Abstract
Background:
Callithrix jacchus, generally known as the common marmoset, has recently garnered interest as an experimental primate model for better understanding the basis of human social behavior, architecture and function. Modelling human neurological and psychological diseases in marmosets can enhance the knowledge obtained from rodent research for future pre-clinical studies. Hence, comprehensive and quantitative assessments of marmoset behaviors are crucial. However, systems for monitoring and analyzing marmoset behaviors have yet to be established.
New method:
In this paper, we present a novel multimodal system, MarmoDetector, for the automated 3D analysis of marmoset behavior under freely moving conditions. MarmoDetector allows the quantitative assessment of marmoset behaviors using computerised tracking analysis techniques that are based on a Kinect system equipped with video recordings, infrared images and depth analysis.
Results:
Using MarmoDetector, we assessed behavioral circadian rhythms continuously over several days in home cages. In addition, MarmoDetector detected acute, transient complex behaviors of alcohol injected marmosets.
Comparison to existing method:
Compared to 2D recording, MarmoDetector detects activities more precisely and is very sensitive as we could detect behavioral defects specifically induced by alcohol administration.
Conclusion:
MarmoDetector facilitates the rapid and accurate analysis of marmoset behavior and will enhance research on the neural basis of brain disorders.
Keywords: Marmoset (Callithrix jacchus, Behavioral neuroscience, Kinect, Alcohol
1. Introduction
Research on animal models has successfully guided neuroscience. Using animal models to study human diseases is a powerful approach for studying neurological and psychological disorders. Rodents such as mice and rats are most frequently used in neurobiological studies. However, compared to rodents, non-human primates show cognitive function and social behavior more similar to that of humans (Roelfsema and Treue, 2014). Among non-human primates, the common marmoset has recently gained attention as a promising model for neuroscience research (Miller et al., 2016; Okano et al., 2012). Marmosets are small animals; the adults reach an average height of 20–30 cm and an average weight of 350–400 g, which makes them advantageous for laboratory usage (Schiel and Souto, 2017). The breeding of marmosets is also faster and more effective compared to that of macaque monkeys. It takes 1.5 years for marmosets to mature, whereas 5years for macaques (Fischer and Austad, 2011). Significantly, genetic techniques, including transgenesis, have been established in marmoset research (Sasaki et al., 2009; Tomioka et al., 2017). Hence, modelling diseases in marmosets is a promising strategy for neurological and neuropsychological research.
Marmosets are very sensitive to stress and should be treated stress-freely during certain behavioral tests (Prins et al., 2017). Infrared photocells and motion sensors were previously applied for recording marmoset behaviors in a stress-free condition (Ronald et al., 1998). In this method, the infrared photocells and sensors were equipped inside of the cage housing marmoset and frequency of interruption of the infrared beams was signified as locomotor activity of marmosets. Alternatively, an infrared motion sensor was applied for the stress-free recording (Ando et al., 2017). Monitoring marmosets with the video tracking system has also been used in a conventional method (Walton et al., 2006). However, these video tracking system can monitor only two-dimensioal (2D) movements of marmosets and has a disadvantage in detecting precise position and activities of the marmoset.
Automatic quantification of three-dimensional (3D) behavioral analysis is a powerful technique for comprehensive behavioral and physiological assessment (Barcia et al., 2004; Chassain et al., 2001; Moussaoui et al., 2000; Togasaki et al., 2005). The Kinect system, which conducts computerised behavioral analyses using video recordings, has been used to assess human and animal behavior (Han et al., 2013; Kulikov et al., 2014; Lyons et al., 2015; Nakamura et al., 2015; Salau et al., 2016). The system has been generally used to automate processes, which has the advantage of requiring less labour. However, Kinect has not been used to quantitatively analyze marmoset behavior.
Here, we describe a novel multimodal system, MarmoDetector, for conducting behavioral assessment on common marmosets. MarmoDetector is integrated with Kinect and can quantitatively evaluate the three-dimensional behavior of marmosets under stress-free conditions. MarmoDetector enables us to analyze moving patterns and calculate activities of marmosets. Our results show that MarmoDetector can be used to evaluate acute and chronic behavioral changes under a variety of conditions and to analyze the behavior of marmosets as models for human disease.
2. Methods
2.1. Animals and housing
A total of 6 adult (16–32 months) wild-type male (n = 3, individual identification Nos. I6395, N560 and I6080) and female (n = 3, individual identification Nos. I5801, I5818 and I5800) marmosets (280–350 g) were purchased from a commercial distributor (CLEA Japan, Inc., Tokyo, Japan). All marmosets were allowed at least 14 days to adjust to the laboratory environment and were housed alone in cages (775 mm height ×386 mm width ×525 mm depth) with light provided via fluorescent light bulbs on a 12-h cycle (on 8:00; off 20:00). Animals were maintained at a standard temperature of 25.5–27.5 °C. To enrich the housing settings, items such as sticks and tubes were placed in the cages. Animals were provided with primate chow ad libitum and two meals per day consisting of plums, mealworm larvae, grains and eggs under the supervision of a veterinarian. Water was given ad libitum to all animals. All methods were performed in accordance with relevant guidelines and regulations. All animal procedures were approved by the local ethics committee of Osaka University. All animal experiments should comply with the ARRIVE guidelines and should be carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). All efforts were made to minimize the number of animals used and their suffering.
2.2. Behavioral testing
The animals were allowed to adapt to the experimental cage for at least 16 h prior to the experiment. Marmoset movement was recorded from 8:00 the following morning to 8:00 five days later.
An intraperitoneal alcohol-administration study was performed between 12:00 and 16:00. A total of 1.5 g of ethanol (99.5; #057–00456, Wako Pure Chemical Industries, Ltd.) per kg of body weight was diluted three-fold with saline solution and injected intraperitoneally (Wright et al., 2013). The same amount of saline was injected as a control. The recording of marmoset movements began soon after the marmoset was placed in the cage and lasted for 1 h.
2.3. Automated tracking procedures using MarmoDetector
To evaluate the motor activities and behaviors of marmosets under stress-free conditions, a novel recording system, MarmoDetector, based on the Kinect system has been created. MarmoDetector enables us to record freely moving marmosets without placing any devices on them.
As shown in Fig. 1, a single front-view Kinect camera (Microsoft Kinect Sensor for Xbox One) (Han et al., 2013) was equipped in an experimental cage (Fig. 1a). The cage was 525 mm long, 356 mm wide and 776 mm high and was designed to be large enough for marmosets to move freely (Fig. 1a, label 1). A Kinect device was located 50 cm away from the cage. The camera was placed at a height that allowed visualisation of the entire cage (Fig. 1a, labels 1 and 2) to fully capture marmoset activity (Fig. 1b). The Kinect camera was equipped with an infrared (IR) camera and a depth sensor. The IR camera monitored marmoset movements on-screen (Fig. 1c–d). The depth sensor captured the marmoset’s exact position (Fig. 1e–f). Images of the empty cage were recorded with the IR camera and the depth sensor to characterize the cage background (Fig. 1c and e). The marmoset was placed in the cage and recording was started. The depth sensor of the Kinect device acquired depth data for the animals with 5 frames per second. These data had a spatial resolution of 512 × 424 with 16 bits per pixel. Accessing an element of the sequence allowed the depth data to be acquired. Microsoft Kinect SDK Version 2.0.1410.19000 was used to collect Kinect data. The depth data was stored as binary data in a database. Using the depth data stored in the database, the software subtracted the background from the image, highlighting the marmosets (Fig. 1g and h). The depth data was transformed into the gray scale image to prepare for the acquisition of the coordinates of the contour of the marmoset. The gray data image was transformed into binary data image with the median filter to remove the noise. Each pixel of the image data from the depth sensor camera was equivalent to one point on the subject. These subject point group data determined the 2D center of gravity of the marmoset. The coordinates of the center of the marmoset were acquired and the moment of the contour of the marmoset was calculated based on the Green’s theorem. The coordinate of moment was defined as the 2D center of gravity of the marmoset. Images of marmosets captured using the depth camera were plotted on the screen at point P. The coordinate system included a horizontal X-axis, a vertical Y-axis and the origin (0, 0) in the upper-left corner of the screen. The resolution of the screen was 512 pixels along the X-axis and 424 pixels along the Y-axis (Supplementary Figure S1). One pixel was equivalent to 2 × 10000/512×tan35 mm in the horizontal direction because the depth sensor horizontal angle was 70 ° (Supplementary Figure S2). Similarly, 1 pixel was equivalent to 2 × 10000/424×tan30 mm in the vertical direction because the depth sensor vertical angle was 60 °. Thus, the X–Y position of the marmoset was determined based on its screen coordinates, and the pixels were converted to distance. The position of the marmoset was converted to the distance.
Fig. 1.
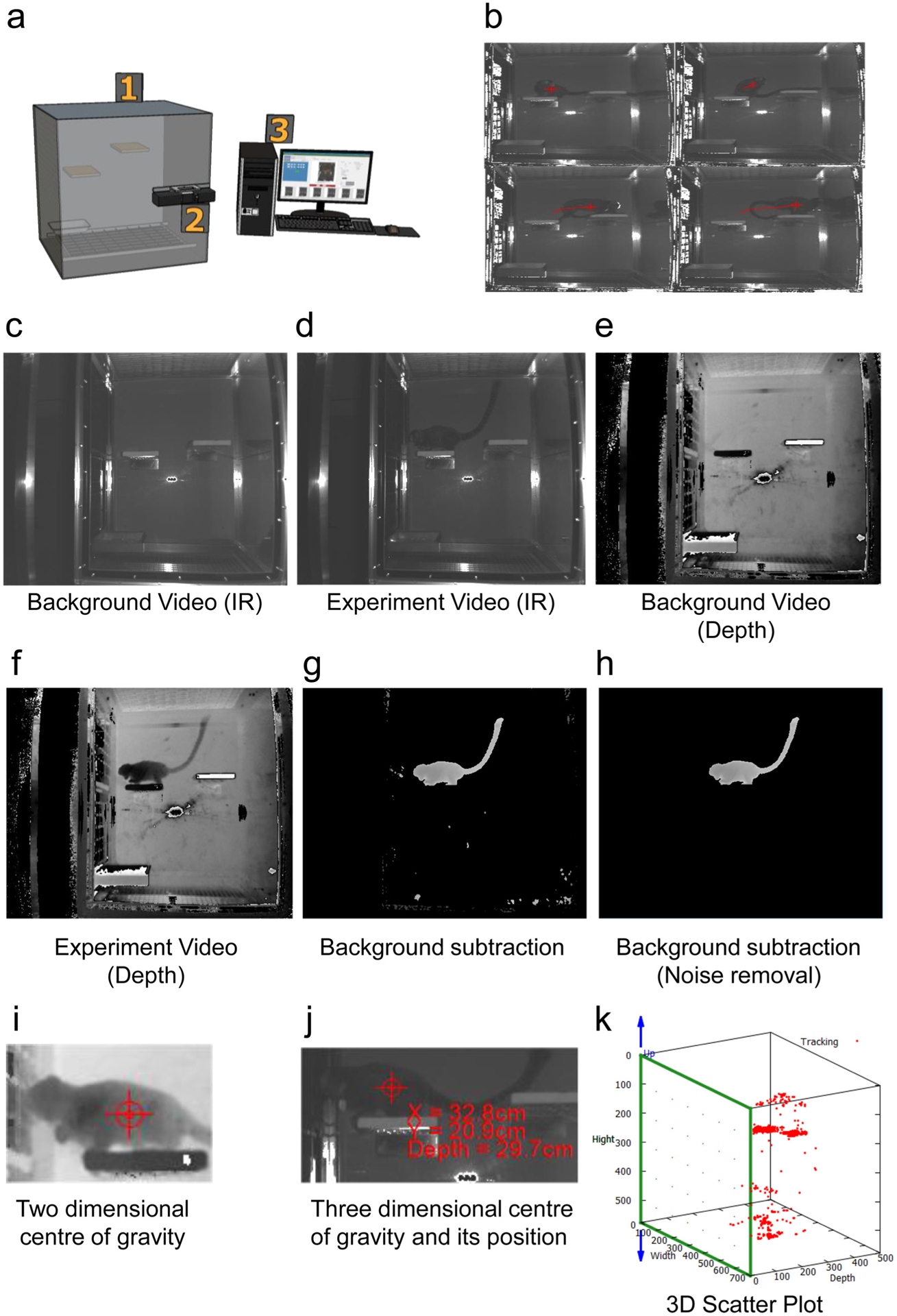
Marmo Detector is capable of evaluating the motor activities of marmosets under stress-free conditions.
(a) Overview of MarmoDetector showing the observation cage (1), the video camera for recording marmoset behavior (2), and the computer and software module (3).
(b) An example of sequential images captured by MarmoDetector, which determines a marmoset’s center of gravity every second.
(c) An IR frame of the empty cage to determine the background.
(d) An IR frame of a marmoset in the cage.
(e) A depth data frame of the empty cage to determine the background.
(f) A depth data frame of a marmoset in the cage.
(g) Processed image highlighting a marmoset after subtracting the background (Fig. 1e) from the marmoset images (Fig. 1f).
(h) The processed image in Fig. 1g after noise removal to highlight the marmoset.
(i) The position of the marmoset is determined based on its two-dimensional center of gravity.
(j) Using depth analysis, we converted the two-dimensional center of gravity of the marmoset to its three-dimensional center of gravity and determined its position.
(k) 3D scatter plot of the location of the marmoset.
The image in 2D screen coordinates was plotted (Supplementary Figure S1) and identifying 2D center of gravity helped us determine the location of a marmoset (Fig. 1i and Supplementary Figure S1). The point of 2D center of gravity of marmosets was used to detect 2D movements of marmosets. To map the movements of a marmoset in 3D space, the distance from the depth sensor to the marmoset was defined as the depth. The depth of the marmoset was equal to the distance between the depth sensor and the point on the line connecting the depth sensor to the 2D center of gravity of the marmoset. These measurements allowed us to define the marmoset’s exact position (Fig. 1j and Supplementary Figure S2).
After data processing, MarmoDetector software generated an automated integration of the traces. Each coordinate value was output in. CSV files, which were used to calculate movement distances. When missing data happened, the latest data was allotted instead. To minimise 3D reconstruction error, the background was established for each animal every second. The cage environment was recorded as the background, before starting the experiment. When the contour defined as the marmoset moved, a new background was created. The new background replaced the depth data of the contour of the marmoset with the corresponding depth data of the new background. If the marmoset did not move, the same background was used.
MarmoDetector monitors a marmoset’s location by mapping the position of the marmoset to the 3D scatter plot every second (Fig. 1k). Hence, MarmoDetector can identify the positions of freely moving marmosets without installing any devices on their bodies.
2.4. Location measurement precision
A dome-like object turning around on the board was measured with the marmoset tracking algorithm. The center of gravity of this object was the center of the hemisphere. Precise monitoring of a moving object with known movement pattern allowed us to assume the idea that the center of gravity of the marmoset was being monitored. (Samson et al., 2015). The object turned around by an electric motor at the upper left, upper right, lower left, lower right, front and back center (Supplementary Figure S3b). The motor rotated about 8 times per minutes. MarmoDetector measured the movement for 6 min enough to draw a circle. The diameter x was defined as Xmax – Xmin. The diameter y was defined as Ymax – Ymin. The calculated diameter was defined as the average of the diameter x and y. The estimated diameter, movement distance and mean speed were compared with each one calculated by MarmoDetector. The estimated diameter was 30 cm while the average diameter of this circle calculated by MarmoDetector was 30.4 cm, indicating an error of only 1.2%. Movement distance and mean speed showed an average error of 3.3% and 3.3% each (Supplementary Figure S3c).
2.5. Statistical analyses
Data were analyzed using one-way ANOVA (results in Fig. 3b), two-way ANOVA with repeated measures (results in Figs. 3c, 4 b and 5 g) and principal component analysis (Fig. 5).
Fig. 3.
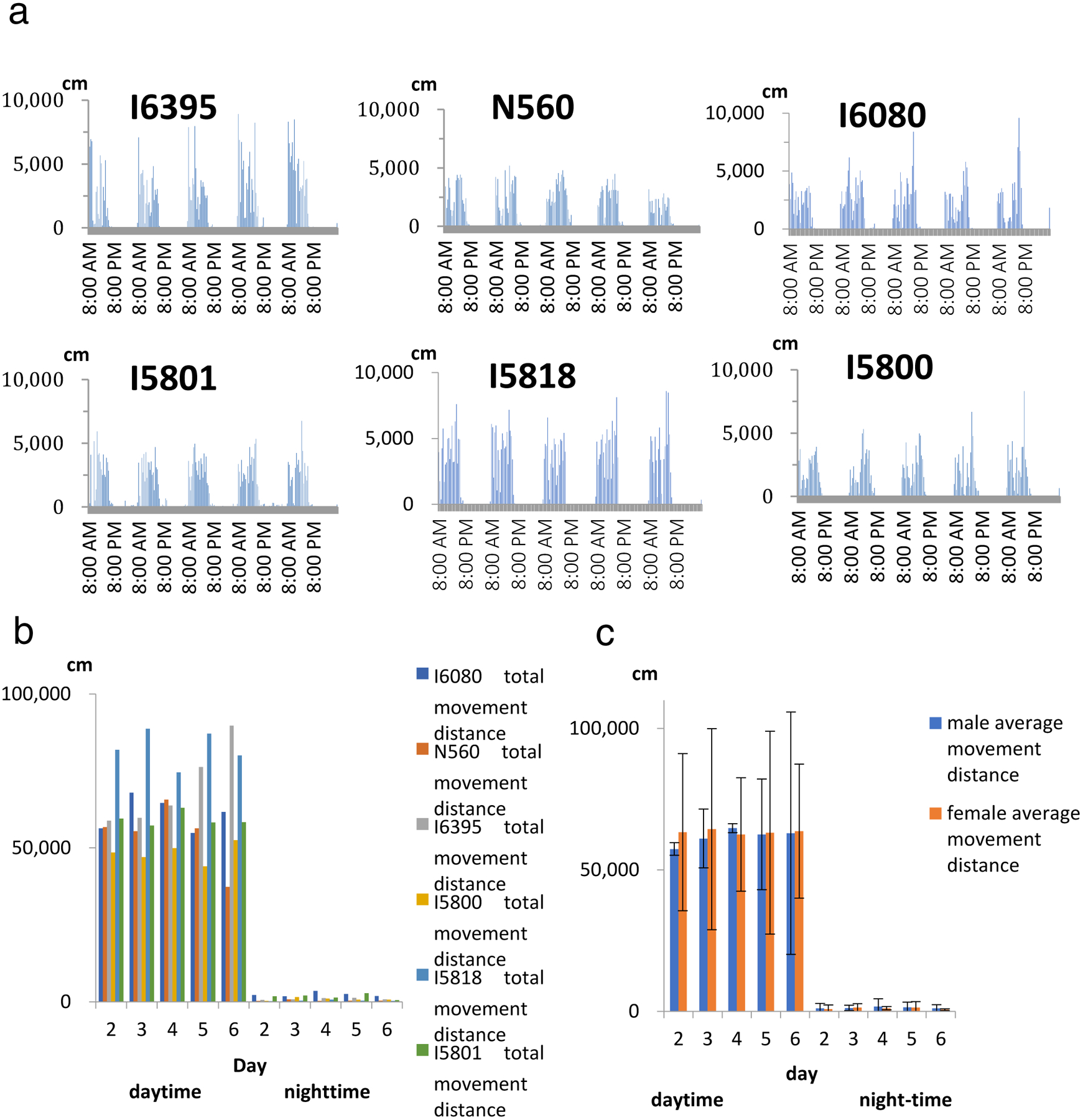
MarmoDetector is effective for analyzing circadian behaviors.
(a) The circadian rhythm of marmoset movement. The general patterns of movement for six marmosets (ID: I6395, N560, I6080, I5801, I5818, I5800) were recorded over 120-hour periods. The movement distance was quantified every 30 min.
(b) The total movement distance of the six marmosets during the day and night for each 24-hour period. There were no differences in daytime or night-time movement patterns among the five days.
(c) Average movement distances of three male marmosets and three female marmosets. There were no significant differences between the movements of male and female marmosets.
Fig. 4.
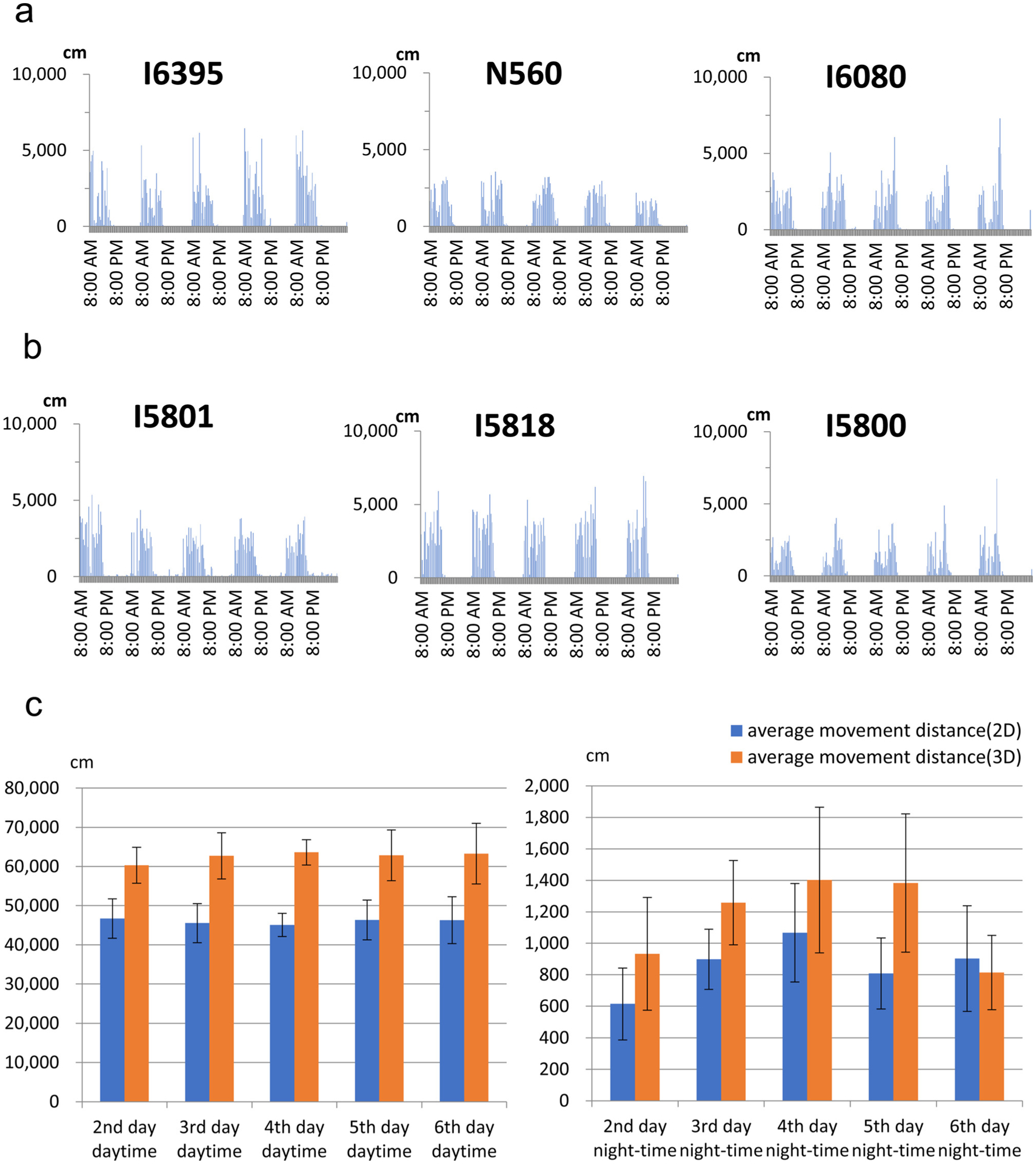
MarmoDetector, 3D analysis, can detect marmoset movements more precisely than 2D analysis.
(a) The general patterns of movement for six marmosets were shown over 120-hour periods with 2D analysis. The movement distance of each direction was quantified every 30 min. The graph showed that all recorded marmosets were active during the daytime but not the night- time, reflecting the circadian activities of marmosets.
(b and c) MarmoDetector, 3D analysis could detect hidden activities recorded with 2D analysis. Average movement distance of 2D and 3D analysis during daytime (b) and night-time (c) were shown. In each period, total movement distance of 3D analysis was almost longer than that of 2D analysis. There was significant difference between 2D and 3D analysis during daytime (two-way ANOVA with repeated measures, F (1, 50) = 23. 784, p = 0.0000114). Values represent mean ± SEM.
Fig. 5.
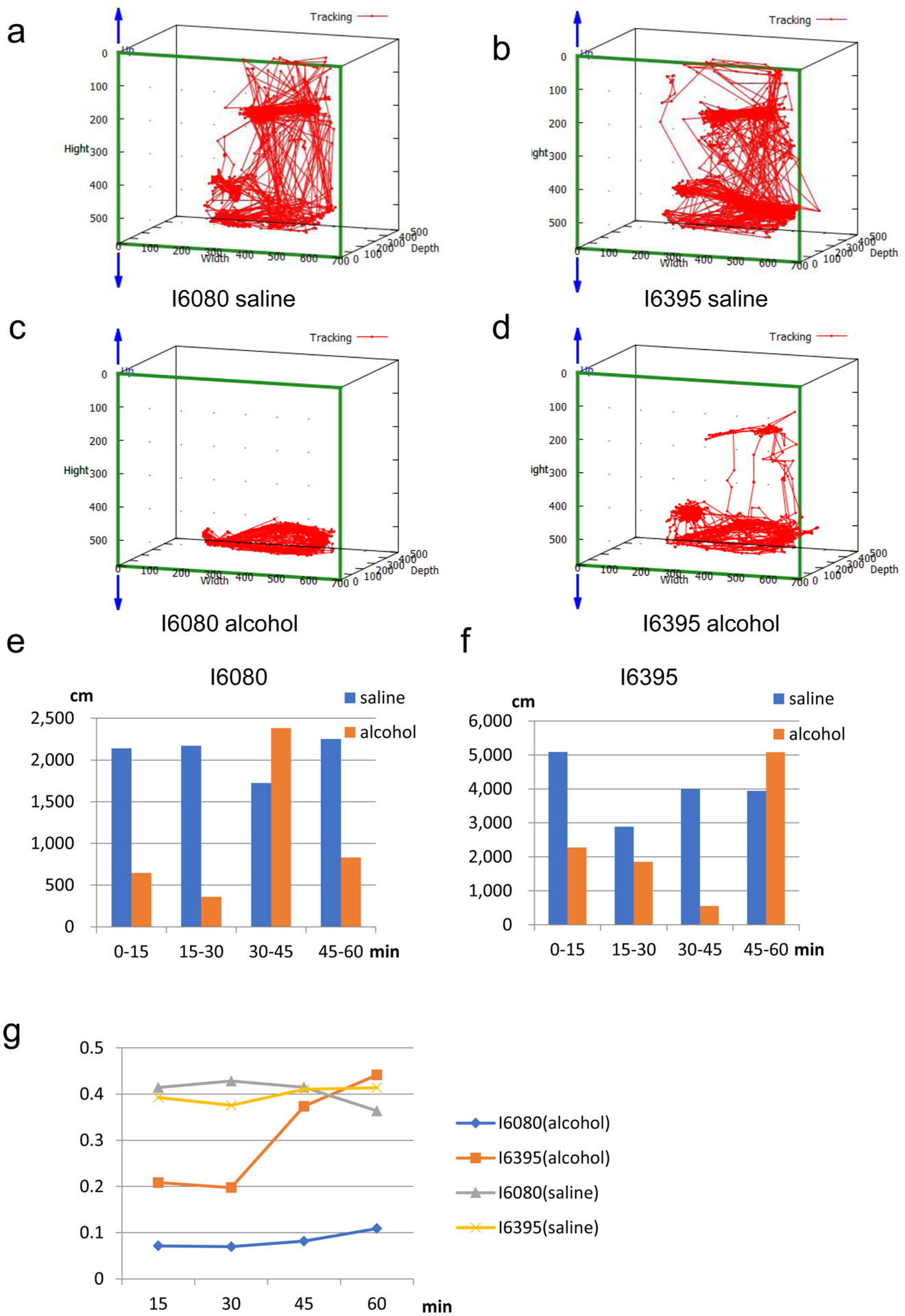
MarmoDetector can analyze transient behavioral changes caused by alcohol administration.
(a–d) One-hour cumulative trajectories of marmosets following saline administration (a and b) and intraperitoneal alcohol (c and d).
(e and f) Quantification of the movement distances in the 1 h following intraperitoneal alcohol and saline administration. The movement distances were averaged over a 15-minute window (900 recordings /window).
(g) The ratio of the vertical movement distance to total movement one over a 15-minute window (900 recordings /window). There was significant difference between saline injected marmosets and alcohol injected ones (two-way ANOVA with repeated measures, F (1,8) = 11.659, p = 0.00916)). Marmosets (especially I6395) restored the vertical movement 30 min after we injected alcohol.
3. Results
3.1. MarmoDetector can trace marmoset movements and assess their kinesis in temporal sequences under stress-free conditions
To analyze marmoset behavior chronologically, we optimised the video recordings and processed multitemporal images. The IR camera and depth sensor took images with a resolution of 512 × 424 pixels per frame. We acquired sequential images of the marmosets at 5 frames per second and used the depth data as binary data that encoded spatial and temporal information for marmoset localisation. To analyze marmoset behavior using these temporal and spatial sequences, we used MarmoDetector to process the binary data and connect each point in time and space (Fig. 2a,1–3), resulting in a 3D trajectory (Fig. 2b–c). In addition, we quantified marmoset activity by analyzing how far each animal moved during various periods (Fig. 2d). Fig. 2d shows the distances that three male marmosets moved every half-hour for 24 h. All marmosets were active during the daytime and rested at night. We conclude that MarmoDetector can perform quantitative assessment of marmoset kinesis in temporal sequences.
Fig. 2.
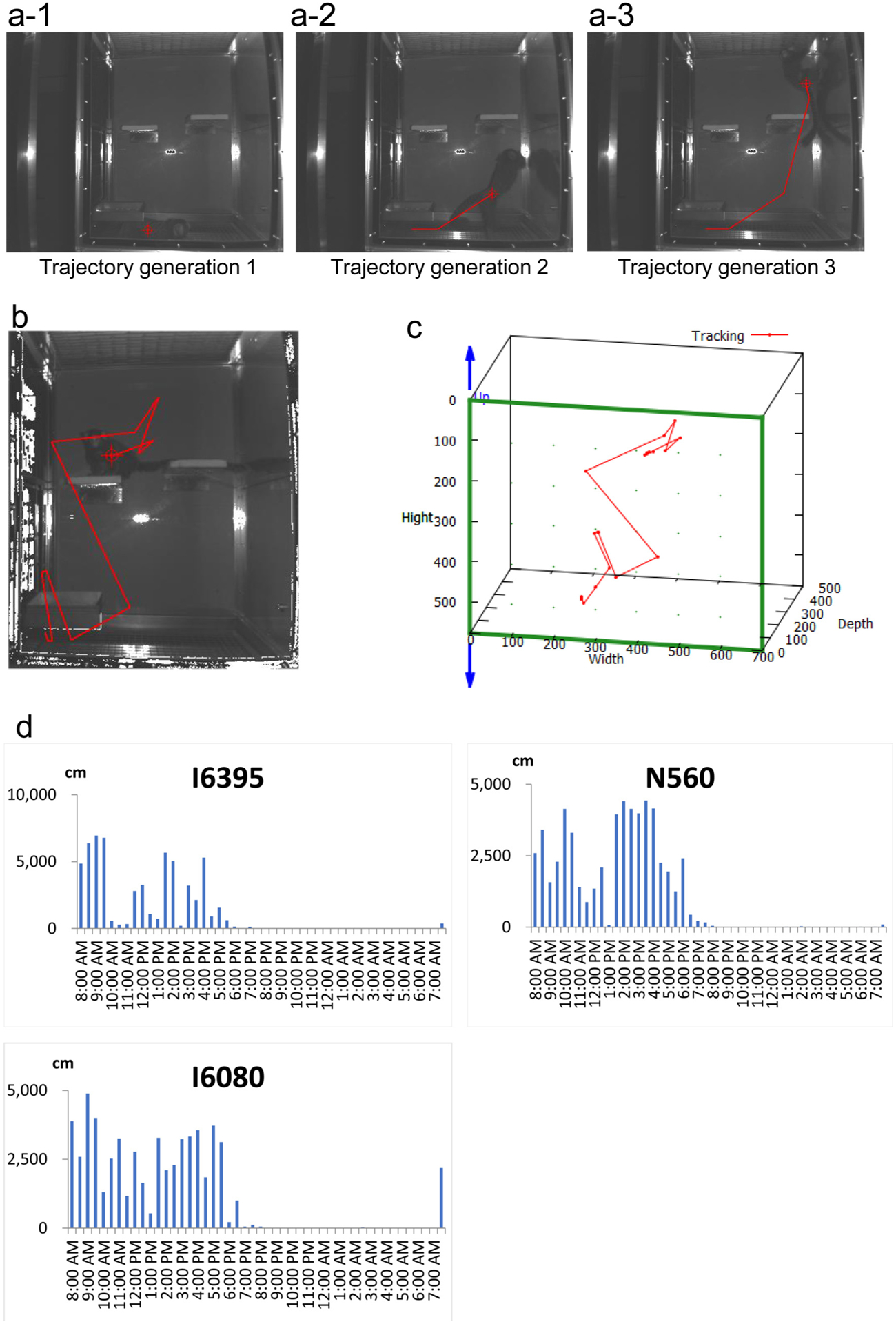
MarmoDetector processes 3D marmoset movements in temporal sequence.
(a1–3) Trajectory generation process. MarmoDetector connects the marmoset locations to make a trajectory. Images a-1, a-2, and a-3 were obtained every two seconds.
(b and c) Representative traces for a marmoset (chosen from n = 6). b: Representative trace in the experimental cage. c: An automated integration of traces using MarmoDetector software (red line).
(d) Twenty-four hour recordings of motor behavior in the experimental cage. The daily activities of three marmosets are shown. Each recording was averaged over a half-hour window (1800 recordings/window).
3.2. MarmoDetector is effective for analyzing circadian behaviors
To determine whether MarmoDetector could evaluate marmoset activity continuously over a long period of time, we analyzed the circadian behavior of marmosets with MarmoDetector. We measured the movement distances of six marmosets (three 16–32 month-old males and three 16–22 month-old females) by collecting data every second over a 120-hour period (Fig. 3a). The recordings showed no variation in circadian activity among the tested marmosets (Fig. 3b, one-way ANOVA, P > 0.05). We recorded general patterns of the X-axis, the Y-axis and the depth movement for three male marmosets reflected the circadian activities of marmosets (Supplementary Figure S4). We also analyzed average movement distances for each sex and found no significant differences between the sexes (Fig. 3c, two-way ANOVA with repeated measures, P > 0.05). These results suggest that MarmoDetector can consistently evaluate the circadian behaviors of both marmoset sexes.
MarmoDetector can detect where marmosets spent time during the daytime and the night-time in the cage. MarmoDetector software can divide the cage equally into twelve areas and measure seconds marmosets spent in each area of the cage (Supplementary Figure S5). In the night-time, each marmoset tends to stay in a specific place. Occasional decreases in movement during the daytime happened and this could mainly be attributed to the sleeping and resting of the marmoset, as revealed by video recording, at the time when MarmoDetector detected decreased levels in moving distance. The decrease in movement distance corresponded to the time when the marmosets stayed inactive (Supplementary Figure S6).
3.3. MarmoDetector, 3D analysis, can detect marmoset movements more precisely than 2D analysis
To determine how precisely MarmoDetector can detect marmoset movement, we compared 2D and 3D analysis of marmoset circadian activities. Using the center of the gravity of the screen coordinate, we showed the general patterns of 2D movement for six marmosets over 120-hour periods (Fig. 4a). Both 2D (Fig. 4a) and 3D (Fig. 3a) analyses showed that all recorded marmosets were active during the daytime but not the night-time, reflecting the circadian activities of marmosets. However, in each period, total movement distance of 3D analysis was also longer than that of 2D analysis, suggesting that 3D analysis can detect activities unrevealed in 2D analysis (Fig. 4b, two-way ANOVA with repeated measures, F (1, 50) = 23. 784, p = 0.0000114). Hence, 3D analysis using MarmoDetector is suitable to analyzing the circadian behaviors of marmosets.
3.4. MarmoDetector can analyze sudden behavioral changes caused by alcohol administration
To determine if MarmoDetector could be used to analyze transient behavioral defects, we administered alcohol (1.5 g per kg of body weight) to the marmosets via intraperitoneal injection. We recorded the motor activities of two marmosets for one hour after alcohol administration using MarmoDetector. We also recorded the motor activities of marmosets injected with saline as a control. Compared to the marmosets injected with saline (Fig. 5 a–b, quantification in Fig. 5e–f), the marmosets injected with alcohol had smaller movement distances as an inhibitory effect (Fig. 5c–d, quantification in Fig. 5e–f). Interestingly, when alcohol was administered to marmosets, they spent more time in the bottom of their cages (Supplementary Figure S7) and tended to move horizontally rather than vertically and showed unique circular movements as an excitatory effect, suggesting that alcohol administration causes complex behaviors including inhibitory and excitatory effects as observed in human. We compared 2D and 3D analysis of behaviors of alcohol injected marmosets (Fig. 5a–b and Supplementary Figure S8). 2D analysis could detect only sedative effects of alcohol a decrease in moving distance caused by acute alcohol administration (Supplementary Figure S8). In marked contrast, MarmoDetector shows that alcohol injected marmosets moved in a circular manner in the horizontal plane (Supplementary Figure S9). Interestingly, alcohol injected marmosets show decreased activity in the vertical axis and marmosets (especially I6395) restored vertical movement gradually (Fig. 5g). We conclude that MarmoDetector could monitor acute, transient complex behaviors of alcohol injected marmoset including the excitatory and inhibitory effects.
3.5. MarmoDetector can detect time-varying behavioral features of alcohol injected marmosets
We applied principal component analysis (PCA) to the alcohol administration study to find time-varying behavioral features of marmosets because we found that the ratio of vertical movement distance to total movement one was clearly different as time passed. We analyzed the first 10 min of saline and alcohol injected marmoset movement data. We also analyzed the last 10 min of alcohol injected marmoset movement data.
The analysis of the first 10 min of saline injected marmosets reflected vertical movement (Fig. 6a). That of the first 10 min of alcohol injected ones showed circular movement similar to 3D movement patterns (Fig. 6b). That of the last 10 min of alcohol injected ones showed that eigenvector of x, y and depth are all positive values (Fig. 6c). It implies the excited state of marmosets caused by alcohol. The PCA classified marmoset movement patterns as normal, drunken (sedative and excited) and excited states. Thus, we can find some features of marmoset behavior from massive data by way of PCA of MarmoDetector data.
Fig. 6.
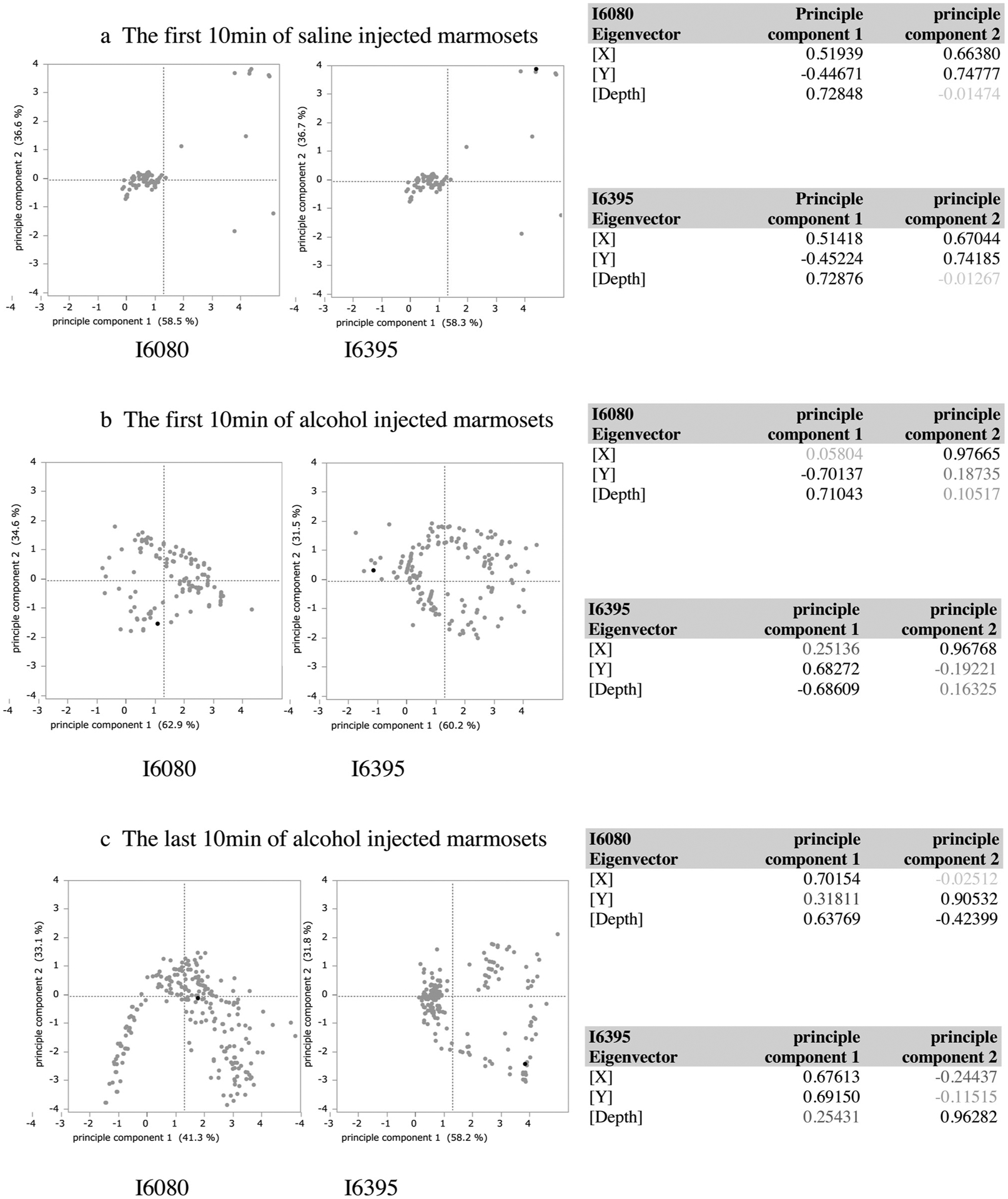
MarmoDetector can detect time-varying features of marmoset movements in alcohol administration study.
We performed the PCA analysis for each coordinate value corresponding to the position of marmosets in alcohol administration study.
(a) The analysis of the first 10 min of alcohol injected marmosets showed circular movement similar to 3D movement patterns. Plots of principle component1 and 2 of each marmoset were shown. The eigenvector of each principle component was also shown.
(b) The analysis of the first 10 min of saline injected ones reflected vertical movement. Plots of principle component1 and 2 of each marmoset were shown. The eigenvector of each principle component was also shown.
(c) The analysis of the last 10 min of alcohol injected marmosets showed that the eigenvector of x, y and depth are all positive values. Plots of principle component1 and 2 of each marmoset were shown. The eigenvector of each principle component was also shown.
4. Discussion
In this study, we described a novel monitoring system, MarmoDetector, which enables us to evaluate marmoset behavior under stress-free conditions. MarmoDetector is the first system adapting the Kinect to record marmoset behavior. Compared to 2D monitoring, 3D analysis using MarmoDetector is more suitable to evaluate movement patterns as observed in alcohol administered marmosets (Fig. 5a–d, Supplementary Figure S8). Alcohol is thought to have complex effect on the central nervous system. Alcohol has inhibitory and stimulant effects. Inhibitory effects have an influence on a smaller movement distance (Karlsson and Roman, 2016; Tran and Gerlai, 2013). In our study, alcohol injected marmosets showed very unique circular movements as the effect of the alcohol. In addition, MarmoDetector even finds marmoset behavioral features in alcohol administration study by way of PCA analysis. MarmoDetector can detect time-varying features such as normal, drunken and excited states of marmosets. Thus MarmoDetector will be useful for dissecting complicated behaviors of the marmosets. It also implies the application possibility that MarmoDetector can detect features of disease model of marmosets.
MarmoDetector does not impose stress on marmosets because it can monitor a freely moving animal without requiring any device on or in the body. This feature has great practical importance for analyzing psychological or neurological defects. In contrast, recording marmoset motor activity using accelerometers (Hoffmann et al., 2012; Santana et al., 2015) may stress the animals because they must carry devices such as vests or belts on their bodies. The presence of the accelerometers may affect their spontaneous behaviors. The non-contact nature of MarmoDetector avoids this problem and enables marmosets to be recorded at any location in the cage, thus providing greater flexibility in experimental design. Furthermore, it is less expensive than other existing sensor systems, such as those equipped with high-speed cameras or multiple IR cameras (Beare et al., 2009; Salau et al., 2016). Microsoft has stopped manufacturing the Kinect Sensor, but other economical Kinect sensors such as Intel RealSense are available. Another depth sensor also detects the center of gravity of the moving object, even if it has different properties, such as its working range and its spatial resolution and sometimes there is a need for adjustment. Our methodology is not specific to Kinect. Detecting the center of gravity of the moving object is applicable to other depth sensors. Our methodology to map marmoset positions is probably applicable to other species such as rodents, even if some adjustments are necessary.
We use only the depth channel so that shadows have no effect on the measurements. As for reflections, we exclude them from the side wall by the ROI which is set before the marmoset enters the MarmoDetector cage. Reflections from the back side wall are hidden behind the marmoset itself so that it does not affect the measurements.
MarmoDetector does not have restrictions related to lighting conditions. This feature enables the recording of marmoset movement in the dark, as well as the monitoring of circadian rhythms and other conditions during sleep. This would enable the use of marmosets for modelling sleep disorders, including REM sleep behavior disorders (Hoffmann et al., 2012; Verhave et al., 2011).
In addition, the length of monitoring can be adjusted to suit the purposes of an experiment. In this study, we recorded marmoset movement for five successive days to analyze circadian behavior. The maximum recording length depends on the availability of data storage. To analyze acute effects of alcohol on marmoset behavior, we monitored marmoset activity for only one hour, which nonetheless enabled us to capture dramatic changes in movement.
Compared with a high-speed camera, five frames per second is not fast enough to be precise; however, as we performed a “location measurement precision” experiment in supplementary Fig. S3, MarmoDetector could detect the moving object turning around on the circle board. In addition, as shown in Fig. 2a, even with measurement every 2 s, MarmoDetector followed the marmoset movement practically. When we measured the gross moving distance in interval units of minutes, hours, or days, we can safely say that MarmoDetector could quantify the movement distance of the object.
Concerning the stress of the marmosets in the experimental cage, we transferred marmosets to the cage one day prior to recording. Habituation to the experimental cage can decrease the stress of the animals. In this study, we confirmed the utility of MarmoDetector to record short time intervals by examining the effects of alcohol on the marmosets. As shown in Fig. 5, we were able to detect movement differences between marmosets in the control and alcohol-administration groups.
The Kinect sensor currently used in MarmoDetector focuses on mapping the location of a marmoset but cannot detect movements in the distal limbs. Therefore, MarmoDetector may be limited in its ability to record fine or involuntary movement of the distal limbs. However, we could monitor movement distance and behavioral change. These features are useful to evaluate neurological disease symptoms such as bradykinesia observed in Parkinson’s disease. MarmoDetector will be effective for analyzing marmosets established as models of neurological and psychological disorders.
5. Conclusion
To quantify the kinesis of marmosets, we created a new recording system, MarmoDetector, which automatically generates 3D trajectories and analyses their motion over time. MarmoDetector can be used to evaluate acute and chronic behavioral changes under a variety of conditions. This system will enable analysis of changes in the movements or behavior of marmosets being used to model human diseases.
Supplementary Material
Acknowledgements
This research was partially supported by the Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) project of the Japan Agency for Medical Research and Development (AMED; to HM), a Grant-in-Aid for Scientific Research on Innovative Areas (Brain Protein Aging and Dementia Control; No. 15H01558), KAKENHI (No. JP17K17866 to FY) from MEXT, Japan Science and Technology Agency PRESTO, Nihon Kohden Corporation, Ultra-thin Electronic Sensor System for Marmoset Brain Signal measurements, the Strategic Research Program for Brain Science from the Japan AMED, Research and development of technologies for high-speed wireless communication from inside to outside of the body and large scale data analyses of brain information and their application for BMI from the Japan’s Commissioned Research of the National Institute of Information and Communications Technology (NICT).
Footnotes
Competing financial interests
The authors have no conflicts of interest to declare.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jneumeth.2019.03.016.
References
- Ando K, et al. , 2017. Diffrential effects of dopaminergic drugs on spontaneous motor activity in the common marmoset following pretreatment with a bilateral brain infusion of 6-hydroxydopmaine. Behav. Pharmacol 28, 670–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcia C, et al. , 2004. Measurement of motor disability in MPTP-treated macaques using a telemetry system for estimating circadian motor activity. J. Neurosci. Methods 134, 59–64. [DOI] [PubMed] [Google Scholar]
- Beare JE, et al. , 2009. Gait analysis in normal and spinal contused mice using the TreadScan system. J. Neurotrauma 26, 2045–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassain C, et al. , 2001. Assessment of motor behavior using a video system and a clinical rating scale in Parkinsonian monkeys lesioned by MPTP. J. Neurosci. Methods 111, 9–16. [DOI] [PubMed] [Google Scholar]
- Fischer KE, Austad SN, 2011. The development of small primate models for aging research. ILAR J. 52, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, et al. , 2013. Enhanced computer vision with Microsoft Kinect sensor: a review. IEEE Trans. Cybern 43, 1318–1334. [DOI] [PubMed] [Google Scholar]
- Hoffmann K, et al. , 2012. Remote long-term registrations of sleep-wake rhythms, core body temperature and activity in marmoset monkeys. Behav. Brain Res 235, 113–123. [DOI] [PubMed] [Google Scholar]
- Karlsson Oskar, Roman Erika, 2016. Dose-dependent effects of alcohol administration on behavioral profiles in the MCSF test. Alcohol 50, 51–56. [DOI] [PubMed] [Google Scholar]
- Kulikov VA, et al. , 2014. Application of 3-D imaging sensor for tracking minipigs in the open field test. J. Neurosci. Methods 235, 219–225. [DOI] [PubMed] [Google Scholar]
- Lyons DM, et al. , 2015. A Kinect-based system for automatic recording of some pigeon behaviors. Behav. Res. Methods 47, 1044–1054. [DOI] [PubMed] [Google Scholar]
- Miller CT, et al. , 2016. Marmosets: a neuroscientific model of human social behavior. Neuron 90, 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaoui S, et al. , 2000. The antioxidant ebselen prevents neurotoxicity and clinical symptoms in a primate model of Parkinson’s disease. Exp. Neurol 166, 235–245. [DOI] [PubMed] [Google Scholar]
- Nakamura A, et al. , 2015. Low-cost three-dimensional gait analysis system for mice with an infrared depth sensor. Neurosci. Res 100, 55–62. [DOI] [PubMed] [Google Scholar]
- Okano H, et al. , 2012. The common marmoset as a novel animal model system for biomedical and neuroscience research applications. Semin. Fetal Neonat. Med 17, 336–340. [DOI] [PubMed] [Google Scholar]
- Prins NW, et al. , 2017. Common marmoset (Callithrix jacchus) as a primate model for behavioral neuroscience studies. J. Neurosci. Methods 284, 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema PR, Treue S, 2014. Basic neuroscience research with nonhuman primates: a small but indispensable component of biomedical research. Neuron 82, 1200–1204. [DOI] [PubMed] [Google Scholar]
- Ronald KB, et al. , 1998. De novo administration of ropinirole and bromocriptine induces less dyskinesia than L-DOPA in the MPTP-treated marmoset. Mov. Disord 13, 234–241. [DOI] [PubMed] [Google Scholar]
- Salau J, et al. , 2016. Developing a multi-kinect-system for monitoring in dairy cows: object recognition and surface analysis using wavelets. Animal 10, 1513–1524. [DOI] [PubMed] [Google Scholar]
- Samson Andre L., et al. , 2015. MouseMove: an open source program for semi-automated analysis of movement and cognitive testing in rodents. Sci. Rep 5, 16171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana M, et al. , 2015. Characterization of long-term motor deficits in the 6-OHDA model of Parkinson’s disease in the common marmoset. Behav. Brain Res 290, 90–101. [DOI] [PubMed] [Google Scholar]
- Sasaki E, et al. , 2009. Generation of transgenic non-human primates with germline transmission. Nature 459, 523–527. [DOI] [PubMed] [Google Scholar]
- Schiel N, Souto A, 2017. The common marmoset: an overview of its natural history, ecology and behavior. Dev. Neurobiol 77, 244–262. [DOI] [PubMed] [Google Scholar]
- Togasaki DM, et al. , 2005. The webcam system: a simple, automated, computer-based video system for quantitative measurement of movement in nonhuman primates. J. Neurosci. Methods 145, 159–166. [DOI] [PubMed] [Google Scholar]
- Tomioka I, et al. , 2017. Transgenic monkey model of the polyglutamine diseases recapitulating progressive neurological symptoms. eNeuro 4, 0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Steven, Gerlai Robert, 2013. Time-course of behavioral changes induced by ethanol in zebrafish (Danio rerio). Behav. Brain Res 252, 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhave PS, et al. , 2011. REM sleep behavior disorder in the marmoset MPTP model of early Parkinson disease. Sleep 34, 1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton Ashley, et al. , 2006. Automated video analysis of age-related motor deficits in monkeys using Etho Vision. Neurobiol. Aging 27, 1477–1483. [DOI] [PubMed] [Google Scholar]
- Wright MJ, et al. , 2013. Acute ethanol reduces reversal cost in discrimination learning by reducing perseverance in adolescent rhesus macaques. Alcohol. Clin. Exp. Res 37, 952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


