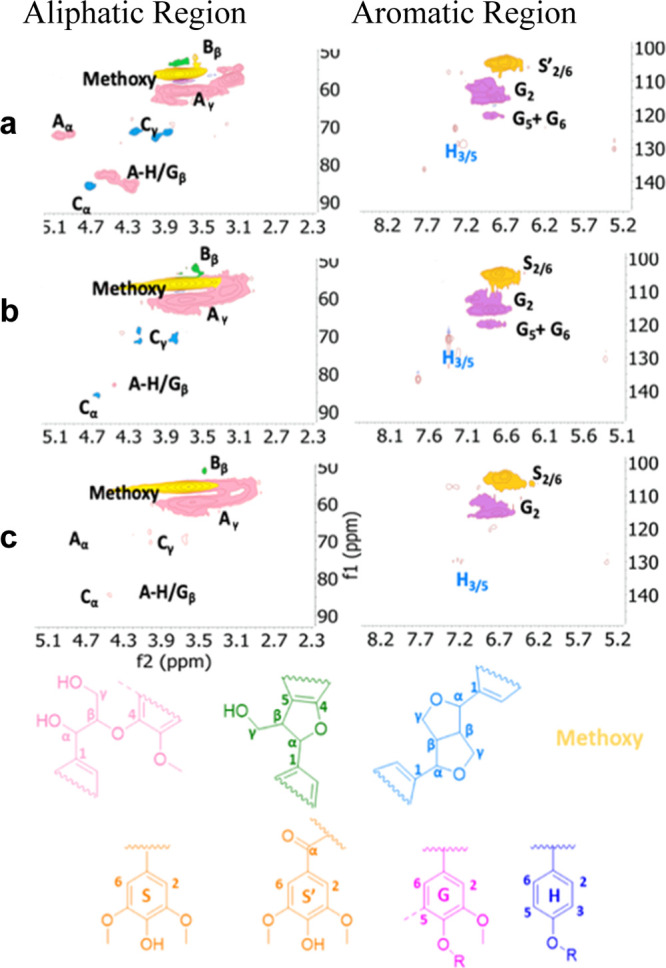
HSQC-NMR spectra for System C organosolv products at (a) 160, (b) 170, and (c) 180 °C. Horizontal axes correspond to 1H chemical shift and vertical to 13C. To see formaldehyde incorporation in aliphatic region, refer to Figure S4.
The spectra seen in Figure 5c display monomer and linkage peaks which are far less prominent than those in Figure 5a,b. It follows that, while the higher heat aided delignification, it must have also accelerated degradation/recondensation reactions. Higher heat also led to larger mass loss, which means that more cellulose and hemicellulose is extracted at higher temperatures.
These findings align with those reported by Jiang et al., where it is stated that harsh temperature and acidic conditions for organosolv ethanol pretreatment of various lignocellulosic biomass samples often result in high extraction by mass, but significant recondensation of lignin.54 Longer exposure to heat, 160 °C for 1 h, also produced a lignin product with non-native HSQC-NMR peaks and a more charred physical appearance. It was concluded that 170 °C for 1/2 h is optimal for yield and quality for the System C organosolv process.
For quantitative analysis, the β-O-4, β–β, and β-5 linkages for (a) 160, (b) 170, and (c) 180 °C were calculated by integrating the contour signals (Table 5). Based on the integration of the β-aryl ether (Aα), phenylcoumaran (Bα), and resinol (Cα) contour signals, the following percentages were found (Table 5): For β-O-4, 66, 70, and 25% were found for (a) 160, (b) 170, and (c) 180 °C, respectively. For β–β, 34, 30, and 73% were found for (a) 160, (b) 170, and (c) 180 °C, respectively.
Table 5. Characteristics of the Organosolv WS Lignin Based on System Ca.
S: syringyl units, G: guaiacyl units, H: p-hydroxyphenyl units.
Too small to ascertain.
%X = X/(S2/6 + S′2/6 + G2 + G5 + G6), X = S2/6 + S′2/6, G2 + G5 + G6.
%Y = Y/(Aα + Bα + Cα), Y = Aα, Bα, Cα.
The distribution of H/G/S was also determined based on the aromatic region of the spectra (see Table 5). Lignin isolated at 170 °C showed higher connectivity for β-O-4 in comparison to 160 and 180 °C. The quantitative analysis shown in Table 5 confirms that at 180 °C, there is significant decrease in intact β-O-4 and β–β linkages. Thus, System C at 170 °C is considered optimal for both yield and quality.
Crude WSL from System B in EtOH, which gave a bimodal GPC distribution, took nearly 24 h to dissolve in the 5:1 deuterated dimethylsulfoxide-d6/pyridine-d5 solution for NMR analysis. Other samples dissolved in less than 10 min. Upon dissolution of the recalcitrant System B with EtOH-derived lignin, the NMR solution became viscous and gel-like. The sample returned HSQC-NMR spectra which contained unidentified peaks and were not reminiscent of native lignin spectra. Examination of the HSQC-NMR spectra in Figure S3, specifically the β-O-4 region, reveals that there is modification of the lignin due to significant incorporation of the alcoholic solvent, which is consistent with previous reports.28,59
To investigate in parallel to the GPC experiments, the spectra of the reprecipitated product from System B in EtOH WSL were taken. The resulting spectra had peaks as expected for native organosolv lignin and similar to those corresponding to System C at 160 and 170 °C. Notably, the defined β-O-4 peaks, which were not observed in the crude product spectra, were visible in the purified product spectra. Additionally, S, G, β–β, and β-5 peaks appear in a higher volume in the spectra for the purified lignin samples than in those for the crude product (see Supporting Information Figure S2 and Table S2). Presumably, the native characteristic peaks were overwhelmed by modified lignin peaks. Modified lignin peaks were observed in a high volume between 3 and 3.5 ppm in the 1H spectrum and 65–75 ppm in the 13C spectrum for the crude sample HSQC-NMR (Figures S2 and S3).
3.6. Reductive Catalytic Upgrading of Lignin
Isolated lignin must be selectively depolymerized to yield a narrow range of monomeric products for complete biomass valorization. Nickel is an attractive catalyst for this process due to its earth abundance and subsequently low cost, tendency to catalyze hydrogenolysis reactions, and selectivity for carbon–oxygen bonds and allyl alcohols.53−56 Because of these properties, nickel catalysts have been thoroughly studied and utilized in the field of biomass valorization, with notable advances in RCF, which offers an industrially viable biomass valorization process.62−65
The selected WSL samples were subjected to hydrogenolysis over the Ni/C catalyst to evaluate the potential of isolated WSL for obtaining monomeric phenol products. These experiments also demonstrate the benefit of isolating lignin before upgrading reactions on top of the benefit which lignin removal provides to cellulose-based processes. Samples from Systems B and C were selected such that MeOH/H2SO4 and EtOH/HCl pretreatments were both represented. WSL samples which showed the highest β-O-4 frequency from NMR analysis were used for the comparative catalytic upgrading study because the hydrogenolysis mechanism targets this linkage specifically.66
Catalysis with 10 wt % Ni/C resulted in conversion of System C at 170 °C with MeOH lignin to an oil product, comprising two major phenolic products (Figure 6, Table 6, entry 1).49,50 For Systems B and C with EtOH, the yield of lignin monomers as well as selectivity for G and S phenolic monomers were significantly lower (Table 6). The success of organosolv lignin from MeOH/H2SO4/H2CO (System C) is attributed to successful protection of the α-carbon in the β-O-4 linkage reducing C–C bond formation (recondensation).
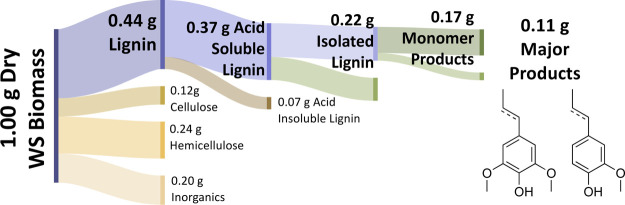
Figure 6.
Sankey diagram of lignocellulosic WS mass to illustrate mass flow throughout the entire reported process. Major products are also depicted.
Table 6. Hydrogenolysis of WSL over Ni/Ca.
| entry | feedstock sample | monomer products (wt %) | G and S Phenol monomers % yieldb |
|---|---|---|---|
| 1 | WSL: System C, MeOH, 170 °C | 46 | 29 |
| 2 | WSL: System C, EtOH, 170 °C | 15 | 2 |
| 3 | WSL: System B, 85 °C, 95/5 EtOH/HCl | 23 | 5 |
Conditions: 10 wt % Ni/C in MeOH, 225 °C, 12 h. Yield of monomer products is relative to total theoretical ASL.
Propyl guaiacol and propyl syringol based on theoretical lignin content in WS.
4. Conclusions
In this study, the valorization potential of WSL was studied by three different organosolv processes. Organosolv pretreatment was explored because direct catalytic hydrogenolysis of raw WS was deemed non-viable due to relatively low product selectivity. EtOH and HCl under ambient pressure proved to effectively fractionate lignin from WS but failed to adequately protect the extracted lignin. Recalcitrant, recondensed lignin made up over half the mass of lignin isolated by this method. The significant size of the fraction indicates that EtOH is unfit for isolation of protected and upgradable organosolv WSL.
Our study shows that an aqueous H2SO4, MeOH, and formaldehyde solvent system is optimal for WSL isolation. The optimized method resulted in WSL recovered in 60–64% yields, based on theoretical lignin content in WS, with a typical molecular weight for organosolv lignin (5 kg mol–1). HSQC-NMR confirmed that the native structure was preserved. Subsequent batch catalytic hydrogenolysis using the earth-abundant Ni/C catalyst afforded 77% of the WSL lignin as select monomeric phenolic products.
Acknowledgments
This work was supported by the University of California, Santa Barbara (UCSB); Edison International and UCSB McNair for an Undergraduate Summer Research Fellowship to R.N.N., and the Mellichamp Initiative for Sustainable Manufacturing graduate fellowship to J.H.T.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c05936.
Color key for HSQC-NMR; HSQC-NMR for Systems B and C; HPLC of WS bio-oil from RCF; linkage quantification from HSQC-NMR; GPC; and RCF products from WS (PDF)
Author Contributions
§ R.N.N. and J.H.T. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Levi P. G.; Cullen J. M. Mapping Global Flows of Chemicals: From Fossil Fuel Feedstocks to Chemical Products. Environ. Sci. Technol. 2018, 52, 1725–1734. 10.1021/acs.est.7b04573. [DOI] [PubMed] [Google Scholar]
- IPCC . Global warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty, 2019. [V. Masson-Delmotte, P. Zhai, H. ). Portner, D. Robers, J. Skea, P. R. Shukla, A., Pirani, W. Moufouma-Okia, C. Pean, R. Pidcock, S. Connors, J. B. R. Matthews, Y. Chen, X., Zhou, M. I. Gomis, E. Lonnoy, T. Maycock, M. Tignor, T. Waterfield, in press.report [Google Scholar]
- Yang H.; Yan R.; Chen H.; Zheng C.; Lee D. H.; Liang D. T. In-Depth Investigation of Biomass Pyrolysis Based on Three major Components: Hemicellulose, Cellulose and Lignin. Energy Fuels 2006, 20, 388–393. 10.1021/ef0580117. [DOI] [Google Scholar]
- Pandey M. P.; Kim C. S. Lignin Depolymerization and Conversion: A Reveiew of Thermochemical Method. Chem. Eng. Technol. 2011, 34, 29–41. 10.1002/ceat.201000270. [DOI] [Google Scholar]
- Sun Z.; Fridrich B.; de Santi A.; Elangovan S.; Barta K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. 10.1021/acs.chemrev.7b00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillet S.; Aguedo M.; Petitjean L.; Morais A. R. C.; da Costa Lopes A. M.; Łukasik R. M.; Anastas P. T. Lignin transformations for high value applications: towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. 10.1039/c7gc01479a. [DOI] [Google Scholar]
- Van Heningen A.Converting a Kraft Pulp Mill into an Integrated Forest Products Biorefinery; Pulp & Paper Canada, 2006; Vol. 107, pp 141–146. [Google Scholar]
- Xiao Z.; Xu Y.; Fan Y.; Zhang Q.; Mao J.; Ji J. Plant lignocellulose-based feedstocks hydrogenolysis into polyols over a new efficient nickel-tungsten catalyst. Asia Pac. J. Chem. 2018, 13, e2153 10.1002/apj.2153. [DOI] [Google Scholar]
- Li C.; Zheng M.; Wang A.; Zhang T. One-pot catalytic hydrocracking of raw woody biomass into chemicals over supported carbide catalysts: simultaneous conversion of cellulose, hemicellulose and lignin. Energy Environ. Sci. 2012, 5, 6383–6390. 10.1039/c1ee02684d. [DOI] [Google Scholar]
- Kim Y.; Ximenes E.; Mosier N. S.; Ladisch M. R. Soluble inhibitors/deactivators of cellulose enzymes from lignocellulosic biomass. Enzyme Microb. Technol. 2011, 48, 408–415. 10.1016/j.enzmictec.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Cheng K.; Liu D. Organosolv pretreatment of lignocellulosicbiomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. 10.1007/s00253-009-1883-1. [DOI] [PubMed] [Google Scholar]
- Liu W.-J.; Jiang H.; Yu H.-Q. Thermochemical conversion of lignin to functional materials: a review and future directions. Green Chem. 2015, 17, 4888–4907. 10.1039/c5gc01054c. [DOI] [Google Scholar]
- Mohan D.; Pittman C. U.; Steele P. H. Pyrolysis of Wood/Biomass for Bio-oil: A Critical Review. Energy Fuels 2006, 20, 848–889. 10.1021/ef0502397. [DOI] [Google Scholar]
- Sunde K.; Brekke A.; Solberg B. Environmental impacts and costs of woody Biomass-to-Liquid (BTL) production and use- A review. For. Policy Econ. 2011, 13, 591–602. 10.1016/j.forpol.2011.05.008. [DOI] [Google Scholar]
- Morvan D.; Rauchfuss T. B.; Wilson S. R. Π- Complexes of Lignols with Manganese (I) and Ruthenium (II). Organometallics 2009, 28, 3161–3166. 10.1021/om9001445. [DOI] [Google Scholar]
- Hatfield R. D.; Ralph J.; Grabber J. H. Cell wall cross-linking by ferulates and diferulates in grasses. J. Sci. Food Agric. 1999, 79, 403–407. . [DOI] [Google Scholar]
- Rouau X.; Cheynier V.; Surget A.; Gloux D.; Barron C.; Meudec E.; Louis-Montero J.; Criton M. A dehydrotrimer of ferulic acid from maize bran. Phytochemistry 2003, 63, 899–903. 10.1016/s0031-9422(03)00297-8. [DOI] [PubMed] [Google Scholar]
- Bharthare P.; Shrivastava P.; Singh P.; Ttiwari A.. Peanut Shell as a Renewable Energy Source and Their Utility in Production of Ethanol, 2014; Vol. 2. [Google Scholar]
- Kerr T. J.; Windham W. R.; Woodward J. H.; Benner R. Chemical Composition and In-vitro Digestibility of Thermochemically Treated Peanut Hulls. J. Sci. Food Agric. 1986, 37, 632–636. 10.1002/jsfa.2740370706. [DOI] [Google Scholar]
- Yılmaz S.; Selim H. A Review on the methods for biomass to energy conversion systems design. Renew. Sustain. Energy Rev. 2013, 25, 420–430. 10.1016/j.rser.2013.05.015. [DOI] [Google Scholar]
- USGS . Investigating the Environmental Effects of Agriculture Practices on Natural Resources. 2007, https://pubs.usgs.gov/fs/2007/3001/pdf/508FS2007_3001.pdf (accessed March 26, 2020).web
- Quick Stats . 2019 State Agriculture Overview: California. 2019, https://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=CALIFORNIA (accessed Aug 24, 2020).web
- Renders T.; Van den Bosch S.; Koelewijn S.-F.; Schutyser W.; Sels B. F. Lignin-first biomass fractionation: the advent of active stabilization strategies. Energy Environ. Sci. 2017, 10, 1551–1557. 10.1039/c7ee01298e. [DOI] [Google Scholar]
- CDFA . California Agricultural Production Statistics. 2018, http://www.cdfa.ca.gov/statistics/ (accessed March 26, 2020).web
- Lancefield C. S.; Panovic I.; Deuss P. J.; Barta K.; Westwood N. J. Pre-treatment of lignocellulosic feedstocks using renewable alcohols: towards complete biomass valorization. Green Chem. 2017, 19, 202–214. 10.1039/c6gc02739c. [DOI] [Google Scholar]
- Chen L.; Wang X.; Yang H.; Lu Q.; Li D.; Yang Q.; Chen H. Study on the pyrolysis behaviors of non-woody lignins with TG_FTIR and Py-GC/MS. J. Anal. Appl. Pyrolysis 2015, 113, 499–507. 10.1016/j.jaap.2015.03.018. [DOI] [Google Scholar]
- Liu A.; Park Y.; Huang Z.; Wang B.; Ankumah R. O.; Biswas P. K. Product Identification and Distribution from Hydrothermal Conversion of Walnut Shells. J. Anal. Appl. Pyrolysis 2006, 20, 446–454. 10.1021/ef050192p. [DOI] [Google Scholar]
- Zijlstra D. S.; Analbers C. A.; de Korte J.; Wilbers E.; Deuss P. J. Efficient Mild Organosolv Lignin Extraction in a Flow-Through Setup Yielding Lignin with High β-O-4 Content. Polymers 2019, 11, 1913. 10.3390/polym11121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuss P. J.; Lancefield C. S.; Narani A.; de Vries J. G.; Westwood N. J.; Barta K. Phenolic acetals from lignins of varying compositions via iron (III) triflate catalyzed depolymerization. Green Chem. 2017, 19, 2774–2782. 10.1039/c7gc00195a. [DOI] [Google Scholar]
- Zijlstra D. S.; Lahive C. W.; Analbers C. A.; Figueredo M. B.; Wang Z.; Lancefield C. S.; Duess P. J. Mild Organosolv Lignin Extraction with Alcohols: The Importance of Benzylic Alkoxylation. ACS Sustainable Chem. Eng. 2020, 8, 5119. 10.1021/acssuschemeng.9b07222. [DOI] [Google Scholar]
- Yan N.; Zhao C.; Dyson P. J.; Wang C.; Liu L. t.; Kou Y. Selective degradation of wood lignin over nobel-metal catalysts in a two-step process. ChemSusChem 2008, 1, 626–629. 10.1002/cssc.200800080. [DOI] [PubMed] [Google Scholar]
- Song Q.; Wang F.; Cai J.; Wang Y.; Zhang J.; Yu W.; Xu J. Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation-hydrogenolysis process. Energy Environ. Sci. 2013, 6, 994–1007. 10.1039/c2ee23741e. [DOI] [Google Scholar]
- Ferrini P.; Rinaldi R. Catalytic biorefining of plant biomass to non-pyrolytic lignin bio-oil and carbohydrates through hydrogen transfer reactions. Angew. Chem. Int. Ed. 2014, 53, 8634–8639. 10.1002/anie.201403747. [DOI] [PubMed] [Google Scholar]
- Parsell T.; Yohe S.; Degenstein J.; Jarrell T.; Klein I.; Gencer E.; Hewetson B.; Hurt M.; Kim J. I.; Choudhari H.; Saha B.; Meilan R.; Mosier N.; Ribeiro F.; Delgass W. N.; Chapple C.; Kenttämaa H. I.; Agrawal R.; Abu-Omar M. M. A synergistic biorefinery based on catalytic conversion of lignin prior to cellulose starting from lignocellulosic biomass. Green Chem. 2015, 17, 1492–1499. 10.1039/c4gc01911c. [DOI] [Google Scholar]
- Van den Bosch S.; Schutyser W.; Vanholme R.; Driessen T.; Koelewijn S.-F.; Renders T.; De Meester B.; Huijgen W. J. J.; Dehaen W.; Courtin C. M.; Lagrain B.; Boerjan W.; Sels B. F. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 2015, 8, 1748–1763. 10.1039/c5ee00204d. [DOI] [Google Scholar]
- Naderi M.; Vesali-Naseh M. Hydrochar-derived fuels from waste walnut shell through hydrothermal carbonization: characterization and effect of processing parameters. Biomass Convers. Biorefin. 2019, 10.1007/s13399-019-00513-2. [DOI] [Google Scholar]
- Kar Y. Co-pyrolysis of Walnut Shell and tar sand in a fixed-bed reactor. Bioresour. Technol. 2011, 102, 9800–9805. 10.1016/j.biortech.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Tarrés Q.; Espinosa E.; Domínguez-Robles J.; Rodríguez A.; Mutjé P.; Delgado-Aguilar M. The suitability of banana leaf residue as raw material for the production of high lignin content micro/nano fibers: From residue to value-added products. Chem. Rev. 2017, 99, 27–33. 10.1016/j.indcrop.2017.01.021. [DOI] [Google Scholar]
- McDonough T. J.The chemistry of organosolv delignification. TAPPI Solvent Pulping Seminar: Boston, Massachusetts, 1992, Nov 6–7, 1992.
- Ragauskas A. J.; Beckham G. T.; Biddy M. J.; Chandra R.; Chen F.; Davis M. F.; Davison B. H.; Dixon R. A.; Gilna P.; Keller M.; Landan P.; Naskar A. K.; Saddler J. N.; Tschaplinksi T. J.; Tuskan G. A.; Wyman C. E. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. 10.1126/science.1246843. [DOI] [PubMed] [Google Scholar]
- Pan X.; Saddler J. N. Effect of Replacing Polyol by Organosolv and Kraft Lignin on the Property and Structure of Rigid Polyurethane Foam. Biotechnol. Biofuels 2013, 6, 12. 10.1186/1754-6834-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.; Harrison M. D.; Rackemann D. W.; Doherty W. O. S.; O’Hara I. M. Organosolv Pretreatment of Plant Biomass for Enhanced Enzymatic Saccharification. Green Chem. 2016, 18, 360–381. 10.1039/c5gc02034d. [DOI] [Google Scholar]
- Hu G.; Cateto C.; Pu Y.; Samuel R.; Ragauskas A. J. Structural Characterization of Switchgrass Lignin after Ethanol Organosolv Pretreatment. Energy Fuels 2012, 26, 740–745. 10.1021/ef201477p. [DOI] [Google Scholar]
- Pan X.; Kadla J. F.; Ehara K.; Gilkes N.; Saddler J. N. Organosolv Ethanol Lignin from Hybrid Poplar as a Radical Scavenger: Relationship between Lignin Structure, Extraction Conditions, and Antioxidant Activity. J. Agric. Food Chem. 2006, 54, 5806–5813. 10.1021/jf0605392. [DOI] [PubMed] [Google Scholar]
- Wen J.-L.; Sun S.-L.; Yuan T.-Q.; Xu F.; Sun R.-C. Structural elucidation of lignin polymers of Eucalyptus chips during organosolv pretreatment and extended delignification. J. Agric. Food Chem. 2013, 61, 11067–11075. 10.1021/jf403717q. [DOI] [PubMed] [Google Scholar]
- Pan X.; Arato C.; Gilkes N.; Gregg D.; Mabee W.; Pye K.; Xiao Z.; Zhang X.; Saddler J. Organosolv Pulping: Preliminary Evaluation of Process Streams for Manufacture of Fuel-grade Ethanol and co-products. Biotechnol. Bioeng. 2005, 90, 473–481. 10.1002/bit.20453. [DOI] [PubMed] [Google Scholar]
- Shuai L.; Amiri M. T.; Questell-Santiago Y. M.; Héroguel F.; Li Y.; Kim H.; Meilan R.; Chapple C.; Ralph J.; Luterbacher J. S. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 2016, 354, 329–333. 10.1126/science.aaf7810. [DOI] [PubMed] [Google Scholar]
- Klein I.; Saha B.; Abu-Omar M. M. Lignin depolymerization over Ni/C catalyst in methanol, a continuation: effect of substrate and catalyst loading. Catal. Sci. Technol. 2015, 5, 3242–3245. 10.1039/c5cy00490j. [DOI] [Google Scholar]
- Sluiter A.; Hames B.; Ruis R.; Scarlata C.. Determination of Structural Carbohydrates in Lignin in Biomass; NREL: Golden, Colorado, 2012. [Google Scholar]
- Luo H.; Abu-Omar M. M. Lignin extraction and catalytic upgrading from genetically modified poplar. Green Chem. 2018, 20, 745–753. 10.1039/c7gc03417b. [DOI] [Google Scholar]
- Luo H.; Klein I. M.; Jiang Y.; Zhu H.; Liu B.; Kenttämaa H. I.; Abu-Omar M. M. Total Utilization of Miscanthus Biomass, Lignin and Carbohydrates Using Earth Abundant Nickel Catalyst. ACS Sustainable Chem. Eng. 2016, 4, 2316–2322. 10.1021/acssuschemeng.5b01776. [DOI] [Google Scholar]
- Sluiter A.; Ruiz R.; Scarlata C.; Sluiter J.; Templeton D.. Determination of Extractives in Biomass; NREL: Golden, Colorado, 2008. [Google Scholar]
- Li X.; Liu Y.; Hao J.; Wang W. Study of Almond Shell Characteristics. Materials 2018, 11, 1782. 10.3390/ma11091782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.; Lei F.; Li P.; Jiang J. Lignocellulosic biomass to biofuels and biochemicals: A comprehensive review with a focus on ethanol organosolv pretreatment technology. Biotechnol. Bioeng. 2018, 115, 2683–2702. 10.1002/bit.26788. [DOI] [PubMed] [Google Scholar]
- Glasser W. G.; Davé V.; Frazier C. E. Molecular Weight Distribution of (Semi-) Commercial Lignin Derivatives. J. Wood Chem. Technol. 1993, 13, 545–559. 10.1080/02773819308020533. [DOI] [Google Scholar]
- Galkin M. V.; Smit A. T.; Subbotina E.; Artemenko K. A.; Bergquist J.; Huijgen W. J. J.; Samec J. S. M. Hydrogen-free catalytic fractionation of woody biomass. ChemSusChem 2016, 9, 3280–3287. 10.1002/cssc.201600648. [DOI] [PubMed] [Google Scholar]
- Kim H.; Ralph J. Solution-state 2D NMR of ball-milled plant cell wall gels in DMOS-d6/pyridine-d5. Org. Biomol. Chem. 2010, 8, 576–591. 10.1039/b916070a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeev A. G.; Hartwig J. F. Selective, Nickel-Catalyzed Hydrogenolysis of Aryl Ethers. Science 2011, 332, 439–443. 10.1126/science.1200437. [DOI] [PubMed] [Google Scholar]
- Renders T.; Van den Bosch S.; Vangeel T.; Ennaert T.; Koelewijn S.-F.; Van den Bossche G.; Courtin C. M.; Schutyser W.; Sels B. F. Selective Conversion of Lignin-Derivable 4-Alkylguaiacols to 4-Alkylcyclohexanols over Noble and Non-Noble-Metal Catalysts. ACS Sustainable Chem. Eng. 2016, 4, 6894–6904. 10.1021/acssuschemeng.6b01844. [DOI] [Google Scholar]
- Ananikov V. P. Nickel: The ″Spirited Horse″ of Transition Metal Catalysis. ACS Catal. 2015, 5, 1964–1971. 10.1021/acscatal.5b00072. [DOI] [Google Scholar]
- Wenkert E.; Michelotti E. L.; Swindell C. S. Nickel-Induced Conversion of Carbon-Oxygen into Carbon-Carbon Bonds. One-Step Transformations of Enol Ethers into Olefins and Aryl Ethers into Biaryls. ACS Commun. 1979, 101, 2246–2247. 10.1021/ja00502a074. [DOI] [Google Scholar]
- Felkin H.; Swierczewski G. Activation of Gringard Reagents by Transition Metal Compounds. Tetrahedron 1975, 31, 2735–2748. 10.1016/0040-4020(75)80283-3. [DOI] [Google Scholar]
- Anderson E. M.; Katahira R.; Reed M.; Resch M. G.; Karp E. M.; Beckham G. T.; Román-Leshkov Y. Reductive Catalytic Fractionation of Corn Stover Lignin. ACS Sustainable Chem. Eng. 2016, 4, 6940–6950. 10.1021/acssuschemeng.6b01858. [DOI] [Google Scholar]
- Anderson E. M.; Stone M. L.; Katahira R.; Reed M.; Beckham G. T.; Roman Leshkov Y. Flowthrough Reductive Catalytic Fractionation of Biomass. Joule 2017, 1, 613–622. 10.1016/j.joule.2017.10.004. [DOI] [Google Scholar]
- https://www.nass.usda.gov/Statistics_by_State/California/Publications/Specialty_and_Other_Releases/Walnut/Objective-Measurement/201908walom.pdf (accessed Jan 15, 2021) (for walnut shell production).web
- Zijlstra D. S.; de Santi A.; Oldenburger B.; de Vires J.; Barta K.; Deuss P. Extraction of Lignin with High β-O-4 Content by Mild Ethanol Extraction and Its Effect on the Depolymerization Yield. J. Vis. Exp. 2019, 143, e58575 10.3791/58575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.