Abstract
Background
The fraction of inspired oxygen (FiO2) administered during general anaesthesia varies widely despite international recommendations to administer FiO2 0.8 to all anaesthetised patients to reduce surgical site infections (SSIs). Anaesthetists remain concerned that high FiO2 administration intraoperatively may increase harm, possibly through increased oxidative damage and inflammation, resulting in more complications and worse outcomes. In previous systematic reviews associations between FiO2 and SSIs have been inconsistent, but none have examined how FiO2 affects perioperative oxidative stress. We aimed to address this uncertainty by reviewing the available literature.
Methods
EMBASE, MEDLINE, and Cochrane databases were searched from inception to March 9, 2020 for RCTs comparing higher with lower perioperative FiO2 and quantifying oxidative stress in adults undergoing noncardiac surgery. Candidate studies were independently screened by two reviewers and references hand-searched. Methodological quality was assessed using the Cochrane Collaboration Risk of Bias tool.
Results
From 19 438 initial results, seven trials (n=422) were included. Four studies reported markers of oxidative stress during Caesarean section (n=328) and three reported oxidative stress during elective colon surgery (n=94). Risk of bias was low (four studies) to moderate (three studies). Pooled results suggested high FiO2 was associated with greater malondialdehyde, protein-carbonyl concentrations and reduced xanthine oxidase concentrations, together with reduced antioxidant markers such as superoxide dismutase and total sulfhydryl levels although total antioxidant status was unchanged.
Conclusions
Higher FiO2 may be associated with elevated oxidative stress during surgery. However, limited studies have specifically reported biomarkers of oxidation. Given the current clinical controversy concerning perioperative oxygen therapy, further research is urgently needed in this area.
Keywords: anaesthesia, hyperoxia, inflammation, oxidative stress, oxygen, perioperative care, surgery
Editor's key points.
-
•
Recommendations to administer 80% oxygen throughout anaesthesia remain controversial.
-
•
Systemic, detrimental effects of hyperoxaemia are often thought to be mediated through increased oxidative stress.
-
•
Despite broad searches, this review could only include seven small single-centre RCTs comparing oxidative stress at higher and lower FiO2 levels.
-
•
High intraoperative FiO2 may be associated with elevated oxidative stress during surgery but further studies are needed to confirm this.
In 2016 the WHO recommended administering a fractional inspired oxygen concentration (FiO2) of 0.8 to all intubated patients undergoing surgery, in order to reduce instances of surgical site infections (SSIs).1,2 In the first revision of these guidelines in 2018, this recommendation remained unaltered but its strength was downgraded from ‘strong’ to ‘conditional’.3 The 2016 recommendation was based on a meta-analysis of 15 RCTs of perioperative oxygen therapy performed by members of the WHO guideline development group,4 and remain controversial amongst the international anaesthetic community.5, 6, 7 Notably, the findings of the single largest trial available when the 2016 recommendation was published (the PeRioperative OXygen Fraction - effect on surgical site Infection and pulmonary complications after abdominal surgery (PROXI) study, n=13788) were deemed biologically implausible by the guideline development group for reasons that remain obscure.2 Post hoc analyses from the PROXI study have suggested that higher intraoperative FiO2 could be associated with higher long-term mortality in patients with cardiac disease, cancer, or both.9,10 A better understanding of the mechanisms underlying such outcome differences is essential to successfully resolve this debate.
Systemic detrimental effects of oxygen are often thought to be mediated through ‘oxidative stress’ – an imbalance between the production of highly reactive by-products of metabolism (reactive oxygen species [ROS]) and endogenous antioxidant defence mechanisms.11, 12, 13 ROS are largely formed during oxidative phosphorylation in the mitochondrial electron transport chain, or within neutrophils/macrophages and non-phagocytic cells.14, 15, 16 Interaction of these chemical species with cellular constituents can irreversibly damage lipids, proteins, and DNA; injuring cells, tissues or end organs and ultimately resulting in cell death through apoptosis or necrosis.14,15 Oxidative stress can be beneficial (e.g. the innate immune system uses this process to attack and destroy invading pathogens) but can also lead to tissue damage and organ failure.17,18
Direct detection of ROS remains a challenge because of their high reactivity and short half-life. Alternative biomarkers are therefore used as indirect measures of ROS activity, for example:
-
1.
Markers of oxidation (after interactions with ROS that alter the cell microenvironment).19,20
-
2.
Antioxidants and markers of cellular redox status, which change biochemical status after exposure to redox stress.21
Common markers of oxidation include lipid peroxides (e.g. malondialdehyde [MDA], F2-isoprostanes, and organic hydroperoxide [OHP]), which indicate the levels of cellular lipid oxidation.19,22 8-Isoprostane is a lipid peroxidation product of arachidonic, widely utilised by redox scientists.23 MDA is also formed from peroxidation of fatty acids and used historically to detect ROS, which degrade lipids to form MDA. MDA is itself toxic to cells; binding and oxidising DNA to cause cross-linking of nucleic acid bases, and reacting with other cellular amine groups.24 Similarly, protein carbonyl moieties (PCO) or methionine sulfoxide can be measured to reflect levels of cellular protein oxidation,25 and DNA oxidation can be assessed by measuring concentrations of 8-oxo-2′-deoxyguanosine.
Across the surgical literature, xanthine oxidase (XO), an enzyme which generates ROS, has been widely used by researchers to quantify ischaemic/reperfusion injury in patients perioperatively; with tissue damage thought to be mediated by adenosine diphosphate catabolism, acidosis, and subsequent XO production and neutrophil mediation.26, 27, 28
Antioxidants act as the cellular counterbalance to oxidation reactions. They can be measured both individually and cumulatively, as their individual effects are additive.29 Well-studied antioxidant enzymes include superoxide dismutase (SOD), glutathione peroxidase, and catalase. Chain-breaking antioxidants (including vitamin E, thiols, nitric oxide, and ubiquinol) act by attenuating ROS-triggered chain reactions by transferring electrons across aqueous or lipid cellular compartments.30,31 Thiols (proteins or non-protein compounds with free sulfhydryl groups) are also major targets of ROS-induced oxidation. Reactive aldehydes (e.g. MDA) can react further with sulfhydryl and amino moieties of proteins and transcription factors to modulate a variety of cell functions and interfere with redox signalling.32 The ratio of reduced over oxidised glutathione (GSG/GSSG) is a common marker of cellular redox status intracellularly, whereas extracellularly (e.g. in plasma), total free thiols are more convenient markers of oxidative status as serum albumin (with one single free sulfhydryl group) accounts for the majority of thiols and free glutathione concentrations are much lower.32
Total antioxidant status (TAS) represents the additive function of antioxidants through a colorimetric assay using a specific test solution. The value produced represents the solution's antioxidant capacity and can act as a figure with which to compare levels of antioxidation across different clinical samples.29,33
Although anaesthetists are becoming increasingly aware of the role oxidative stress plays in the inflammatory surgical stress response, how intraoperative oxygen affects this remains unclear.34,35 The aim of this review is to determine whether a lower FiO2 during general anaesthesia reduces the magnitude of perioperative oxidative stress.
Methods
This review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA),36 and was prospectively registered online at International Prospective Register of Systematic Reviews (PROSPERO, ID: CRD42017078995).
Selection criteria
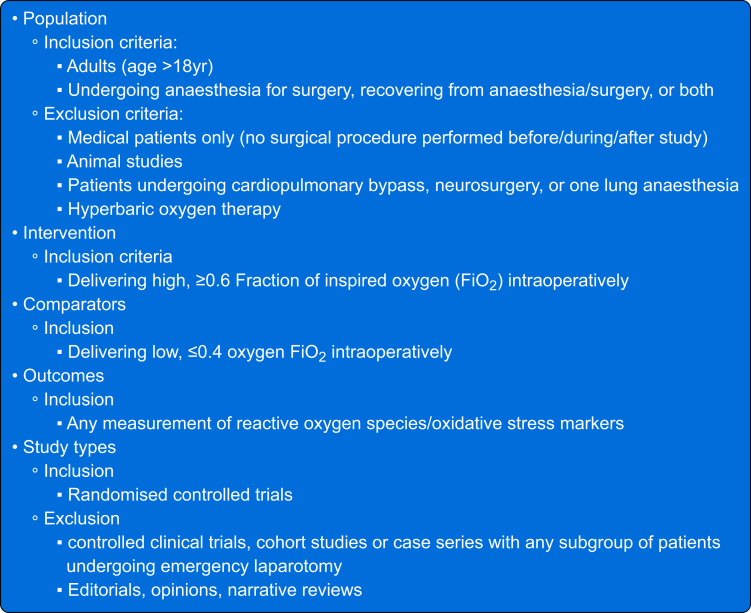
RCTs, published in English, in adult (aged 18 yr or older) patients undergoing any noncardiac procedure in an operating theatre under general anaesthesia and not requiring one lung ventilation, neurosurgery or hyperbaric oxygen therapy were eligible. All included studies reported biochemical levels of oxidative stress (as agreed by all authors) in response to administration of either a high or low intraoperative FiO2 (>0.6 vs <0.4, or ≥20% difference between interventional groups) (Fig. 1).
Fig 1.
Inclusion and exclusion criteria for studies.
Search strategy
EMBASE, MEDLINE, and Cochrane databases were searched from inception until March 9, 2020 for keywords relating to ROS, oxidative stress, oxygen, hyperoxia, anaesthesia, and surgery. Full search strategies are detailed in Appendix A. Two authors (AO and AC) independently identified potentially eligible studies by screening all titles and abstracts using Rayyan (systematic review web application37). Any disagreements were resolved by discussion with all other authors. Full texts of potentially eligible studies were obtained and reviewed by two authors (AO and AC). Review by other authors was available if consensus could not be reached, but not necessary. Included articles' references were then hand-searched for completeness.
Data extraction and assessment of methodological quality
Data were extracted, placed in an analysis table, and independently cross-checked by two authors (AO and AC). One author (AO) used the Cochrane Collaboration Tool (The Nordic Cochrane Centre, Copenhagen, Denmark) to assess Risk of Bias to assess methodological quality. Studies were scored as high, low, or unclear risk in each of the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, complete outcome data, selective reporting, and other biases. Because of the high level of heterogeneity in the small number of results, a meta-analysis was not performed.
Results
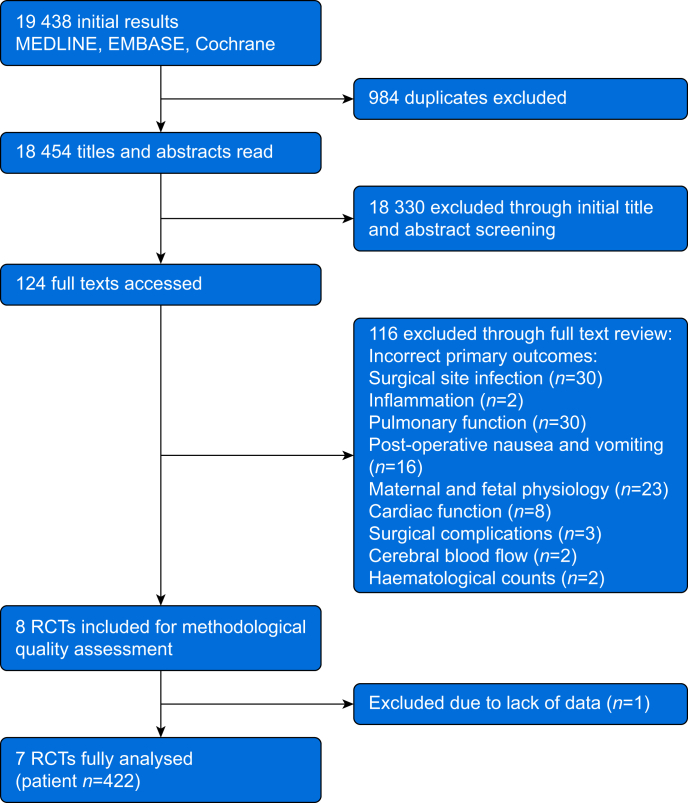
The initial search yielded 19 438 results, of which 984 were duplicates. Overall, 124 were deemed potentially eligible after title and abstract review; however, 116 were excluded on reviewing the full texts, leaving eight eligible studies (Fig. 2). The most common reasons for exclusion were only reporting clinical outcomes and not specifically reporting on biochemical measures. One article was subsequently excluded from the analysis owing to missing data despite attempts to contact the authors.38 Data from 422 patients in seven studies were included in the final analysis.
Fig 2.
CONSORT diagram. CONSORT, Consolidated Standards of Reporting Trials.
Characteristics of included studies
From available data across the seven studies with a total of 422 participants, mean age was 38 (standard deviation [sd], 13.9) yr and weight 66.9 (3.1) kg. Of the six trials reporting participants' sex (n=392 total), only 47 (12%) participants were male. All seven RCTs included in the analysis reported different biomarkers of oxidative stress in surgical patients (Table 1).39, 40, 41, 42, 43, 44, 45 Four studies (three of which were from the same group) reported oxidative stress in maternal and fetal blood samples collected during either elective or emergency Caesarean section.41, 42, 43,45 One trial reported markers of oxidative stress in serum and bronchoalveolar lavage (BAL) samples collected from 40 patients undergoing a hemicolectomy procedure under general anaesthesia,44 and the final two studies (both from the same group) studied both mucosal and arterial levels of MDA intraoperatively and postoperatively during colon surgery.39,40
Table 1.
Combined results of RCTs reporting on markers of oxidative stress in arterial blood, fetal blood, and bronchial lavage samples. ∗P<0.05; ∗∗P<0.01. EL C/S elective Caesarean section; EM C/S: emergency Caesarean section.
| Authors | Patient no. | Control vs intervention (FiO2) | Sample | Isoprostane (various) | Organic hydroperoxides (μmol L−1) | Malondialdehyde, MDA (various) | Protein carbonyl, PCO (nmol mg−1) | Xanthine oxidase, XO (mU mg protein−1) |
|---|---|---|---|---|---|---|---|---|
| Khaw and colleagues41 | 44 | 0.21 vs 0.6 | Maternal arterial | 121.8 vs 200.6∗∗(μmol L−1) | 0.14 vs 0.14 | 0.89 vs 1.2∗∗ (μmol L−1) | – | – |
| Umbilical venous | 135.3 vs 403.0∗∗ (μmol L−1) | 0.15 vs 0.5∗ | 0.47 vs 0.78∗ (μmol L−1) | – | – | |||
| Umbilical arterial | 122.1 vs 215∗∗ (μmol L−1) | 0.18 vs 0.39∗∗ | 0.4 vs 0.4∗∗ (μmol L−1) | – | – | |||
| Khaw and colleagues42 | 125 | 0.21 vs 0.6 | Maternal venous | 225 vs 240.7 (pg ml−1) | – | – | – | – |
| Umbilical venous | 427 vs 471 (pg ml−1) | – | – | – | – | |||
| Umbilical arterial | 457 vs 473 (pg ml−1) | – | – | – | – | |||
| Khaw and colleagues43 | 39 | 0.3 vs 0.5 vs 1.0 | Maternal arterial | 154 vs 156 vs 158 (pg ml−1) | – | – | – | – |
| Umbilical venous | 480 vs 416 vs 441 (pg ml−1) | – | – | – | – | |||
| Umbilical arterial | 410 vs 368 vs 468 (pg ml−1) | – | – | – | – | |||
| Koksal and colleagues44 | 40 | 0.4 vs 0.8 | Subject arterial | – | – | 8.1 vs 8.1 (nmol mg−1) | 5.8 vs 7.5 | – |
| Subject bronchial lavage | – | – | 7.7 vs 12.6∗∗ (nmol mg−1) | 10.1 vs 4.5∗∗ | – | |||
| Ahuja and colleagues45 | 60 (EL C/S) | 0.21 vs 0.5 | Maternal arterial | – | – | 6.1 vs 6.2 (μmol) | – | – |
| Umbilical venous | – | – | 5.3 vs 4.8 (μmol) | – | – | |||
| Umbilical arterial | – | – | 5.4 vs 4.3 (μmol) | – | – | |||
| 60 (EM C/S) | 0.21 vs 0.5 | Maternal arterial | – | – | 6.1 vs 6.2 (μmol) | – | – | |
| Umbilical arterial | – | – | 5.1 vs 5.5 (μmol) | – | – | |||
| Umbilical venous | – | – | 5.4 vs 4.8 (μmol) | – | – | |||
| Garcia de la Asuncion and colleagues39 | 30 | 0.3 vs 0.8 | Subject arterial 1 h after induction | – | – | 0.6 vs 0.5 (nmol ml−1) | – | – |
| Subject arterial 6 h postoperatively | – | – | 0.65 vs 0.4∗ (nmol ml−1) | – | – | |||
| Garcia de la Asuncion and colleagues40 | 24 | 0.3 vs 0.8 | Subject mucosal | – | – | 2.0 vs 1.0∗∗ (nmol mg−1 protein−1) | 595 vs 310∗ | |
| Subject arterial | – | – | 1.5 vs 0.4∗∗ (nmol mg−1 ml−1) |
Risk of bias in included studies
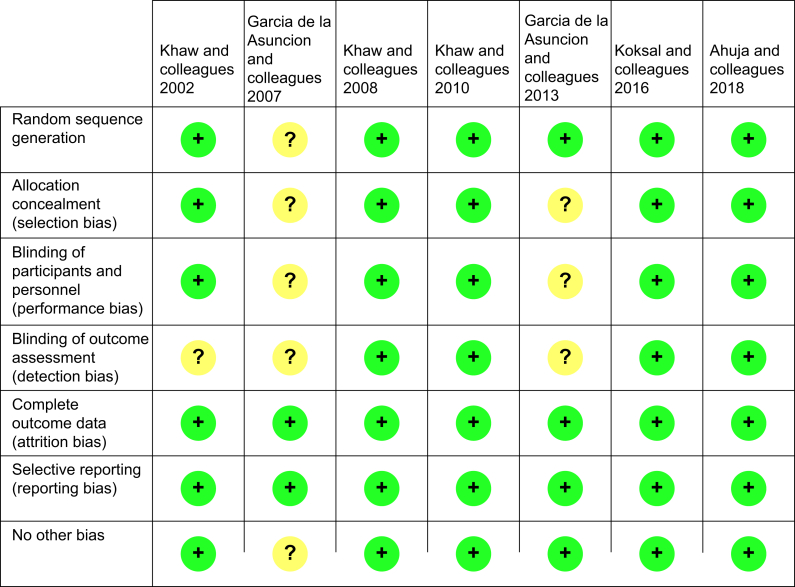
Of the seven studies analysed, four were deemed to have low risk of bias across all domains,42, 43, 44, 45 and three articles were deemed to have a moderate risk of bias because of no reporting on blinding and patient group allocation concealment.39, 40, 41 A risk bias summary grid depicting these results is shown in Figure 3. Combined results are listed in Table 1, Table 2.
Fig 3.
Bias grid.
Table 2.
Results of RCTs reporting on antioxidant levels in blood and bronchial lavage. ∗P<0.05; ∗∗P<0.01.
| Author | Patient no. | Control vs intervention (FiO2) | Sample | Superoxide dismutase (nmol mg−1) | Non-protein sulfhydryl (nmol mg−1) | Protein sulfhydryl (nmol mg−1) | Reduced glutathione (μmol ml−1) | Oxidised glutathione | Total antioxidant status (mM) |
|---|---|---|---|---|---|---|---|---|---|
| Koksal and colleagues44 | 40 | 0.4 vs 0.8 | Subject arterial | 3.6 vs 1.4∗∗ | 2.56 vs 2.7 | 3.2 vs 2.6∗ | – | – | |
| Subject bronchial lavage | 13.7 vs 13.4∗∗ | 2.2 vs 1.2∗∗ | 11.8 vs 6.7∗∗ | – | – | ||||
| Garcia de la Asuncion and colleagues39 | 30 | 0.3 vs 0.8 | Subject arterial 1 h after induction | – | – | – | 0.68 vs 0.58 | 20 vs 30 | |
| Subject atrial 6 h postoperatively | – | – | – | 0.78 vs 0.7 | 42 vs 30∗∗ | ||||
| Garcia de la Asuncion and colleagues40 | 24 | 0.3 vs 0.8 | Subject arterial | – | – | – | – | 42 vs 30 | |
| Ahuja and colleagues45 | 60 (EL C/S) | 0.21 vs 0.5 | Maternal arterial | – | – | – | – | – | 1.1 vs 1.1 |
| Umbilical arterial | – | – | – | – | – | 1.2 vs 1.3 | |||
| Umbilical venous | – | – | – | – | – | 1.3 vs 1.3 | |||
| 60 (EM C/S) | 0.21 vs 0.5 | Umbilical arterial | – | – | – | – | – | 1.1 vs 1.1 | |
| Umbilical venous | – | – | – | – | – | 1.6 vs 1.5 |
Markers of oxidation
MDA was the most commonly reported biomarker of oxidative stress, reported in five of the seven studies.39, 40, 41,44,45 Two studies demonstrated significant increases in MDA with higher FiO2 in maternal and umbilical serum,41 and bronchial lavage.44 Two other studies (from the same group) reported significantly lower mucosal and postoperative arterial MDA concentrations with an FiO2 of 0.8,39,40 and neither maternal nor umbilical MDA concentrations changed in the remaining study.45
Three separate studies (from the same group) reported maternal and umbilical isoprostane concentrations.41, 42, 43 Although the earliest of these reported significant increases in the higher FiO2 (=0.6) group,41 no significant differences were demonstrated in the latter two studies.42,43
High FiO2 was also associated with higher fetal OHP concentrations,41 lower bronchial PCO concentrations,44 and lower mucosal XO concentrations40 in three separate studies.
Antioxidant and cellular redox status
No differences in oxidised and reduced glutathione were demonstrated, either intraoperatively (1 h after induction) or 6 h after surgery, in two separate studies (both FiO2 0.3 vs 0.8) from the same group.39,40
Only two RCTs reported on other markers of antioxidant status (Table 2). Koksal and colleagues44 reported significant decreases in arterial and BAL SOD and PSH, and also BAL non-protein sulfhydryl (NPSH), with lower FiO2 (0.4 vs 0.8) in 40 patients having colorectal surgery, and Ahuja and colleagues45 reported no changes in TAS between control (FiO2 0.21) and intervention (FiO2 0.5) and in maternal arterial, fetal arterial, or fetal venous blood during elective and emergency Caesarean section.
Discussion
Evidence from this systematic review suggests that higher intraoperative FiO2 could be associated with increased perioperative oxidative stress. Evidence from 138 patients across four studies demonstrated increased biomarkers of oxidative stress in serum and alveolar samples collected from patients receiving high FiO2.39, 40, 41,44 However, the number and size of all of these studies were small, and considerable uncertainty remains about which redox pathways might be most affected by intraoperative oxygen administration.
Oxygen is one of the most commonly administered perioperative drugs, yet paradoxically the debate about how much oxygen patients should receive whilst undergoing surgery remains highly controversial. Even though studies and publications frequently state that hyperoxia increases levels of oxidative stress during surgery, direct mechanistic evidence during the perioperative period appears limited. We believe this to be the first systematic review reporting oxidative stress in response to different FiO2s during surgery and our findings show few (all small single-centre) trials have explored this during surgery to date. This is even more interesting given one of the most contentious aspects of this debate amongst anaesthetists is that the WHO's guideline development group considered the PROXI trial's findings to be ‘mechanistically implausible’.2 This is surprising given that excess oxygen administration is well documented to be associated with a lack of benefit or increased harm in a variety of related clinical settings, including acute illness,46 critical illness,47,48 cardiac disease,49 post resuscitation,50 stroke,51 and traumatic brain injury.52, 53, 54 High FiO2 is also thought to cause acute cardio-pulmonary complications including pulmonary oedema, atelectasis, and fibrosis in the critical care setting.55,56
Similarly redox biomarkers have increasingly been associated with adverse clinical outcomes in a range of clinical conditions. High cysteine/glutathione ratios are associated with increased mortality in coronary artery disease.57 Total free thiol concentrations were tightly inversely correlated both with all-cause mortality in renal transplant patients, and with adverse features in patients with chronic heart failure.58,59 A pro-oxidant change in the free thiol ratio has also been demonstrated in patients with active malignancy,60 myocardial infarction,61 atrial fibrillation,62 chronic obstructive pulmonary disease,63 and asthma.64 Many risk factors known to increase perioperative risk have also been associated with thiol oxidation, including ageing, smoking, alcohol abuse, and obesity.65 It is also plausible that ROS produced under hyperoxic conditions may contribute to cellular carcinogenesis, damage DNA, and impair DNA polymerase activity, negatively affecting DNA synthesis and repair.66,67
Significant increases in MDA (used as a serum and tissue marker in four of the seven included trials) were observed across neonatal cord blood, arterial, bronchial, and colon mucosal samples given high FiO2. MDA and isoprostane represent the final oxidation products of polyunsaturated fatty acids, suggesting FiO2 might affect lipid membrane composition during surgery. Interestingly, serum MDA concentrations showed no change between different FiO2 levels (0.4 and 0.8) in one study, but did increase within BAL and arterial samples, suggesting most oxidative stress may occur within the pulmonary vasculature.44 ROS induced hyperoxia-induced acute lung injury, a state of increased permeability of the alveolar/vascular interface and endothelial disruption (also mediated by interleukins, cytokines, and chemokines) is well described,68 and direct disruption of type 2 epithelial cells by oxidative and inflammatory mediators promotes cellular apoptotic and necrotic pathways.69
In contrast, during elective C-section, isoprostane and MDA concentrations in both maternal and umbilical serum increased up to two-fold with FiO2 0.6,41 supporting other research showing that redox mediators can cross the placenta.70 However, MDA concentrations did not change in a second study where mothers received FiO2 of 0.21 or 0.5 during both elective and emergency operations,45 possibly because of either the lower FiO2 or shorter duration (<10 vs >52 min) of oxygen exposure. Oxidative stress has been implicated in multiple obstetric complications including preterm labour, maternal vascular disease, and miscarriage, with ROS formation causing lipid peroxidation, membrane disruption of placental tissue, and dysregulation of fetal growth and development.71, 72, 73 It is worth noting that all participants in two of the trials conducted by Khaw and colleagues41, 42 received spinal (regional) anaesthesia alone, so these results may not be directly comparable with patients undergoing endotracheal intubation and general anaesthesia.
Only one trial reported XO expression, an enzyme family known to directly generate ROS,40 suggesting that inspiring high FiO2 may attenuate XO activity at a tissue level and reduce ROS production. Given that urate, a common product of XO activity, is also one of the main constituents of many assays used to measure total antioxidant capacity,74 other measures of antioxidant activity might also be expected to respond similarly to hyperoxia. Lack of consistency as to how antioxidant status is reported makes direct comparison challenging – two studies only reported oxidised and reduced glutathione concentrations,39,40 whereas two other trials reported alternative markers of activity including SOD, NPSH, PSH, and TAS.44,45 In one of these latter trials, SOD expression and both NPSH and PSH concentrations were significantly reduced with high FiO2 administration,44 suggesting that lower concentrations of oxygen may stimulate a greater antioxidant response or that excess oxygen might ‘consume’ cellular antioxidant capacity. In contrast, the other trial reported no significant differences in TAS,45 suggesting oxidative stress was not associated with reciprocal anti-oxidation responses in these procedures performed under regional (as opposed to general) anaesthesia.
Hyperoxia-induced vasoconstriction is recognised to increase afterload and reduce cardiac output,75,76 as well as reduce coronary blood flow.77 Moreover, high flow oxygen administration is no longer routinely used to manage myocardial infarction.49,78 A meta-analysis looking at all in vivo and ex vivo animal studies of oxygen-induced vasoconstriction concluded that vasoconstriction was directly proportional to the degree of oxygen exposure, and greatest within the vascular smooth muscle.79 High FiO2 may also contribute to peripheral and coronary vasoconstriction during anaesthesia, increasing tissue ischaemia.
Our analysis is limited by the quantity and quality of research conducted in this area. Of the seven studies identified in the current systematic review, only four studies were deemed to have low risk of reporting bias in all domains (Fig. 3). Furthermore, a high proportion of participants were young females as four of the seven included studies only recruited participants having Caesarean section procedures. It is not known how perioperative redox changes might differ between obstetric and non-obstetric surgery, but redox markers are known to vary with age, sex, body habitus and pregnancy.35 Another limitation that has hampered progress in this field is the lack of a conceptual framework for what oxidative stress actually means in vivo. Many different readouts have been proposed and are currently being used as indicators of the involvement of ROS in clinical setting without a clear understanding what any of these analytes actually ‘mark’ or how these different ‘readouts of cellular activity’ may interact with each other.80
Taken together, our findings evidence a striking lack of high-quality research exploring the cellular consequences of perioperative oxygen administration. Historically, perioperative oxygen research has focused on the effects of hyperoxia on SSI rates as well as nausea and vomiting.81, 82, 83 However, larger trials (such as PROXI) and meta-analyses demonstrate that the presumed association between hyper-oxygenation and reduction in SSI rates is uncertain,8,84 and there remains strong evidence to suggest that ROS formation increases perioperative tissue inflammation.35 Understanding whether oxygen causes shifts in the production of ROS and antioxidants has considerable implications for clinical practice, and further work is urgently needed to explore these mechanisms that underlie so many current practices in perioperative medicine.
Authors' contributions
Conception: all authors
Design: all authors
Data collection: AHO, AC
Writing of manuscript: AHO, AC
Editing of manuscript: all authors
Acknowledgements
The authors acknowledge the significant contribution of Paula Sands for helping the authors to develop and run literature searches for this project over a period of 48 months. Without her expert help this work would not have been possible.
Handling editor: Jonathan Hardman
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.09.050.
Declarations of interest
DM has received honoraria for speaking and consultancy work from Siemens Healthineers and Edwards Lifesciences, and is a director of Oxygen Control Systems Ltd. MPWG serves on the medical advisory board of Sphere Medical Ltd. and is a director of Oxygen Control Systems Ltd. He has received honoraria for speaking and/or travel expenses from BOC Medical (Linde Group), Edwards Lifesciences, and Cortex GmBH. MPWG leads the Xtreme Everest Oxygen Research Consortium and the Fit-4-Surgery research collaboration. Some of this work was undertaken at University Southampton NHS Foundation Trust–University of Southampton NIHR Biomedical Research Centre. MPWG serves as the UK NIHR CRN national specialty group lead for Anaesthesia Perioperative Medicine and Pain and is an elected council member of the Royal College of Anaesthetists and president of the Critical Care Medicine Section of the Royal Society of Medicine. All other authors declare that they have no conflicts of interests.
Funding
National Institute for Health Research Southampton Biomedical Research Centre (to AHO and AFC) and National Institute for Health Research Senior Investigator award (to MPWG).
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Allegranzi B., Zayed B., Bischoff P. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16:e288–303. doi: 10.1016/S1473-3099(16)30402-9. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . 1st ed. 2016. Global guidelines for the prevention of surgical site infection.http://www.ncbi.nlm.nih.gov/books/NBK401132/ Available from: [PubMed] [Google Scholar]
- 3.World Health Organization . 2nd ed. World Health Organisation; Geneva: 2018. Global guidelines for the prevention of surgical site infection.https://www.ncbi.nlm.nih.gov/books/NBK536404/ Available from: [Google Scholar]
- 4.World Health Organisation Global guidelines for the prevention of surgical site infection web appendix13.pdf. https://www.who.int/gpsc/appendix13.pdf?ua=1 Available from:
- 5.Myles P.S., Kurz A. Supplemental oxygen and surgical site infection: getting to the truth. Br J Anaesth. 2017;119:13–15. doi: 10.1093/bja/aex096. [DOI] [PubMed] [Google Scholar]
- 6.Oldman A.H., Cumpstey A.F., Martin D.S., Grocott M.P.W. Data integrity issues: catalyst for a more robust approach to research on perioperative oxygen therapy? Perioper Med. 2019;8:7. doi: 10.1186/s13741-019-0118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munshi L., Ferguson N.D. Evolving issues in oxygen therapy in acute care medicine. JAMA. 2020;323:607–608. doi: 10.1001/jama.2019.22029. [DOI] [PubMed] [Google Scholar]
- 8.Meyhoff C.S., Wetterslev J., Jorgensen L.N. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA. 2009;302:1543–1550. doi: 10.1001/jama.2009.1452. [DOI] [PubMed] [Google Scholar]
- 9.Fonnes S., Gögenur I., Søndergaard E.S. Perioperative hyperoxia — long-term impact on cardiovascular complications after abdominal surgery, a post hoc analysis of the PROXI trial. Int J Cardiol. 2016;215:238–243. doi: 10.1016/j.ijcard.2016.04.104. [DOI] [PubMed] [Google Scholar]
- 10.Meyhoff C.S., Jorgensen L.N., Wetterslev J., Christensen K.B., Rasmussen L.S., PROXI Trial Group Increased long-term mortality after a high perioperative inspiratory oxygen fraction during abdominal surgery: follow-up of a randomized clinical trial. Anesth Analg. 2012;115:849–854. doi: 10.1213/ANE.0b013e3182652a51. [DOI] [PubMed] [Google Scholar]
- 11.Jamieson D., Chance B., Cadenas E., Boveris A. The relation of free radical production to hyperoxia. Annu Rev Physiol. 1986;48:703–719. doi: 10.1146/annurev.ph.48.030186.003415. [DOI] [PubMed] [Google Scholar]
- 12.Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sies H., Berndt C., Jones D.P. Oxidative stress. Annu Rev Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 14.Auten R.L., Davis J.M. Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr Res. 2009;66:121–127. doi: 10.1203/PDR.0b013e3181a9eafb. [DOI] [PubMed] [Google Scholar]
- 15.Helmerhorst H.J.F., Schultz M.J., van der Voort P.H.J., de Jonge E., van Westerloo D.J. Bench-to-bedside review: the effects of hyperoxia during critical illness. Crit Care. 2015;19:284. doi: 10.1186/s13054-015-0996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panday A., Sahoo M.K., Osorio D., Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell Mol Immunol. 2015;12:5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cumpstey A., Feelisch M. Free radicals in inflammation. In: Cavaillon J.-M., Singer M., editors. Inflammation: from molecular and cellular mechanisms to the clinic, 4 volume set. Wiley; Weinheim: 2017. pp. 695–726. [Google Scholar]
- 18.Chen Y., Zhou Z., Min W. Mitochondria, Oxidative stress and innate immunity. Front Physiol. 2018;9:1487. doi: 10.3389/fphys.2018.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho E., Karimi K.G., Liu C.C., Bhindi R., Figtree G.A. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol. 2013;1:483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sies H. Oxidative stress: eustress and distress in redox homeostasis. In: Fink G., editor. Stress: physiology, biochemistry, and pathology. Academic Press; London: 2019. pp. 153–163. [Google Scholar]
- 21.Halliwell B., Gutteridge J.M.C. Clarendon Press; Oxford: 2015. Free radicals in biology and medicine. [Google Scholar]
- 22.Gutteridge J.M. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–1828. [PubMed] [Google Scholar]
- 23.Montuschi P., Barnes P.J., Roberts L.J. Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 24.Del Rio D., Stewart A.J., Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Dalle-Donne I., Rossi R., Giustarini D., Milzani A., Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 26.Chung H.Y., Baek B.S., Song S.H. Xanthine dehydrogenase/xanthine oxidase and oxidative stress. Age (Omaha) 1997;20:127–140. doi: 10.1007/s11357-997-0012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anup R., Aparna V., Pulimood A., Balasubramanian K.A. Surgical stress and the small intestine: role of oxygen free radicals. Surgery. 1999;125:560–569. [PubMed] [Google Scholar]
- 28.Gabriel E.A., Mazza C.A., Mello MAJ de. Use of antioxidants in cardiovascular surgery. Int J Nutrol. 2018;11:80–86. [Google Scholar]
- 29.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Wink D.A., Miranda K.M., Espey M.G. Mechanisms of the antioxidant effects of nitric oxide. Antioxid Redox Signal. 2001;3:203–213. doi: 10.1089/152308601300185179. [DOI] [PubMed] [Google Scholar]
- 31.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris C., Hansen J.M. Oxidative stress, thiols, and redox profiles. Methods Mol Biol. 2012;889:325–346. doi: 10.1007/978-1-61779-867-2_21. [DOI] [PubMed] [Google Scholar]
- 33.Rice-Evans C., Miller N.J. Total antioxidant status in plasma and body fluids. Methods Enzymol. 1994;234:279–293. doi: 10.1016/0076-6879(94)34095-1. [DOI] [PubMed] [Google Scholar]
- 34.Rosenfeldt F., Wilson M., Lee G. Oxidative stress in surgery in an ageing population: pathophysiology and therapy. Exp Gerontol. 2013;48:45–54. doi: 10.1016/j.exger.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Stevens J.L., Feelisch M., Martin D.S. Perioperative oxidative stress: the unseen enemy. Anesth Analg. 2019;129:1749–1760. doi: 10.1213/ANE.0000000000004455. [DOI] [PubMed] [Google Scholar]
- 36.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting Items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 37.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh V., Hooda S., Dahiya K., Sharma R. Effect of different inspired oxygen concentrations during caesarean section under spinal anaesthesia on maternal and foetal oxygenation and lipid peroxidation. Bombay Hosp J. 2006;4:561–566. [Google Scholar]
- 39.García de la Asunción J., Belda F.J., Greif R., Barber G., Viña J., Sastre J. Inspired supplemental oxygen reduces markers of oxidative stress during elective colon surgery. Br J Surg. 2007;94:475–477. doi: 10.1002/bjs.5497. [DOI] [PubMed] [Google Scholar]
- 40.García-de-la-Asunción J., Barber G., Rus D. Hyperoxia during colon surgery is associated with a reduction of xanthine oxidase activity and oxidative stress in colonic mucosa. Redox Rep. 2013;16:121–128. doi: 10.1179/174329211X13049558293632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khaw K.S., Wang C.C., Ngan Kee W.D., Pang C.P., Rogers M.S. Effects of high inspired oxygen fraction during elective caesarean section under spinal anaesthesia on maternal and fetal oxygenation and lipid peroxidation. Br J Anaesth. 2002;88:18–23. doi: 10.1093/bja/88.1.18. [DOI] [PubMed] [Google Scholar]
- 42.Khaw K.S., Wang C.C., Ngan Kee W.D. Supplementary oxygen for emergency Caesarean section under regional anaesthesia. Br J Anaesth. 2009;102:90–96. doi: 10.1093/bja/aen321. [DOI] [PubMed] [Google Scholar]
- 43.Khaw K.S., Ngan Kee W.D., Chu C.Y. Effects of different inspired oxygen fractions on lipid peroxidation during general anaesthesia for elective Caesarean section. Br J Anaesth. 2010;105:355–360. doi: 10.1093/bja/aeq154. [DOI] [PubMed] [Google Scholar]
- 44.Koksal G.M., Dikmen Y., Erbabacan E. Hyperoxic oxidative stress during abdominal surgery: a randomized trial. J Anesth. 2016;30:610–619. doi: 10.1007/s00540-016-2164-7. [DOI] [PubMed] [Google Scholar]
- 45.Ahuja V., Gombar S., Jaswal S. Effect of maternal oxygen inhalation on foetal free radical activity: a prospective, randomized trial. Acta Anaesthesiol Scand. 2018;62:26–37. doi: 10.1111/aas.13007. [DOI] [PubMed] [Google Scholar]
- 46.Chu D.K., Kim L.H.-Y., Young P.J. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391:1693–1705. doi: 10.1016/S0140-6736(18)30479-3. [DOI] [PubMed] [Google Scholar]
- 47.de Jonge E., Peelen L., Keijzers P.J. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care. 2008;12:R156. doi: 10.1186/cc7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Damiani E., Adrario E., Girardis M. Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit Care. 2014;18:711. doi: 10.1186/s13054-014-0711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cabello J.B., Burls A., Emparanza J.I., Bayliss S., Quinn T. Oxygen therapy for acute myocardial infarction. Cochrane Database Syst Rev. 2016;12:CD007160. doi: 10.1002/14651858.CD007160.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kilgannon J., Jones A.E., Shapiro N.I. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303:2165–2171. doi: 10.1001/jama.2010.707. [DOI] [PubMed] [Google Scholar]
- 51.Rincon F., Kang J., Maltenfort M. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med. 2014;42:387–396. doi: 10.1097/CCM.0b013e3182a27732. [DOI] [PubMed] [Google Scholar]
- 52.Davis D.P., Meade W., Sise M.J. Both hypoxemia and extreme hyperoxemia may be detrimental in patients with severe traumatic brain injury. J Neurotrauma. 2009;26:2217–2223. doi: 10.1089/neu.2009.0940. [DOI] [PubMed] [Google Scholar]
- 53.Brenner M., Stein D., Hu P., Kufera J., Wooford M., Scalea T. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch Surg. 2012;147:1042–1046. doi: 10.1001/archsurg.2012.1560. [DOI] [PubMed] [Google Scholar]
- 54.Rincon F., Kang J., Vibbert M., Urtecho J., Athar M.K., Jallo J. Significance of arterial hyperoxia and relationship with case fatality in traumatic brain injury: a multicentre cohort study. J Neurol Neurosurg Psychiatry. 2014;85:799–805. doi: 10.1136/jnnp-2013-305505. [DOI] [PubMed] [Google Scholar]
- 55.Martin DSBs, Grocott M.P.W.M. Oxygen therapy in critical illness: precise control of arterial oxygenation and permissive hypoxemia. Crit Care Med. 2013;41:423–432. doi: 10.1097/CCM.0b013e31826a44f6. [DOI] [PubMed] [Google Scholar]
- 56.Lumb A.B., Walton L.J. Perioperative oxygen toxicity. Anesthesiol Clin. 2012;30:591–605. doi: 10.1016/j.anclin.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 57.Patel R.S., Ghasemzadeh N., Eapen D.J. Novel biomarker of oxidative stress is associated with risk of death in patients with coronary artery disease. Circulation. 2016;133:361–369. doi: 10.1161/CIRCULATIONAHA.115.019790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frenay A.-R.S., de Borst M.H., Bachtler M. Serum free sulfhydryl status is associated with patient and graft survival in renal transplant recipients. Free Radic Biol Med. 2016;99:345–351. doi: 10.1016/j.freeradbiomed.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 59.Koning A.M., Meijers W.C., Pasch A. Serum free thiols in chronic heart failure. Pharmacol Res. 2016;111:452–458. doi: 10.1016/j.phrs.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 60.Banne A.F., Amiri A., Pero R.W. Reduced level of serum thiols in patients with a diagnosis of active disease. J Anti Aging Med. 2003;6:327–334. doi: 10.1089/109454503323028920. [DOI] [PubMed] [Google Scholar]
- 61.Kundi H., Ates I., Kiziltunc E. A novel oxidative stress marker in acute myocardial infarction; thiol/disulphide homeostasis. Am J Emerg Med. 2015;33:1567–1571. doi: 10.1016/j.ajem.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 62.Neuman R.B., Bloom H.L., Shukrullah I. Oxidative stress markers are associated with persistent atrial fibrillation. Clin Chem. 2007;53:1652–1657. doi: 10.1373/clinchem.2006.083923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kopčinović L.M., Domijan A.-M., Posavac K., Čepelak I., Grubišić T.Ž., Rumora L. Systemic redox imbalance in stable chronic obstructive pulmonary disease. Biomarkers. 2016;21:692–698. doi: 10.3109/1354750X.2016.1172110. [DOI] [PubMed] [Google Scholar]
- 64.Stephenson S.T., Brown L.A.S., Helms M.N. Cysteine oxidation impairs systemic glucocorticoid responsiveness in children with difficult-to-treat asthma. J Allergy Clin Immunol. 2015;136:454–461. doi: 10.1016/j.jaci.2015.01.023. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Go Y.-M., Jones D.P. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic Biol Med. 2011;50:495–509. doi: 10.1016/j.freeradbiomed.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liou G.-Y., Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wiseman H., Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhandari V. Molecular mechanisms of hyperoxia-induced acute lung injury. Front Biosci. 2008;13:6653–6661. doi: 10.2741/3179. [DOI] [PubMed] [Google Scholar]
- 69.Yee M., Vitiello P.F., Roper J.M. Type II epithelial cells are critical target for hyperoxia-mediated impairment of postnatal lung development. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1101–L1111. doi: 10.1152/ajplung.00126.2006. [DOI] [PubMed] [Google Scholar]
- 70.Argüelles S., Machado M.J., Ayala A., Machado A., Hervías B. Correlation between circulating biomarkers of oxidative stress of maternal and umbilical cord blood at birth. Free Radic Res. 2006;40:565–570. doi: 10.1080/10715760500519834. [DOI] [PubMed] [Google Scholar]
- 71.Duhig K., Chappell L.C., Shennan A.H. Oxidative stress in pregnancy and reproduction. Obstet Med. 2016;9:113–116. doi: 10.1177/1753495X16648495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vural P., Akgül C., Yildirim A., Canbaz M. Antioxidant defence in recurrent abortion. Clin Chim Acta. 2000;295:169–177. doi: 10.1016/s0009-8981(99)00255-7. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y., Walsh S.W. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta. 1998;19:581–586. doi: 10.1016/s0143-4004(98)90018-2. [DOI] [PubMed] [Google Scholar]
- 74.Ryan M., Grayson L., Clarke D.J. The total antioxidant capacity of human serum measured using enhanced chemiluminescence is almost completely accounted for by urate. Ann Clin Biochem. 1997;34:688–689. doi: 10.1177/000456329703400615. [DOI] [PubMed] [Google Scholar]
- 75.Haque W.A., Boehmer J., Clemson B.S., Leuenberger U.A., Silber D.H., Sinoway L.I. Hemodynamic effects of supplemental oxygen administration in congestive heart failure. J Am Coll Cardiol. 1996;27:353–357. doi: 10.1016/0735-1097(95)00474-2. [DOI] [PubMed] [Google Scholar]
- 76.Mak S., Azevedo E.R., Liu P.P., Newton G.E. Effect of hyperoxia on left ventricular function and filling pressures in patients with and without congestive heart failure. Chest. 2001;120:467–473. doi: 10.1378/chest.120.2.467. [DOI] [PubMed] [Google Scholar]
- 77.Farquhar H., Weatherall M., Wijesinghe M. Systematic review of studies of the effect of hyperoxia on coronary blood flow. Am Heart J. 2009;158:371–377. doi: 10.1016/j.ahj.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 78.Blitzer M.L., Lee S.D., Creager M.A. Endothelium-derived nitric oxide mediates hypoxic vasodilation of resistance vessels in humans. Am J Physiol Heart Circ Physiol. 1996;271:H1182–H1185. doi: 10.1152/ajpheart.1996.271.3.H1182. [DOI] [PubMed] [Google Scholar]
- 79.Smit B., Smulders Y.M., Eringa E.C. Effects of hyperoxia on vascular tone in animal models: systematic review and meta-analysis. Crit Care. 2018;22:189. doi: 10.1186/s13054-018-2123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cortese-Krott M.M., Koning A., Kuhnle G.G.C. The reactive species interactome: evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxid Redox Signal. 2017;27:684–712. doi: 10.1089/ars.2017.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Greif R., Akça O., Horn E.-P., Kurz A., Sessler D.I. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. New Engl J Med. 2000;342:161–167. doi: 10.1056/NEJM200001203420303. [DOI] [PubMed] [Google Scholar]
- 82.Belda F.J., Aguilera L., García de la Asunción J. Supplemental perioperative oxygen and the risk of surgical wound infection: a randomized controlled trial. JAMA. 2005;294:2035–2042. doi: 10.1001/jama.294.16.2035. [DOI] [PubMed] [Google Scholar]
- 83.Myles P.S., Leslie K., Chan M.T.V. Avoidance of nitrous oxide for patients undergoing major surgery: a randomized controlled trial. Anesthesiology. 2007;107:221–231. doi: 10.1097/01.anes.0000270723.30772.da. [DOI] [PubMed] [Google Scholar]
- 84.Jonge S de, Egger M., Latif A. Effectiveness of 80% vs 30–35% fraction of inspired oxygen in patients undergoing surgery: an updated systematic review and meta-analysis. Br J Anaesth. 2019;122:325–334. doi: 10.1016/j.bja.2018.11.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.