Abstract
Background
Postoperative delirium is associated with increases in the neuronal injury biomarker, neurofilament light (NfL). Here we tested whether two other biomarkers, glial fibrillary acidic protein (GFAP) and tau, are associated with postoperative delirium.
Methods
A total of 114 surgical patients were recruited into two prospective biomarker cohort studies with assessment of delirium severity and incidence. Plasma samples were sent for biomarker analysis including tau, NfL, and GFAP, and a panel of 10 cytokines. We determined a priori to adjust for interleukin-8 (IL-8), a marker of inflammation, when assessing associations between biomarkers and delirium incidence and severity.
Results
GFAP concentrations showed no relationship to delirium. The change in tau from preoperative concentrations to postoperative Day 1 was greater in patients with postoperative delirium (P<0.001) and correlated with delirium severity (ρ=0.39, P<0.001). The change in tau correlated with increases in IL-8 (P<0.001) and IL-10 (P=0.0029). Linear regression showed that the relevant clinical predictors of tau changes were age (P=0.037), prior stroke/transient ischaemic attack (P=0.001), and surgical blood loss (P<0.001). After adjusting for age, sex, preoperative cognition, and change in IL-8, tau remained significantly associated with delirium severity (P=0.026). Using linear mixed effect models, only tau (not NfL or IL-8) predicted recovery from delirium (P<0.001).
Conclusions
The change in plasma tau was associated with delirium incidence and severity, and resolved over time in parallel with delirium features. The impact of this putative perioperative neuronal injury biomarker on long-term cognition merits further investigation.
Clinical trial registration
NCT02926417 and NCT03124303.
Keywords: biomarker, delirium, glial fibrillary acidic protein, inflammation, neuronal injury, postoperative, surgery, tau
Editor's key points.
-
•
There are many different putative causes of delirium, including stroke, drugs with actions on the brain, and major organ dysfunction.
-
•
This prospective observational study assessed the relationship between postoperative delirium and plasma biomarkers associated with neuronal injury and synaptic activity.
-
•
A significant increase in plasma tau was related to delirium incidence and severity, and a subsequent decrease was related to delirium resolution.
-
•
Plasma tau measured over time might provide helpful biomarker information regarding both the cause and prognosis of delirium.
Postoperative delirium is a major healthcare issue affecting thousands of patients each year. Not only is delirium unpleasant, it is associated with increased morbidity and mortality, and increased costs and subsequent cognitive decline.1,2 However, establishing that long-term changes in cognitive decline are causally attributable to delirium is challenging. A major hurdle is that pre-delirium cognitive impairment is associated with increased risk for delirium, and patients with this preoperative cognitive decline are likely declining faster than subjects who do not incur delirium.3,4 Hence, any postoperative changes in cognition could be attributable to preoperative differences rather than the perioperative event itself. We, and others, have supplied important evidence for a small, long-term effect of surgery on cognition, when accounting for the preoperative cognitive trajectory.5, 6, 7 However, it is unclear whether this small effect is attributable—wholly or in part—to delirium. In order to address whether delirium may itself impart some pathogenic neurotoxic effect, we recently undertook studies of postoperative covert strokes8 and the plasma biomarker, neurofilament light (NfL).9 We found that delirium was associated with postoperative covert strokes8 and increases in postoperative NfL.9 Although it remains to be seen if these increases in NfL correlate with changes in long-term cognition, there are additional possibly relevant biomarkers to consider. We therefore undertook further analyses to identify if two alternate plasma biomarkers, tau and glial fibrillary acidic protein (GFAP), may show similar relationships with delirium.
Plasma concentrations of tau and GFAP have been associated with neuronal injury. Tau is a microtubule-associated protein that is highly expressed in neurons. It modulates the stability of axonal microtubules. Plasma concentrations of tau are increased in various neurodegenerative states,10 and after stroke.11 Although NfL appears to solely track neuronal injury, tau concentrations may increase after injury, synaptic activity, or synaptic pruning,12, 13, 14 making interpretation of changes more complex. However, as delirium is associated with EEG slowing,15 we hypothesised that tau is unlikely to represent increases in synaptic activity. Hence, it seems likely that any measurable tau represents either neuronal injury or synaptic pruning. Importantly, tau has not been well studied in the perioperative period and its rapid clearance from the blood, relative to NfL, may be advantageous for studying acute changes.10 GFAP is an intermediate filament protein that is expressed in astrocytes. It is often used in microscopy for their cellular identification. Despite being relatively specific for astrocytes in the CNS, expression outside of the CNS does occur, however in the plasma it is often considered a peripheral marker of astrogliosis. This limitation aside, plasma concentrations of GFAP have been proposed as a nervous system injury biomarker in traumatic brain injury and stroke, and for postoperative cognitive decline.16
As postoperative delirium is frequently considered to be a response of the brain to inflammation, we hypothesised that any neuronal marker of injury will correlate to some extent with inflammation. Therefore, we tested a panel of 10 cytokines to identify markers that correlate with delirium9 and pursued the cytokine with the strongest relationship to peak delirium severity. In our prior work, we investigated increases in NfL on postoperative Day 1 (POD1). However, NfL concentrations continued to increase with time, and it was unclear if this reflected selection bias (as healthier patients were discharged earlier), ongoing injury, or accumulation in the plasma. In this paper, we pursued POD1 changes for similar logic, and we also report information on preoperative biomarkers.
Methods
The data were derived from two ongoing prospective perioperative cohort studies registered with ClinicalTrials.gov (NCT02926417 and NCT03124303) and approved by the University of Wisconsin-Madison Institutional Review Board (2015-0960 and 2015-0374). The inclusion and exclusion criteria are listed in Supplementary Tables S1 and S2. A total of 114 adult patients were recruited who were undergoing major elective non-intracranial, noncardiac surgery which was defined as requiring at least a 2 day hospital stay (described in our recent publication on NfL9). All patients received general anaesthesia during surgery. All but one subject had volatile anaesthesia (one total i.v. anaesthesia) and 11 subjects had depth of anaesthesia monitoring. Fourteen subjects had regional anaesthesia for postoperative analgesia. See Supplementary Figure S1 for the STROBE diagram.
Participants had blood draws preoperatively and on each of the first (up to) four postoperative hospital days. Preoperatively, and twice daily postoperatively, participants underwent delirium assessments with the Confusion Assessment Method (CAM)/3D-CAM, or the CAM-ICU if the patient was intubated. Delirium severity was assessed with the validated Delirium Rating Scale-98 (DRS). No patient was intubated for greater than 96 h postoperatively.
Plasma samples were collected in EDTA-containing tubes preoperatively, and in the morning (06.00–10.00) of each postoperative day, stored at −80°C, and sent for cytokine multiplex assay for interleukin 1 (IL-1) beta, IL-1 receptor antagonist (IL-1ra), IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, monocyte chemoattractant protein 1, and tumour necrosis factor-alpha (Eve Technologies, Montreal, Canada). In addition, samples were sent to the University of Gothenburg for analysis of tau and GFAP. Tau (targeting the mid region of tau and detecting full and truncated forms of tau) and GFAP (targeting the central alpha helical rod) concentrations were measured using commercially available kits (the Tau Advantage and GFAP Discovery kits, respectively) on the Single molecule array (Simoa) HD-1 Analyzer (Quanterix, Billerica, MA, USA). Samples (78 μl) were diluted four-fold and run as singlicates, along with duplicate internal quality control (QC) samples with clinically relevant biomarker concentrations. For a QC sample with a tau concentration of 7.1 pg ml−1, repeatability was 7.1 % and intermediate precision was 11.5%. For a QC sample with a tau concentration of 24.9 pg ml−1, repeatability was 2.7% and intermediate precision was 4.3%. For a QC sample with a GFAP concentration of 82.2 pg ml−1, repeatability was 5.6% and intermediate precision was 11.2%. For a QC sample with a GFAP concentration of 221 pg ml−1, repeatability was 5% and intermediate precision was 8.6%.
We report some additional analyses with NfL that were not conducted for our prior publication. NfL concentration was also measured using a Simoa method, as previously described in detail.17 Measured values below the range of detection were imputed as 0.001 for cytokines, 1.22 pg ml−1 for tau, 0.69 pg ml−1 for GFAP, and 6.7 pg ml−1 for NfL based on the lower limit of quantification of each assay. All biomarker data were then log-transformed to correct the strong rightward skew. In total, 103 participants had both preoperative and POD1 blood samples analysable for GFAP and tau. When the difference between preoperative and POD1 samples was calculated we logged both data sets and took the difference.
Calculation of hippocampal volume
To test whether GFAP or tau could detect preoperative neurodegeneration, we correlated concentrations with preoperative hippocampal volume obtained via research scans (n=48). Hippocampal volume was calculated using high resolution T1-weighted scans, and T2-fluid attenuated inversion recovery as we have conducted previously.9,18 Cortical thickness (mm) measures were obtained using FreeSurfer v6.0 (https://surfer.nmr.mgh.harvard.edu/). All scans were processed using T1+T2-FLAIR multimodal recon-all processing stream. This includes motion correction, skull-stripping, registration, segmentation, smoothing, and parcellation mapping. Slices were manually inspected for segmentation errors and artefacts. Hippocampal volumes were defined based on the automated labelling procedure outlined by Fischl and extracted using the asegstats2table tool.4 Left and right hippocampal volumes were averaged per participant.
Statistical analysis
The sample size was determined based on a prior study that showed an increase in postoperative tau concentrations.19 Assuming that delirium harboured the burden of the tau increase from preoperative concentrations, 87 patients would provide 80% power (P=0.025) to show a difference in tau concentrations of 2.1 pg ml−1 (standard deviation 3 pg ml−1) assuming a delirium incidence of 33%. This report represents a secondary analysis of this dataset to verify whether we should test any other biomarkers in addition to NfL. The significance level for the primary outcome measure was set at P<0.025 for testing both the change in POD1 concentrations of GFAP or tau based on delirium incidence (Bonferroni correction for two tests). However, a priori we determined that for additional stringency, for any putative change in biomarker of delirium to be considered meaningful, it also had to vary proportionately to delirium severity (Spearman correlation) meaning the P-value was equivalent to 0.0252.
As a mechanistic/exploratory secondary step, a priori we determined that we would correlate GFAP or tau with each of the 10 cytokines and adjust P-values across cytokines for testing a correlation with GFAP or tau; we did not adjust for multiple comparisons across the 10 cytokines. Hence the P-value was again set at P<0.025, and these analyses should be considered hypothesis-generating. For mechanistic studies, IL-8 was selected as the marker of the inflammation, as it demonstrated the strongest correlation to delirium severity based on our prior work, and it allows direct comparison to our previous results with NfL.9 Spearman correlations of POD1 change in tau or GFAP and peak DRS were conducted. Linear regression to predict peak postoperative delirium severity was conducted in R with age, sex, preoperative Trail Making Test B (a measure of cognitive executive function), POD1 change in tau, and POD1 change in IL-8 as covariates. We also investigated clinical predictors of tau (and NfL) using a linear regression model including age, sex, ASA class, previous stroke or transient ischaemic attack (stroke/TIA), peripheral vascular disease, renal failure, type of surgery, operative time, blood loss, and area under the curve for intraoperative MAP<10% from preoperative values. Finally, we conducted linear mixed effect modelling to investigate whether change in any of the biomarkers may predict resolution of delirium symptom severity from POD1 to POD4. Variables included random intercepts for subjects and fixed effects for age, sex, time, biomarker, and a biomarker∗time interaction. All analyses were conducted in R.
Results
The cohort patient characteristics are reported in Supplementary Table S3. As this is the same cohort as our previous publication, the patient characteristics are nearly identical to those reported previously.9 However, we have updated values for Trail Making Test B (TMTB), surgery type, and blood loss. Of the 110 patients who completed surgery, 40 (36.4%) were delirious postoperatively. Delirious patients' mean peak delirium severity score, as assessed by the DRS, was 21.8, whereas non-delirious patients' mean was 7.6. As reported previously, delirious and non-delirious patients did not differ in age (69.2 vs 71.4 yr old, t=1.54, P=0.13) or sex (48% female vs 41% female, χ2=0.51, P=0.47), but did differ in whether they had vascular surgery (60% vs 37%, χ2=5.81, P=0.016) and in their National Surgical Quality Improvement Program Risk of Death (5% vs 2%, t=−2.19, P=0.033).9
Preoperative biomarker associations with postoperative delirium
Neither preoperative GFAP nor tau were associated with postoperative delirium incidence (Table 1). As we previously showed that preoperative NfL correlated with hippocampal volume, we tested whether GFAP or tau showed similar relationships. Of the subjects who had available hippocampal volume data, there were nine who later became delirious and 37 who did not.
Table 1.
Biomarker concentrations by delirium status. All preoperative values were log10-transformed. All preoperative to POD1 change values were calculated as the log10-transformed POD1 value minus the log10-transformed preoperative value. P-values show results of Wilcoxon rank sum tests with continuity correction. Four patients are missing from the Preoperative columns as a result of a blood sample not being collected before surgery. Seven additional patients are missing from the Preoperative to POD1 change columns as a result of surgery not being completed (n=3), POD1 blood draw not being completed (n=3), or sample processing error resulting in incomplete/invalid assay runs (n=1). GFAP, glial fibrillary acidic protein; IL, interleukin; IL-1ra, IL-1 receptor antagonist; IQR, inter-quartile range; MCP-1, monocyte chemoattractant protein 1; POD1, postoperative Day 1; TNFa, tumour necrosis factor-alpha.
| Preoperative |
Preoperative to POD1 change |
|||||
|---|---|---|---|---|---|---|
| No delirium median (IQR) (n=72) | Delirium median (IQR) (n=38) | P-value | No delirium median (IQR) Primary outcomes (n=66) Secondary outcomes (n=67) | Delirium median (IQR) (n=37) | P-value | |
| Primary outcomes | ||||||
| GFAP | 2.02 (0.30) | 1.91 (0.51) | 0.52 | 0.097 (0.23) | 0.17 (0.25) | 0.56 |
| Tau | 0.46 (0.25) | 0.51 (0.29) | 0.83 | 0.068 (0.29) | 0.32 (0.94) | <0.001 |
| Secondary outcomes | ||||||
| IL-1B | 0.93 (0.33) | 0.76 (1.22) | 0.014 | −0.11 (0.46) | 0.028 (0.58) | 0.037 |
| IL-1ra | 2.26 (0.41) | 2.07 (0.70) | 0.049 | −0.093 (0.31) | 0.12 (0.57) | 0.0055 |
| IL-2 | 0.73 (0.35) | 0.60 (0.84) | 0.029 | −0.082 (0.37) | 0.084 (0.59) | 0.036 |
| IL-4 | 0.89 (0.98) | 0.55 (1.15) | 0.066 | −0.064 (0.62) | 0.00 (1.07) | 0.12 |
| IL-6 | 0.47 (0.54) | 0.47 (0.47) | 0.67 | 0.88 (0.63) | 0.99 (0.93) | 0.061 |
| IL-8 | 0.98 (0.47) | 0.92 (0.44) | 0.82 | 0.15 (0.32) | 0.65 (0.68) | <0.001 |
| IL-10 | 0.97 (0.39) | 0.85 (0.49) | 0.086 | 0.089 (0.35) | 0.80 (1.13) | <0.001 |
| IL-12p70 | 1.09 (0.42) | 1.01 (0.59) | 0.12 | −0.15 (0.41) | −0.041 (0.48) | 0.16 |
| MCP-1 | 2.42 (0.17) | 2.36 (0.30) | 0.24 | 0.015 (0.22) | 0.10 (0.37) | 0.0048 |
| TNFa | 1.46 (0.29) | 1.46 (0.24) | 0.56 | −0.058 (0.26) | −0.056 (0.26) | 0.32 |
Neither preoperative GFAP (P=0.063) or tau (P=0.880) correlated with hippocampal volume (Supplementary Fig. S2, n=48).
As exploratory analyses, we investigated preoperative differences in cytokines. Preoperatively IL-1B, IL-1ra, and IL-2 may be lower in patients who subsequently incur delirium (note these are reported at an uncorrected P-value threshold of P<0.05).
Changes in perioperative biomarkers with delirium incidence
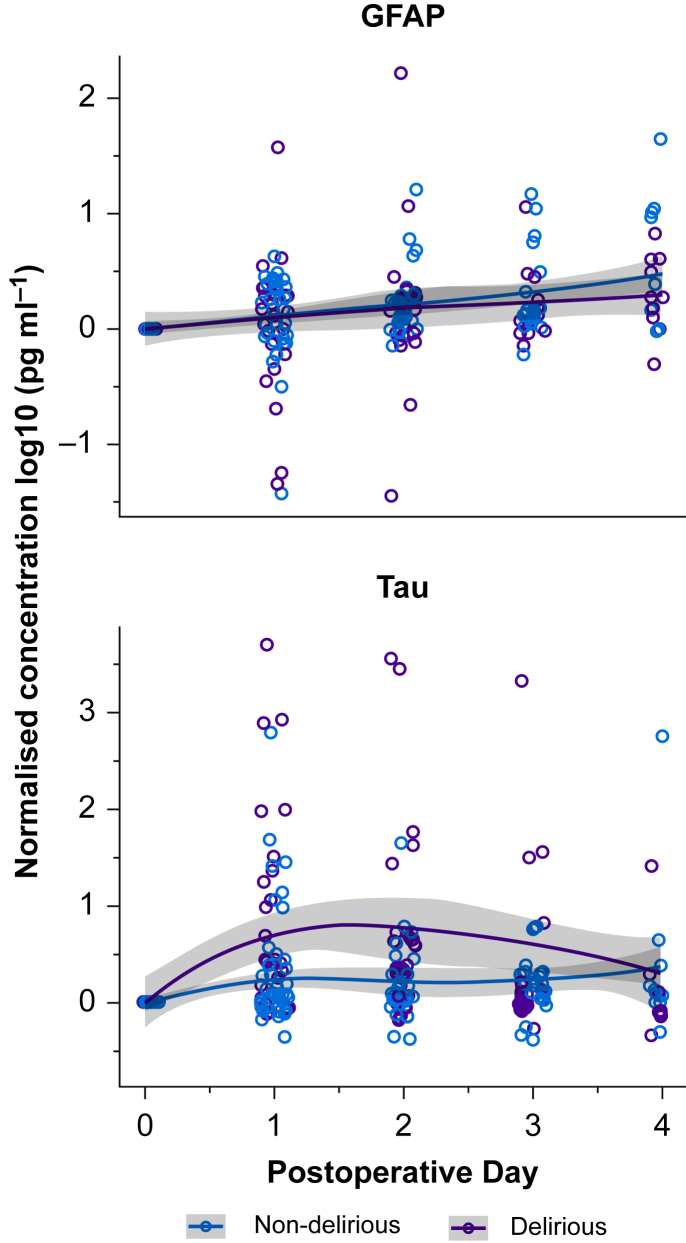
Figure 1 shows the time course for perioperative changes in tau or GFAP compared with preoperative concentrations and grouped by delirium status. Supplementary Figure S3 shows the tau and GFAP results that are not normalised to baseline. Only tau showed clear changes from preoperative to postoperative concentrations. Plasma concentrations of tau increased in both delirious and non-delirious subjects, although the increase was greater in delirious subjects (P<0.001, Table 1).
Fig 1.
Perioperative time course of GFAP and tau divided by delirium status. GFAP and tau, from the preoperative visit through the fourth postoperative day are plotted by whether patients were delirious postoperatively or not. Tau and GFAP were normalised by log10-transforming the postoperative value and the baseline value, and then subtracting the transformed baseline value from the transformed postoperative value. n=114. GFAP, glial fibrillary acidic protein; Post-op, postoperative.
As exploratory analyses, we also investigated whether any cytokines rose on POD1 to a greater degree in delirious patients compared with non-delirious patients. Delirious patients also experienced increases in IL-8 (univariate P<0.001), IL-10 (univariate P<0.001), and monocyte chemoattractant protein 1 (univariate P=0.0048). All of these changes survive Bonferroni correction at P<0.005 for adjustment for testing the 10 cytokines. Univariate changes in IL-1B, IL-1ra, and IL-2 were also detected but these would not survive Bonferroni correction.
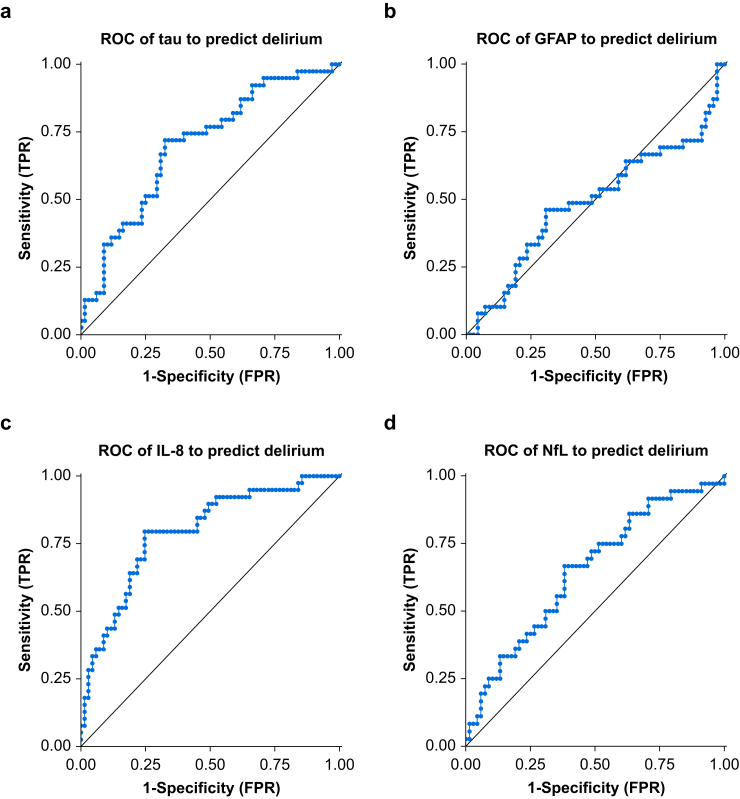
Next, we tested whether biomarkers can classify the incidence of delirium (a binary diagnosis of yes/no for delirium) using the area under the receiver operating characteristic curve (AUROC). Tau showed a significant classification of delirium status (AUROC 0.70, 95% confidence interval [CI] 0.60–0.81, P<0.001) whereas GFAP did not (AUROC 0.49, 95% CI 0.38–0.60, P=0.85; Fig. 2). IL-8 (AUROC 0.79, 95% CI 0.70–0.89, P<0.001) and NfL (AUROC 0.65, 95% CI 0.54–0.76, P=0.011) could also classify delirium.
Fig 2.
Receiver operating curves for classifying delirium. Postoperative Day 1 (POD1) values were used for all biomarkers. Empirical receiver operating characteristic (ROC) curves were generated by calculating the true positive rates (TPR) and false positive rates (FPR) using each observed value of a biomarker as a classification threshold. Prediction performance was assessed using the area under the ROC (AUROC) and statistically tested against chance performance (0.5) using a two-tailed Z-test as described for GraphPad Prism 8.0. The sample sizes for each plot are: (a) and (b): n=107, (c): n=108, and (d): n=104. Six patients are missing POD1 biomarker data as a result of surgery not being completed (n=3) or blood sample not being collected (n=3). For plots (a), (b), and (d), additional patients are missing because of a sample processing error resulting in incomplete/invalid assay runs. GFAP, glial fibrillary acidic protein; IL-8, interleukin-8; NfL, neurofilament light.
Correlations of changes in perioperative biomarkers with delirium severity
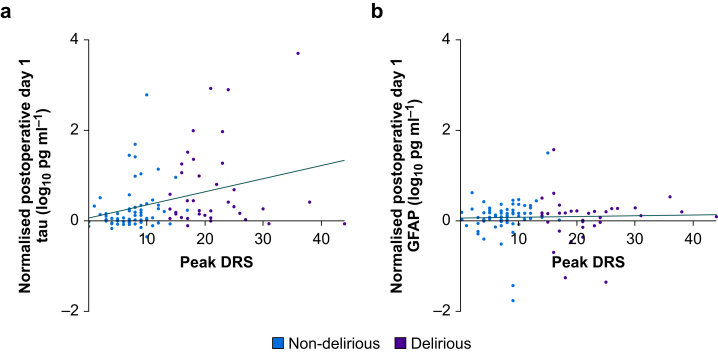
Changes in delirium severity correlated with changes in tau but not GFAP (Fig. 3). We previously showed that POD1 increases in NfL correlated with delirium severity, therefore we tested whether NfL or tau showed stronger correlations. There was no difference in the strength of correlation of tau and delirium severity and NfL and delirium severity (Williams' Test P=0.90).
Fig 3.
Correlations of preoperative-POD1 change in tau or GFAP with peak delirium severity. Plots show the difference between log10-transformed preoperative and log10-transformed POD1 concentrations of tau or GFAP vs the highest delirium severity recorded postoperatively as measured by the Delirium Rating Scale-98. The plots are colour-coded by whether patients were delirious postoperatively. Spearman's rank correlation rho and P-value for the tau plot are ρ=0.39, P<0.001, and for the GFAP plot are ρ=0.13, P=0.20. N=103. Four patients are missing preoperative biomarker data as a result of a blood sample not being collected preoperatively. Seven patients are missing POD1 biomarker data as a result of a blood sample not being collected on POD1 (n=3), surgery not being completed (n=3), or because of a sample processing error resulting in incomplete/invalid assay runs (n=1). DRS, Delirium Rating Scale-98 score; GFAP, glial fibrillary acidic protein; POD1, postoperative Day 1.
Relationships of tau and IL-8
Next, we tested the relationships of tau and IL-8 (and other cytokines, Table 2). We previously reported that NfL and IL-8 are correlated.9 In our exploratory analyses, we found that POD1 change in tau and POD1 change in IL-8 and IL-10 are correlated at a threshold of P<0.025 (Table 2). Importantly, these changes would also survive Bonferroni correction across testing the 10 cytokines.
Table 2.
Correlations of preoperative-POD1 change in GFAP or tau with preoperative-POD1 change in cytokines. Spearman's rank correlation rho and P-values are displayed for all biomarkers. The biomarker change values used in the correlations are the difference between the log10-transformed POD1 values and the log10-transformed preoperative values. N=103. Four patients did not have a preoperative blood sample collected, and seven patients did not have POD1 biomarker data because a POD1 blood sample was not collected (n=3), the patient's surgery was not completed (n=3), or there was a sample processing error resulting in incomplete/invalid assay runs (n=1). GFAP, glial fibrillary acidic protein; IL, interleukin; IL-1ra, IL-1 receptor antagonist; MCP-1, monocyte chemoattractant protein 1; POD1, postoperative day 1; TNFa, tumour necrosis factor-alpha.
| GFAP | P-value | Tau | P-value | |
|---|---|---|---|---|
| Cytokine | ||||
| IL-1B | 0.038 | 0.70 | 0.0018 | 0.99 |
| IL-1ra | 0.060 | 0.55 | 0.15 | 0.13 |
| IL-2 | −0.030 | 0.77 | −0.029 | 0.77 |
| IL-4 | −0.024 | 0.81 | 0.11 | 0.29 |
| IL-6 | 0.096 | 0.33 | 0.074 | 0.46 |
| IL-8 | 0.14 | 0.17 | 0.36 | <0.001 |
| IL-10 | 0.040 | 0.69 | 0.29 | 0.0029 |
| IL-12p70 | −0.096 | 0.34 | −0.080 | 0.42 |
| MCP-1 | 0.16 | 0.11 | 0.18 | 0.063 |
| TNFa | −0.017 | 0.86 | 0.073 | 0.46 |
In order to probe whether tau contributed to the pathogenesis of delirium independently of inflammation, we conducted linear regression for the change in IL-8, age, sex, and preoperative Trail Making Test B to identify predictors of peak delirium severity (DRS). In this model, preoperative Trail Making Test B (P=0.0016), and change in tau (P=0.026) were predictors of peak DRS (adjusted r2=0.22, P<0.001, Supplementary Table S4).
Clinical predictors of the increase in tau
We also investigated clinical predictors of tau (and NfL) using a linear regression model including age, sex, ASA class, previous stroke/TIA, peripheral vascular disease, renal failure, type of surgery, operative time, blood loss, and area under the curve for intraoperative mean arterial pressures <10% from baseline. Age (P=0.037), prior stroke/TIA (P=0.001), and blood loss (P<0.001) were all associated with increased POD1 plasma tau concentrations (adjusted r2=0.49, P<0.001, Supplementary Table S5). The same model with NfL as an outcome showed that prior stroke/TIA (P=0.029) and blood loss (P<0.001) were associated with increased POD1 plasma concentrations (adjusted r2=0.46, P<0.001, Supplementary Table S6).
Biomarker correlations with the resolution of delirium symptoms
Finally, we investigated whether changes in tau, NfL, or IL-8 over time may correlate with resolution of delirium features (as shown in Supplementary Fig. S4). Linear mixed effect models with an outcome of delirium severity were constructed with age, sex, biomarker, time (POD from Days 1–4) and an interaction for biomarker and time. Of the three models tested, tau was the only biomarker that showed a significant effect (P<0.001). Tau also showed a significant negative interaction with time (P=0.003; Supplementary Table S7) suggesting that as time progresses, the impact of tau on delirium severity wanes. This may be consistent with an injury or synaptic event occurring on the day of surgery, rather than be an on-going process.
In similar linear mixed effect models, the effects for NfL (P=0.512) or IL-8 (P=0.379) were not significant (Supplementary Tables S8 and S9). Using complete datasets for all three models, we compared the models using the Akaike Information Criterion; this showed that the model with tau (1091) was superior to NfL (1100) or IL-8 (1100). The linear mixed effects model including tau was then re-run with additional covariates of IL-8 and NFL and their respective interactions with time. The effects of tau (P<0.001) and the interaction of tau and time were unchanged (P=0.004). GFAP was not tested as it showed no relationship to delirium.
Discussion
Plasma tau changes were associated with delirium incidence and severity, and they resolved with delirium features. These findings are consistent with a causal role for neuronal injury, synaptic changes in delirium pathogenesis, or both. Interestingly, linear mixed effect model analysis showed that tau, but not NfL and IL-8, predicted the changes in delirium severity over time. This suggests that it tracks the resolution of features, which is further evidence for a causal relationship. Whether this is as a result of more rapid plasma clearance, rather than its ability to detect changes in neuronal injury or synaptic function, is unclear; further studies are required to disambiguate these alternatives. However, our analysis of associations between preoperative tau and hippocampal volume did not show a relationship, unlike our prior analysis with NfL,9 perhaps suggesting that tau may not be as robust a marker of neurodegeneration. Overall, we favour an interpretation that tau is a marker of synaptic changes, rather than cell death. Future studies are warranted to investigate the possible role of perioperative tau on inducing any long-term cognitive decline.5 In contrast, GFAP changes were not associated with delirium. We suggest further studies would be useful to confirm that astrogliosis is not a feature of delirium pathogenesis.
Although associations of delirium and inflammatory cytokines have been known for some time, there are limited data on contemporaneous changes in neuronal injury biomarkers.9 In particular, we are unaware of prior studies showing resolution of delirium features over time with biomarker changes. Our data therefore provide a novel lens for considering delirium pathogenesis. We recently showed that delirium severity is associated with inflammation and EEG slowing15 and future studies should test whether tau changes are also associated with this slowing. It will be intriguing to test how changes in neurophysiology contribute to the release of tau and whether tau release is associated with other aetiologies of neuroinflammation. Given the associations of inflammation and tau and NfL, it will also be intriguing to investigate whether modulation of inflammation may affect their release. Possible mechanisms could include protecting the blood brain barrier or direct prevention of neurotoxicity.20 It is also likely that neuronal injury occurs through alternative mechanisms, such as covert stroke,8 and that multimodal approaches will be required to prevent perioperative neuronal injury to a meaningful extent.
In terms of clinical predictors, age, prior stroke/TIA, and blood loss predicted tau concentrations. Interestingly, the subjects with the three highest concentrations of tau were patients with prior stroke/TIA. In contrast, vascular surgery was not associated with tau release. This may have been because of the strong impact of other variables that are associated with vascular surgery, such as blood loss, and inclusion of the important vascular risk factor, prior stroke/TIA, in the model. Each of these variables was strongly associated with tau release and, after adjustment for these factors, the impact of vascular surgery was reduced.
Although tau has been considered a marker of cell death for many years, largely as a result of associations between chronic neurodegeneration and dementia, recent data are challenging whether tau is merely a marker of cell death or not. The lack of association of preoperative tau with hippocampal volume, that we observed, would argue against tau being a robust marker of neurodegeneration. Our tau assay uses an antibody directed at the mid-region of the tau molecule, capturing both full length and truncated forms of tau. This is relevant as recent data suggest that the majority of tau detected in CSF and plasma is not full length (the form that may be expected to be released on cell lysis).21 Rather they are truncated forms which are actively secreted from neurons,14 perhaps in response to glutamatergic neurotransmission.13 This latter finding is intriguing as EEG analyses suggest that postoperative delirium is associated with slowing in EEG frequencies which may be suggestive of fewer excitatory postsynaptic potentials rather than increased excitatory activity.15
The link between inflammation and postoperative EEG slowing in delirium in our recent study,15 may prompt an alternative explanation for the tau changes. Microglial activation leads to the phagocytosis of dying cells, redundant synapses, or both, releasing tau. Furthermore tau that is released from microglia in exosomes, has been shown to dampen excitatory activity.12 This would be consistent with the reduction in activity in the EEG in delirium.
Given that our linear mixed effects modelling showed that recovery of delirium features are associated with normalisation of plasma tau, we favour an interpretation that this reflects changes in synaptic activity, rather than an acute recovery from neuronal injury. This logic also rationalises the differences we have seen with NfL, a more definitive neuronal injury marker, that does not correlate with recovery of delirium features in our dataset.
Overall, we propose that surgery and delirium are associated with changes in tau, that may be synaptically driven, as a separate (recovery) process to neuronal injury as detected by NfL. While the disease associations of tau with cognitive decline and dementia are well established, the physiological function (if any) of secreted tau remains obscure. However, recent evidence suggests that extracellular tau may be associated with the spread of pathological tau in the brain and hence may contribute to longer-term cognitive decline. In turn, this could explain associations of delirium with long-term cognitive decline. Future work should probe associations of tau release associated with delirium and long-term changes in cognition.
Our study has many strengths, including modelling approaches and relatively stringent P-values. In particular, the requirement that a biomarker changes with delirium incidence and proportionately to delirium severity lends credence to our findings. We also adjusted for preoperative cognitive impairment where necessary, using preoperative Trail Making Test B, that we have previously shown is an important clinical predictor in this cohort.22 However, there are also multiple limitations. Although we have attempted to control for confounding, unmeasured confounding is an issue in any observational study. Nonetheless we have established that changes in plasma tau are associated with delirium severity and with the resolution of delirium features, even when adjusting for inflammation as measured by IL-8. Of course, IL-8 may not capture all relevant information on inflammation, and further studies investigating potential biomarkers of delirium are also warranted. A further limitation is that our study was of modest size; larger studies are required, particularly if they are to probe associations with long-term cognition. Most importantly, we are unable to establish causality with our observational research, and experimental studies are required to probe causal relationships between changes in neuronal injury biomarkers and delirium. It is also worth us emphasising that the patient characteristics for this group of subjects have been reported before,9,15 but the biomarker data we describe herein have been updated and have not been reported previously.
In summary, our findings support tau as a potential biomarker for both the incidence and severity of delirium. Tau appears to be superior to NfL or IL-8 for tracking delirium features. Further studies are required to determine whether changes in tau represent significant neuronal injury with consequences for long-term cognition.
Authors' contributions
Designed the study: RDS
Collected and analysed data: RDS, MW, AB, AK, MP, HL, CC, ZF
Collected data and performed the majority of the analyses: TB
Contributed to study design, and interpretation of data: RL, RAP
Wrote the paper with input from all co-authors: TB, RDS
Declarations of interest
All authors declare no competing interests that may be relevant to the submitted work. HZ has served at scientific advisory boards for Roche Diagnostics, Wave, Samumed and CogRx, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg (all unrelated to the submitted work). KB has served as a consultant or at advisory boards for Alector, Alzheon, CogRx, Biogen, Lilly, Novartis and Roche Diagnostics, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Venture-based platform company at the University of Gothenburg, all unrelated to the work presented in this paper. RDS is a member of the board of the British Journal of Anaesthesia.
Funding
RDS is supported by US National institutes of Health grants K23 AG055700 and R01 AG063849-01.
Handling editor: Michael Avidan
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.08.061.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sanders R.D., Pandharipande P.P., Davidson A.J., Ma D., Maze M. Anticipating and managing postoperative delirium and cognitive decline in adults. BMJ. 2011;343:d4331. doi: 10.1136/bmj.d4331. [DOI] [PubMed] [Google Scholar]
- 2.Witlox J., Eurelings L.S., de Jonghe J.F., Kalisvaart K.J., Eikelenboom P., van Gool W.A. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 3.Inouye S.K., Marcantonio E.R., Kosar C.M. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12:766–775. doi: 10.1016/j.jalz.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasunilashorn S.M., Fong T.G., Albuquerque A. Delirium severity post-surgery and its relationship with long-term cognitive decline in a cohort of patients without dementia. J Alzheimers Dis. 2018;61:347–358. doi: 10.3233/JAD-170288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krause B.M., Sabia S., Manning H.J., Singh-Manoux A., Sanders R.D. Association between major surgical admissions and the cognitive trajectory: 19 year follow-up of Whitehall II cohort study. BMJ. 2019;366:l4466. doi: 10.1136/bmj.l4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulte P.J., Roberts R.O., Knopman D.S. Association between exposure to anaesthesia and surgery and long-term cognitive trajectories in older adults: report from the Mayo Clinic Study of Aging. Br J Anaesth. 2018;121:398–405. doi: 10.1016/j.bja.2018.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitlock E.L., Diaz-Ramirez L.G., Smith A.K., Boscardin W.J., Avidan M.S., Glymour M.M. Cognitive change after cardiac surgery versus cardiac catheterization: a population-based study. Ann Thorac Surg. 2019;107:1119–1125. doi: 10.1016/j.athoracsur.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neuro V.I. Perioperative covert stroke in patients undergoing non-cardiac surgery (NeuroVISION): a prospective cohort study. Lancet. 2019;394:1022–1029. doi: 10.1016/S0140-6736(19)31795-7. [DOI] [PubMed] [Google Scholar]
- 9.Casey C.P., Lindroth H., Mohanty R. Postoperative delirium is associated with increased plasma neurofilament light. Brain. 2019;143:47–54. doi: 10.1093/brain/awz354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bateman R.J., Blennow K., Doody R. Plasma biomarkers of AD emerging as essential tools for drug development: an EU/US CTAD Task Force Report. J Prev Alzheimers Dis. 2019;6:169–173. doi: 10.14283/jpad.2019.21. [DOI] [PubMed] [Google Scholar]
- 11.Pase M.P., Himali J.J., Aparicio H.J. Plasma total-tau as a biomarker of stroke risk in the community. Ann Neurol. 2019;86:463–467. doi: 10.1002/ana.25542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asai H., Ikezu S., Tsunoda S. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci. 2015;18:1584–1593. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada K., Holth J.K., Liao F. Neuronal activity regulates extracellular tau in vivo. J Exp Med. 2014;211:387–393. doi: 10.1084/jem.20131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato C., Barthelemy N.R., Mawuenyega K.G. Tau kinetics in neurons and the human central nervous system. Neuron. 2018;97:1284–1298. doi: 10.1016/j.neuron.2018.02.015. e1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanabe S., Mohanty R., Lindroth H. Cohort study into the neural correlates of postoperative delirium: the role of connectivity and slow-wave activity. Br J Anaesth. 2020;125:55–66. doi: 10.1016/j.bja.2020.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rappold T., Laflam A., Hori D. Evidence of an association between brain cellular injury and cognitive decline after non-cardiac surgery. Br J Anaesth. 2016;116:83–89. doi: 10.1093/bja/aev415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gisslen M., Price R.W., Andreasson U. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine. 2016;3:135–140. doi: 10.1016/j.ebiom.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindroth H., Nair V.A., Stanfield C. Examining the identification of age-related atrophy between T1 and T1 + T2-FLAIR cortical thickness measurements. Sci Rep. 2019;9:11288. doi: 10.1038/s41598-019-47294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evered L., Silbert B., Scott D.A., Zetterberg H., Blennow K. Association of changes in plasma neurofilament light and tau levels with anesthesia and surgery: results from the CAPACITY and ARCADIAN studies. JAMA Neurol. 2018;75:542–547. doi: 10.1001/jamaneurol.2017.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu J., Feng X., Valdearcos M. Interleukin-6 is both necessary and sufficient to produce perioperative neurocognitive disorder in mice. Br J Anaesth. 2018;120:537–545. doi: 10.1016/j.bja.2017.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Z., Mengel D., Keshavan A. Learnings about the complexity of extracellular tau aid development of a blood-based screen for Alzheimer's disease. Alzheimers Dement. 2019;15:487–496. doi: 10.1016/j.jalz.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindroth H., Bratzke L., Twadell S. Predicting postoperative delirium severity in older adults: the role of surgical risk and executive function. Int J Geriatr Psychiatry. 2019;34:1018–1028. doi: 10.1002/gps.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.