Abstract
Background
Increased intravascular volume has been associated with protection from acute kidney injury (AKI), but in patients with congestive heart failure, venous congestion is associated with increased AKI. We tested the hypothesis that intraoperative venous congestion is associated with AKI after cardiac surgery.
Methods
In patients enrolled in the Statin AKI Cardiac Surgery trial, venous congestion was quantified as the area under the curve (AUC) of central venous pressure (CVP) >12, 16, or 20 mm Hg during surgery (mm Hg min). AKI was defined using Kidney Disease Improving Global Outcomes (KDIGO) criteria and urine concentrations of tissue inhibitor of metalloproteinase-2 and insulin-like growth factor binding protein 7 ([TIMP-2]⋅[IGFBP7]), a marker of renal stress. We measured associations between venous congestion, AKI and [TIMP-2]⋅[IGFBP7], adjusted for potential confounders. Values are reported as median (25th–75th percentile).
Results
Based on KDIGO criteria, 104 of 425 (24.5%) patients developed AKI. The venous congestion AUCs were 273 mm Hg min (81–567) for CVP >12 mm Hg, 66 mm Hg min (12–221) for CVP >16 mm Hg, and 11 mm Hg min (1–54) for CVP >20 mm Hg. A 60 mm Hg min increase above the median venous congestion AUC above each threshold was independently associated with increased AKI (odds ratio=1.06; 95% confidence interval [CI], 1.02–1.10; P=0.008; odds ratio=1.12; 95% CI, 1.02–1.23; P=0.013; and odds ratio=1.30; 95% CI, 1.06–1.59; P=0.012 for CVP>12, >16, and >20 mm Hg, respectively). Venous congestion before cardiopulmonary bypass was also associated with increased [TIMP-2]⋅[IGFBP7] measured during cardiopulmonary bypass and after surgery, but neither venous congestion after cardiopulmonary bypass nor venous congestion throughout surgery was associated with postoperative [TIMP-2]⋅[IGFBP7].
Conclusion
Intraoperative venous congestion was independently associated with increased AKI after cardiac surgery.
Keywords: acute kidney injury, cardiac surgery, central venous pressure, urine biomarkers, venous congestion
Editor's key points.
-
•
Venous congestion is associated with higher rates of acute kidney injury in nonsurgical patients with heart failure.
-
•
The authors used pre-existing trial data to test the hypothesis that intraoperative venous congestion was associated with acute kidney injury (using KDIGO criteria) after cardiac surgery.
-
•
Intraoperative venous congestion was independently associated with increased postoperative AKI.
Acute kidney injury (AKI) affects 25% of patients undergoing cardiac surgery and is associated with increased duration of mechanical ventilation, wound infection, length of hospitalisation, and mortality.1,2 The limited understanding of the mechanisms underlying perioperative AKI has led to few successful interventions to prevent and treat it.3,4
Increased central venous pressure is a known risk factor for worsening renal function in non-surgical patients with cardiovascular disease and heart failure.5,6 During surgery, central venous pressure may be impacted by venous blood volume, vascular tone, cardiac output, right ventricular compliance, and intrathoracic pressure,7 but it is unclear if high intraoperative central venous pressure – venous congestion – impacts AKI. In preclinical studies and non-surgical patient populations, venous congestion increases inflammation, oxidative stress, endothelial activation, and sympathetic activation, mechanisms which have each been implicated in development of postoperative AKI.8,9
Intraoperative contributors to venous congestion may include positive pressure ventilation, administration of intravenous fluids, blood product transfusion, oxidative stress, and the use of cardiopulmonary bypass (CPB).10, 11, 12 Increased central venous pressure increases renal venous pressure,13 and increased renal venous pressure decreases renal perfusion and glomerular filtration rate in experimental models and small patient cohorts.14,15 There is a paucity of data regarding the contribution of intraoperative venous congestion to postoperative renal events, and during surgery there are frequent and dynamic changes in the factors that affect venous congestion.16, 17, 18 We tested the hypothesis that increased intraoperative venous congestion is associated with development of postoperative AKI.
Methods
Study design
This was an observational cohort study performed using prospectively collected clinical data and urine specimens from patients undergoing cardiac surgery with CPB enrolled from 2009 to 2014 in the Statin AKI Cardiac Surgery RCT (NCT00791648), a published randomised trial that tested the efficacy of perioperative atorvastatin to reduce postoperative AKI.19 The study was approved by the Vanderbilt University Institutional Review Board, was conducted in accordance with the Declaration of Helsinki, and all patients provided written informed consent for study.
Patients received protocolised anaesthetic, surgical, CPB, and critical care management as previously described. Protocolised mechanical ventilation dictated 6–8 ml kg−1 ideal body weight tidal volumes and 5 cm H2O positive end expiratory pressure.
Inclusion criteria
Patients who underwent elective cardiac surgery (coronary artery bypass grafting, heart valve surgery, or surgery on the ascending aorta) with CPB were eligible for analysis.
Exclusion criteria
Exclusion criteria as defined by the original trial were statin intolerance, acute coronary syndrome, liver dysfunction or use of cytochrome P450 3A4 inhibitors, use of cyclosporine, current renal replacement therapy, history of kidney transplantation, emergency surgery, and pregnancy.
Central venous pressure measurement and venous congestion quantification
Central venous pressure was continuously transduced from the right atrial port of a pulmonary artery catheter placed immediately after induction of anaesthesia, and the value was recorded and stored by automated software every minute during surgery. The recorded value is the average value over 1 min and is comparable with manually measured central venous pressure at end expiration.20 The central venous pressure transducer was placed in a fixed holder on the right side of the surgical table at the level of the right atrium and set to 0 mm Hg while exposed to atmospheric pressure before transducing the patient central venous pressure. Venous congestion was quantified as the area under the curve (AUC) of central venous pressure values above 12, 16, and 20 mm Hg and reported in mm Hg min. We chose multiple central venous pressure thresholds to define venous congestion because the threshold at which elevated central venous pressure causes congestion is unclear. The central venous pressure in a spontaneously breathing human is typically 0 to 10 mm Hg and may decrease below 0 mm Hg.21 During cardiac surgery, positive pressure ventilation increases intrathoracic pressure and central venous pressure.22 We chose central venous pressure thresholds of 12, 16, and 20 mm Hg to define venous congestion due to the effects of positive pressure ventilation, because these values are often encountered during surgery, because previous studies also examined central venous pressure values in this range, and to determine if higher thresholds for defining venous congestion are more or less detrimental than lower thresholds. Intraoperative venous congestion was considered to have occurred only in the periods before or after CPB because during CPB an applied vacuum on the venous drainage cannula creates a negative central venous pressure.
Data collection
We studied all patients who completed the Statin AKI Cardiac Surgery RCT and had CPB during surgery (n=435). Technical issues in vital sign data transfer from intraoperative monitors to perioperative data warehouse computer servers eliminated data in 10 patients. For [TIMP-2]⋅[IGFBP7] analyses, we studied all patients in whom we collected urine (n=267). Patient characteristics including age, sex and race, and preoperative and intraoperative data were recorded. Serum creatinine was measured daily after surgery until hospital discharge. We defined baseline creatinine as the preoperative value closest to the time of surgery. This measurement was obtained during the preoperative anaesthesiology appointment for outpatients or the day before surgery for inpatients. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.23
Primary outcome
The primary outcome was AKI quantified using Kidney Disease Improving Global Outcomes (KDIGO) consensus criteria, which define stage I AKI as a 26 μmol l−1 increase in 48 h or 50% increase within 7 days, stage II as a 100% increase within 7 days, and stage III as a 200% increase, a value greater than 354 μmol l−1 with a 26 μmol l−1 increase, or initiation of dialysis within 7 days of surgery.24 We did not use urine output criteria because of potential confounding by intravascular hypovolaemia and diuretic use,25 both common among cardiac surgery patients.
Secondary outcome
We measured renal stress throughout the perioperative period using urinary concentrations of tissue inhibitor of metalloproteinases 2 and insulin-like growth factor binding protein 7 ([TIMP-2]⋅[IGFBP7]). These proteins are produced in renal tubular cells in response to stress and induce cell-cycle arrest.26,27 [TIMP-2]⋅[IGFBP7] is an early and sensitive predictor of AKI.28,29
Urine was collected at baseline, 30 min after initiation of CPB, immediately after CPB, at ICU unit admission, 6 h after intensive care unit admission, and on the morning of postoperative day 1 to measure [TIMP-2]⋅[IGFBP7]. Samples were immediately placed on ice, centrifuged at 1000 g for 15 min, and the supernatant frozen at –80°C until thawed for measurement by blinded laboratory personnel at Astute Medical (San Diego, CA, USA). We previously reported the relationships between [TIMP-2]⋅[IGFBP7] and KDIGO criteria AKI among these subjects.28
Statistical analysis
The association between intraoperative venous congestion and the development of postoperative AKI was estimated using logistic regression. Analyses were adjusted for potential confounders of the relationship between venous congestion and AKI and for risk factors of AKI and included age, baseline eGFR, Cleveland Clinic risk for acute renal failure score,30 history of atrial fibrillation (yes/no), history of congestive heart failure (yes/no), perioperative atorvastatin treatment (yes/no), duration of CPB, total intraoperative crystalloid volume administered, and duration of surgery. The AUC of venous congestion for each threshold was fourth root transformed to reduce the effect of any outliers. The effect of intraoperative venous congestion above each pre-specified central venous pressure threshold on the incidence of postoperative AKI was summarised using an odds ratio comparing an increase of 60 mm Hg min above the median venous congestion AUC to the median venous congestion AUC. This comparison was made because venous congestion AUC has a non-linear effect on the odds of postoperative AKI.
During peer review, we also performed a proportional odds logistic regression analysis to examine the association between the AUC of central venous pressure above each threshold throughout surgery and increasing severity of AKI. This ordinal outcome included the following levels: no AKI, stage I AKI, stage II AKI, stage III AKI, use of dialysis, and death from all causes. If two or more of these outcomes was observed, then the most severe outcome prevailed. Statistical significance was tested using the associated Wald test. Binary characteristics are presented as n (%) and continuous characteristics as median (25th, 75th percentile).
The effects of intraoperative venous congestion on log-transformed urine [TIMP-2]⋅[IGFBP7] were quantified using linear regression, adjusting for the same set of factors listed previously. For assessments between pre-CPB venous congestion and [TIMP-2]⋅[IGFBP7] measured during and immediately after CPB, we did not include intraoperative crystalloid volume or duration of surgery variables since these variables occurred after CPB. The effect of intraoperative venous congestion above each pre-specified central venous pressure threshold on mean urine [TIMP-2]⋅[IGFBP7] was summarised using an estimate of the fold change and 95% confidence interval (CI) and was tested for statistical significance using the associated Wald test.
Graphical diagnostics were examined for all regression analyses. P-values less than 0.05 were considered statistically significant. All analyses were performed using R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
Sample size estimation
Assuming a 25% incidence of AKI in the cohort and a type I error rate of 5%, we would have 84% power to detect a 20±60 mm Hg min difference in the AUC of central venous pressure above a given threshold between patients who did and who did not develop AKI.
Results
Participant characteristics
A total of 425 patients were included in the study cohort (Table 1). The median central venous pressure at the time of central venous catheter placement was 13 (10, 17) mm Hg.
Table 1.
Subject characteristics. Binary characteristics are reported as n (%) and continuous characteristics as median (25th percentile, 75th percentile). CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; COPD, chronic obstructive pulmonary disease; ACEi, ace-converting enzyme inhibitor; CPB, cardiopulmonary bypass.
| Characteristic | Total (n=425) |
|---|---|
| Age (yr) | 68 (50, 76) |
| Age range (yr) | 23–92 |
| Female sex | 148 (34.8%) |
| Height (cm) | 173 (164, 180) |
| Weight (kg) | 82.0 (70.3, 96.2) |
| Body mass index (kg m−2) | 28 (24, 31) |
| African American ancestry | 12 (2.8%) |
| Medical history | |
| Estimated glomerular filtration rate, ml min−1 1.73 m−2 | 72.1 (51.1, 86.4) |
| CKD stage 1 (eGFR, >90 ml min−1 1.73 m−2) | 80 (18.8%) |
| CKD stage 2 (eGFR, 60–90 ml min−1 1.73 m−2) | 197 (46.4%) |
| CKD stage 3 (eGFR, 30–60 ml min−1 1.73 m−2) | 131 (30.8%) |
| CKD stage 4 (eGFR, 15–30 ml min−1 1.73 m−2) | 17 (4.0%) |
| CKD stage 5 (eGFR, <15 ml min−1 1.73 m−2 or dialysis) | 0 (0.0%) |
| Cleveland Clinic renal failure score | 1 (1, 3) |
| Congestive heart failure | 196 (46.1%) |
| Left ventricular ejection fraction (%) | 60 (50, 60) |
| Atrial fibrillation | 124 (29.2%) |
| Hypertension | 360 (84.7%) |
| COPD | 50 (11.8%) |
| Obstructive sleep apnoea | 64 (15.1%) |
| Charlson comorbidity index | 2 (1, 4) |
| Diabetes mellitus | 121 (28.5%) |
| Medication | |
| Preoperative ACEi use | 113 (26.6%) |
| Perioperative statin treatment | 211 (49.6%) |
| Baseline laboratory and haemodynamic data | |
| Heart rate (beats min−1) | 72 (63, 83) |
| Mean arterial pressure (mm Hg) | 89 (79, 97) |
| Central venous pressure (mm Hg) | 13 (10, 17) |
| Cardiac index (L min−1 m−1) | 2.2 (1.8, 2.6) |
| Haematocrit (%) | 36 (33, 40) |
| Arterial pH | 7.40 (7.36, 7.43) |
| PaCO2 (mm Hg) | 41 (37, 45) |
| Arterial lactate (mg dl−1) | 0.7 (0.6, 1.0) |
| Procedure characteristics | |
| Duration of surgery (min) | 336 (271, 416) |
| Coronary artery bypass surgery | 120 (28.2%) |
| Aortic valve replacement | 231 (54.4%) |
| Mitral valve replacement | 166 (39.1%) |
| Tricuspid valve replacement | 40 (9.5%) |
| Ascending aorta surgery | 52 (12.2%) |
| Aortic cross clamp used | 284 (66.8%) |
| CPB time (min) | 138 (105, 186) |
| Intraoperative mean arterial pressure (mm Hg) | 70 (62, 78) |
| Norepinephrine use | 337 (79.3%) |
| Norepinephrine dose (μg min−1) | 5 (3, 8) |
| Vasopressin use | 50 (11.7%) |
| Vasopressin dose (units min−1) | 0.04 (0.04, 0.04) |
A total of 104 (24.5%) patients developed AKI. Eighty-four patients developed KDIGO stage 1 AKI, 20 developed KDIGO stage 2 or stage 3 AKI, and six patients required postoperative dialysis. Patients who developed AKI were more likely to develop intensive care unit delirium (36.5% vs 24.0%), atrial fibrillation (45.2% vs. 38.3%), and had a longer intensive care unit (5 vs. 3 days) and hospital (9 vs. 7 days) stay than patients who did not develop AKI. Four patients in the cohort died during their hospitalization.
The median (25th, 75th percentile) urine [TIMP-2]⋅[IGFBP7] was 0.12 (ng/ml)2/1000 (0.04, 0.31) prior to surgery, 0.16 (0.02, 0.80) 30 minutes after initiation of CPB, 0.21 (0.08, 0.51) after cessation of CPB, 0.10 (0.04, 0.24) at the time of intensive care unit admission, 0.23 (0.10, 0.47) six hours postoperatively, and 0.19 (0.04, 0.46) on the first postoperative day. Increased perioperative urinary concentrations of [TIMP-2]⋅[IGFBP7] were independently associated with increased moderate and severe AKI in this cohort as previously reported.28
Primary outcome: intraoperative venous congestion and postoperative AKI
The median (25th, 75th percentile) AUC of venous congestion was 273 (81, 567), 66 (12, 221) and 11 (1, 54) mm Hg min for the 12, 16, and 20 mm Hg central venous pressure thresholds, respectively. For clinical context, a central venous pressure of 15 mmHg for 90 minutes is equal to an AUC of 270 mm Hg Min for the 12 mmHg venous congestion threshold. The median (25th, 75th percentile) AUC of venous congestion that occurred prior to CPB was 99 (23, 314), 17 (2, 103), and 2 (0, 16) mm Hg min for the 12, 16, and 20 mmHg central venous pressure thresholds, respectively, and the median AUC of venous congestion that occurred following CPB was 100 (20, 282), 15 (0, 88), and 1 (0, 16).
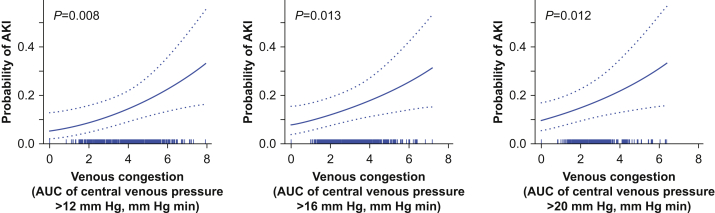
Increased venous congestion was associated with increased odds of AKI for each central venous pressure threshold. A 60 mm Hg min increase above the median AUC of intraoperative venous congestion above 12 mm Hg was independently associated with a 6% increase in the odds of AKI (odds ratio=1.06; 95% CI, 1.02–1.10; P=0.008; Table 2; Fig. 1). A 60 mm Hg min increase above the median AUC of intraoperative venous congestion above 16 mm Hg had a 12% increase in the odds of AKI (odds ratio=1.12; 95% CI, 1.02–1.23; P=0.013). Patients with a 60 mm Hg min increase above the median AUC of intraoperative venous congestion above 20 mm Hg had an 30% increase in the odds of AKI (odds ratio=1.30; 95% CI, 1.06–1.59; P=0.012).
Table 2.
Independent associations between increased intraoperative venous congestion, quantified as the area under the curve of central venous pressure above 12, 16, and 20 mm Hg, and acute kidney injury. Odds ratios represent the independent association between a 60 mm Hg min increase in the area under the curve above and the corresponding central venous pressure threshold median value. CI, confidence interval.
| Central venous pressure threshold (mm Hg) | Acute kidney injury Odds ratio (95% CI) |
P-value | |
|---|---|---|---|
| Venous congestion throughout surgery | 12 | 1.06 (1.02–1.10) | 0.008 |
| 16 | 1.12 (1.02–1.23) | 0.013 | |
| 20 | 1.30 (1.06–1.59) | 0.012 | |
| Pre-cardiopulmonary bypass venous congestion | 12 | 1.11 (1.03–1.20) | 0.004 |
| 16 | 1.25 (1.06–1.47) | 0.009 | |
| 20 | 1.44 (1.06–1.96) | 0.018 | |
| Post-cardiopulmonary bypass venous congestion | 12 | 1.07 (0.996–1.14) | 0.065 |
| 16 | 1.18 (1.00–1.38) | 0.046 | |
| 20 | 1.45 (1.02–2.09) | 0.041 |
Fig 1.
Independent associations between intraoperative venous congestion and postoperative acute kidney injury (AKI). The probability of acute kidney injury was increased for venous congestion above central venous pressure thresholds of 12, 16, or 20 mm Hg, adjusted for potential confounders. The rug plot above the x-axis indicates the observed venous congestion values for each patient in the cohort, fourth-root transformed to achieve normality. AUC, area under the curve.
In the proportional odds logistic regression analysis of the ordinal endpoint, a 60 mm Hg min increase above the median of the AUC of central venous pressure above each threshold was associated with a 6% (odds ratio=1.06; 95% CI, 1.02–1.10; P=0.006), 13% (odds ratio=1.13; 95% CI, 1.03–1.23; P=0.009), and 32% (odds ratio=1.32; 95% CI, 1.08–1.60; P=0.006) increase in the odds of each level of AKI severity compared with the less severe level for the 12, 16, and 20 mm Hg thresholds, respectively.
To determine if intraoperative venous congestion occurring in the period before CPB or the period after CPB was more strongly associated with AKI, we examined the AUC of congestion in these periods and their association with AKI. A 60 mm Hg min increase above the median AUC of venous congestion above 12 mm Hg before CPB had an 11% increase in the odds of postoperative AKI (odds ratio=1.11; 95% CI, 1.03–1.20; P=0.004) compared with those at the median. A 60 mm Hg min increase above the median AUC of pre-CPB venous congestion above 16 mm Hg had a 25% increase in the odds of postoperative AKI (odds ratio=1.25; 95% CI, 1.06–1.47; P=0.009), and a 60 mm Hg min increase above the median AUC of pre-CPB venous congestion above 20 mm Hg had a 44% increase in the odds of AKI (odds ratio=1.44; 95% CI, 1.06–1.96; P=0.018), compared with those at the median.
After CPB, however, the association between venous congestion and AKI was less clear. A 60 mm Hg min increase above the median of post-CPB venous congestion above 12 mm Hg had a 7% increase in the odds AKI that was not statistically significant (odds ratio=1.07; 95% CI, 0.99–1.14; P=0.065) compared with those at the median. A 60 mm Hg min increase above the median of post-CPB venous congestion above 16 mm Hg had a 18% increase in the odds of postoperative compared with the median (odds ratio=1.18; 95% CI, 1.00–1.38; P=0.046), and a 60 mm Hg min increase above the median of post-CPB venous congestion above 20 mm Hg had a 46% increase in the odds of AKI (odds ratio=1.46; 95% CI, 1.02–2.08; P=0.041) compared with those at the median.
Secondary outcome: intraoperative venous congestion and perioperative urinary concentrations of [TIMP-2]•[IGFBP7]
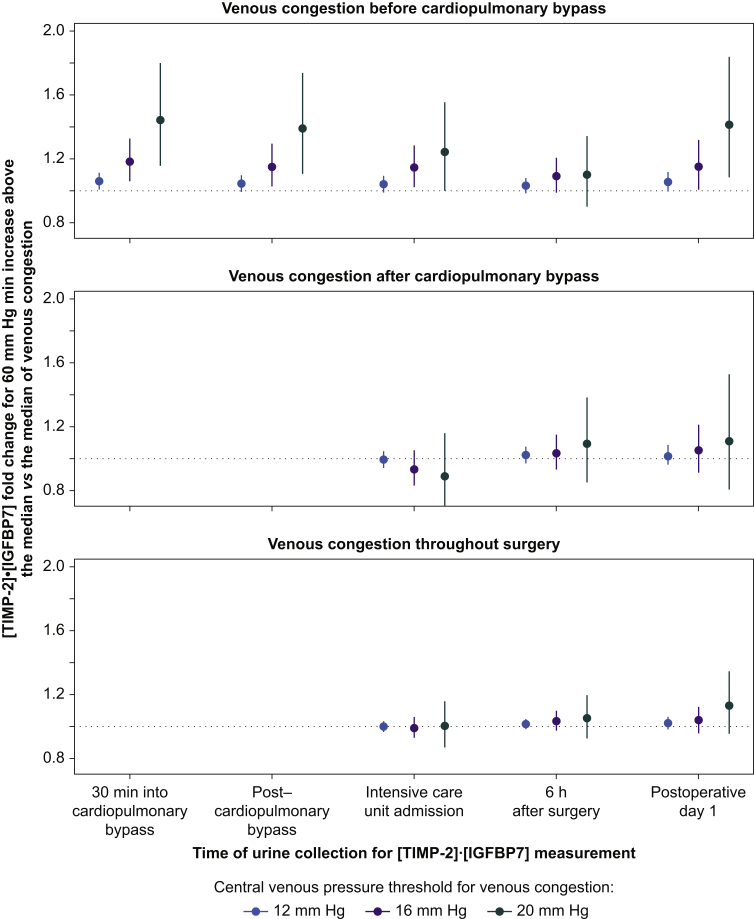
A 60 mm Hg min increase above the median AUC of venous congestion before CPB was independently associated with higher urine [TIMP-2]•[IGFBP7] concentration measured 30 min into CPB, compared with median values for the 12, 16, and 20 mm Hg thresholds (1.06-fold [95% CI, 1.01–1.11; P=0.014], 1.18-fold [95% CI, 1.06–1.32; P=0.004], and 1.44-fold [95% CI, 1.15–1.80; P=0.001], respectively) (Fig. 2). A 60 mm Hg min increase above the median AUC of venous congestion before CPB was associated with 1.04-fold (95% CI, 0.99–1.09; P=0.065), 1.15-fold (95% CI, 1.03–1.29; P=0.012), and 1.39-fold (95% CI, 1.11–1.73; P=0.004) greater urine [TIMP-2]•[IGFBP7] concentrations at the end of CPB, compared with median values for the 12, 16, and 20 mm Hg thresholds, respectively. Venous congestion after CPB was not associated with postoperative urinary concentrations of [TIMP-2]⋅[IGFBP7]. Similarly, venous congestion throughout surgery (the combination of venous congestion before and after CPB) was not associated with postoperative urinary concentrations of [TIMP-2]•[IGFBP7].
Fig 2.
Independent associations between intraoperative venous congestion before cardiopulmonary bypass (CPB; top panel), after CPB (middle panel), and before and after CPB (bottom panel) and urinary concentrations of [TIMP-2]⋅[IGFBP7]. Dots indicate the fold increase in log transformed urine [TIMP-2]⋅[IGFBP7] for a value 60 mm Hg min greater than the median of the area under the curve of venous congestion above each indicated central venous pressure threshold, compared with the median, and error bars indicate the 95% confidence interval. An association is statistically significant if the error bar does not cross the dotted line. [TIMP-2]⋅[IGFBP7], tissue inhibitor of metalloproteinase-2 and insulin-like growth factor binding protein 7.
Discussion
Venous congestion during cardiac surgery was independently associated with higher odds of AKI and increased severity of AKI after surgery. Venous congestion between the start of surgery and initiation of CPB was associated with increased urinary concentrations of [TIMP-2]⋅[IGFBP7] throughout the perioperative period and as early as 30 min later, but venous congestion after CPB was not associated with postoperative urinary concentrations of [TIMP-2]⋅[IGFBP7]. Taken together, these findings support the hypothesis that intraoperative venous congestion, estimated with measurements of central venous pressure, is associated with kidney stress and postoperative kidney injury.
Prior studies have primarily focused on the association between postoperative, as opposed to intraoperative, central venous pressure measurements and AKI, and have not quantified the magnitude of venous congestion over time. Palomba and colleagues,16 for example reported that a central venous pressure greater than 14 mm Hg at ICU admission after cardiac surgery was associated with a 92% increase in the odds of AKI after cardiac surgery, Williams and colleagues17 reported that a central venous pressure greater than 9 mm Hg after surgery was associated with a 30% increase in the rate of renal failure in a cohort of patients undergoing coronary artery bypass grafting, and Yang and colleagues18 reported that patients with a central venous pressure greater than 10 mm Hg after coronary artery bypass grafting had a 6-fold higher rate of AKI than patients with a central venous pressure less than 10 mm Hg. These studies did not examine the intraoperative period, a time when many factors contribute to venous congestion and during which anaesthesiologists may intervene to affect central venous pressure, nor did these studies evaluate different pressure thresholds. It remains possible that an absolute central venous pressure threshold value does not exist to quantify venous congestion and that other factors, such as arterial pressure and inflammation, may alter the critical venous pressure threshold for individual patients and for different intraoperative events. We observed a dose–response relationship between escalating central venous pressure thresholds for venous congestion and AKI. Furthermore, increased AUC of venous congestion above each threshold was consistent with increased severity of AKI in the proportional odds logistic regression. These findings support the idea that higher intraoperative central venous pressure may reflect more severe venous congestion, and more severe venous congestion may contribute to kidney injury. However, the rarity of severe kidney injury in this sample of patients and the associated uncertainty warrant additional confirmatory research.
Analyses of congestion before CPB and after CPB indicated that venous congestion during each of these periods may increase risk for AKI, although after CPB the association between venous congestion and AKI was less clear, and only venous congestion before CPB was associated with increased urine expression of [TIMP-2]⋅[IGFBP7]. [TIMP-2]⋅[IGFBP7] concentrations as early as 30 min after initiation of CPB increased in proportion to pre-CPB venous congestion, highlighting a potentially rapid effect of intraoperative venous congestion on kidney injury and urinary expression of these markers. This finding may impact consideration of therapies to reduce AKI in the early intraoperative period. Decreased associations between post-CPB venous congestion and urinary [TIMP-2]⋅[IGFBP7] may indicate that venous congestion later during surgery does not increase renal stress or that factors aside from late intraoperative venous congestion have more of an impact on increased postoperative [TIMP-2]⋅[IGFBP7] expression.
Increased central venous pressure is the result of excess intravascular volume, systolic and diastolic heart dysfunction, increased vascular resistance, and increased intrathoracic pressure. The results from this study do not indicate which of the contributing factors may explain the association between increased central venous pressure and AKI, do not reveal the specific pathophysiology linking venous congestion and AKI, nor convey any causal relationship between venous congestion and AKI. There are several plausible explanations for an effect on AKI, however, if one exists. Venous congestion could decrease renal perfusion, decrease transglomerular pressure gradients via increases in tubular lumen pressure, alter tubuloglomerular feedback mechanisms such as the renin–angiotensin–aldosterone system, induce systemic and renal inflammation, increase endothelial permeability, and increase sympathetic outflow. All of these physiologic alterations could impact perioperative AKI.31, 32, 33, 34, 35, 36 More specific targeting of intraoperative venous congestion by the use of inotropes, vasodilators, ventilator and fluid management, and venous bypass systems are warranted to determine if these interventions decrease venous congestion and if they decrease AKI.
This study has several strengths and limitations. The observational nature of this study prevents any statements of causality and renders it subject to unknown confounding. For example decreased arterial pressure, decreased cardiac output, intraoperative renal emboli, and myoglobinuria may increase postoperative AKI. We did, however, adjust analyses for a number of potentially important confounders and risk factors for AKI. This study was conducted in a rigorously protocolised clinical trial cohort which provided high-fidelity intraoperative haemodynamic data, perioperative urine sampling for [TIMP-2]⋅[IGFBP7] quantification, and AKI phenotyping, but results may not be generalisable to other cohorts. We examined several central venous pressure thresholds to define venous congestion because there is not a clear value to indicate congestion in this setting and because we sought to capture a dose effect of venous congestion. We noted an increase in the magnitude of the association between venous congestion AUC and AKI at higher central venous pressure thresholds for quantifying venous congestion. We also note that central venous pressure thresholds even lower than 12 mm Hg may be clinically important. Fortunately, central venous pressure is commonly measured during surgery, and there are many potential therapies that likely could reduce venous congestion.
In summary, increased intraoperative venous congestion was independently associated with the development of postoperative AKI but inconsistently associated with increased urinary expression of [TIMP-2]⋅[IGFBP7]. These findings support the hypothesis that venous congestion during surgery may contribute to postoperative kidney injury and dysfunction, but studies that target intraoperative venous congestion are necessary to further evaluate the relationships between this potential mechanism of kidney injury and to determine if reducing intraoperative venous congestion decreases postoperative kidney injury and associated morbidities.
Authors' contributions
Study conception and design: MGL, YL, FTB
Data analysis: MGL, MSS, JM, FTB
Data interpretation: MGL, MSS, YL, JM, TSA, KRB, MML, ASS, AH, FTB
Data collection: YL, JPW, FTB
Drafting of the manuscript: MGL, MSS, YL, JM
Revisions to the manuscript: JM, JPW, TSA, KRB, MML, ASS, AH
All authors approved the submitted version of the manuscript and agreed to be personally accountable for their own contributions and to ensure that questions related to the accuracy and integrity of the work are resolved and documented.
Acknowledgements
The authors acknowledge Patricia Hendricks from the Department of Anesthesiology at Vanderbilt University Medical Center for nursing support and the Vanderbilt Anesthesiology Perioperative Informatics Research division for automated central venous pressure measurement data capture and archiving.
Handling editor: Gareth Ackland
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
National Institutes of Health (K23GM129662 to MGL; K23GM102676 and R01GM112871 to F. T. Billings; UL1TR000445) and by the Vanderbilt University Medical Center Department of Anesthesiology.
References
- 1.Rosner M.H., Okusa M.D. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1:19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 2.Meersch M., Schmidt C., Zarbock A. Perioperative acute kidney injury: an under-recognized problem. Anesth Analg. 2017;125:1223–1232. doi: 10.1213/ANE.0000000000002369. [DOI] [PubMed] [Google Scholar]
- 3.McIlroy D.R., Lopez M.G., Billings F.T.T. Perioperative clinical trials in AKI. Semin Nephrol. 2020;40:173–187. doi: 10.1016/j.semnephrol.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gumbert S.D., Kork F., Jackson M.L. Perioperative acute kidney injury. Anesthesiology. 2020;132:180–204. doi: 10.1097/ALN.0000000000002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullens W., Abrahams Z., Francis G.S. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damman K., van Deursen V.M., Navis G., Voors A.A., van Veldhuisen D.J., Hillege H.L. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 7.Gelman S. Venous function and central venous pressure: a physiologic story. Anesthesiology. 2008;108:735–748. doi: 10.1097/ALN.0b013e3181672607. [DOI] [PubMed] [Google Scholar]
- 8.Colombo P.C., Rastogi S., Onat D. Activation of endothelial cells in conduit veins of dogs with heart failure and veins of normal dogs after vascular stretch by acute volume loading. J Card Fail. 2009;15:457–463. doi: 10.1016/j.cardfail.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka M., Yoshida H., Furuhashi M. Deterioration of renal function by chronic heart failure is associated with congestion and oxidative stress in the tubulointerstitium. Intern Med. 2011;50:2877–2887. doi: 10.2169/internalmedicine.50.5925. [DOI] [PubMed] [Google Scholar]
- 10.Mitaka C., Nagura T., Saknishi N., Tsunoda Y., Amaha K. Two-dimensional echocardiographic evaluation of inferior vena cava, right ventricle, and left ventricle during positive-pressure ventilation with varying levels of positive end-expiratory pressure. Crit Care Med. 1989;17:205–210. doi: 10.1097/00003246-198903000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Matot I., Paskaleva R., Eid L. Effect of the volume of fluids administered on intraoperative oliguria in laparoscopic bariatric surgery: a randomized controlled trial. Arch Surg. 2012;147:228–234. doi: 10.1001/archsurg.2011.308. [DOI] [PubMed] [Google Scholar]
- 12.Elijaiek R., Cavayas Y., Rodrigue E. High postoperative portal venous flow pulsatility indicates right ventricular dysfunction and predicts complications in cardiac surgery patients. Br J Anaesth. 2018;122:206–214. doi: 10.1016/j.bja.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 13.Chen X., Wang X., Honore P.M., Spapen H.D., Liu D. Renal failure in critically ill patients, beware of applying (central venous) pressure on the kidney. Ann Intensive Care. 2018;8:91. doi: 10.1186/s13613-018-0439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burnett J.C., Jr., Knox F.G. Renal interstitial pressure and sodium excretion during renal vein constriction. Am J Physiol. 1980;238:F279–F282. doi: 10.1152/ajprenal.1980.238.4.F279. [DOI] [PubMed] [Google Scholar]
- 15.Hadimioglu N., Ertug Z., Yegin A., Sanli S., Gurkan A., Demirbas A. Correlation of peripheral venous pressure and central venous pressure in kidney recipients. Transplant Proc. 2006;38:440–442. doi: 10.1016/j.transproceed.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 16.Palomba H., de Castro I., Neto A.L., Lage S., Yu L. Acute kidney injury prediction following elective cardiac surgery: AKICS score. Kidney Int. 2007;72:624–631. doi: 10.1038/sj.ki.5002419. [DOI] [PubMed] [Google Scholar]
- 17.Williams J.B., Peterson E.D., Wojdyla D. Central venous pressure after coronary artery bypass surgery: does it predict postoperative mortality or renal failure? J Crit Care. 2014;29:1006–1010. doi: 10.1016/j.jcrc.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y., Ma J., Zhao L. High central venous pressure is associated with acute kidney injury and mortality in patients underwent cardiopulmonary bypass surgery. J Crit Care. 2018;48:211–215. doi: 10.1016/j.jcrc.2018.08.034. [DOI] [PubMed] [Google Scholar]
- 19.Billings I.V.F.T., Hendricks P.A., Schildcrout J.S. High-dose perioperative atorvastatin and acute kidney injury following cardiac surgery: a randomized clinical trial. JAMA. 2016;315:877–888. doi: 10.1001/jama.2016.0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roger C., Muller L., Riou B. Comparison of different techniques of central venous pressure measurement in mechanically ventilated critically ill patients. Br J Anaesth. 2017;118:223–231. doi: 10.1093/bja/aew386. [DOI] [PubMed] [Google Scholar]
- 21.Friedman E., Grable E., Fine J. Central venous pressure and direct serial measurements as guides in blood-volume replacement. Lancet. 1966;2:609–614. doi: 10.1016/s0140-6736(66)91926-x. [DOI] [PubMed] [Google Scholar]
- 22.Mahmood S.S., Pinsky M.R. Heart-lung interactions during mechanical ventilation: the basics. Ann Transl Med. 2018;6:349. doi: 10.21037/atm.2018.04.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 25.Macedo E., Malhotra R., Claure-Del Granado R., Fedullo P., Mehta R.L. Defining urine output criterion for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2011;26:509–515. doi: 10.1093/ndt/gfq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kashani K., Al-Khafaji A., Ardiles T. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meersch M., Schmidt C., Van Aken H. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PloS One. 2014;9 doi: 10.1371/journal.pone.0093460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cummings J.J., Shaw A.D., Shi J., Lopez M.G., O'Neal J.B., Billings F.T.T. Intraoperative prediction of cardiac surgery-associated acute kidney injury using urinary biomarkers of cell cycle arrest. J Thorac Cardiovasc Surg. 2019;157:1545–1553. doi: 10.1016/j.jtcvs.2018.08.090. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilarczyk K., Edayadiyil-Dudasova M., Wendt D. Urinary [TIMP-2]∗[IGFBP7] for early prediction of acute kidney injury after coronary artery bypass surgery. Ann Intensive Care. 2015;5:50. doi: 10.1186/s13613-015-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thakar C.V., Arrigain S., Worley S., Yared J.P., Paganini E.P. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–168. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 31.Gottschalk C.W., Mylle M. Micropuncture study of pressures in proximal tubules and peritubular capillaries of the rat kidney and their relation to ureteral and renal venous pressures. Am J Physiol. 1956;185:430–439. doi: 10.1152/ajplegacy.1956.185.2.430. [DOI] [PubMed] [Google Scholar]
- 32.Kishimoto T., Maekawa M., Abe Y., Yamamoto K. Intrarenal distribution of blood flow and renin release during renal venous pressure elevation. Kidney Int. 1973;4:259–266. doi: 10.1038/ki.1973.112. [DOI] [PubMed] [Google Scholar]
- 33.Kranzhofer R., Schmidt J., Pfeiffer C.A., Hagl S., Libby P., Kubler W. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1623–1629. doi: 10.1161/01.atv.19.7.1623. [DOI] [PubMed] [Google Scholar]
- 34.Colombo P.C., Doran A.C., Onat D. Venous congestion, endothelial and neurohormonal activation in acute decompensated heart failure: cause or effect? Curr Heart Fail Rep. 2015;12:215–222. doi: 10.1007/s11897-015-0254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kostreva D.R., Seagard J.L., Castaner A., Kampine J.P. Reflex effects of renal afferents on the heart and kidney. Am J Physiol. 1981;241:R286–R292. doi: 10.1152/ajpregu.1981.241.5.R286. [DOI] [PubMed] [Google Scholar]
- 36.Afsar B., Ortiz A., Covic A., Solak Y., Goldsmith D., Kanbay M. Focus on renal congestion in heart failure. Clin Kidney J. 2016;9:39–47. doi: 10.1093/ckj/sfv124. [DOI] [PMC free article] [PubMed] [Google Scholar]