Abstract
Arylsulfatase and β-glucuronidase are two important enzymes in humans, which play an important role in the dynamic equilibrium of steroidal estrogens. This work probably for the first time reported that hydrogen peroxide (H2O2), hypochlorite, and peracetic acid (PAA) could effectively inhibit the activities of arylsulfatase and/or β-glucuronidase. The 50% of inhibitions (IC50) of H2O2, hypochlorite, and PAA on arylsulfatase were found to be 142.90 ± 9.00, 91.83 ± 10.01, and 43.46 ± 2.92 μM, respectively. The corresponding IC50 values of hypochlorite and PAA on β-glucuronidase were 704.90 ± 41.40 and 23.26 ± 0.82 μM, whereas H2O2 showed no inhibition on β-glucuronidase. The inhibitions of arylsulfatase and/or β-glucuronidase by these three chemicals were pH-dependent. It was further revealed that the inhibitions of hypochlorite on both arylsulfatase and β-glucuronidase were irreversible. On the contrary, the inhibitions by H2O2 and PAA were reversible. In addition, the inhibition by H2O2 was competitive and that by PAA was noncompetitive. In general, H2O2 and hypochlorite can be endogenously produced in humans, which suggested that the two compounds are potential endocrine disruption compounds (EDCs) as they can cause endocrine disruption via the inhibition of arylsulfatase and β-glucuronidase. This work further indicated that any agent that can induce the production of H2O2 or hypochlorite in humans is a potential EDC, which explains why some EDCs with very weak or no estrogenic potency can cause endocrine disruption, which is confirmed in epidemiological studies.
Introduction
Arylsulfatase and β-glucuronidase have been widely present in many organisms including humans, which are able to catalyze the hydrolysis of sulfates/glucuronides.1−4 The important physiological functions of the two enzymes are to maintain the dynamic equilibrium of natural estrogens in human body. Normally, only about 2–3% of natural estrogens are free estrogens in human, whereas the rest are in sulfate or glucuronide form.5,6 The free natural estrogens play important roles in stimulating growth, blood flow, water retention in sexual organs, neuro- and vasoprotection, and reduction of bone loss, whereas the conjugated estrogens act as reservoirs.5,7 Natural free estrogens in humans are accurately regulated, and once the equilibrium is disrupted, it may trigger a severe outcome. For example, humans with a significantly higher urinary arylsulfatase/β-glucuronidase activity have higher free estrogens in their body, which have been thought to be the main reason for the development of many cancers, including breast cancer, stomach cancer, ovarian cancer, colonic cancer, and so forth.8−11 Therefore, the inhibition of arylsulfatase/β-glucuronidase has been explored as one of the effective strategies for such cancer treatment, among which STX64 has been applied in clinical trials.7,12
Arylsulfatase and β-glucuronidase have been reported to be two extreme enzymes, which can endure high concentrations of mercury dichloride, sodium azide, ethanol, and ethylenediaminetetraacetic acid.13 H2O2, hypochlorite, and peracetic acid (PAA) have been commonly used as sanitizers for various medical and nonmedical applications.14−17 Among these, H2O2 and hypochlorite are also important compounds which can be endogenously produced by cellular enzymatic reactions in humans and are important signal biomarkers in humans.18−21 This work is the first to report that H2O2, hypochlorite, and PAA can effectively inhibit arylsulfatase and/or β-glucuronidase, which may indicate important physiological functions.
Materials and Methods
Enzymes and Chemicals
Potassium 4-nitrophenyl sulfate (pNPS, purity > 98%), 4-nitrophenyl-β-d-glucuronide (pNPG, purity > 98%), p-nitrophenol (pNP, spectrophotometric grade), arylsulfatase (≥10,000 units/g solid) from Helix pomatia (type H-1, catalog number: S9626), and β-glucuronidase (≥100,000 units/g solid) from H. pomatia (type H-1, catalog number: G0751) were purchased from Sigma-Aldrich (Shanghai, China). H2O2 (30%) was purchased from Guangzhou Chemical Reagent Factory (Guangzhou, China). NaClO (available chlorine, 8.0%, w/w) was purchased from ANPEL (Shanghai, China). The NaClO concentration was calibrated with the standard method of GB/T 19106.22 PAA (13%, w/w) including solutions I and II was purchased from GHTECH (Shantou, China). Before using, fresh PAA was obtained by mixing the solutions I and II in the proportion of 1:1 (v/v), and it was used within 2 days. This PAA concentration was calibrated according to the standard method of GB/T 19104.23 The other reagents not listed were purchased from Aladdin (Shanghai, China).
Stock solutions of arylsulfatase and β-glucuronidase were prepared at the concentration of 10 U/mL by dissolving the enzyme powder into 0.5 M Tris-HCl buffer (pH 7.0). The pNPS stock solution was prepared with 0.5 M acetate buffer (pH 5.8), whereas the pNPG stock solution was prepared with 0.5 M phosphate buffer (pH 7.0), both of which were at concentrations of 5 mM. All solutions were prepared with reagent-grade chemicals and ultrapure water (18.2 MΩ/cm).
Enzyme Activity
The activity of arylsulfatase/β-glucuronidase was measured according to Zhang et al.13 The arylsulfatase/β-glucuronidase enzyme activity (U) was defined as the absorbance equivalent of 1 μmol pNP produced per hour per milliliter of enzyme solution at 37 °C. For the convenience of comparison, relative activity is used, and the maximal enzyme activity is arbitrarily set to 100%. The limit of detection (LOD) of the spectrophotometric method was calculated according to three times of the standard deviation, which was obtained from nine repeated determinations of the lowest pNP concentration used for the standard calibration curve.24,25 The LOD of this method for pNP was 0.22 μM, which is equal to 0.22 U/L for arylsulfatase and β-glucuronidase.
The Michaelis–Menten constant (Km) and maximal reaction rate (Vmax) of the two enzymes, arylsulfatase and β-glucuronidase, were obtained by fixing the enzyme concentration in the reaction and then analyzing the initial speed at several different substrate concentrations. The concentrations of the substrate in the reaction mixture were 0.02, 0.04, 0.08, 0.1, 0.2, 0.4, 0.8, 1.0, and 1.2 mM, respectively. The rest of the steps were the same as those for measuring the enzyme activity.
Km and Vmax were expressed by the Michaelis equation,26 that is
| 1 |
Km and Vmax are calculated by the Lineweaver–Burk equation,26 that is
| 2 |
where V0 is the production rate of the substrate, Km is called the Michaelis constant, Vmax is the reaction rate when the enzyme is saturated with the substrate, and [s] is the concentration of the substrate.
Inhibitory Assays
Inhibition experiments on arylsulfatase or β-glucuronidase by different inhibitors were performed with different concentrations, and the inhibitor solution was freshly prepared before use. For the arylsulfatase inhibition experiment, the reaction mixture solution (3.5 mL) contained 2 mL of 0.1 M acetate buffer (pH 5.8), 0.5 mL of dilute arylsulfatase enzyme, 0.5 mL of 5 mM pNPS, and 0.5 mL of water as the control or 0.5 mL of the inhibitor (H2O2, NaClO, or PAA) with different concentrations. The addition concentrations of H2O2 and NaClO in the reaction mixture were 1, 5, 10, 50, 100, 500, 1000, 5000, and 10,000 μM, respectively, whereas the concentrations of PAA were 1, 5, 10, 50, 100, 500, and 1000 μM, respectively. The reaction mixture solution was mixed and incubated for 1 h at 37 °C. pNP deconjugated from pNPS was measured at a wavelength of 400 nm after the reaction was stopped by adding 1.5 mL of 0.5 M NaOH. Each experiment was performed in triplicate.
For β-glucuronidase inhibition experiment, the reaction mixture solution (1.2 mL) contained 0.6 mL of 0.1 M phosphate buffer (pH 7.0), 0.2 mL of diluted β-glucuronidase enzyme, 0.2 mL of 5 mM pNPG, and 0.2 mL of water as the control or 0.2 mL of H2O2, NaClO, or PAA. The addition concentrations of H2O2 in the reaction mixture were 1, 5, 10, 50, 100, 500, 1000, 5000, and 10,000 μM, respectively, whereas the concentrations of NaClO and PAA were the same with 1, 5, 10, 50, 100, 500, and 1000 μM, respectively. The reaction mixture solution was mixed and incubated for 1 h at 37 °C. pNP deconjugated from pNPG was measured at a wavelength of 400 nm after the reaction was stopped by adding 0.8 mL of 0.5 M NaOH. Each experiment was performed in triplicate.
To check the inhibition differences at different pH conditions, different buffer solutions were used as listed: 0.1 M acetate buffer (pH 5.0–5.8), 0.1 M citric acid/0.1 M sodium citrate (pH 5.0–6.0), 0.5 M Tris-HCl (pH 7.0–8.0), and 0.1 M phosphate buffer (pH 6.0–8.0).
In order to explore the types of enzyme inhibition by different inhibitors, we used four different concentrations of inhibitors to perform inhibition experiments at different enzyme concentrations. The enzyme activities in the reaction solution were 0.01, 0.02, 0.03, 0.04, 0.05 and 0.06 U/mL, respectively. For arylsulfatase, the reaction concentrations of H2O2 were 0, 50, 150, and 500 μM, and the reaction concentrations of NaClO were 0, 50, 100, and 200 μM, whereas the reaction concentrations of PAA were 0, 10, 50, and 100 μM. For β-glucuronidase, the reaction concentrations of NaClO were 0, 500, 1000, and 2000 μM, whereas the reaction concentrations of PAA were 0, 50, 100, and 500 μM. The other conditions were consistent with the enzyme inhibition experiments.
Inhibition percentage was calculated as shown in eq 3
| 3 |
where I is the inhibition percentage of enzyme activity by an inhibitor, A is the measured enzyme activity with an inhibitor at different concentrations, and C is the measured activity without the addition of an inhibitor. Inhibition dose–response curves were plotted with GraphPad Prism 8 software, and IC50 was simultaneously obtained, which means the half inhibition concentration of an inhibitor.
Results and Discussion
Inhibition Dose–Response Curves
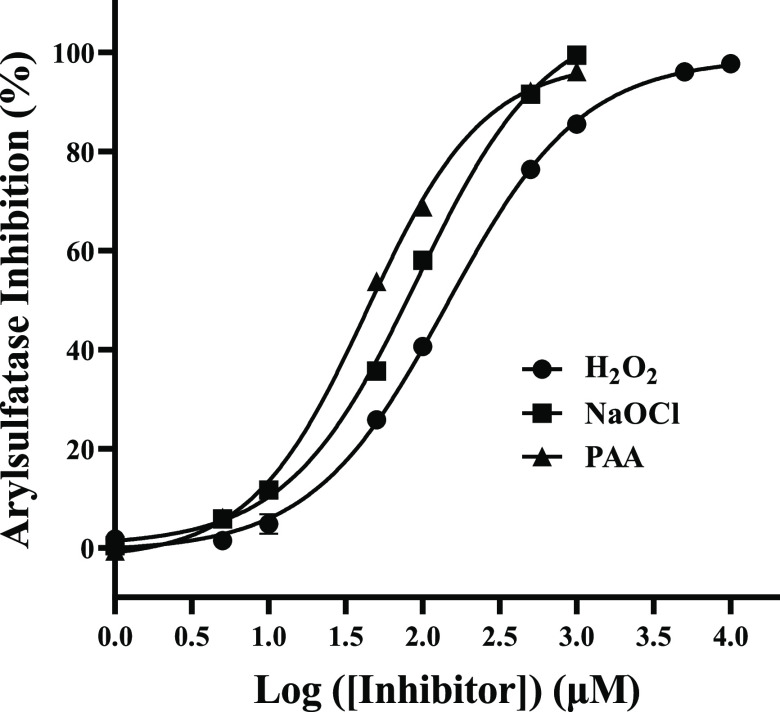
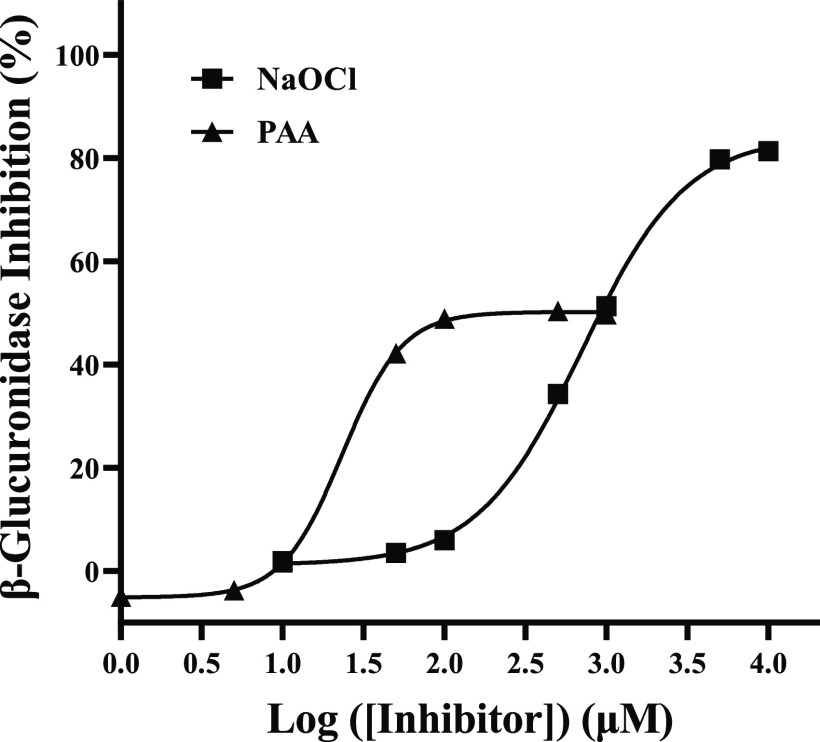
Figures 1 and 2 show the inhibition dose–response curves of H2O2, hypochlorite, and PAA on arylsulfatase and β-glucuronidase. It is evident that hypochlorite and PAA could effectively inhibit both arylsulfatase and β-glucuronidase, whereas H2O2 was only effective for inhibiting arylsulfatase. The respective IC50 values of H2O2, hypochlorite, and PAA on arylsulfatase were determined to be 142.90 ± 9.00, 91.83 ± 10.01, and 43.46 ± 2.92 μM, whereas they were 704.90 ± 41.40 and 23.26 ± 0.82 μM for the inhibition of β-glucuronidase for hypochlorite and PAA. These clearly suggested that PAA had the strongest inhibitory effect on arylsulfatase, followed by hypochlorite, with H2O2 being the least. Meanwhile, the inhibitory effect of PAA on β-glucuronidase was much stronger than that of hypochlorite. Interestingly, the inhibitory effect of hypochlorite on arylsulfatase was significantly stronger than that on β-glucuronidase. In contrast, the inhibitory effect of PAA on β-glucuronidase appeared to be stronger than that on arylsulfatase. However, it should be noted that the maximum inhibition degree of PAA on β-glucuronidase was only about 60%.
Figure 1.
Dose–response curves of H2O2, NaClO, and PAA on arylsulfatase.
Figure 2.
Dose–response curves of NaClO and PAA on β-glucuronidase. Data for H2O2 are not drawn as it showed no inhibition on β-glucuronidase.
Effect of pH on Enzyme Inhibition
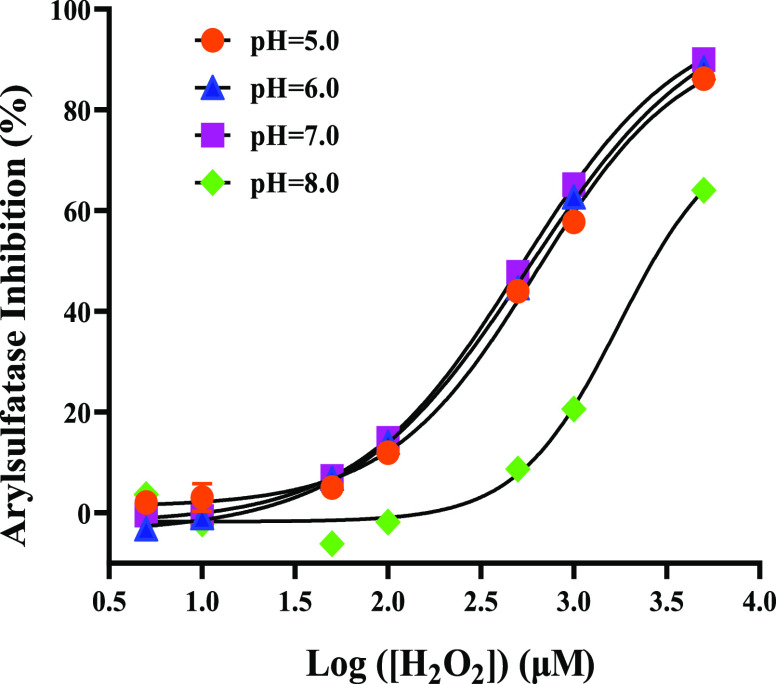
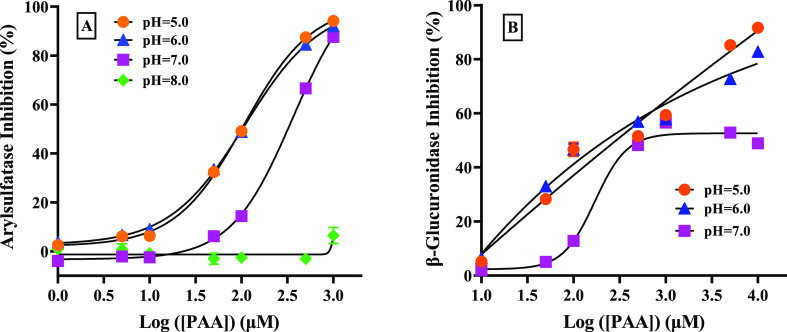
As H2O2 and PAA belong to weak acids and hypochlorite is a weak alkali, it is reasonable to consider that their dissociation degrees are all pH-dependent and subsequently affect their inhibitory powers to arylsulfatase and β-glucuronidase. On the other hand, it has been known that both arylsulfatase and β-glucuronidase could be completely inhibited at a high pH above 11 or below 3.13 Thus, in this study, the pH studied was controlled in the range of 5–8. As can be seen in Figure 3, there were no significant differences in the inhibition of arylsulfatase by H2O2 in the pH range of 5–7, whereas reduced inhibition was observed under the weak alkaline condition of pH 8.
Figure 3.
Dose–response curves of H2O2 on arylsulfatase under different pH conditions.
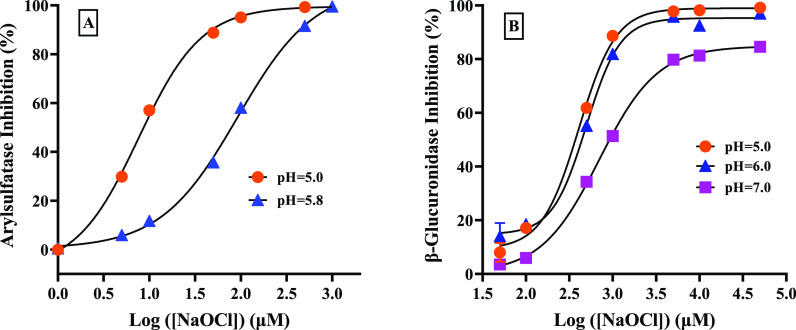
Under acidic conditions, hypochlorite can react with hydrogen ions by forming hypochlorous acid (HOCl), which may have different inhibition powers to the two enzymes (data are not shown). It appears from Figure 4A that the inhibition of hypochlorite on arylsulfatase was much stronger at pH 5.0 than at pH 5.8. Moreover, its IC50 at pH 5.0 (i.e., 7.64 ± 0.61 μM) was 12 times lower than that at pH 5.8 (i.e., 91.83 ± 10.01 μM). A similar trend was observed in Figure 4B for β-glucuronidase. These seemed to suggest that the acidic condition was making hypochlorite exhibit more inhibitory potential toward arylsulfatase and β-glucuronidase. The inhibitory effects of PAA on arylsulfatase at different pH are shown in Figure 5. It seemed that inhibition tended to decrease with increasing pH (Figure 5B), for example, nearly no inhibition was observed at pH 8 with a PAA concentration below 1 mM (data are not shown). Similar to arylsulfatase, the inhibitory effect of PAA on β-glucuronidase was insignificant at pH 5 and 6 (Figure 5B).
Figure 4.
Dose–response curves of NaClO on arylsulfatase (A) and β-glucuronidase (B) under different pH conditions.
Figure 5.
Dose–response curves of PAA on arylsulfatase (A) and β-glucuronidase (B) under different pH conditions.
Inhibition Mechanisms
To explore the possible inhibition mechanisms of arylsulfatase and β-glucuronidase by H2O2, hypochlorite, and PAA, the relationship between enzyme activity and individual inhibitor concentration is presented in Figure 6. It was found that the inhibitions of H2O2 and PAA on arylsulfatase was reversible, whereas the inhibition of hypochlorite on arylsulfatase was irreversible. Similar to β-glucuronidase, the inhibition of PAA on β-glucuronidase was reversible, whereas the inhibition of hypochlorite was irreversible. The Km values of arylsulfatase and β-glucuronidase were 0.217 and 0.736 mM, respectively. To further determine the inhibition kinetics, the Lineweaver–Burk plot was plotted in Figure 7. In Figure 7A, compared with the control group, the experimental group Km increased and Vmax did not change, whereas in Figure 7B,C, Km did not change but Vmax decreased, showing that the inhibition on arylsulfatase by H2O2 was competitive, whereas the inhibitions of PAA on arylsulfatase and β-glucuronidase were noncompetitive.
Figure 6.
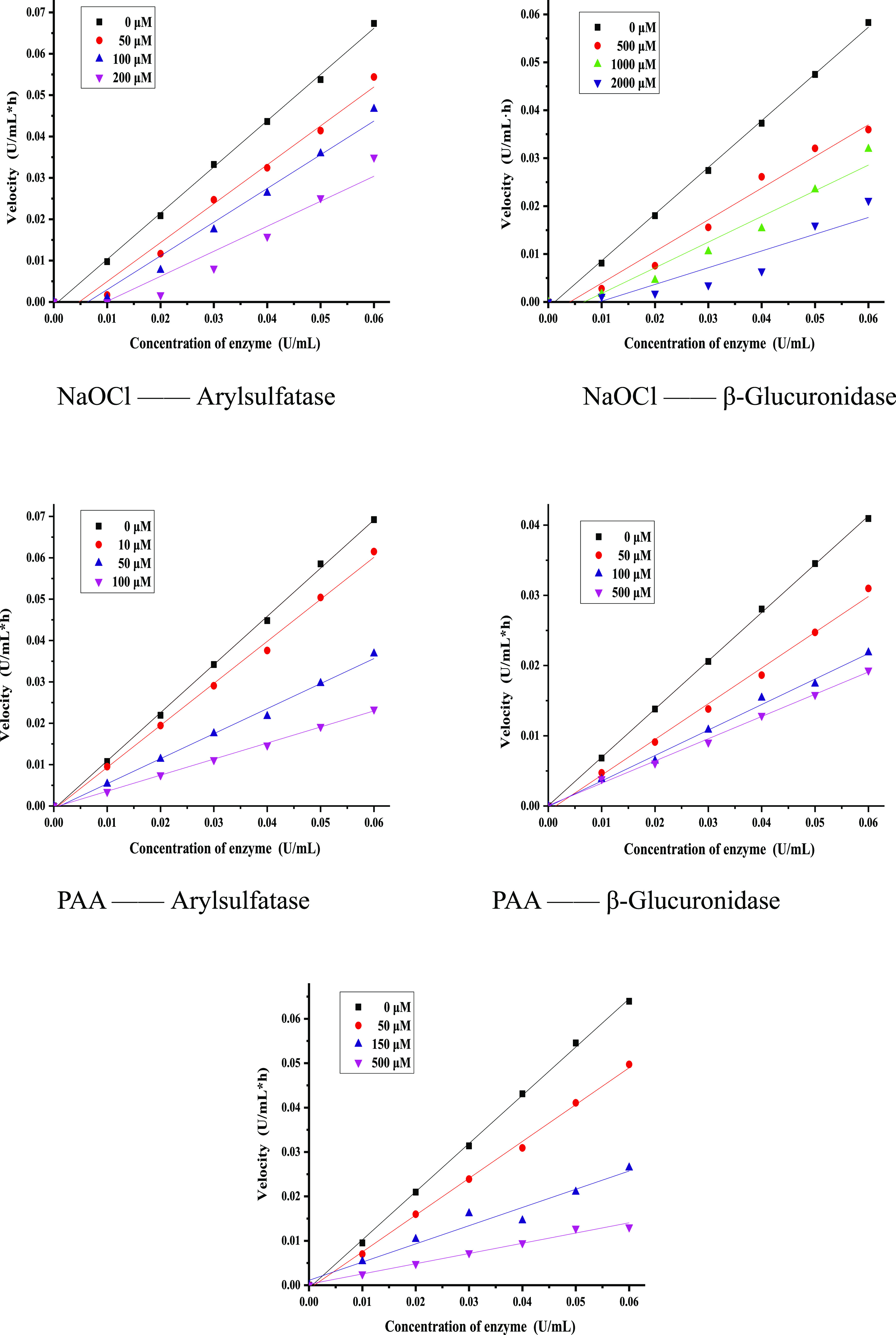
Inhibitions of H2O2, NaClO, and PAA with different concentrations on arylsulfatase and/or β-glucuronidase.
Figure 7.
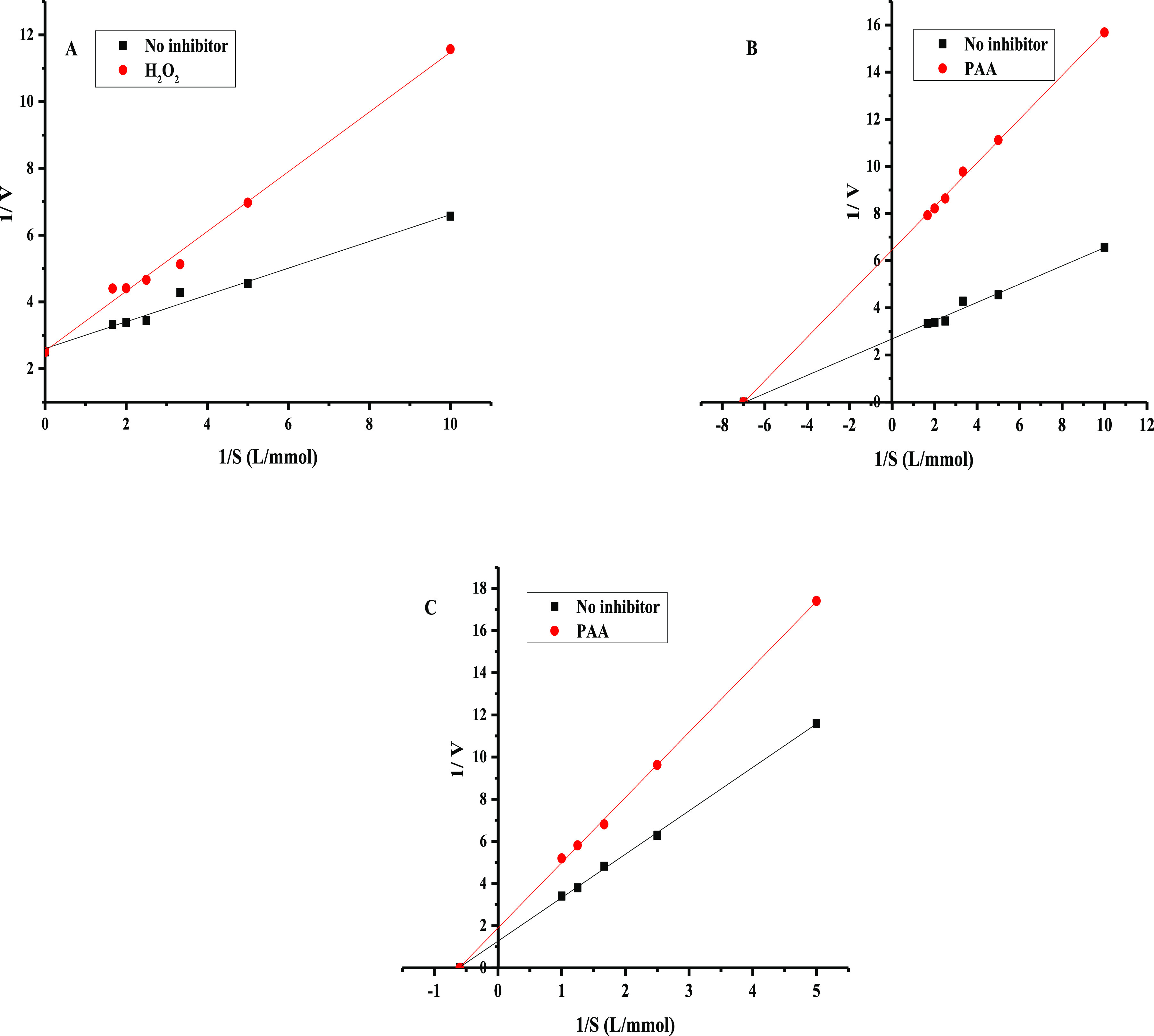
Lineweaver–Burk plots of the inhibition reaction of arylsulfatase in the presence of H2O2 (A) and PAA (B). Lineweaver–Burk plots of the inhibition reaction of β-glucuronidase in the presence of PAA (C).
Significance of This Work
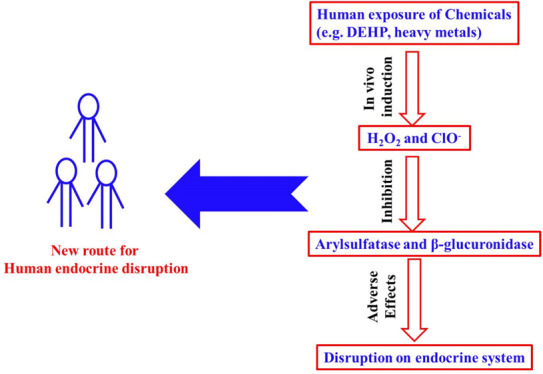
Endocrine-disrupting compounds (EDCs) are, by definition, natural or synthetic agents that can mimic, enhance, or inhibit the action of endogenous hormones that are responsible for maintaining homeostasis and controlling normal development.27,28,59 The most commonly investigated EDCs are those that can mimic the effects of steroid hormones, which often show different estrogenic potencies that can be determined with different bioassays.29−31 With the definition of estrogenic potency, it is convenient to identify which are strong EDCs and which are weak. The potential adverse effect of each EDC depends on both its estrogenic potency and concentration; thus, the concept of estrogen equivalence has been widely adopted.32−34,60 This above criterion well describes the potential adverse effect of one individual EDC at any given concentration. However, many EDCs cannot be well explained with the above conception. For example, di-2-(ethyl hexyl) phthalate, well known as DEHP, the estrogenic potency of which is as low as 10–7, is suggested as an extremely weak EDC.32 Considering its possible human exposure level to normal population, DEHP as an EDC unlikely poses potential adverse effects to humans. Nevertheless, epidemiological studies have shown that DEHP can pose different adverse effects on humans, including reproductive, developmental, and cardiovascular systems.35−38 Another example is cadmium, which possesses no estrogenic potency at all, but it is a well-known EDC.39−41
Many studies have shown that both DEHP and cadmium could induce the production of H2O2 in humans,42−46 whereas the existence of H2O2 can further induce hypochlorite.47 Based on the fact that H2O2 and hypochlorite can effectively inhibit arylsulfatase and/or β-glucuronidase, this may suggest that DEHP and cadmium can disturb the human endocrine system via the production of H2O2 and hypochlorite. This may well explain why DEHP and cadmium with very weak or no estrogenic potency can act as two EDCs that can pose adverse effects on humans. Not limited to DEHP and cadmium, any agent that can induce the production of H2O2 and hypochlorite in humans or animals is a potential EDC.
Considering the importance of H2O2 and hypochlorite in human body, studies relating their human blood concentration were summarized. As shown in Table 1, the reported concentrations of H2O2 in human blood samples varied greatly, which ranged from 0.1 to 6050 μM. Among these, some exceeded the IC50 value that is reported in this work for arylsulfatase and β-glucuronidase, which clearly suggests that H2O2 in human body can act as an EDC via inhibition of arylsulfatase. Compared to H2O2, hypochlorite showed a much stronger inhibition to arylsulfatase, simultaneously showing inhibition to β-glucuronidase, which are all pH-dependent. As hypochlorite can also be endogenously produced through peroxidation of chloride ion by the catalysis of the enzyme myeloperoxidase in leukocytes including macrophages, monocytes, and neutrophils, the adverse effect of any potential EDC, via induction of H2O2 and hypochlorite, is likely enhanced when the production of hypochlorite is at a more favorable condition. It should be noted that because of the instability of H2O2 in human blood as well as the matrix interferences, not all the concentration data summarized in Table 1 are correct.19 However, it is still believable that H2O2 and hypochlorite in humans can act as two special EDCs via the inhibition of arylsulfatase and/or β-glucuronidase, which further suggests that any agent that can induce the production of H2O2 or hypochlorite is a potential EDC. To the best of our knowledge, this is the first report that demonstrates that H2O2, hypochlorite, and PAA can effectively inhibit arylsulfatase and/or β-glucuronidase, which further suggests that some EDCs may act as endocrine disruptors via the induction of H2O2 and hypochlorite.
Table 1. Hydrogen Peroxide in Humans Reported by Different Studies.
| number | objective | sample type | sample size | concentration (μM) | reference |
|---|---|---|---|---|---|
| 1 | man | plasma | 1 | 4.825 | (48) |
| 2 | man | plasma | 1 | 5.5 | (49) |
| 3 | men | plasma | 17 | 5.85–7.15 | (50) |
| 4 | men | plasma | 50 | 2.14–3.15 | (51) |
| 5 | men and women | plasma | 236 | 0.61–6.79 | (52) |
| 6 | children | plasma | 1.4–2 | (53) | |
| 7 | men and women | plasma | 60 | 2.5–6.2 | (54) |
| 8 | men and women | plasma | 30 | 21–113 | (55) |
| 9 | men and women | plasma | 53 | 30.5–50.3 | (56) |
| 10 | pregnant women | plasma | 31 | 50.1–66.9 | (57) |
| 11 | men | blood | 6 | 114–577 (288) | (58) |
| 12 | men | plasma | 6 | 13–57 (34) | (58) |
Conclusions
It was demonstrated for the first time that H2O2, hypochlorite, and PAA could effectively inhibit arylsulfatase and/or β-glucuronidase. The following conclusions can be drawn.
-
(1)
Hypochlorite and PAA at the concentration level of μM could significantly inhibit both arylsulfatase and β-glucuronidase, whereas H2O2 was only effective for inhibiting arylsulfatase.
-
(2)
The inhibition of H2O2 and PAA to arylsulfatase and β-glucuronidase was found to be reversible, whereas the inhibition was found to be irreversible for hypochlorite.
-
(3)
H2O2, hypochlorite, and PAA may behave as special endocrine disruptors through the inhibition of arylsulfatase and/or β-glucuronidase. This indeed led to an extended family of known endocrine disruptors.
Acknowledgments
This work was financially supported by the Program for National Natural Science Foundation of China (nos. 21577040; 21107025); Science and Technology Program of Guangzhou, China (nos. 201904010100 and 201510010162); and Special funds for public welfare research and capacity building in Guangdong Province (no. 2015A020215003) as well as the Guangdong Science and Technology Program (2020B121201003).
The authors declare no competing financial interest.
References
- Dzialoszynski L. M.; Frohlich A.; Kuczynski J.; Pydzik T. The activity of arylsulfatase in some tumors of the genital organs in women. Arch. Immunol. Ther. Exp. 1967, 15, 97–99. [PubMed] [Google Scholar]
- Posey L. E.; Roy Morgan L. Urine enzyme activities in patients with transitional cell carcinoma of the bladder. Clin. Chim. Acta 1977, 74, 7–10. 10.1016/0009-8981(77)90380-1. [DOI] [PubMed] [Google Scholar]
- Taha M.; Almandil N. B.; Rashid U.; Ali M.; Ibrahim M.; Gollapalli M.; Mosaddik A.; Khan K. M. 2,5-disubstituted thiadiazoles as potent β-glucuronidase inhibitors; Synthesis, in vitro and in silico studies. Bioorg. Chem. 2019, 91, 103126. 10.1016/j.bioorg.2019.103126. [DOI] [PubMed] [Google Scholar]
- Parenti G.; Meroni G.; Ballabio A. The sulfatase gene family. Curr. Opin. Genet. Dev. 1997, 7, 386–391. 10.1016/s0959-437x(97)80153-0. [DOI] [PubMed] [Google Scholar]
- Gruber C. J.; Tschugguel W.; Schneeberger C.; Huber J. C. Production and actions of estrogens. N. Engl. J. Med. 2002, 346, 340–352. 10.1056/nejmra000471. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Boyd J. M.; Sawyer M. B.; Li X.-F. Liquid chromatography tandem mass spectrometry determination of free and conjugated estrogens in breast cancer patients before and after exemestane treatment. Anal. Chim. Acta 2014, 806, 172–179. 10.1016/j.aca.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Reed M. J.; Purohit A.; Woo L. W. L.; Newman S. P.; Potter B. V. L. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr. Rev. 2005, 26, 171–202. 10.1210/er.2004-0003. [DOI] [PubMed] [Google Scholar]
- Almandil N. B.; Taha M.; Gollapalli M.; Rahim F.; Ibrahim M.; Mosaddik A.; Anouar E. Indole bearing thiadiazole analogs: synthesis, β-glucuronidase inhibition and molecular docking study. BMC Chem. 2019, 13, 14. 10.1186/s13065-019-0522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E.; Ding S.; Schultz P. G.; Wong C.-H. A potent and highly selective sulfotransferase inhibitor. J. Am. Chem. Soc. 2002, 124, 14524–14525. 10.1021/ja021086u. [DOI] [PubMed] [Google Scholar]
- Rath V. L.; Verdugo D.; Hemmerich S. Sulfotransferase structural biology and inhibitor discovery. Drug Discovery Today 2004, 9, 1003–1011. 10.1016/s1359-6446(04)03273-8. [DOI] [PubMed] [Google Scholar]
- Taha M.; Imran S.; Alomari M.; Rahim F.; Wadood A.; Mosaddik A.; Uddin N.; Gollapalli M.; Alqahtani M. A.; Bamarouf Y. A. Synthesis of oxadiazole-coupled-thiadiazole derivatives as a potent β-glucuronidase inhibitors and their molecular docking study. Bioorg. Med. Chem. 2019b, 27, 3145–3155. 10.1016/j.bmc.2019.05.049. [DOI] [PubMed] [Google Scholar]
- Walaszek Z. Potential use of D-glucaric acid derivatives in cancer prevention. Cancer Lett. 1990, 54, 1–8. 10.1016/0304-3835(90)90083-a. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Liu Z.-h.; Zhong S.-s.; Wang H.; Caidan B.; Yin H.; Dang Z. Strategy for effective inhibition of arylsulfatase/β-glucuronidase to prevent deconjugation of sulfate and glucuronide conjugates in wastewater during sample collection and storage. Sci. Total Environ. 2020, 703, 135536. 10.1016/j.scitotenv.2019.135536. [DOI] [PubMed] [Google Scholar]
- Hidalgo E.; Bartolome R.; Dominguez C. Cytotoxicity mechanisms of sodium hypochlorite in cultured human dermal fibroblasts and its bactericidal effectiveness. Chem.-Biol. Interact. 2002, 139, 265–282. 10.1016/s0009-2797(02)00003-0. [DOI] [PubMed] [Google Scholar]
- Huang Q.; Dawson R. A.; Pegg D. E.; Kearney J. N.; Macneil S. Use of peracetic acid to sterilize human donor skin for production of acellular dermal matrices for clinical use. Wound Repair Regen. 2004, 12, 276–287. 10.1111/j.1067-1927.2004.012312.x. [DOI] [PubMed] [Google Scholar]
- Hugo W. B. A brief history of heat, chemical and radiation preservation and disinfection. Int. Biodeterior. Biodegrad. 1995, 36, 197–217. 10.1016/0964-8305(95)00055-0. [DOI] [Google Scholar]
- Loo A. E. K.; Halliwell B. Effects of hydrogen peroxide in a keratinocyte-fibroblast co-culture model of wound healing. Biochem. Biophys. Res. Commun. 2012, 423, 253–258. 10.1016/j.bbrc.2012.05.100. [DOI] [PubMed] [Google Scholar]
- Bekeschus S.; Kolata J.; Winterbourn C.; Kramer A.; Turner R.; Weltmann K. D.; Bröker B.; Masur K. Hydrogen peroxide: A central player in physical plasma-induced oxidative stress in human blood cells. Free Radical Res. 2014, 48, 542–549. 10.3109/10715762.2014.892937. [DOI] [PubMed] [Google Scholar]
- Forman H. J.; Bernardo A.; Davies K. J. A. What is the concentration of hydrogen peroxide in blood and plasma?. Arch. Biochem. Biophys. 2016, 603, 48–53. 10.1016/j.abb.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Koide Y.; Urano Y.; Hanaoka K.; Terai T.; Nagano T. Development of an Si-Rhodamine-based far-red to near-infrared fluorescence probe selective for hypochlorous acid and its applications for biological imaging. J. Am. Chem. Soc. 2011, 133, 5680–5682. 10.1021/ja111470n. [DOI] [PubMed] [Google Scholar]
- Sato Y.; Ogino K.; Sakano N.; Wang D. H.; Yoshida J.; Akazawa Y.; Kanbara S.; Inoue K.; Kubo M.; Takahashi H. Evaluation of urinary hydrogen peroxide as an oxidative stress biomarker in a healthy Japanese population. Free Radical Res. 2013, 47, 181–191. 10.3109/10715762.2012.759218. [DOI] [PubMed] [Google Scholar]
- GB/T 19106 . Solution of Sodium Hypochlorite, 2013.
- GB/T 19104 . Peracetic Acid Solution, 2008.
- Yuan S.-f.; Liu Z.-h.; Lian H.-X.; Yang C.; Lin Q.; Yin H.; Dang Z. Simultaneous determination of estrogenic odorant alkylphenols, chlorophenols, and their derivatives in water using online headspace solid phase microextraction coupled with gas chromatography-mass spectrometry. Environ. Sci. Pollut. Res. 2016, 23, 19116–19125. 10.1007/s11356-016-7107-1. [DOI] [PubMed] [Google Scholar]
- Yuan S.-f.; Liu Z.-h.; Lian H.-x.; Yang C.-t.; Lin Q.; Yin H.; Dang Z. Simultaneous determination of eleven estrogenic and odorous chloro- and bromo-phenolic compounds in surface water through an automated online headspace SPME followed by on-fiber derivatization coupled with GC-MS. Anal. Methods 2017, 9, 4819–4827. 10.1039/c7ay00641a. [DOI] [Google Scholar]
- Johnson K. A.; Goody R. S. The Original Michaelis Constant: Translation of the 1913 Michaelis-Menten Paper. Biochemistry 2011, 50, 8264–8269. 10.1021/bi201284u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick R. Introduction--workshop on characterizing the effects of endocrine disruptors on human health at environmental exposure levels. Environ. Health Perspect. 1999, 107, 603–604. 10.1289/ehp.99107s4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA . Special Report on Environmental Endocrine Disruption: An Effect as Assessment and Analysis. EPA/630/r-96/012, 1997.
- Bovee T. F. H.; Helsdingen R. J. R.; Rietjens I. M. C. M.; Keijer J.; Hoogenboom R. L. A. P. Rapid yeast bioassays stably expressing human estrogen receptors α and β and green fluorescent protein: A comparison of different compounds with both receptor types. J. Steroid Biochem. Mol. Biol. 2004, 91, 99–109. 10.1016/j.jsbmb.2004.03.118. [DOI] [PubMed] [Google Scholar]
- Liu Z.-h.; Kanjo Y.; Mizutani S. Urinary excretion rates of natural estrogens and androgens from humans, and their occurrence and fate in the environment: a review. Sci. Total Environ. 2009, 407, 4975–4985. 10.1016/j.scitotenv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Liu Z.-h.; Kanjo Y.; Mizutani S. A review of phytoestrogens: their occurrence and fate in the environment. Water Res. 2010, 44, 567–577. 10.1016/j.watres.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Luo Q.; Liu Z.-h.; Yin H.; Dang Z.; Wu P.-x.; Zhu N.-w.; Lin Z.; Liu Y. Migration and potential risk of trace phthalates in bottled water: A global situation. Water Res. 2018, 147, 362–372. 10.1016/j.watres.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Luo Q.; Liu Z.-h.; Yin H.; Dang Z.; Wu P.-x.; Zhu N.-w.; Lin Z.; Liu Y. Global review of phthalates in edible oil: An emerging and nonnegligible exposure source to human. Sci. Total Environ. 2020, 704, 135369. 10.1016/j.scitotenv.2019.135369. [DOI] [PubMed] [Google Scholar]
- Wang H.; Liu Z.-h.; Tang Z.; Zhang J.; Yin H.; Dang Z.; Wu P.-x.; Liu Y. Bisphenol analogues in Chinese bottled water: Quantification and potential risk analysis. Sci. Total Environ. 2020, 713, 136583. 10.1016/j.scitotenv.2020.136583. [DOI] [PubMed] [Google Scholar]
- Benjamin S.; Masai E.; Kamimura N.; Takahashi K.; Anderson R. C.; Faisal P. A. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard. Mater. 2017, 340, 360–383. 10.1016/j.jhazmat.2017.06.036. [DOI] [PubMed] [Google Scholar]
- Kay V. R.; Chambers C.; Foster W. G. Reproductive and developmental effects of phthalate diesters in females. Crit. Rev. Toxicol. 2013, 43, 200–219. 10.3109/10408444.2013.766149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariana M.; Feiteiro J.; Verde I.; Cairrao E. The effects of phthalates in the cardiovascular and reproductive systems: A review. Environ. Int. 2016, 94, 758–776. 10.1016/j.envint.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Mathieu-Denoncourt J.; Wallace S. J.; de Solla S. R.; Langlois V. S. Plasticizer endocrine disruption: Highlighting developmental and reproductive effects in mammals and non-mammalian aquatic species. Gen. Comp. Endocrinol. 2015, 219, 74–88. 10.1016/j.ygcen.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Luo J.; Hendryx M. Relationship between blood cadmium, lead, and serum thyroid measures in US adults—the National Health and Nutrition Examination Survey (NHANES) 2007–2010. Int. J. Environ. Health Res. 2014, 24, 125–136. 10.1080/09603123.2013.800962. [DOI] [PubMed] [Google Scholar]
- Paschoalini A. L.; Savassi L. A.; Arantes F. P.; Rizzo E.; Bazzoli N. Heavy metals accumulation and endocrine disruption in Prochilodus argenteus from a polluted neotropical river. Ecotoxicol. Environ. Saf. 2019, 169, 539–550. 10.1016/j.ecoenv.2018.11.047. [DOI] [PubMed] [Google Scholar]
- Stasenko S.; Bradford E. M.; Piasek M.; Henson M. C.; Varnai V. M.; Jurasović J.; Kušec V. Metals in human placenta: focus on the effects of cadmium on steroid hormones and leptin. J. Appl. Toxicol. 2010, 30, 242–253. 10.1002/jat.1490. [DOI] [PubMed] [Google Scholar]
- Ghosh J.; Das J.; Manna P.; Sil P. C. Hepatotoxicity of di-(2-ethylhexyl)phthalate is attributed to calcium aggravation, ROS-mediated mitochondrial depolarization, and ERK/NF-κB pathway activation. Free Radical Biol. Med. 2010, 49, 1779–1791. 10.1016/j.freeradbiomed.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Matés J. M.; Segura J. A.; Alonso F. J.; Márquez J. Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radical Biol. Med. 2010, 49, 1328–1341. 10.1016/j.freeradbiomed.2010.07.028. [DOI] [PubMed] [Google Scholar]
- Matović V.; Buha A.; D̵ukić-Ćosić D.; Bulat Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015, 78, 130–140. 10.1016/j.fct.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Shen C.; Wang Y.; Shen Q.; Wang L.; Lu Y.; Li X.; Wei J. Di-(2-ethylhexyl) phthalate induced the growth inhibition and oxidative damage in the microalga Chlorella vulgaris. IOP Conf. Ser. Earth Environ. Sci. 2019, 227, 052054. 10.1088/1755-1315/227/5/052054. [DOI] [Google Scholar]
- Szymańska-Chabowska A.; Beck A.; Poręba R.; Andrezejak R.; Antonowicz-Juchniewicz J. Evaluation of DNA damage in people occupationally exposed to arsenic and some heavy metals. Pol. J. Environ. Stud. 2009, 18, 1131–1139. [Google Scholar]
- Yap Y. W.; Whiteman M.; Cheung N. S. Chlorinative stress: An under appreciated mediator of neurodegeneration?. Cell. Signalling 2007, 19, 219–228. 10.1016/j.cellsig.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y.; Brodsky M. H.; Baker J. C.; Ames B. N. Detection and characterization of lipid hydroperoxides at picomole levels by high-performance liquid chromatography. Anal. Biochem. 1987, 160, 7–13. 10.1016/0003-2697(87)90606-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y.; Ames B. N. Detection of lipid hydroperoxides and hydrogen peroxide at picamole levels by an HPLC and isoluminol chemiluminescence assay. Free Radical Biol. Med. 1987, 3, 359–361. 10.1016/s0891-5849(87)80048-5. [DOI] [PubMed] [Google Scholar]
- Deskur E.; Przywarska L.; Dylewicz P.; Szczęśniak Ł.; Rychlewski T.; Wilk M.; Wysocki H. Exercise-induced increase in hydrogen peroxide plasma levels is diminished by endurance training after myocardial infarction. Int. J. Cardiol. 1998, 67, 219–224. 10.1016/s0167-5273(98)00231-9. [DOI] [PubMed] [Google Scholar]
- Lacy F.; OʼConnor D. T.; Schmid-Schönbein G. W. Plasma hydrogen peroxide production in hypertensives and normotensive subjects at genetic risk of hypertension. J. Hypertens. 1998, 16, 291–303. 10.1097/00004872-199816030-00006. [DOI] [PubMed] [Google Scholar]
- Lacy F.; Kailasam M. T.; O’Connor D. T.; Schmid-Schönbein G. W.; Parmer R. J. Plasma hydrogen peroxide production in human essential hypertension. Role of heredity, gender, and ethnicity. Hypertension 2000, 36, 878–884. 10.1161/01.hyp.36.5.878. [DOI] [PubMed] [Google Scholar]
- Li D.; Sun W. P.; Zhou Y. M.; Liu Q. G.; Zhou S. S.; Luo N.; Bian F. N.; Zhao Z. G.; Guo M. Chronic niacin overload may be involved in the increased prevalence of obesity in US children. World J. Gastroenterol. 2010, 16, 2378–2387. 10.3748/wjg.v16.i19.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee D.; Madhusoodanan U. K.; Nayak S.; Jacob J. Urinary hydrogen peroxide: a probable marker of oxidative stress in malignancy. Clin. Chim. Acta 2003, 334, 205–209. 10.1016/s0009-8981(03)00236-5. [DOI] [PubMed] [Google Scholar]
- Wierusz-Wysocka B.; Wysocki H.; Byks H.; Zozulińska D.; Wykrȩtowicz A.; Kaźmierczak M. Metabolic control quality and free radical activity in diabetic patients. Diabetes Res. Clin. Pract. 1995, 27, 193–197. 10.1016/0168-8227(95)01043-d. [DOI] [PubMed] [Google Scholar]
- Kazmierczak M.; Wysocki H.; Wykretowicz A.; Minczykowski A. Estimation of hydrogen peroxide plasma levels in patients evaluated for coronary heart disease using dipyridamole infusion followed by SPECT. Coron. Artery Dis. 1995, 6, 65–70. 10.1097/00019501-199501000-00010. [DOI] [PubMed] [Google Scholar]
- Tsukimori K.; Yoshitomi T.; Morokuma S.; Fukushima K.; Wake N. Serum uric acid levels correlate with plasma hydrogen peroxide and protein carbonyl levels in preeclampsia. Am. J. Hypertens. 2008, 21, 1343–1346. 10.1038/ajh.2008.289. [DOI] [PubMed] [Google Scholar]
- Varma S. D.; Devamanoharan P. S. Hydrogen peroxide in human blood. Free Radical Res. Commun. 1991, 14, 125–131. 10.3109/10715769109094124. [DOI] [PubMed] [Google Scholar]
- Liu Z.-h.; Dang Z.; Yin H.; Liu Y. Making waves: Improving removal performance of conventional wastewater treatment plants on endocrine disrupting compounds (EDCs): their conjugates matter. Water Res. 2021, 188, 116469. 10.1016/j.watres.2020.116469. [DOI] [PubMed] [Google Scholar]
- Tang Z.; Liu Z.-h.; Wang H.; Dang Z.; Yin H.; Zhou Y.; Liu Y. Trace determination of eleven natural estrogens and insights from their occurrence in a municipal wastewater treatment plant and river water. Water Res. 2020, 182, 115976. 10.1016/j.watres.2020.115976. [DOI] [PubMed] [Google Scholar]