Abstract
The ability of macrophages to promote vascular growth has been associated with the secretion and local delivery of classic proangiogenic factors (e.g., VEGF-A and proteases). More recently, a series of studies have also revealed that physical contact of macrophages with growing blood vessels coordinates vascular fusion of emerging sprouts. Interestingly, the interactions between macrophages and vascular endothelial cells (ECs) appear to be bidirectional, such that activated ECs also support the expansion and differentiation of proangiogenic macrophages from myeloid progenitors. Here, we discuss recent findings suggesting that dynamic angiogenic vascular niches might also exist in vivo, e.g. in tumors, where sprouting blood vessels and immature myeloid cells like monocytes engage in heterotypic interactions that are required for angiogenesis. Finally, we provide an account of emerging mechanisms of cell-to-cell communication that rely on secreted microvesicles, such as exosomes, which can offer a vehicle for the rapid exchange of molecules and genetic information between macrophages and ECs engaged in angiogenesis.
Keywords: Macrophage, Monocyte, Angiogenesis, Blood vessel, Heterotypic cell interaction, Microvesicle, Exosome
Introduction
Macrophages are an important component of the innate arm of the immune system, as they constitute a first line of defense against invading pathogens. They engulf microbes and can present antigens to T-cells to promote specific (adaptive) immune responses. In addition, macrophages perform a broad array of functions beyond immune surveillance, and regulate tissue and organ growth, remodeling, and homeostasis. This is primarily achieved via their secretion of cytokines, growth factors, proteolytic enzymes, and expression of scavenger receptors that recognize multiple components of the extra-cellular matrix (ECM) [1,2]. Thus, macrophages play crucial roles in tissue morphogenesis (e.g., vascular and neuronal patterning) and patho-physiological conditions like inflammation and organ healing [3,4].
It has long been recognized that macrophages can support angiogenesis [5], the formation or expansion of new blood vessels during development and post-natal life [6,7]. Early reports showed that activated macrophages inoculated in the cornea of guinea pigs induced robust vascular proliferation [8], hence suggesting that these cells are an important source of proangiogenic signals. More recent studies have indeed revealed that (activated) macrophages are a source of multiple growth factors that can enhance endothelial cell (EC) proliferation and/or survival [9]. Furthermore, macrophages promote the remodeling of the ECM and provide survival and/or “guidance” cues to the ECs, both of which are important for EC migration and the (directional) growth of new blood vessels [10,11]. Finally, macrophages engage in tight cell-to-cell contacts with sprouting blood vessels to facilitate the “bridging” of endothelial sprouts during vascular anastomosis [12].
The proangiogenic functions of macrophages have been actively studied in the context of retinal and tumor angiogenesis. For example, abundant macrophages are observed in tumors, often in direct contact with the ECs of uncoated or partially coated tumor blood vessels [9,13–16]. These findings are consistent with the evaluation of human cancer specimens, in which high numbers of macrophages often correlate with increased angiogenesis [17,18]. Together, these data suggest that macrophages may exert direct proangiogenic functions in tumors. Indeed, mechanistic studies showed that depletion of monocytes/macrophages in tumor-bearing mice inhibits tumor angiogenesis and may delay cancer growth and progression [13,15;19–22].
In this review, we collate recent findings on the role of macrophages as regulators of angiogenesis. We further discuss the novel concept that EC-macrophage interactions are reciprocal: while macrophages regulate angiogenesis, ECs in turn provide signals that promote the expansion of proangiogenic macrophages within the perivascular microenvironment.
Proangiogenic functions of macrophages: role of secreted factors
Activated macrophages, such as tumor-associated macrophages (TAMs), secrete growth factors and inflammatory cytokines that can enhance EC activation, proliferation and survival (Fig. 1A). These include key proangiogenic mediators, such as vascular-endothelial growth factor (VEGF)-A, which activates the VEGF receptor (VEGFR)-2. Macrophages also express other VEGF family members, including placental growth factor (PlGF), which activates VEGFR-1; and VEGF-C, which activates VEGFR-3 and downstream NOTCH signaling [9,23]. Additional angiogenesis regulators expressed by macrophages include CXCL8, interleukin (IL)-1β, tumor-necrosis factor-α (TNFα), and basic fibroblast growth factor (FGF2). The functions of these cytokines, and the angiogenic responses triggered upon their binding to cognate receptors expressed on ECs, have been extensively reviewed elsewhere [9,24].
Fig. 1 –
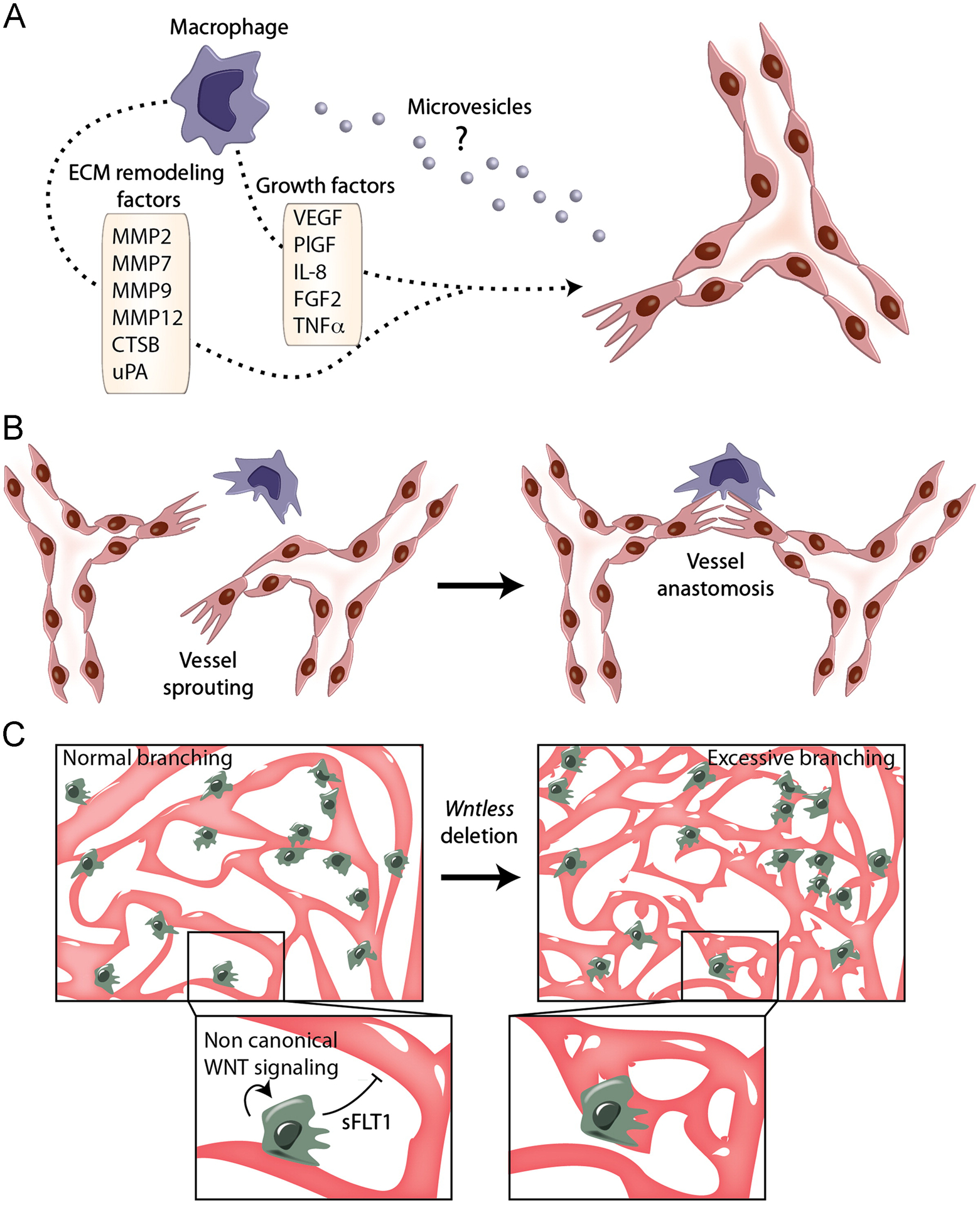
Indirect and direct communication between ECs and macrophages. (A) M2-like macrophages (violet) secrete proangiogenic growth factors and proteases that facilitate EC migration and vascular growth. Furthermore, macrophages secrete microvesicles, such as exosomes, that may fuse with and deliver their cargo of proteins and RNAs to ECs, possibly influencing the angiogenic response of the blood vessel. (B) M2-like macrophages (violet) associate with adjacent vascular sprouts and “bridge” endothelial tip cells to facilitate vascular anastomosis in the developing mouse hindbrain. (C) Macrophages associate with growing blood vessels in the deep vascular plexus of the retina. Non canonical WNT signaling stimulates macrophage secretion of sFLT1, which limits vessel branching (left). In the absence of WNT signaling, excessive vessels branches are formed (right).
Macrophages activated during angiogenesis also secrete membrane-bound or soluble proteases that, via proteolytic digestion of the ECM, can mobilize proangiogenic growth factors embedded in the perivascular matrix. These include enzymes such as matrix-metalloproteinases (MMP-2, MMP-7, MMP-9, MMP-12) and serine/cysteine proteinases (urokinase, cathepsins, plasminogen activator) [24,25]. Furthermore, macrophages are thought to express “vascular guidance” molecules, like semaphorins, which modulate EC migration and survival [11,26]. Together with the matrix-remodeling activity of the many secreted proteases, the activity of macrophage-derived “vascular guidance” molecules may facilitate blood vessel sprouting and directional vascular growth through the ECM in tissues undergoing angiogenesis.
Recent findings also suggest that macrophages secrete microvesicles (MVs) that can deliver their cargo of macromolecules upon fusion with “acceptor” cells [27,28], possibly including ECs (Fig. 1A). Macrophage-derived MVs may thus have the potential to influence EC behavior via direct transfer of functional RNAs or proteins (see below).
Vascular-modulatory functions of macrophages: role of cell-to-cell contacts
Recent studies have suggested that, during fetal development, macrophages regulate vascular morphogenesis in the central nervous system by establishing cell-to-cell contacts with ECs (Fig. 1B, C).
Hindbrain angiogenesis
In the developing hindbrain, vascular growth follows two main steps, the first being the sprouting of pre-existing blood vessels and the second the fusion of such vascular sprouts with adjacent capillaries (a process known as vascular anastomosis). Whereas it is well-known that vessel sprouting is largely induced by VEGF-A gradients, less is known about how the ECs present at the leading edge of the sprout (tip cells) fuse to form new circuits that expand the vascular network. Fantin and co-workers showed that hindbrain macrophages (the yolk-sac derived precursors of adult microglia) and vascular ECs closely interact during the process of vascular anastomosis, both in mouse and zebrafish embryos [12]. Remarkably, mice carrying a loss-of-function mutation in PU.1—a transcription factor required for macrophage differentiation [29]—had fewer vessel intersections than wild-type mice [12]. These findings were also confirmed using colony stimulating factor-1 (CSF1) mutant mice (Csf1op/op), which have impaired macrophage development [30].
The aforementioned data suggest that macrophages are required for vascular development, at least in the embryonic hindbrain [12]. Of note, macrophage deficiency did not affect the formation of the vascular sprouts, a phenomenon that, as mentioned previously, is largely VEGF-dependent and is impaired in VEGF-mutant mice [31,32]. Rather, tissue macrophages were important to support anastomosis of the endothelial tip cells, which occurs subsequent to VEGF-induced vessel sprouting. Imaging of macrophages and ECs revealed that the former physically associate with sprouting ECs and bridge them to form new vascular intersections (Fig. 1B). Interestingly, these “bridging” macrophages express the angiopoietin (ANG) receptor, TIE2, and the VEGF co-receptor, neuropilin-1 (NRP1), thus they resemble the perivascular macrophages that promote angiogenesis in tumors [20,33,34] (see below). These fascinating observations support the concept that, in addition to the well-established secretion of proangiogenic (growth) factors and matrix-remodeling enzymes, communication cues between macrophages and vascular ECs also encompass direct cell-to-cell contacts.
Retinal angiogenesis
Kubota and co-workers described a similar phenomenon in the mouse retina [35]. The development of the retinal vasculature is initiated post-natally at the optic nerve head with the radial expansion of a primary (or superficial) vascular plexus(post-natal days 0–7 [P0–7] in C57Bl/6 mice). The superficial plexus then projects vertical sprouts into the retina, which expand radially inside the retina to form the deep (P7–12) and intermediate (P12–15) vascular plexuses [36,37]. Kubota and co-workers showed that macrophages in the retina are essential for normal vessel network formation in the first week of the mouse life. Indeed, macrophage deficiency in Csf-1op/op mice led to reduced branching in the vascular plexus of the retina at P2. Interestingly, macrophage deficiency did not affect the number of tip cells or filopodia. The vascular defects observed in Csf-1op/op mice subsided as development progressed; in fact, the vasculature in the mutant mice was comparable to wild-type mice at three months after birth [35].
Rymo and co-workers confirmed that macrophages play an Important role in the induction of endothelial tip cells in the developing mouse retina [38]. These investigators observed abundant retinal macrophages (microglia) at the forefront of the growing primary vascular plexus. In particular, the retinal macrophages were frequently present at sites where endothelial tip cells contact each other through their filopodia. Morphometric analyses further indicated that each macrophage theoretically contacts two neighboring endothelial tip cells. Interestingly, the authors confirmed findings by Kubota by demonstrating that macrophage deficiency in Csf-1op/op mice leads to a sparser vascular plexus. They also observed that the vascular network displayed reduced numbers of filopodia-bearing sprouts [38]. In particular, the retinal vascular sprouts of Csf-1op/op mice were fewer and mostly radially-oriented, whereas those of control mice were both radially- and forward-oriented, and formed more intersections [38]. Overall, these data are consistent with findings of Fantin and co-workers [12] and Kubota and co-workers [35], and imply that macrophage-EC contacts support vascular morphogenesis in the central nervous system. However, the regulation of endothelial tip cells by macrophages remains controversial [12,35,38]. Indeed, whereas the study by Rymo and co-workers provides evidence that macrophages induce endothelial tip cells, Fantin and co-workers [12] and Kubota and co-workers [35] found no change in endothelial tip cell numbers upon macrophage depletion in either hindbrain or retina.
Rymo and co-workers also asked whether direct cell-to-cell contacts between retinal macrophages and ECs are necessary to promote blood vessel branching. By using mouse aortic ring assays, they showed that macrophages induce significant vascular sprouting before establishing physical contact with the nascent vessels [38]. While these data may imply that direct macrophage-EC contacts are not essential for vascular sprouting, at least in the mouse aortic ring assay, it is very likely that a combination of cell-to-cell contacts and secreted proangiogenic factors is required for normal vascular morphogenesis [4,12,38]. Along these lines, a recent study shed light on the mechanism involved in macrophage-endothelial tip cell contacts at vascular fusion sites [23]. Tammela and co-workers found that EC deletion of Vegfr3 leads to excessive angiogenic sprouting and branching in the retina, as well as decreased NOTCH signaling. Interestingly, TIE2-positive macrophages secreting the VEGFR-3 ligand, VEGF-C, localized at vessel branch points, and VEGF-C heterozygous mice exhibited decreased vascular branching in the retina. The results indicate that VEGF-C/VEGFR-3 signaling controls, at least in part, the fusion of vascular sprouts and implicate macrophages in the phenotypic conversion of ECs at vascular fusion points [23].
Close spatial relationships between macrophages and endothelial tip cells are also observed in the deep layers of the post-natal retina. At that site, macrophages and ECs engage in heterotypic signaling interactions that activate WNT signaling in the endothelium [39]. WNT signaling has been shown to play a key role in the regulation of cell proliferation and differentiation during development, and has been implicated in several other processes such as cancer progression and regulation of stem cells [40]. In the canonical WNT pathway, WNT proteins bind to cell-surface receptors and activate downstream mediators that lead to the nuclear translocation of β-catenin, which then associates with other transcription factors to control the expression of WNT target genes. Interestingly, deletion of the essential WNT transporter, Wntless, in retinal macrophages augmented vessel branching, suggesting that macrophage-derived WNT ligands suppress (excessive) branch formation in the deep retinal layer. Furthermore, it was found that non-canonical WNT signaling in the macrophages stimulates secretion of soluble VEGFR-1 (sFLT1) [39] (Fig. 1C), a potent anti-angiogenic factor able to sequester VEGF-A and hence limit its bioavailability [6]. These findings are consistent with earlier data showing that monocytic cells can exert anti-angiogenic functions via secretion of sFLT1 [41]. Thus, whereas macrophages promote the growth of retinal blood vessels in the primary plexus [35,38], they also appear to limit excessive branching of the deep vascular plexus [39].
A role for macrophages in the negative regulation of vascular viability was also revealed by studies in the hyaloid vessels, a temporary vascular bed that supports the lens during development [42]. In the mouse, regression of the hyaloid vasculature occurs post-natally and involves the activity of vessel-associated, WNT7B-secreting macrophages [43]. In the presence of angiopoietin-2 (ANG2) produced by ECs, macrophage-derived WNT7B stimulates EC entry into the cell cycle, therefore sensitizing them to ANG2 [44]. Together with the aforementioned findings of Stefater III and co-workers [39], these interesting data support the notion that, at selected anatomical sites and/or developmental stages, perivascular macrophages finely regulate angiogenesis also by limiting the formation of (extra-numeral) vessel branches. This process appears to be tightly determined through spatially controlled cell-to-cell contacts and induction of EC apoptosis mediated by ANG2 or sFLT.
Pathological retinal angiogenesis is also regulated by macrophages. For example, Checchin and co-workers investigated the role of microglia in retinal blood vessel formation in a model of ischemic retinopathy induced by hyperoxia or hypercapnia [45]. Interestingly, ischemic retinas exhibit reduced microglia and vessel density. Depletion of resident retinal microglia using clodronate liposomes further reduced retinal vessel development and density, an effect that was rescued by adoptive transfer of microglial cells. Therefore, these data suggest that pathological retinal vascularization requires the activity of microglial cells [45].
Tumor angiogenesis
While ANG2 and non-canonical WNT signaling have been implicated in the negative regulation of angiogenesis by perivascular retinal macrophages, the molecular determinants of proangiogenic macrophage-EC contacts in growing blood vessels are yet to be identified. Moreover, the EC-derived factors that tether macrophages in close contact with the angiogenic ECs are unknown. Mazzieri and co-workers suggested that the ANG2/TIE2 axis may modulate, at least in part, such heterotypic cell-to-cell interactions during tumor angiogenesis [46]. TIE2 is a receptor tyrosine kinase constitutively expressed on ECs and upregulated on a subset of perivascular and proangiogenic TAMs, also known as TIE2-expressing macrophages (TEMs) [15;20]. To study the putative role of TIE2 in mediating macrophage-EC association in tumors, Mazzieri and co-workers employed a conditional knock-down strategy to silence TIE2 expression in TAMs. They found that suppression of TIE2 in perivascular TAMs impedes their association with the angiogenic tumor blood vessels. Of note, the pharmacological blockade of the TIE2 ligand, ANG2, also disrupted TAM-EC association; in both cases, tumor angiogenesis was inhibited [46]. Together, these observations suggest that ANG2-TIE2 signaling, or the upregulation of TIE2 on the surface of perivascular TAMs per se, are important to enable the association of macrophages with blood vessels and sustain tumor angiogenesis. The data support a tentative model in which activated ECs release ANG2 [47], which in turn stimulates macrophages to associate with sprouting blood vessels by (i) upregulating macrophage expression of TIE2, either directly or indirectly; and (ii) inducing the formation of EC-macrophage contacts mediated by TIE2 and ANG2 [48]. Of note, recent studies have shown that ANG2 mediates the formation of ANG2-TIE2 complexes at EC junctions [49], possibly indicating that similar complexes may also form at heterotypic cell-to-cell contacts, i.e. between TIE2+ macrophages and ECs. Another ligand/receptor pair that may be involved in mediating macrophage-EC interactions is CXCL12/CXCR4. Indeed, CXCL12 secreted by angiogenic blood vessels is responsible for the “perivascular retention” of CXCR4+ monocytes recruited in response to VEGF [50].
Taken together, the aforementioned studies suggest that pro and anti-angiogenic interactions between macrophages and ECs may entail, or even require, physical contact between the two cell types. Interestingly, perivascular macrophages have also been recently implicated in the regulation of lymphangiogenesis, both in development and disease [4,51]. For example, Gordon and co-workers identified a subset of macrophages that intimately associate with the lymphatic vessels and regulate their growth in the mouse embryonic skin [52]. In this study, cell-to-cell contacts, and not the secretion of classic lymphangiogenic growth factors like VEGF-C, underlay the lymphangiogenic activity of the perivascular macrophages in the developing skin.
Diversification of macrophages: different subtypes for distinct functions?
The fact that macrophages can promote both vascular growth and vascular regression prompts the obvious question as to whether these functions are executed by distinct cellular subtypes or activation states. Indeed, macrophages can be either classically (M1) and alternatively (M2) activated, in analogy to the T-helper (Th) type-1 (Th1) and Th2 classification of lymphocytes [53;54]. In vivo, classically activated (or M1-like) macrophages are mostly found in inflamed tissues and help combat infections, whereas alternatively activated (or M2-like) macrophages regulate the resolution of inflammation and promote tissue healing [1;53;54]. M1 and M2-like macrophages are also found in tumors and have been portrayed to exert distinct functions, with M1-like being antitumoral and M2-like being proangiogenic and protumoral [53;54].
Macrophage activation/polarization toward the M1 and M2 phenotype is mediated by specific and distinct cytokines; thus, microenvironmental signals tune the spectrum and relative abundance of one versus the other subtype. Moreover, the complexity of microenvironmental signals is such that M1 and M2 phenotypes represent two ends of a continuum of phenotypes, and intermediate states of activation are likely to predominate in vivo [54]. Interestingly, the gene expression signature of perivascular macrophages found in tumors and fetal tissues is largely consistent with an M2-like phenotype [4,33,51,55], perhaps suggesting that defined blood vessel-derived factors contribute to shape the phenotype and functional activation of macrophages in perivascular niches. However, it is currently unclear whether the macrophages that mediate pruning of the hyaloid vessels [43] or limit uncontrolled branching of the retinal vessels [39] are phenotypically distinct from those that promote vascular anastomosis during hindbrain [12] or retinal development [35,38].
Another relevant question is whether the yolk-sac derived macrophages that associate with developing blood and lymphatic vessels [12,52] have the same developmental origin and function as other yolk-sac derived macrophages that populate fetal tissues and persist in adult organs [56]. Interestingly, perivascular fetal macrophages [12,38,52] share many phenotypic similarities with the perivascular TAMs that promote angiogenesis in tumors [33], a concept that is primarily supported by the strikingly convergent gene expression signatures of the two macrophage populations [33,55]. Indeed, both fetal and tumor-derived perivascular macrophages were reported to express markers that are seldom expressed by other tissue macrophages. These include TIE2 [12,33,52], NRP1 [12,33] and several ECM receptors, including macrophage mannose receptor (MRC1/CD206), hyaluronan receptor-1 (LYVE1), stabilin-1 (STAB1), and the aptoglobin/hemoglobin scavenger receptor (CD163) [33,52]. These analogies likely reflect functional convergence dictated by microenvironmental cues present in the perivascular niches rather than bona fide developmental relationships. Indeed, whereas fetal macrophages are derived from the yolk-sac before the emergence of hematopoietic stem cells (HSCs), the perivascular TAMs are derived from bone marrow (BM)-derived HSCs [15].
Macrophages and angiogenesis in human tumors
There is increasing evidence that angiogenesis in human cancer is promoted by perivascular macrophages that are phenotypically similar to their mouse counterpart. For example, a recent study documented a correlation between the number of CD163+ TAMs and that of angiogenic sprouts in follicular lymphoma patients that had a poor prognosis [57]. Interestingly, CD163+ TAMs (but not CD163-negative macrophages) were frequently observed within the immediate microenvironment surrounding the neovascular sprouts in the human tumor biopsies. Another recent study showed that the frequency of TIE2+ macrophages (TEMs) was higher in the vascular-rich areas of human hepatocellular carcinomas [58], again suggesting the preferential involvement of a specific macrophage subtype in the regulation of human tumor angiogenesis.
However, it should be noted that proangiogenic macrophages are not exclusively found in perivascular tumor areas. For example, Leek and co-workers found that macrophage density in human breast cancer specimens was higher in poorly than highly vascularized tumor areas [17]. Nevertheless, macrophage infiltration correlated with the overall vascular grade of the tumors. These findings imply that the proangiogenic activity of TAMs does not necessarily depend on their location around tumor blood vessels. Indeed, VEGF-expressing macrophages were mostly observed in hypoxic, avascular tumor areas of breast cancer specimens [59].
The crosstalk is reciprocal: ECs provide a vascular niche for the differentiation of perivascular, proangiogenic macrophages
A large body of data thus far indicates that perivascular macrophages modulate the biology of ECs during angiogenesis and vascular remodeling. However, these macrophage-EC interactions are bidirectional, as ECs were recently shown to support the differentiation of alternatively activated/M2-like macrophages [60]. He and co-workers showed that EC monolayers provide a niche for the expansion and differentiation of M2-like macrophages directly from mouse hematopoietic progenitor cells (HPCs) (Fig.2A). In contact with ECs, HPCs formed colonies that exhibited a progressive differentiation, from the center to the periphery, toward a proangiogenic, M2-like phenotype, characterized by increasing expression of TIE2, MRC1 and other typical “M2 markers”. Of note, direct contact between HPCs and ECs was necessary for the formation and maintenance of the macrophage colonies, as the latter failed to form in transwell assays. Mechanistically, it was found that EC-derived CSF1 supported HPC expansion and macrophage survival in the co-cultures, whereas VEGF-A produced by the macrophages likely enhanced the long-termed stability of the EC monolayer. Of note, EC-induced, M2-like macrophages were found to associate with tumor blood vessels and to enhance vascular density and tumor growth when co-injected with cancer cells in mice [60].
Fig. 2 –
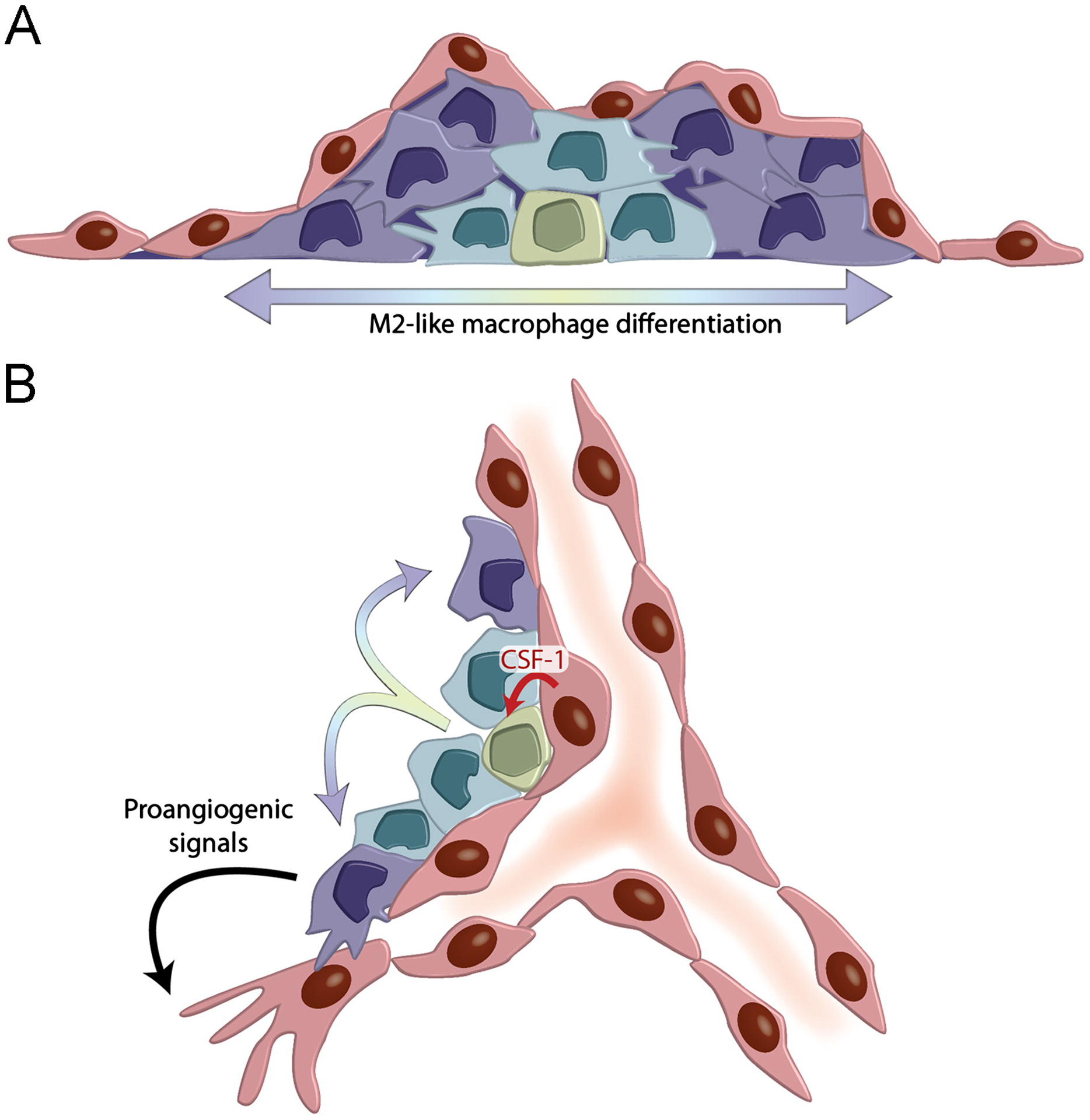
ECs support M2-like macrophage differentiation. (A) EC monolayers support the expansion of HPCs, which form myeloid cell colonies characterized by the progressive differentiation of M2-like macrophages. (B). Hypothetical model of the angiogenic vascular niche, which supports reciprocal interactions between ECs and proangiogenic, M2-like macrophages. ECs support HPC expansion (red arrow) and differentiation into M2-like macrophages (rainbow arrows); M2-like macrophages support angiogenesis by delivering proangiogenic signals to sprouting ECs.
Based on the aforementioned findings, it is tempting to speculate that, in growing/angiogenic tissues, monocytes/macrophages and ECs form an instructive vascular niche that supports (i) proangiogenic macrophage development and differentiation; (ii) macrophage-dependent angiogenesis (Fig. 2B). It is currently unclear whether the EC niche that supports M2-like macrophage differentiation exists at different anatomical sites, such as in the BM or at metastatic sites. It is likely that, in addition to CSF1, other EC-derived factors orchestrate the functional differentiation of M2-like macrophages from immature myeloid-cell progenitors in angiogenic vascular niches. Secreted factors that have been shown previously to instruct M2-like macrophage differentiation include the Th2 cytokines, IL-4 and IL-13 [61,62]. Thus, it will be worth to investigate whether these T-cell derived cytokines are also secreted by ECs and can promote the perivascular differentiation of proangiogenic macrophages in ad hoc experimental systems.
The findings of He et al. [60] may also imply that the terminal differentiation of perivascular, M2-like macrophages, such as TEMs, might be mediated by cell-cell interactions with the endothelium as these cells (or their precursors) extravasate and gain residence in the vessel wall. Indeed, gene marking studies using Tie2-GFP reporter lentiviral vectors showed the frequent occurrence of clusters of Tie2-GFP+ cells with the morphology of immature monocytes in close association with sprouting blood vessels [15,20,63]. It is still unknown whether these TIE2+ monocytic cells proliferate upon their extravasation in perivascular spaces of tumors and before acquire the morphology and phenotype of bona fide macrophages. Nevertheless, the observation that TIE2+ monocytes, when engineered to express the Herpes symplex thymidine kinase gene, are efficiently depleted by ganciclovir suggests that they may proliferate while upregulating the expression of Tie2 in the peri-endothelial microenvironment [15,20,63]. Consistent with this hypothesis, recent studies have shown that, during inflammation and tumor growth, BM-derived HPCs accumulate at extramedullary sites, which can become important sites of monocyte/macrophage production and differentiation [64,65].
Cell-to-cell communication mediated by secreted microvesicles (MVs)
In addition to their production of canonical proangiogenic growth factors, macrophages might stimulate angiogenesis by secreting MVs, such as exosomes [27] (Fig. 1A). Exosomes are small MVs (40–100 nm) that contain proteins, lipids, and RNAs. They are thought to originate from the inward budding of vesicles within endosomes. These large endosomes then fuse to the plasma membrane and release free exosomes. MVs therefore contain cytosol enclosed by a lipid bilayer. Exosomes and other MVs are secreted by many different cell types, including epithelial cells, ECs, inflammatory (immune) cells, and neurons [27]. Several recent reports have shown that secreted MVs can be taken up by neighboring cells upon MV fusion with the acceptor cell’s plasma membrane [28]. As MVs contain donor-cell derived molecules, their cargo may affect the phenotype/behavior of the acceptor cells. This form of cell-to-cell communication may also be long-ranging. Indeed, MVs circulate in the peripheral blood and, in this manner, they are systemically distributed [28,66].
Macrophages and dendritic cells (DCs) release abundant amounts of exosomes and other MVs in vitro [67,68]. In some of these studies, macrophage/DC-derived MVs were found to contain microRNAs (miRNAs) that might be transferred to acceptor cells [69–72]. For example, Zhang and co-workers showed that THP1 human monocyte-like, leukemic cells secrete exosomes enriched in miR-150 [69]. Interestingly, THP1-derived exosomes were shown to fuse with human HMEC1 ECs and to increase their endogenous miR-150 levels by several-folds. Although the rigorous demonstration of active-miRNA transfer from the monocytes to the ECs requires further studies, the work by Zhang provides circumstantial evidence that macrophage-derived exosomes have the potential to influence EC behavior in vitro. Indeed, purified THP1 exosomes could increase HMEC1 cell migration in a transwell assay [69].
These intriguing findings suggest that perivascular macrophages may secrete MVs/exosomes that are enriched for specific proteins or RNAs, which in turn could modulate gene expression and function of ECs engaged in angiogenesis. Of note, angiogenic and tumor blood vessels in particular, differ substantially from the quiescent vasculature. Peri-endothelial layers of vascular mural cells (pericytes and smooth muscle cells) and the integrity of endothelial basement membranes are often disrupted during angiogenesis to enable EC migration and growth [6,7]. These microanatomical changes make it conceivable that perivascular macrophages recruited during angiogenesis could influence vascular biology and patterning also through MV secretion and transfer to ECs. Further studies are now warranted to explore the significance of this phenomenon for angiogenesis in vivo.
Concluding remarks
The data discussed above indicate that the proangiogenic activity of macrophages (or specific subsets of these cells) encompasses both the production of classic proangiogenic factors, as well as the physical association of macrophages with sprouting blood vessels. The latter requires the direct interaction of ECs with (M2-like) macrophages, a process that appears to be regulated, at least in part, by the ANG2/TIE2 and CXCL12/CXCR4 signaling axes [46,50]. More recent studies further suggest that macrophage-EC interactions are indeed bidirectional, as in vitro co-culture studies showed that EC monolayers can support the differentiation of such M2-like macrophages from myeloid progenitors [60]. These observations suggest that a dynamic angiogenic vascular niche might also exist in vivo, for example in tumors where sprouting blood vessels and immature myeloid cells engage in reciprocal interactions.
Fetal macrophages have been shown to both promote and limit the formation of novel vascular intersections in developing neural tissues [12,35,38,39,43,44]. Although these apparent discrepancies may well reflect the known plasticity of macrophages as well as anatomical-site or developmental-stage specific functions (see section 4 above), several questions currently remain unanswered. Do the macrophages that promote vascular growth and/or anastomosis by bridging sprouting ECs [12,35,38] differ from those that promote vascular pruning in the eye [39,43,44]? What are the microenvironmental cues that modulate such opposite functions in ostensibly similar tissues? Do signals released by differently activated ECs instruct such divergent macrophage phenotypes? Addressing these questions, together with the identification of (EC-derived) signals that regulate proversus anti-angiogenic activity of macrophages, may ultimately provide the means to reprogram perivascular macrophages toward an angiostatic or even anti-angiogenic function in tumors.
Acknowledgments
Work in the authors’ laboratories was supported by grants from the European Research Council (TIE2+MONOCYTES), the Swiss National Centers of Competence in Research (NCCR), the Swiss National Science Foundation (SNSF), Anna Fuller Fund (to MDP); and the California Institute for Regenerative Medicine Award (RB1-01328, to MLIA).
Abbreviations:
- ANG
Angiopoietin
- CSF1
Colony stimulating factor-1
- EC
Endothelial cells
- ECM
Extracellular matrix
- FGF2
Basic fibroblast growth factor
- HPC
Hematopoietic progenitor cell
- IL
Interleukin
- MV
Microvesicles
- NRP1
Neuropilin-1
- PlGF
Placental growth factor
- TAM
Tumor-associated macrophage
- TEM
TIE2-expressing macrophage
- TNFα
Tumor-necrosis factor-α
- VEGF
Vascular-endothelial growth factor
References
- 1.Gordon S, Martinez FO, Alternative activation of macrophages: mechanism and functions, Immunity 32 (2010) 593–604. [DOI] [PubMed] [Google Scholar]
- 2.Murray PJ, Wynn TA, Protective and pathogenic functions of macrophage subsets, Nat. Rev. Immunol 11 (2011) 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollard JW, Trophic macrophages in development and disease, Nat. Rev. Immunol 9 (2009) 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nucera S, Biziato D, DePalma M, The interplay between macrophages and angiogenesis in development, tissue injury and regeneration, Int. J. Dev. Biol 55 (2011) 495–503. [DOI] [PubMed] [Google Scholar]
- 5.Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C, Macrophages and angiogenesis, J. Leukoc. Biol 55 (1994) 410–422. [DOI] [PubMed] [Google Scholar]
- 6.Potente M, Gerhardt H, Carmeliet P, Basic and therapeutic aspects of angiogenesis, Cell 146 (2011) 873–887. [DOI] [PubMed] [Google Scholar]
- 7.Weis SM, Cheresh DA, Tumor angiogenesis: molecular pathways and therapeutic targets, Nat. Med 17 (2011) 1359–1370. [DOI] [PubMed] [Google Scholar]
- 8.Polverini PJ, Cotran PS, Gimbrone MA Jr., E.R. Unanue, Activated macrophages induce vascular proliferation, Nature 269 (1977) 804–806. [DOI] [PubMed] [Google Scholar]
- 9.Squadrito ML, De Palma M, Macrophage regulation of tumor angiogenesis: implications for cancer therapy, Mol. Aspects Med 32 (2011) 123–145. [DOI] [PubMed] [Google Scholar]
- 10.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D, Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis, Nat. Cell Biol 2 (2000) 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sierra JR, Corso S, Caione L, Cepero V, Conrotto P, Cignetti A, Piacibello W, Kumanogoh A, Kikutani H, Comoglio PM, Tamagnone L, Giordano S, Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages, J.Exp. Med 205 (2008) 1673–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C, Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction, Blood 116 (2010) 829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW, Macrophages regulate the angiogenic switch in a mouse model of breast cancer, Cancer Res. 66 (2006) 11238–11246. [DOI] [PubMed] [Google Scholar]
- 14.Lewis CE, Pollard JW, Distinct role of macrophages in different tumor microenvironments, Cancer Res. 66 (2006) 605–612. [DOI] [PubMed] [Google Scholar]
- 15.DePalma M, Venneri MA, Roca C, Naldini L, Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells, Nat. Med 9 (2003) 789–795. [DOI] [PubMed] [Google Scholar]
- 16.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S, Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth, Nat. Med 7 (2001) 1194–1201. [DOI] [PubMed] [Google Scholar]
- 17.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL, Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma, Cancer Res. 56 (1996) 4625–4629. [PubMed] [Google Scholar]
- 18.Leek RD, Landers RJ, Harris AL, Lewis CE, Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast, Br. J. Cancer 79 (1999) 991–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giraudo E, Inoue M, Hanahan D, An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis, J. Clin. Invest 114 (2004) 623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DePalma M, Venneri MA, Galli R, Sergi SL, Politi LS, Sampaolesi M, Naldini L, Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors, Cancer Cell 8 (2005) 211–226. [DOI] [PubMed] [Google Scholar]
- 21.Bingle L, Lewis CE, Corke KP, Reed MW, Brown NJ, Macrophages promote angiogenesis in human breast tumour spheroids in vivo, Br. J. Cancer 94 (2006) 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA, Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach, Br. J. Cancer 95 (2006) 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tammela T, Zarkada G, Nurmi H, Jakobsson L, Heinolainen K, Tvorogov D, Zheng W, Franco CA, Murtomaki A, Aranda E, Miura N, Yla-Herttuala S, Fruttiger M, Makinen T, Eichmann A, Pollard JW, Gerhardt H, Alitalo K, VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling, Nat. Cell Biol 13 (2011) 1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murdoch C, Muthana M, Coffelt SB, Lewis CE, The role of myeloid cells in the promotion of tumour angiogenesis, Nat. Rev. Cancer 8 (2008) 618–631. [DOI] [PubMed] [Google Scholar]
- 25.De Palma M, Coussens LM, Immune cells and inflammatory mediators as regulators of tumor angiogenesis, in: Figg W, Folkman J (Eds.), Angiogenesis: An integrative Approach from Science to Medicine, Springer Science, New York, 2008, pp. 225–238. [Google Scholar]
- 26.Tamagnone L, Emerging role of semaphorins as major regulatory signals and potential therapeutic targets in cancer, Cancer Cell 22 (2012) 145–152. [DOI] [PubMed] [Google Scholar]
- 27.Thery C, Zitvogel L, Amigorena S, Exosomes: composition, biogenesis and function, Nat. Rev. Immunol 2 (2002) 569–579. [DOI] [PubMed] [Google Scholar]
- 28.Simons M, Raposo G, Exosomes—vesicular carriers for intercellular communication, Curr. Opin. Cell Biol 21 (2009) 575–581. [DOI] [PubMed] [Google Scholar]
- 29.Tondravi MM, McKercher SR, Anderson K, Erdmann JM, Quiroz M, Maki R, Teitelbaum SL, Osteopetrosis in mice lacking haematopoietic transcription factor PU.1, Nature 386 (1997) 81–84. [DOI] [PubMed] [Google Scholar]
- 30.Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW Jr., A. Ahmed-Ansari, K.W. Sell, J.W. Pollard, E.R. Stanley, Total absence of colony-stimulating factor 1 in the macrophage-deficient osteo-petrotic (op/op) mouse, Proc. Natl. Acad. Sci. USA 87 (1990) 4828–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A, Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele, Nature 380 (1996) 435–439. [DOI] [PubMed] [Google Scholar]
- 32.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW, Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene, Nature 380 (1996) 439–442. [DOI] [PubMed] [Google Scholar]
- 33.Pucci F, Venneri MA, Biziato D, Nonis A, Moi D, Sica A, Di SC, Naldini L, De Palma M, A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood resident monocytes, and embryonic macrophages suggests common functions and developmental relationships, Blood 114 (2009) 901–914. [DOI] [PubMed] [Google Scholar]
- 34.Fantin A, Vieira JM, Plein A, Denti L, Fruttiger M, Pollard JW, Ruhrberg C, NRP1 acts cell autonomously in endothelium to promote tip cell function during sprouting angiogenesis, Blood (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, Saya H, Suda T, M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis, J. Exp. Med 206 (2009) 1089–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fruttiger M, Development of the retinal vasculature, Angiogenesis 10 (2007) 77–88. [DOI] [PubMed] [Google Scholar]
- 37.Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, Hua J, Lofqvist C, Hellstrom A, Smith LE, The mouse retina as an angiogenesis model, Invest. Ophthalmol. Vis. Sci 51 (2010) 2813–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rymo SF, Gerhardt H, Wolfhagen SF, Lang R, Uv A, Betsholtz C, A two-way communication between microglial cells and angiogenic sprouts regulates angiogenesis in aortic ring cultures, PLoS.One 6 (2011) e15846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stefater III JA, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, Fan J, Ajima R, Molkentin JD, Williams BO, Wills-Karp M, Pollard JW, Yamaguchi T, Ferrara N, Gerhardt H, Lang RA, Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells, Nature 474 (2011) 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smalley MJ, Dale TC, Wnt signalling in mammalian development and cancer, Cancer Metastasis Rev. 18 (1999) 215–230. [DOI] [PubMed] [Google Scholar]
- 41.Eubank TD, Roberts R, Galloway M, Wang Y, Cohn DE, Marsh CB, GM-CSF induces expression of soluble VEGF receptor-1 from human monocytes and inhibits angiogenesis in mice, Immunity 21 (2004) 831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saint-Geniez M, D’Amore PA, Development and pathology of the hyaloid, choroidal and retinal vasculature, Int. J. Dev. Biol 48 (2004) 1045–1058. [DOI] [PubMed] [Google Scholar]
- 43.Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, Morrisey EE, McMahon AP, Karsenty G, Lang RA, WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature, Nature 437 (2005) 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao S, Lobov IB, Vallance JE, Tsujikawa K, Shiojima I, Akunuru S, Walsh K, Benjamin LE, Lang RA, Obligatory participation of macrophages in an angiopoietin 2-mediated cell death switch, Development 134 (2007) 4449–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Checchin D, Sennlaub F, Levavasseur E, Leduc M, Chemtob S, Potential role of microglia in retinal blood vessel formation, Invest. Ophthalmol. Vis. Sci 47 (2006) 3595–3602. [DOI] [PubMed] [Google Scholar]
- 46.Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L, De Palma M, Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells, Cancer Cell 19 (2011) 512–526. [DOI] [PubMed] [Google Scholar]
- 47.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG, The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies, Blood 103 (2004) 4150–4156. [DOI] [PubMed] [Google Scholar]
- 48.De Palma M, Naldini L, Angiopoietin-2 TIEs up macrophages in tumor angiogenesis, Clin. Cancer Res 17 (2011) 5226–5232. [DOI] [PubMed] [Google Scholar]
- 49.Holopainen T, Saharinen P, D’Amico G, Lampinen A, Eklund L, Sormunen R, Anisimov A, Zarkada G, Lohela M, Helotera H, Tammela T, Benjamin LE, Yla-Herttuala S, Leow CC, Koh GY, Alitalo K, Effects of angiopoietin-2-blocking antibody on endothelial cell-cell junctions and lung metastasis, J. Natl. Cancer Inst 104 (2012) 461–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E, VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells, Cell 124 (2006) 175–189. [DOI] [PubMed] [Google Scholar]
- 51.Harvey NL, Gordon EJ, Deciphering the roles of macrophages in developmental and inflammation stimulated lymphangiogenesis, Vasc. Cell 4 (2012) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gordon EJ, Rao S, Pollard JW, Nutt SL, Lang RA, Harvey NL, Macrophages define dermal lymphatic vessel calibre during development by regulating lymphatic endothelial cell proliferation, Development 137 (2010) 3899–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A, Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes, Trends Immunol. 23 (2002) 549–555. [DOI] [PubMed] [Google Scholar]
- 54.Sica A, Mantovani A, Macrophage plasticity and polarization: in vivo veritas, J. Clin. Invest 122 (2012) 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ojalvo LS, King W, Cox D, Pollard JW, High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors, Am. J. Pathol 174 (2009) 1048–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomez PE, Schulz C, Geissmann F, Development and homeostasis of resident myeloid cells: the case of the microglia, Glia 61 (2013) 112–120. [DOI] [PubMed] [Google Scholar]
- 57.Clear AJ, Lee AM, Calaminici M, Ramsay AG, Morris KJ, Hallam S, Kelly G, Macdougall F, Lister TA, Gribben JG, Increased angiogenic sprouting in poor prognosis FL is associated with elevated numbers of CD163+ macrophages within the immediate sprouting microenvironment, Blood 115 (2010) 5053–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsubara T, Kanto T, Kuroda S, Yoshio S, Higashitani K, Kakita N, Miyazaki M, Sakakibara M, Hiramatsu N, Kasahara A, Tomimaru Y, Tomokuni A, Nagano H, Hayashi N, Takehara T, TIE2-expressing monocytes as a diagnostic marker for hepatocellular carcinoma correlated with angiogenesis, Hepatology (2012). [DOI] [PubMed] [Google Scholar]
- 59.Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE, Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas, J. Pathol 192 (2000) 150–158. [DOI] [PubMed] [Google Scholar]
- 60.He H, Xu J, Warren CM, Duan D, Li X, Wu L, Iruela-Arispe ML, Endothelial cells provide an instructive niche for the differentiation and functional polarization of M2-like macrophages, Blood 120 (2012) 3152–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Squadrito ML, Pucci F, Magri L, Moi D, Gilfillan GD, Ranghetti A, Casazza A, Mazzone M, Lyle R, Naldini L, De Palma M, miR-511–3p modulates genetic programs of tumor-associated macrophages, Cell Rep 1 (2012) 141–154. [DOI] [PubMed] [Google Scholar]
- 62.De Palma M, Partners in crime: VEGF and IL-4 conscript tumour-promoting macrophages, J. Pathol 227 (2012) 4–7. [DOI] [PubMed] [Google Scholar]
- 63.De Palma M, Mazzieri R, Politi LS, Pucci F, Zonari E, Sitia G, Mazzoleni S, Moi D, Venneri MA, Indraccolo S, Falini A, Guidotti LG, Galli R, Naldini L, Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis, Cancer Cell 14 (2008) 299–311. [DOI] [PubMed] [Google Scholar]
- 64.Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C, Ryan RJ, Iwamoto Y, Marinelli B, Gorbatov R, Forghani R, Novobrantseva TI, Koteliansky V, Figueiredo JL, Chen JW, Anderson DG, Nahrendorf M, Swirski FK, Weissleder R, Pittet MJ, Origins of tumor-associated macrophages and neutrophils, Proc. Natl. Acad. Sci.USA 109 (2012) 2491–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van RN, MacDonald AS, Allen JE, Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation, Science 332 (2011) 1284–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pucci F, Pittet MJ, Molecular pathways: tumor-derived micro-vesicles and their interactions with immune cells in vivo. Clin. Cancer Res (2013). DOI: 10.1158/1078-0432.CCR-12-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qu Y, Franchi L, Nunez G, Dubyak GR, Nonclassical IL-1 beta secretion stimulated by P2 × 7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages, J. Immunol 179 (2007) 1913–1925. [DOI] [PubMed] [Google Scholar]
- 68.Johansson SM, Admyre C, Scheynius A, Gabrielsson S, Different types of in vitro generated human monocyte-derived dendritic cells release exosomes with distinct phenotypes, Immunology 123 (2008) 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, Sun F, Lu J, Yin Y, Cai X, Sun Q, Wang K, Ba Y, Wang Q, Wang D, Yang J, Liu P, Xu T, Yan Q, Zhang J, Zen K, Zhang CY, Secreted monocytic miR-150 enhances targeted endothelial cell migration, Mol. Cell 39 (2010) 133–144. [DOI] [PubMed] [Google Scholar]
- 70.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons-Weiler J, Watkins SC, Morelli AE, Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes, Blood 119 (2012) 756–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang M, Chen J, Su F, Yu B, Su F, Lin L, Liu Y, Huang JD, Song E, Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells, Mol. Cancer 10 (2011) 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Squadrito ML, Etzrodt M, De Palma M, Pittet MJ, MicroRNA-mediated control of macrophages and its implications for cancer, Trends Immunol. (2013) doi:pii:S1471-4906(13)00029-X.10.1016/j.it.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


