Abstract
Background
Preoperative cognitive dysfunction has been associated with adverse postoperative outcomes. There are limited data characterising the epidemiology of preoperative cognitive dysfunction in older surgical patients.
Methods
This retrospective cohort included all patients ≥65 yr old seen at the Washington University preoperative clinic between January 2013 and June 2018. Cognitive screening was performed using the Short-Blessed Test (SBT) and Eight-Item Interview to Differentiate Aging and Dementia (AD8) screen. The primary outcome of abnormal cognitive screening was defined as SBT score ≥5 or AD8 score ≥2. Multivariable logistic regression was used to identify associated factors.
Results
Overall, 21 666 patients ≥65 yr old completed screening during the study period; 23.5% (n=5099) of cognitive screens were abnormal. Abnormal cognitive screening was associated with increasing age, decreasing BMI, male sex, non-Caucasian race, decreased functional independence, and decreased metabolic functional capacity. Patients with a history of stroke or transient ischaemic attack, chronic obstructive pulmonary disease, diabetes mellitus, hepatic cirrhosis, and heavy alcohol use were also more likely to have an abnormal cognitive screen. Predictive modelling showed no combination of patient factors was able to reliably identify patients who had a <10% probability of abnormal cognitive screening.
Conclusions
Routine preoperative cognitive screening of unselected aged surgical patients often revealed deficits consistent with cognitive impairment or dementia. Such deficits were associated with increased age, decreased function, decreased BMI, and several common medical comorbidities. Further research is necessary to characterise the clinical implications of preoperative cognitive dysfunction and identify interventions that may reduce related postoperative complications.
Keywords: cognitive screening, dementia screening, geriatric surgery, neurocognitive dysfunction, perioperative medicine, preoperative assessment
Editor's key points.
-
•
A retrospective analysis of a cohort of patients ≥65 yr old who underwent preoperative cognitive screening at a large US centre using the Short-Blessed Test and Eight Item Interview to Differentiate Aging and Dementia screen was performed.
-
•
Of 21 666 patients who completed screening, 23.5% had abnormal cognitive screens, which were associated with increasing age, decreasing BMI, male sex, non-Caucasian race, decreased functional independence, and decreased functional capacity.
-
•
No combination of patient factors was able to identify patients unlikely to have abnormal cognitive screening.
-
•
Analysis of this large cohort shows that abnormal preoperative cognitive screening is common in older patients presenting for elective surgery, and might provide a signal for interventions to improve outcome.
Preoperative cognitive dysfunction has been associated with adverse postoperative outcomes in several surgical populations.1, 2, 3, 4 Reliable preoperative identification of patients with cognitive dysfunction could be utilised for risk stratification and to implement targeted interventions to mitigate related risks. In the general population, ∼10% of individuals older than 65 yr have clinical dementia,5 with an additional 10–20% having some degree of mild cognitive impairment.6,7 However, the prevalence of cognitive dysfunction in preoperative populations of different age and comorbidity burdens has not been well characterised. An improved epidemiological understanding of this would facilitate efficient preoperative screening in large surgical populations.
We aimed to describe the epidemiology of abnormal preoperative cognition in a large cohort of aged surgical patients at a tertiary academic medical centre in the USA, as defined by the Short-Blessed Test (SBT) and Eight-Item Interview to Differentiate Aging and Dementia (AD8) screening tests.8,9 Routine cognitive screening of aged surgical patients was implemented at our institution in 2012 as part of a broader preoperative screening programme designed to better inform surgical decision-making in geriatric patients, as outlined in the National Surgical Quality Improvement Program and the American Geriatrics Society best practice guidelines for geriatric preoperative assessment.10 These two tests were selected to provide efficient objective and subjective assessment of cognitive dysfunction. The SBT is an objective screening test focused on memory, orientation, and concentration. It consists of six questions with standardised scoring, where a score of 5–9 suggests questionable cognitive impairment and a score ≥10 suggests cognitive impairment consistent with dementia. The AD8 is a brief subjective screening test for dementia that consists of eight questions and is focused on identifying a change in cognitive function. An AD8 score ≥2 suggests that cognitive impairment is likely to be present. Both screening tests have been validated in the outpatient geriatric population and the emergency department as effective screening tools to identify cognitive dysfunction.8,9,11, 12, 13
We also aimed to evaluate associations between positive SBT or AD8 screening results, age, coexisting disease, and other patient factors. The objective of this analysis was to characterise the overall burden of abnormal cognitive screening in aged surgical patients and to identify subpopulations with a higher prevalence of deficits to facilitate targeting of screening programmes and risk mitigation efforts.
Methods
This study was approved by the Institutional Review Board at Washington University in St. Louis, St. Louis, MO, USA (IRB ID# 201909163). This manuscript was written in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.14 Authorship was determined using the International Committee of Medical Journal Editors (ICMJE) definition of authorship.
Study design and patient population
This retrospective observational cohort study included all preoperative assessments performed before elective surgery on patients ≥65 yr of age at the Barnes-Jewish Hospital Center for Preoperative Assessment and Planning (Barnes-Jewish Hospital, St. Louis, MO, USA) between January 2013 and June 2018. Patients with complete results for both the SBT and AD8 tests were included in the demographic analysis, multivariable analysis, and a predictive model for abnormal preoperative cognitive screening. To assess potential bias in screening administration, patients without complete results for both the SBT and AD8 tests were then included in an analysis where they were compared with those patients with complete SBT and AD8 results. For patients who had multiple screenings over the study period, only the first screening was included in the analysis to exclude a possible practice effect. The primary outcome was abnormal cognitive screening as defined by SBT score ≥5 or AD8 score ≥2.
Data collection
Patients presenting for preoperative evaluation at our preoperative clinic who are ≥65 yr of age undergo cognitive screening with the SBT and AD8 instruments administered by a registered nurse. The SBT is administered directly to the patient, and the AD8 is administered preferentially to the patient's cohabitant or caregiver, although it is acceptable to administer the AD8 directly to the patient if no caregiver is present at the time of assessment.15 Nurses in the preoperative assessment clinic receive training in the proper administration of these screening tests.
Variable categorisation
SBT and AD8 results were categorised as above. Barthel index scores were categorised as normal (100), impaired (<100), or missing. History of cerebrovascular accident, stroke, transient ischaemic attack (TIA), and current cerebrovascular disease were combined into a single variable. Because of the low prevalence of non-White non-Black racial categories, race was categorised as White, Black, other, or missing. BMI >60 and <12 kg m−2 were treated as typographic or unit errors and were replaced by the sample median. BMI (in kg m−2) was categorised as underweight (<18.5), normal weight (18.5–24.9), overweight (25–29.9), class 1 obesity (30–34.9), class 2 obesity (35–39.9), and class 3 obesity (≥40). Because of low rates of missingness for all other variables (<0.5%), row-wise deletion was used for other missing data.
Statistical analysis
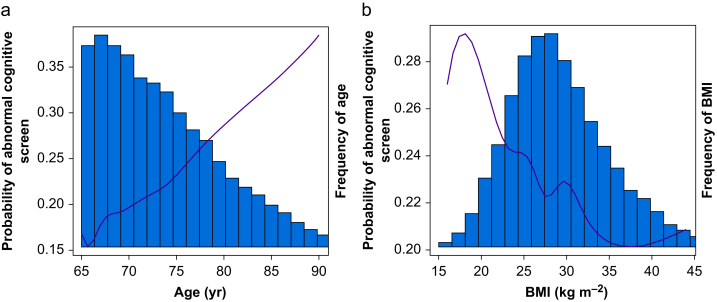
In the group of patients with complete AD8 and SBT screen results, logistic regression was performed where a positive outcome was defined as either measure being abnormal. Because of the large sample size, all predictors were entered simultaneously. BMI and age were the only quantitative predictors, and were modelled by b-splines with 9 degrees of freedom. On examination of partial dependence plots, these terms were both roughly linear and the model was simplified to linear terms for presentation (Fig. 1).
Fig 1.
Predicted probability of abnormal cognitive screen by age (a) and body mass index (b). Columns indicate the relative frequency of each age or body mass index within the screened population.
A second logistic regression was constructed for all patients ≥65 yr of age who underwent preoperative assessment during the study period with the outcome of missing either the AD8 or SBT screening tests. The predictors were identical, again using linear terms for age and BMI. Confidence intervals and P-values were calculated using Wald's methods with an alpha of 0.05. Firth's correction was used to examine for separation, but this was not found to make a noticeable difference.16 All calculations were performed in R 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
As a secondary goal, we sought to develop a predictive model of abnormal cognitive screening using demographic and comorbidity variables, which could be used to identify patient populations unlikely to be screen positive. We fit non-parametric models using gradient boosted decision trees (xgboost package) and Bayesian additive regression trees (BART package).13,17 Hyper-parameters for gradient boosting were selected by 5-fold cross validation. Default parameters were used for BART (k=2, splitting=0.95, 50 trees). Our threshold for ‘low baseline probability’ was 10%.
As noted above, we used only the first completed screening in our analysis. In comparisons of screened vs unscreened patients, we used the first screened visit (if one existed) or the first visit (if no visits were screened). Because this introduces a bias towards earlier visits in the screened group, we conducted sensitivity analyses sampling a single observation from each patient (a) at random, (b) first chronologically regardless of screening status, (c) last chronologically regardless of screening status, and (d) including all screening data (Supplementary Table S1).
Results
Data included 30 388 unique patients ≥65 yr old who underwent preoperative assessment during the study period, of which 21 666 patients completed the SBT and AD8 screening and were included in the demographic, predictive model, and primary multivariable analyses. Descriptive statistics for the screened and unscreened (incomplete or absent AD8 or SBT) populations are shown in Table 1.
Table 1.
Comparison of patients who completed cognitive screening with those who had an incomplete or absent screen. Odds ratio (OR) reported from multivariable regression for missingness. OR for age is presented for each 10 yr increase. CI, confidence interval; COPD, chronic obstructive pulmonary disease; METs, metabolic equivalents; sd, standard deviation; TIA, transient ischaemic attack.
| Total group (n=30 388) | Screen present (n=21 666) | Screen absent (n=8722) | OR missing (95% CI) | |
|---|---|---|---|---|
| Age, mean, yr (sd) | 73.3 (6.40) | 73.4 (6.30) | 72.8 (6.63) | 0.78 (0.74–0.82) |
| Male sex, n (%) | 14 390 (47.4) | 10 200 (47.1) | 4190 (48.0) | 1.09 (1.02–1.16) |
| Race, n (%) | ||||
| White | 28 499 (75.0) | 19 109 (75.0) | 9390 (74.9) | Reference |
| Black | 22 878 (75.3) | 16 254 (75.0) | 6624 (75.9) | 0.95 (0.84–1.07) |
| Other | 1928 (6.3) | 1268 (5.9) | 660 (7.6) | 1.43 (1.28–1.61) |
| Missing | 3653 (12.0) | 2767 (12.8) | 886 (10.2) | 1.05 (0.96–1.15) |
| BMI, kg m−2, n (%) | ||||
| 14–18.5 | 423 (1.4) | 270 (1.2) | 153 (1.8) | 1.34 (1.06–1.71) |
| 18.5–25 | 7131 (23.5) | 5028 (23.2) | 2103 (24.1) | Reference |
| 25–30 | 10 797 (35.5) | 7686 (35.5) | 3111 (35.7) | 0.91 (0.84–0.98) |
| 30–35 | 6991 (23.0) | 5074 (23.4) | 1917 (22.0) | 0.87 (0.79–0.94) |
| 35–40 | 3161 (10.4) | 2306 (10.6) | 855 (9.8) | 0.80 (0.71–0.89) |
| 40–61 | 1827 (6.0) | 1267 (5.8) | 560 (6.4) | 0.93 (0.81–1.06) |
| Barthel index <100, n (%) | 6612 (21.8) | 5498 (25.4) | 1114 (12.8) | 0.83 (0.77–0.90) |
| Missing, n (%) | 4375 (14.4) | 638 (2.9) | 3737 (42.8) | 24.15 (22.02–26.49) |
| Functional capacity <4 METS, n (%) | 13 503 (44.4) | 9662 (44.6) | 3841 (44.0) | 1.11 (1.04–1.19) |
| History of stroke or TIA, n (%) | 4280 (14.1) | 3054 (14.1) | 1226 (14.1) | 1.03 (0.95–1.13) |
| Coronary artery disease, n (%) | 7169 (23.6) | 5195 (24.0) | 1974 (22.6) | 0.94 (0.87–1.01) |
| Congestive heart failure, n (%) | 3152 (10.4) | 2273 (10.5) | 879 (10.1) | 1.01 (0.91–1.13) |
| Atrial fibrillation or flutter history, n (%) | 4512 (14.8) | 3225 (14.9) | 1287 (14.8) | 1.04 (0.95–1.13) |
| COPD, n (%) | 3653 (12.0) | 2652 (12.2) | 1001 (11.5) | 0.90 (0.82–1.00) |
| Asthma, n (%) | 2917 (9.6) | 2078 (9.6) | 839 (9.6) | 0.97 (0.87–1.07) |
| Peripheral vascular disease, n (%) | 1450 (4.8) | 1063 (4.9) | 387 (4.4) | 0.87 (0.75–1.01) |
| Diabetes mellitus, n (%) | 7967 (26.2) | 5708 (26.3) | 2259 (25.9) | 0.97 (0.90–1.04) |
| Current cancer, n (%) | 5647 (18.6) | 4030 (18.6) | 1617 (18.5) | 0.95 (0.88–1.03) |
| History of heavy alcohol use, n (%) | 2061 (6.8) | 1461 (6.7) | 600 (6.9) | 1.08 (0.96–1.21) |
| Hepatic cirrhosis, n (%) | 364 (1.2) | 277 (1.3) | 87 (1.0) | 0.67 (0.50–0.90) |
| Dialysis, n (%) | 517 (1.7) | 360 (1.7) | 157 (1.8) | 0.96 (0.76–1.21) |
The overall prevalence of abnormal cognitive screening among patients with a complete SBT and AD8 screen was 23.5% (n=5099; Table 2). Of these, 4.6% (n=995) had positive results on both screening tests, 4.9% (n=1068) had an isolated abnormal AD8, and 13.9% (n=3036) had an isolated abnormal SBT (Table 3). When comparing the prevalence before surgeries carried out by surgical specialties, abnormal preoperative cognitive screening was most common in patients undergoing vascular surgery (33%) and neurosurgery (28%); it was least common in patients undergoing orthopaedic (19%), gynaecologic (20%), and plastic surgery (20%) procedures (Table 4).
Table 2.
Patient characteristics, comorbidities, and preoperative cognitive screening results in screened patients. Abnormal patients had a Short-Blessed Test (SBT) score ≥5 or an Eight Item Interview to Differentiate Aging and Dementia (AD8) score ≥2. COPD, chronic obstructive pulmonary disease; METs, metabolic equivalents; sd, standard deviation; TIA, transient ischaemic attack.
| Total group (n=21 666) | Normal (n=16 567; 76.5%) | Abnormal (n=5099; 23.5%) | |
|---|---|---|---|
| Age, mean, yr (sd) | 73.4 (6.30) | 72.8 (5.94) | 75.5 (6.94) |
| Male sex, n (%) | 10 200 (47.1) | 7667 (46.3) | 2533 (49.7) |
| Race, n (%) | |||
| White | 16 254 (75.0) | 12 853 (77.6) | 3401 (66.7) |
| Black | 1377 (6.4) | 858 (5.2) | 519 (10.2) |
| Other | 1268 (5.9) | 790 (4.8) | 478 (9.4) |
| Missing | 2767 (12.8) | 2066 (12.5) | 701 (13.7) |
| BMI, kg m−2, n (%) | |||
| 14–18.5 | 270 (1.2) | 179 (1.1) | 91 (1.8) |
| 18.5–25 | 5028 (23.2) | 3708 (22.4) | 1320 (25.9) |
| 25–30 | 7686 (35.5) | 5924 (35.8) | 1762 (34.6) |
| 30–35 | 5074 (23.4) | 3947 (23.8) | 1127 (22.1) |
| 35–40 | 2306 (10.6) | 1809 (10.9) | 497 (9.7) |
| 40–61 | 1267 (5.8) | 975 (5.9) | 292 (5.7) |
| Missing | 35 (0.2) | 25 (0.2) | 10 (0.2) |
| Barthel index <100, n (%) | 5498 (25.4) | 3521 (21.3) | 1977 (38.8) |
| Missing | 638 (2.9) | 477 (2.9) | 161 (3.2) |
| Functional capacity <4 METS, n (%) | 9662 (44.6) | 6597 (39.8) | 3065 (60.1) |
| History of stroke or TIA, n (%) | 3054 (14.1) | 1961 (11.8) | 1092 (21.4) |
| Coronary artery disease, n (%) | 5195 (24.0) | 3667 (22.1) | 1528 (30.0) |
| Congestive heart failure, n (%) | 2273 (10.5) | 1548 (9.3) | 725 (14.2) |
| Atrial fibrillation or flutter history, n (%) | 3225 (14.9) | 2320 (14.0) | 905 (17.7) |
| COPD, n (%) | 2652 (12.2) | 1758 (10.6) | 894 (17.5) |
| Asthma, n (%) | 2078 (9.6) | 1595 (9.6) | 483 (9.5) |
| Peripheral vascular disease, n (%) | 1063 (4.9) | 707 (4.3) | 356 (7.0) |
| Diabetes mellitus, n (%) | 5708 (26.3) | 4070 (24.6) | 1638 (32.1) |
| Current cancer, n (%) | 4030 (18.6) | 3066 (18.5) | 964 (18.9) |
| History of heavy alcohol use, n (%) | 1461 (6.7) | 1063 (6.4) | 398 (7.8) |
| Hepatic cirrhosis, n (%) | 277 (1.3) | 185 (1.1) | 92 (1.8) |
| Dialysis, n (%) | 360 (1.7) | 219 (1.3) | 141 (2.8) |
Table 3.
Cognitive screening results by individual screening test in screened patients. An Eight-Item Interview to Differentiate Aging and Dementia (AD8) score ≥2 is considered abnormal. A Short-Blessed Test (SBT) score of 5–9 suggests questionable cognitive impairment, and a score ≥10 suggests cognitive impairment consistent with dementia. Results reported as number (% of total screened group).
| SBT <4 | SBT 5–9 | SBT ≥10 | |
|---|---|---|---|
| AD8 0–1 | 16 567 (76.5) | 2294 (10.5) | 742 (3.4) |
| AD8 ≥2 | 1068 (4.9) | 466 (2.2) | 529 (2.4) |
Table 4.
Prevalence of abnormal preoperative cognitive screens by surgical service. AD8, Eight Item Interview to Differentiate Aging and Dementia; SBT, Short-Blessed Test.
| Surgical service | n | AD8 ≥2 (%) | SBT ≥5 (%) | AD8 ≥2 or SBT ≥5 (%) |
|---|---|---|---|---|
| General | 3261 | 9 | 19 | 24 |
| Cardiothoracic surgery | 1587 | 10 | 20 | 25 |
| Cardiology/electrophysiology | 939 | 12 | 20 | 26 |
| Gynaecology | 1269 | 7 | 16 | 20 |
| Neurosurgery | 1264 | 16 | 20 | 28 |
| Ophthalmology | 2624 | 10 | 22 | 27 |
| Orthopaedic surgery | 4762 | 8 | 14 | 19 |
| Otolaryngology | 1376 | 9 | 18 | 23 |
| Plastic surgery | 438 | 6 | 16 | 20 |
| Transplant surgery | 327 | 7 | 22 | 23 |
| Urology | 2043 | 9 | 20 | 24 |
| Vascular surgery | 964 | 13 | 28 | 33 |
| Other | 485 | 12 | 17 | 24 |
| Missing surgical service | 327 | 12 | 21 | 27 |
Multivariable analysis identified the strongest predictors of abnormal preoperative cognitive screening as non-Caucasian race (Black race: odds ratio [OR]=2.33; 95% confidence interval [CI], 2.06–2.64; P<0.0001; other race: OR=2.26; 95% CI, 1.99–2.57; P<0.001), impaired functional independence (Barthel index <100; OR=1.85; 95% CI, 1.72–2.00; P<0.0001) and <4 METs (metabolic equivalents) functional capacity (OR=1.57; 95% CI, 1.46–1.70; P<0.0001). Low BMI and older age (OR=1.70 per 10 yr increase; 95% CI, 1.61–1.79; P<0.0001) were also strongly associated with abnormal cognitive screening (Fig. 1). Patients with a history of stroke or TIA, chronic obstructive pulmonary disease, diabetes mellitus, prior heavy alcohol use, or hepatic cirrhosis were all at increased risk for abnormal cognitive screening. ORs for the multivariable analysis are shown in Table 5. Auto-correlation sensitivity analyses (Supplementary Table S2) showed minimal differences when using alternative sampling methods.
Table 5.
Multivariable analysis of factors associated with abnormal preoperative cognitive screening in screened patients. Abnormal patients had a Short-Blessed Test (SBT) score ≥5 or an Eight-Item Interview to Differentiate Aging and Dementia (AD8) score ≥2. CI, confidence interval; METs, metabolic equivalents; TIA, transient ischaemic attack; COPD, chronic obstructive pulmonary disease.
| Variable | Odds ratio (95% CI) | P value |
|---|---|---|
| Age (per decade) | 1.70 (1.61–1.79) | <0.001 |
| Male sex | 1.39 (1.30–1.50) | <0.001 |
| Race | ||
| White | Reference | |
| Black | 2.33 (2.06–2.64) | <0.001 |
| Other | 2.26 (1.99–2.57) | <0.001 |
| Missing | 1.32 (1.19–1.45) | <0.001 |
| BMI, kg m−2, n (%) | ||
| 14–18.5 | 1.16 (0.88–1.54) | 0.161 |
| 18.5–25 | Reference | |
| 25–30 | 0.85 (0.78–0.93) | <0.001 |
| 30–35 | 0.80 (0.73–0.89) | <0.001 |
| 35–40 | 0.73 (0.64–0.83) | <0.001 |
| 40–61 | 0.75 (0.64–0.88) | <0.001 |
| Barthel index <100 | 1.85 (1.72–2.00) | <0.001 |
| Missing | 1.27 (1.05–1.54) | 0.015 |
| Functional capacity <4 METS | 1.57 (1.46–1.70) | <0.001 |
| History of stroke or TIA | 1.47 (1.34–1.61) | <0.001 |
| Coronary artery disease | 1.07 (0.98–1.16) | 0.124 |
| Congestive heart failure | 0.94 (0.84–1.05) | 0.307 |
| Atrial fibrillation or flutter | 0.96 (0.87–1.06) | 0.416 |
| COPD | 1.36 (1.24–1.50) | <0.001 |
| Asthma | 0.95 (0.85–1.06) | 0.372 |
| Peripheral vascular disease | 0.99 (0.85–1.14) | 0.847 |
| Diabetes mellitus | 1.20 (1.11–1.30) | <0.001 |
| Active cancer | 1.09 (1.00–1.18) | 0.057 |
| Prior heavy alcohol use | 1.32 (1.15–1.50) | <0.001 |
| Hepatic cirrhosis | 1.44 (1.11–1.88) | 0.007 |
| Dialysis | 1.19 (0.94–1.50) | 0.15 |
The predictive models fit by gradient boosting and BART identified a small fraction of patients with pre-screening probabilities <10% (2985/21 666 for the gradient boosting model and 2701/21 666 for the BART model). The BART model identified 1197/21 666 (5.5%) of patients as low risk with high confidence (posterior probability of risk >0.1 less than 5%). There were no highly sensitive predictors of low risk which ruled out patients from needing screening. These models were well approximated by a combination of age <75 yr, normal functional capacity, normal Barthel index, lack of a history of heavy drinking, and absence of comorbidities (coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, cerebrovascular accident, peripheral artery disease, cirrhosis) which cumulatively identified patients at the lowest level of risk (388/3425 [11%] screened positive).
Discussion
Analysis of this large cohort shows that abnormal preoperative cognitive screening is common in older patients presenting for elective surgery. In addition to providing large-scale validation of prior work evaluating cognitive screening results in smaller surgical populations,1,4,18, 19, 20 this study also provides evidence that routine cognitive screening of aged surgical patients may be preferable to selective screening based on underlying comorbidities or other characteristics.
The prevalence of abnormal cognitive screening in our population is consistent with other recent findings in smaller, more restricted surgical cohorts. Several prior studies have utilised the Mini-Cog cognitive screen in both selected (by frailty indicators or procedure) and unselected preoperative patients. These trials reported an overall prevalence of abnormal cognitive screening of 10–24%.1,4,20 A pilot implementation trial using a clock-drawing test scored using Mini-Cog and the Montreal Cognitive Assessment scoring paradigm21 and a three-word memory score also found an overall abnormal screening rate of ∼20%.18 In the ambulatory setting, survey data utilising the Telephone Interview for Cognitive Status showed a prevalence of 16.1%, with similar associated comorbidities identified in the multivariable analysis.19 Although these trials all examined either small cohorts or specific patient subpopulations, they were consistent in identifying abnormal cognition as a common comorbidity with a prevalence similar to that of diabetes mellitus, coronary artery disease, and cancer in aged surgical patients.
We observed a linear relationship between increasing age and incidence of abnormal cognitive screening, and an inverse relationship between BMI and abnormal cognition. The relationship between age and abnormal cognitive screening has been described in small surgical cohorts and in the non-perioperative dementia literature, and this study provides large-scale validation of this observation in a perioperative population.1,22,23 Similarly, the inverse relationship between BMI and abnormal cognition has been identified in the non-perioperative dementia literature, though studies in surgical patients are limited.24, 25, 26, 27, 28 Although this relationship is somewhat counterintuitive given the higher incidence of associated comorbidities in patients with elevated BMI, we hypothesise that the association between decreased BMI and abnormal cognitive screening is part of a frailty phenotype (i.e. decreased functional capacity, abnormal cognition, comorbidities, and malnutrition) in some aged surgical patients.29 There is evidence that the relationship between BMI and cognition may vary depending on racial or ethnic background, but we did not have adequate representation in our data to test this hypothesis.25
The relationship between decreased cardiorespiratory fitness and abnormal cognition has been described, but has not been well characterised in the perioperative population. In a previous investigation, low VO2 max strongly correlated with accelerated cognitive decline in a cohort of older adults.30 Although functional capacity was subjectively assessed in this study, there was a strong relationship between decreased functional capacity and abnormal cognitive screening in our patient cohort. Patients with limited ability to carry out activities of daily living, as assessed by the Barthel index, were also much more likely to have an abnormal cognitive screen. Although race was noted to be strongly associated with an abnormal preoperative cognitive screen, the interpretation of this finding is difficult given the absence of reliable education and socioeconomic variables within our dataset. Notably, differential performance between racial groups has been described with a number of other cognitive assessment tools, although we are unaware of any studies specifically evaluating the relationship between race and SBT or AD8 results.31,32
We observed limited correlation between the SBT and AD8 results. We hypothesise several reasons for this discrepancy. Most importantly, the SBT and AD8 evaluate different domains of cognition. Whereas the SBT is an objective test focused on memory, orientation, and concentration, the AD8 is designed to identify subjective changes in function secondary to cognitive decline. As more complex dementia evaluation typically combines both objective measures of abnormal cognition and evaluation of functional deficits, our clinic elected to utilise a similar paradigm when building our cognitive screening process.33 In addition, the SBT and AD8 both have a high sensitivity for dementia (>85% in most populations), but are more limited in their specificity.11,12,34 The limited specificity of both tests likely results in an overestimation of the true rate of clinical dementia or cognitive impairment and is likely responsible for some of the discordance seen in our results. Similarly, these tests likely have a lower sensitivity for mild cognitive impairment, although data for the SBT and AD8 are limited. There are scarce data detailing the sensitivity and specificity of any existing cognitive screening test for dementia specifically in the perioperative patient population.
There is emerging evidence that the addition of preoperative cognitive screening to patient assessment may enhance postoperative outcome prediction.29,35 However, it remains unclear if the presence of abnormal cognition is simply an additional domain marking progressive vulnerability to those adverse outcomes experienced by frail patients or if cognitive deficits specifically predispose patients to certain complications. It is also possible that abnormal cognition may be a marker for the severity of another disease process that may contribute independently to postoperative adverse events. For example, patients with severe cerebrovascular disease that increases the risk of adverse perioperative events may also have abnormal cognition as a result of associated vascular dementia. The above considerations represent important concepts for future investigations in aged surgical populations and could facilitate targeted interventions to reduce perioperative risks.
As a secondary goal, we attempted to identify a group of patients at low risk for abnormal cognitive screening in whom it may be appropriate to defer screening to improve clinic efficiency. Although we did identify a subset of patients without any of the variables associated with abnormal cognitive screening who were at lower risk relative to the entire patient cohort, 11% of this population still had an abnormal cognitive screen. In light of this, we do not believe that filtering patients by age, comorbidity, and function offers a meaningful logistical advantage over the strategy of screening all patients of advanced age.
This study has several strengths. The large sample size may be generalised to the surgical patient populations at other large academic institutions. The nurse-driven model for preoperative cognitive screening may allow for easy integration into existing preoperative workflows. In addition, the SBT and AD8 evaluations are both brief and sensitive tests for preoperative cognitive dysfunction that can be easily administered without significant disruption to preoperative clinic patient throughput.
This study also has notable limitations. It utilised a scoring cut-off for the SBT that favours a high sensitivity for detection of cognitive dysfunction. This could result in a modest overestimation of the actual incidence of abnormal cognition compared with a more comprehensive cognitive evaluation. The included patients were selected in several ways: surgery at an academic medical centre may reflect greater burden of comorbidity, patients attending a preoperative clinic may carry greater comorbidity levels, and not all patients had complete screening performed. The missing outcome data in this study were related primarily to incomplete training of all clinic nursing staff. This was attributable to both temporary staff rotating from other hospital units and new staff who had not yet undergone training in test administration. Our analysis suggests that the screened and unscreened groups were fairly similar, but this does not eliminate the possibility that there may have been selection bias in screening that results in an underestimation or overestimation of the true incidence of abnormal cognitive screening in our population. Finally, the SBT and AD8 do not assess all domains of cognition, which may allow some phenotypes of cognitive dysfunction to go undetected.
In conclusion, this large retrospective cohort study shows that abnormal cognitive screening is quite common in older surgical patients. The presence of abnormal preoperative cognitive screening is strongly associated with a number of common comorbidities and other patient factors. Abnormal cognition may be associated with a greater incidence of adverse outcomes after elective surgery, but further investigation is necessary to better characterise the aetiology of these adverse events and identify potential strategies for risk mitigation.
Authors' contributions
Study design, data analysis, manuscript writing: SG
Study design, data collection, data analysis, manuscript revision: CK
Study design, data analysis, manuscript revision: ABA, TW
Data collection, manuscript revision: AK
Acknowledgements
The authors acknowledge the nursing staff at the Barnes-Jewish Hospital Center for Preoperative Assessment and Planning.
Handling editor: Hugh C Hemmings Jr
Footnotes
Preliminary results of this study were submitted as an abstract to the Society for Perioperative Assessment and Quality Improvement Perioperative Medicine Summit Meeting (Orlando, FL, USA; March 12, 2020).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.08.026.
Declarations of interest
The authors declare that they have no conflicts of interest.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Culley D.J., Flaherty D., Fahey M.C. Poor performance on a preoperative cognitive screening test predicts postoperative complications in older orthopedic surgical patients. Anesthesiology. 2017;127:765–774. doi: 10.1097/ALN.0000000000001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evered L.A., Silbert B.S., Scott D.A., Maruff P., Ames D., Choong P.F. Preexisting cognitive impairment and mild cognitive impairment in subjects presenting for total hip joint replacement. Anesthesiology. 2011;114:1297–1304. doi: 10.1097/ALN.0b013e31821b1aab. [DOI] [PubMed] [Google Scholar]
- 3.Fritz B.A., King C.R., Ben Abdallah A. Preoperative cognitive abnormality, intraoperative electroencephalogram suppression, and postoperative delirium: a mediation analysis. Anesthesiology. 2020;132:1458–1468. doi: 10.1097/ALN.0000000000003181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Reilly-Shah V.N., Hemani S., Davari P. A preoperative cognitive screening test predicts increased length of stay in a frail population: a retrospective case-control study. Anesth Analg. 2019;129:1283–1290. doi: 10.1213/ANE.0000000000004103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langa K.M., Larson E.B., Crimmins E.M. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177:51–58. doi: 10.1001/jamainternmed.2016.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen R.C. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364:2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 7.Petersen R.C., Roberts R.O., Knopman D.S. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75:889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galvin J.E., Roe C.M., Powlishta K.K. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65:559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 9.Katzman R., Brown T., Fuld P., Peck A., Schechter R., Schimmel H. Validation of a short orientation–memory–concentration test of cognitive impairment. Am J Psychiatr. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 10.Chow W.B., Rosenthal R.A., Merkow R.P. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American college of surgeons national surgical quality improvement Program and the American geriatrics society. J Am Coll Surg. 2012;215:453–466. doi: 10.1016/j.jamcollsurg.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Barbic D., Kim B., Salehmohamed Q., Kemplin K., Carpenter C.R., Barbic S.P. Diagnostic accuracy of the Ottawa 3DY and Short Blessed Test to detect cognitive dysfunction in geriatric patients presenting to the emergency department. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-019652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carpenter C.R., Bassett E.R., Fischer G.M., Shirshekan J., Galvin J.E., Morris J.C. Four sensitive screening tools to detect cognitive dysfunction in geriatric emergency department patients: brief Alzheimer's Screen, Short Blessed Test, Ottawa 3DY, and the caregiver-completed AD8. Acad Emerg Med. 2011;18:374–384. doi: 10.1111/j.1553-2712.2011.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H.H., Sun F.J., Yeh T.L. The diagnostic accuracy of the Ascertain Dementia 8 questionnaire for detecting cognitive impairment in primary care in the community, clinics and hospitals: a systematic review and meta-analysis. Fam Pract. 2018;35:239–246. doi: 10.1093/fampra/cmx098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Elm E., Altman D.G., Egger M. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 15.Galvin J.E., Roe C.M., Coats M.A., Morris J.C. Patient's rating of cognitive ability: using the AD8, a brief informant interview, as a self-rating tool to detect dementia. Arch Neurol. 2007;64:725–730. doi: 10.1001/archneur.64.5.725. [DOI] [PubMed] [Google Scholar]
- 16.Heinze G., Schemper M. A solution to the problem of separation in logistic regression. Stat Med. 2002;21:2409–2419. doi: 10.1002/sim.1047. [DOI] [PubMed] [Google Scholar]
- 17.Chipman H.A., George E.I., McCulloch R.E. BART: Bayesian additive regression trees. Ann Appl Stat. 2010;4:266–298. [Google Scholar]
- 18.Amini S., Crowley S., Hizel L. Feasibility and rationale for incorporating frailty and cognitive screening protocols in a preoperative anesthesia clinic. Anesth Analg. 2019;129:830–838. doi: 10.1213/ANE.0000000000004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaulton T.G., Eckenhoff R.G., Neuman M.D. Prevalence and multivariable factors associated with preoperative cognitive impairment in outpatient surgery in the United States. Anesth Analg. 2019;129:e5–e7. doi: 10.1213/ANE.0000000000004035. [DOI] [PubMed] [Google Scholar]
- 20.Sherman J.B., Chatterjee A., Urman R.D. Implementation of routine cognitive screening in the preoperative assessment clinic. A A Pract. 2019;12:125–127. doi: 10.1213/XAA.0000000000000891. [DOI] [PubMed] [Google Scholar]
- 21.Nasreddine Z.S., Phillips N.A., Bedirian V. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 22.Hogue C.W., Jr., Hershey T., Dixon D. Preexisting cognitive impairment in women before cardiac surgery and its relationship with C-reactive protein concentrations. Anesth Analg. 2006;102:1602–1608. doi: 10.1213/01.ANE.0000219591.10826.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plassman B.L., Langa K.M., Fisher G.G. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arvanitakis Z., Capuano A.W., Bennett D.A., Barnes L.L. Body mass index and decline in cognitive function in older black and white persons. J Gerontol A Biol Sci Med Sci. 2018;73:198–203. doi: 10.1093/gerona/glx152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryant A.N., Ford K.L., Kim G. Racial/ethnic variations in the relation between body mass index and cognitive function among older adults. Am J Geriatr Psychiatr. 2014;22:653–660. doi: 10.1016/j.jagp.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Cova I., Clerici F., Maggiore L. Body mass index predicts progression of mild cognitive impairment to dementia. Dement Geriatr Cogn Disord. 2016;41:172–180. doi: 10.1159/000444216. [DOI] [PubMed] [Google Scholar]
- 27.Kim S., Kim Y., Park S.M. Body mass index and decline of cognitive function. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luchsinger J.A., Biggs M.L., Kizer J.R. Adiposity and cognitive decline in the cardiovascular health study. Neuroepidemiology. 2013;40:274–281. doi: 10.1159/000345136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S.W., Han H.S., Jung H.W. Multidimensional frailty score for the prediction of postoperative mortality risk. JAMA Surg. 2014;149:633–640. doi: 10.1001/jamasurg.2014.241. [DOI] [PubMed] [Google Scholar]
- 30.Wendell C.R., Gunstad J., Waldstein S.R., Wright J.G., Ferrucci L., Zonderman A.B. Cardiorespiratory fitness and accelerated cognitive decline with aging. J Gerontol A Biol Sci Med Sci. 2014;69:455–462. doi: 10.1093/gerona/glt144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aiken Morgan A.T., Marsiske M., Dzierzewski J.M. Race-related cognitive test bias in the active study: a mimic model approach. Exp Aging Res. 2010;36:426–452. doi: 10.1080/0361073X.2010.507427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan A.A., Marsiske M., Whitfield K.E. Characterizing and explaining differences in cognitive test performance between African American and European American older adults. Exp Aging Res. 2008;34:80–100. doi: 10.1080/03610730701776427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes C.P., Berg L., Danziger W.L., Coben L.A., Martin R.L. A new clinical scale for the staging of dementia. Br J Psychiatr. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 34.Hendry K., Green C., McShane R. AD-8 for detection of dementia across a variety of healthcare settings. Cochrane Database Syst Rev. 2019;3:CD011121. doi: 10.1002/14651858.CD011121.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makhani S.S., Kim F.Y., Liu Y. Cognitive impairment and overall survival in frail surgical patients. J Am Coll Surg. 2017;225:590–600. doi: 10.1016/j.jamcollsurg.2017.07.1066. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.