Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) is associated with increased cardiovascular risk irrespective of conventional risk factors. The role of gut-liver interaction is implicated in its development. We investigated the effects of VSL#3® probiotic supplementation on biomarkers of cardiovascular risk and liver injury in patients with NAFLD.
Methods
A randomised, double-blinded, placebo-controlled, proof-of-concept study was undertaken. Patients with NAFLD were randomly allocated to take 2 sachets VSL#3® probiotic or placebo twice daily for 10 weeks. Measurements of endothelial function (digital photoplethysmography, sVCAM-1 and cGMP), oxidative stress (glutathione ratio and LHP), inflammation (hsCRP), insulin resistance (HOMA-IR) and liver injury [transaminases, fibrosis risk score and acoustic structure quantification (ASQ)] were undertaken before and after intervention. Difference in baseline characteristics between the treatment groups was analysed using independent t-test or Mann Whitney U test for non-parametric data. Independent t-test was used to compare the outcomes at the end of the study between the two treatment groups. Wilcoxon Signed Rank test was used to determine the difference in fibrosis risk scores before and after treatment. Spearman’s correlation was used to determine any association between cardiovascular and hepatic markers at baseline.
Results
Thirty-five patients completed the study (28 males and 7 females) with a mean age of 57 ± 8 years, body mass index of 32.6 ± 5.0 kg/m2 and a relatively short duration of NAFLD (median duration 0.3 IQR 2.0 years). No significant difference was observed in biomarkers of cardiovascular risk and liver injury following VSL#3® supplementation. Significant correlations were noted between sVCAM-1 and hsCRP (rho = 0.392, p = 0.01), and HOMA-IR and AST (rho = 0.489, p < 0.01) at baseline.
Conclusions
This is the first study to evaluate the effect of VSL#3® on ASQ in patients with NAFLD. VSL#3® did not significantly improve markers of cardiovascular risk and liver injury in patients with NAFLD. However, the study supports an association between endothelial dysfunction and inflammation in patients with NAFLD and suggests that NAFLD is linked with insulin resistance.
Trial registration: ISRCTN05474560 (https://doi.org/10.1186/ISRCTN05474560) Registered 9 August 2012 (retrospectively registered).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-021-01660-5.
Keywords: Fatty liver, Probiotic, Biomarkers
Background
Non-alcoholic fatty liver disease (NAFLD) is commonly encountered with an estimated prevalence of 20–30% in Western countries [1] and 6–35% worldwide [2]. There is a strong relationship between NAFLD and insulin resistance with NAFLD regarded as the hepatic manifestation of the metabolic syndrome [3]. NAFLD is often associated with obesity [4] and type 2 diabetes mellitus (T2DM) [5].
Whilst the pathophysiology of NAFLD is complex and not fully understood, postulated mechanisms include excessive hepatic triglyceride-induced inflammatory responses and generation of reactive oxygen species (ROS) leading to liver injury [6]. More recently, gut microbiota and gut-derived endotoxinaemia have been implicated in the development of NAFLD [7]. Lipopolysaccharide (LPS) is the active component of gut-derived endotoxins which exerts metabolic and inflammatory effects on the liver. A high fat diet is associated with intestinal dysbiosis resulting in higher proportion of LPS-producing bacteria in the gut and a 2–3 fold increase in LPS concentration [8]. LPS compromises intestinal barrier integrity and increases gut permeability, facilitating translocation of endotoxins into the portal circulation [9, 10]. Within the liver, LPS activates the Toll-like receptor 4 (TLR4)-dependent pathway in Kupffer cells resulting in activation of inflammatory pathways and impairment of insulin signaling [9].
Individuals with NAFLD have increased risk of cardiovascular events and mortality independent of conventional risk factors and the metabolic syndrome [5, 11–14]. NAFLD and atherosclerosis are chronic inflammatory conditions that seem to share common pathways (endothelial dysfunction, oxidative stress and inflammation).
Probiotics are non-pathogenic live micro-organisms beneficial for gut health with associated clinical benefits such as improved lipid profile [15], reduced systolic blood pressure [15], improved insulin sensitivity [16], increased antioxidant activity [17] and decreased inflammation [18].
VSL#3® is a highly concentrated probiotic product which contains 8 different strains of live freeze-dried lactic acid bacteria (streptococcus thermophilus, bifidobacterium breve, bifidobacterium longum, bifidobacterium infantis, lactobacillus acidophilus, lactobacillus plantarum, lactobacillus paracasei and lactobacillus bulgaricus). VSL#3®-related studies on insulin resistance [19, 20], vascular inflammation [20], oxidative stress [21], endothelial dysfunction [19] and liver injury [19, 21] are limited. Nonetheless, the gut microbiota should be considered a potential therapeutic target to treat patients with NAFLD.
Methods
The aim of this study was to examine the effects of VSL#3® probiotic supplementation on biomarkers of cardiovascular risk (insulin resistance, inflammation, endothelial function and oxidative stress) and biomarkers of liver injury in patients with NAFLD. We hypothesised that VSL#3® would improve these biomarkers.
Ethical approval was granted by NRES Committee South Central—Hampshire B [Whitefriars, Lewins Mead, Bristol BS1 2NT] (ref: 11/SC/0532) and written informed consent was obtained from all eligible patients. The study was conducted at the Diabetes Centre, QAH. Recruitment began in May 2012 and ended in March 2014 when the study closed. Follow-up of the last patient was completed in January 2014.
A randomised, double blinded, placebo-controlled, proof-of-concept trial was undertaken. The primary outcome was to detect changes in biomarkers of insulin resistance, oxidative stress, endothelial dysfunction, inflammation, and liver injury. The secondary outcome was to examine whether there were any relationships between insulin resistance, oxidative stress, endothelial function, inflammation and liver transaminases at baseline in patients with NAFLD.
Patients aged 25–70 years with confirmed NAFLD (either biopsy proven or based on imaging), at least a 20% risk of a cardiovascular event over the next 10 years (Qrisk2 score) [22] and HbA1c < 86 mmol/mol (10%) were recruited from Hepatology, Diabetes and Radiology departments at Queen Alexandra Hospital (QAH); a community-based obesity clinic and Primary Care in Portsmouth, UK. Qrisk2 score was multiplied by a factor of 1.87 as existing cardiovascular risk calculators do not include NAFLD as a risk factor [14].
Those with established cardiovascular disease, decompensated liver cirrhosis, allergy or intolerance to VSL#3® probiotic, chronic excess alcohol intake (> 24 g per day for men and > 16 g per day for women in the last 2 years), antibiotic treatment 4 weeks prior to the study and/or more than 3 courses of antibiotic treatment over the preceding 6 months, solid organ or bone marrow transplantation, and oral corticosteroid therapy were excluded from the study.
Patients were studied before and after a 10-week intervention period with VSL#3® (Danisco Inc.) probiotic or placebo at a dose of 2 sachets twice a day [20, 21, 23]. They were randomised in a 1:1 ratio between the treatment and placebo arms by a computer-generated code using random permuted blocks of randomly varying size. Investigators and patients were blinded to the intervention throughout the study period. VSL#3® and placebo were supplied by Actial Farmaceutica, Italy directly to the Clinical Trials Pharmacy Service, QAH who were responsible for packaging boxes containing identical, non-identifiable sachets of VSL#3® and placebo.
Patients were fasted at least 12 h prior to their study visit. The day before their visit, foods high in fibre and starch were avoided and those on insulin therapy omitted their insulin dose(s). On the day of their visit, patients were asked not to smoke or exercise 30 min prior. Consumption of other probiotic products was prohibited during the study period.
At each visit, anthropometric data (weight and waist circumference) and blood pressure (Welch Allyn; 52,000 series) were recorded. Venous blood samples were obtained to measure fasting insulin, fasting glucose, fasting lipid profile (total cholesterol, HDL cholesterol, LDL cholesterol and triglycerides), HbA1c, fructosamine, liver enzymes (ALT and AST), albumin, INR, platelet count and high sensitivity C-reactive protein, and markers of endothelial function and oxidative stress. Lactulose hydrogen breath test (LHBT) and digital photoplethysmography were performed. A subset of patients had liver ultrasound using Acoustic Structure Quantification (ASQ) (Additional file 1).
Lactulose hydrogen breath test was undertaken to detect small intestinal bacterial overgrowth (SIBO). Breath samples were measured using a Micro meter H2 (Micro Medical Rochester, Kent, UK). An increase in hydrogen concentration of more than 20 parts per million from baseline within 90 min of the test and a second peak at least 15 min following the initial peak constitute a positive result [24].
Insulin resistance was calculated using HOMA2 (homeostasis model assessment) which provides HOMA-IR (Homeostatic Model Assessment for Insulin Resistance) estimates [25]. Three paired fasting venous glucose and insulin were taken at 5-min intervals, and their mean values were entered into the HOMA2 calculator. Inflammation was measured by high sensitivity C-reactive protein (hsCRP) [26] using immunonephelometry on a Siemens BN Prospec nephelometer at the Biochemical Sciences Department, St Thomas’ Hospital, London.
Endothelial function Digital photoplethysmography is a non-invasive technique for assessment of endothelial function. Measurements were obtained using a Micro Medical Pulse Trace (Rochester, Kent, UK). Reflective index (RI) readings were recorded before and after the administration of 400 μg sublingual glycerol trinitrate (GTN; an endothelium-independent vasodilator). Following a washout, RI readings were repeated before and after inhaled Salbutamol 400 μg (an endothelium-dependent vasodilator). The mean RI readings was used to calculate the change in RI for GTN (∆RI-GTN) and Salbutamol (∆RI-Salb) thus determining endothelium-independent and dependent vasodilator changes respectively [27].
Soluble vascular cell adhesion molecule-1 (sVCAM-1) [28] was measured using the Quantikine Human sVCAM-1 immunoassay kit from R&D Systems. Cyclic guanosine monophosphate (cGMP) [29] was analysed using the cGMP Immunoassay kit from R&D Systems.
Oxidative stress The glutathione ratio (GSH:GSSG) was assessed using the GSSG reductase/5,5′-dithio-bis(2-nitrobenzoic acid) re-circulating method on whole blood described by Tietze [30] and Shaik and Mehvar [31] Total antioxidant capacity (TAC) was assessed using the cupric ion reducing antioxidant capacity—bathocuproinedisulfonic acid disodium salt (CUPRAC-BCS) assay as described by Campos [32]. Quantification of lipid hydroperoxides (LHP), a direct index of oxidative stress, was measured using a method described by Ruiz et al. which involves a coupled glutathione peroxidase-glutathione reductase reaction [33].
Liver injury The NAFLD fibrosis risk score [34] and FIB4 index [35] were used as predictive models of advanced fibrosis in patients with NAFLD. Acoustic Structure Quantification is a novel high definition ultrasonographic modality that processes spatial echopatterns from scanned tissues enabling measurement of fibrous structures that reflect the ultrasound beam [36]. Two Radiologists were designated to perform ASQ liver scan (Toshiba Aplio XG, Toshiba Imaging Systems) on a subset of patients. The mode, average and standard deviation of ASQ data were generated. The mode ASQ score (expressed as C2m) was used to compare the degree of liver fat/fibrosis instead of the average and standard deviation as the latter values can be affected by small vessels within the liver.
Statistical analysis
This was an exploratory proof-of-concept study examining the impact of VSL#3® on cardiovascular and hepatic markers given the absence of previous data upon which to undertake a power calculation.
Data were presented as mean and standard deviation or median and inter-quartile range (IQR) for non-parametric data. Independent t-test (or Mann Whitney U test for non-parametric data) assessed differences in baseline characteristics between treatment groups. Study outcomes between the two treatment groups were compared using independent t-test. Descriptive statistics for ANCOVA were presented as mean and SD. Wilcoxon Signed Rank test was used to establish any difference in fibrosis risk scores before and after treatment. Spearman’s correlation was used to determine possible associations between markers of cardiovascular risk and hepatic liver at baseline.
Statistical software IBM SPSS Statistics 22.0 for windows (IBM Corporation 2013) was used for all analyses and all tests were performed at a 5% level of significance.
Results
Figure 1 summaries patient recruitment. Forty-two patients participated in the study but only 35 completed. One patient was withdrawn as he was due to undergo bariatric surgery whilst 6 patients withdrew from the study for various reasons (taste of product, personal reasons, recurrent infections, nausea or diarrhoea).
Fig. 1.
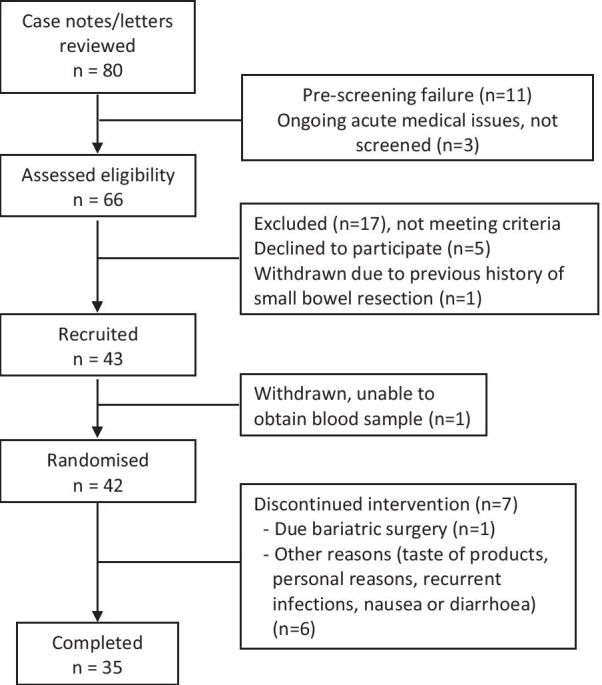
Patient recruitment
Out of the 35 patients who completed the study, most were men (28 males and 7 females) with a mean age of 57 ± 8 years and a relatively short duration of NAFLD (median duration since diagnosis of 0.3 IQR 2.0 years). T2DM or impaired fasting glucose was present in 74%. Most of the patients were obese with a mean of BMI 32.6 ± 5.0 kg/m2 and a mean waist circumference of 111.8 ± 12.6 cm (mean for men was 110.9 ± 11.4 cm and women was 115.3 ± 17.3 cm). Baseline mean blood pressure was 134/82 ± 13/7 mmHg, total cholesterol 4.42 ± 1.15 mmol/l, HDL 1.06 ± 0.29 mmol/l, LDL 2.43 ± 1.06 mmol/l, triglycerides 2.00 ± 0.88 mmol/l and HbA1c 53 ± 14 mmol/mol.
Baseline characteristics were similar between the two treatment groups (Table 1) except HOMA-IR which was significantly lower in the VSL#3®-treated group (1.6 IQR 1.7 v placebo 3.0 IQR 1.8; p = 0.04). This difference persisted despite the exclusion of patients on insulin.
Table 1.
Comparison of baseline characteristics between treatment groups
| Measurements | Mean baseline pre-VSL#3® (n = 19) | Mean baseline pre-placebo (n = 16) | p value |
|---|---|---|---|
| Age (y) | 57 ± 8 | 58 ± 7 | 0.96 |
| Gender (male/female)b | 15/4 | 13/3 | 0.91 |
| Duration NAFLD (y)b | 0.25 IQR 2.0 | 1.0 IQR 1.5 | 0.27 |
| No. of T2DM (or IFG)b | 15 | 11 | 0.61 |
| No. of smokersb | 2 | 2 | 0.94 |
| Alcohol (units/wk)b | 1 IQR 8 | 6 IQR 8 | 0.10 |
| BMI (kg/m2)b | 31.2 IQR 9.1 | 31.9 IQR 4.0 | 0.57 |
| Waist circumference (cm) | 112.2 ± 14.3 | 111.2 ± 10.7 | 0.81 |
| Systolic BP (mmHg) | 133 ± 13 | 135 ± 12 | 0.56 |
| Diastolic BP (mmHg) | 82 ± 8 | 82 ± 7 | 0.91 |
| Total cholesterol (mmol/l) | 4.51 ± 1.38 | 4.31 ± 0.85 | 0.61 |
| HDL (mmol/l)b | 0.98 IQR 0.35 | 0.95 IQR 0.54 | 0.66 |
| LDL (mmo/l) | 2.58 ± 1.18 | 2.42 ± 0.70 | 0.64 |
| Triglycerides (mmol/l)b | 1.80 IQR 0.45 | 1.80 IQR 1.27 | 0.73 |
| HbA1c (mmol/mol)b | 54 IQR 17 | 47 IQR 19 | 0.23 |
| HOMA-IRb | 1.6 IQR 1.7 | 3.0 IQR 1.8 | 0.04a |
| ALT (iu/L)b | 43 IQR 56 | 51 IQR 30 | 0.96 |
| AST (iu/L) | 40 ± 16 | 40 ± 15 | 0.99 |
ap < 0.05
bMann Whitney U test for non-parametric data one missing value on LDL as triglyceride (> 4.5 mmol/l) was too high for LDL calculation
SIBO was present in 6 out of 35 patients (17%). No significant differences were observed in metabolic parameters and markers of insulin resistance, endothelial function, oxidative stress, inflammation and liver transaminases (ALT and AST) with VSL#3® supplementation or placebo (Tables 2, 3). Even after exclusion of patients on insulin therapy, there was no significant difference in insulin resistance between the two treatment groups. Lipid hydroperoxide was excluded from analysis as levels were undetectable in all but one subject.
Table 2.
Summary of results in patients on VSL#3®
| Measurements | Mean pre-VSL#3® | Mean post-VSL#3® | p value |
|---|---|---|---|
| Systolic BP (mmHg) | 133 ± 13 | 130 ± 11 | 0.53 |
| Diastolic BP (mmHg) | 82 ± 8 | 80 ± 7 | 0.42 |
| Total cholesterol (mmol/l) | 4.51 ± 1.38 | 4.42 ± 1.27 | 0.83 |
| HDL (mmol/l) | 1.07 ± 0.26 | 1.09 ± 0.24 | 0.79 |
| LDL (mmol/l) | 2.58 ± 1.18 | 2.56 ± 1.02 | 0.95 |
| Triglycerides (mmol/l) | 1.89 ± 0.57 | 1.91 ± 1.00 | 0.94 |
| HbA1c (mmol/mol) | 54 ± 12 | 55 ± 12 | 0.87 |
| Fructosamine (μmol/l) | 257 ± 44 | 263 ± 49 | 0.67 |
| HOMA-IR | 2.2 ± 1.9 | 2.2 ± 1.5 | 0.95 |
| ∆RI-GTN (%) | 28 ± 6 | 25 ± 8 | 0.20 |
| ∆RI-Salb (%) | 9 ± 8 | 8 ± 4 | 0.62 |
| sVCAM-1 (ng/ml) | 536 ± 305 | 524 ± 262 | 0.90 |
| cGMP (pmol/l) | 178 ± 57 | 159 ± 43 | 0.27 |
| Blood glutathione ratio | 22 ± 10 | 26 ± 13 | 0.21 |
| TAC [mM Asc (AEAC)] | 0.46 ± 0.04 | 0.47 ± 0.07 | 0.75 |
| hsCRP (mg/l) | 3.0 ± 2.5 | 3.9 ± 6.1 | 0.53 |
| ALT (iu/l) | 56 ± 31 | 51 ± 32 | 0.63 |
| AST (iu/l) | 40 ± 16 | 38 ± 20 | 0.78 |
| Mode ASQ (C2m) | 91 ± 14 | 95 ± 16 | 0.57 |
VSL#3® treated group n = 19; placebo treated group n = 16. Data expressed as mean and standard deviation. Four cGMP values were not measurable (3 from placebo group and 1 from VSL#3® group). HOMA-IR missing 1 value from each treatment group as insulin levels were outside the HOMA calculator range. HbA1c in the placebo group had one missing value. LDL cholesterol had missing data on 1 patient from placebo group and 1 from VSL#3® group as triglycerides were > 4.5 mmol/l which preclude LDL cholesterol calculation. SBP and DBP values were missing after intervention on 1 patient in the placebo group. Nineteen patients had completed ASQ (10 in VSL#3® group and 11 in placebo group). Missing data were excluded from respective analyses
Table 3.
Summary of results in patients on placebo
| Measurements | Mean pre-placebo | Mean post-placebo | p value |
|---|---|---|---|
| Systolic BP (mmHg) | 135 ± 13 | 128 ± 17 | 0.19 |
| Diastolic BP (mmHg) | 82 ± 7 | 78 ± 11 | 0.27 |
| Total cholesterol (mmol/l) | 4.31 ± 0.85 | 4.50 ± 1.06 | 0.57 |
| HDL (mmol/l) | 1.05 ± 0.34 | 1.06 ± 0.35 | 0.96 |
| LDL (mmol/l) | 2.42 ± 0.70 | 2.50 ± 0.96 | 0.80 |
| Triglycerides (mmol/l) | 2.11 ± 1.15 | 2.39 ± 1.42 | 0.55 |
| HbA1c (mmol/mol) | 50 ± 15 | 51 ± 15 | 0.92 |
| Fructosamine (μmol/l) | 257 ± 53 | 266 ± 64 | 0.67 |
| HOMA-IR | 3.1 ± 1.8 | 3.0 ± 1.4 | 0.86 |
| ∆RI-GTN (%) | 24 ± 7 | 23 ± 6 | 0.58 |
| ∆RI-Salb (%) | 9 ± 6 | 9 ± 6 | 0.98 |
| sVCAM-1 (ng/ml) | 705 ± 423 | 722 ± 423 | 0.91 |
| cGMP (pmol/l) | 177 ± 46 | 177 ± 59 | 0.98 |
| Blood glutathione ratio | 20 ± 12 | 21 ± 9 | 0.65 |
| TAC (mM Asc [AEAC]) | 0.43 ± 0.06 | 0.44 ± 0.07 | 0.62 |
| hsCRP (mg/l) | 3.2 ± 5.3 | 2.7 ± 2.7 | 0.72 |
| ALT (iu/l) | 51 ± 19 | 49 ± 26 | 0.86 |
| AST (iu/l) | 40 ± 15 | 41 ± 17 | 0.90 |
| Mode ASQ (C2m) | 99 ± 10 | 95 ± 13 | 0.44 |
VSL#3® treated group n = 19; placebo treated group n = 16. Data expressed as mean and standard deviation. Four cGMP values were not measurable (3 from placebo group and 1 from VSL#3® group). HOMA-IR missing 1 value from each treatment group as insulin levels were outside the HOMA calculator range. HbA1c in the placebo group had one missing value. LDL cholesterol had missing data on 1 patient from placebo group and 1 from VSL#3® group as triglycerides were > 4.5 mmol/l which preclude LDL cholesterol calculation. SBP and DBP values were missing after intervention on 1 patient in the placebo group. Nineteen patients had completed ASQ (10 in VSL#3® group and 11 in placebo group). Missing data were excluded from respective analyses
There was no significant change in NAFLD fibrosis risk score or FIB4-index following VSL#3® treatment (Table 4). The placebo-treated group had lower post intervention mode ASQ score than the VSL#3®-treated group (mode ASQ score 91 C2m and 99 C2m respectively; p = 0.048).
Table 4.
Effect of VSL#3® on fibrosis risk scores
| Number of patients pre-VSL#3® | Number of patients post-VSL#3® | p valuea | |||||
|---|---|---|---|---|---|---|---|
| F0–F2 | Indeterminate | F3–F4 | F0–F1 | Indeterminate | F3–F4 | ||
| NAFLD fibrosis risk score | 1 | 11 | 7 | 3 | 9 | 7 | 0.16 |
| FIB4 index | 8 | 7 | 4 | 8 | 9 | 2 | 0.41 |
n = 19
F0–F2 = absence of significant fibrosis
F3–F4 = presence of significant fibrosis
aWilcoxon signed rank test
Moderate correlation was observed between sVCAM-1 and hsCRP (rho = 0.392, p = 0.01); between hsCRP and glutathione ratio (rho = 0.325, p = 0.04); and between HOMA-IR and AST (rho = 0.489, p < 0.01) at baseline. Figure 2 shows the correlation between HOMA-IR and AST. No other associations were found.
Fig. 2.
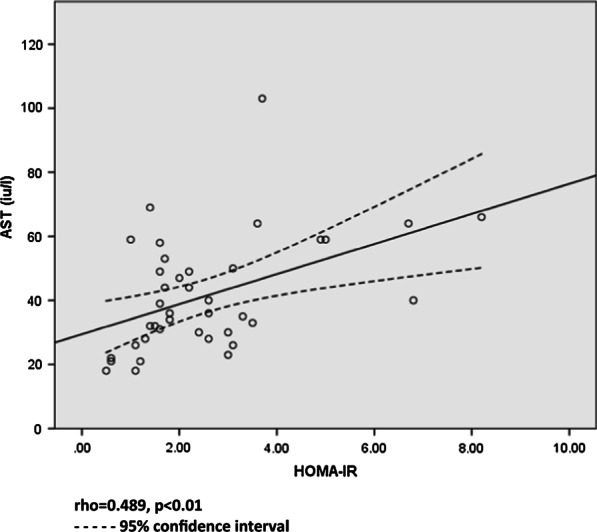
Association between baseline HOMA-IR and AST, rho = 0.489, p < 0.01, 95% confidence interval
No serious adverse events were reported in the study. In the VSL#3®-treated group (n = 19), 3 patients developed a urinary tract infection, 2 had bloating, 2 had nausea, 1 had genital thrush and 1 had perianal rash. In the placebo-treated group, 1 patient had diarrhoea, 1 had abdominal cramps, 1 had back pain and 1 had a traumatic toe infection.
Discussion
In this study, VSL#3® probiotic supplementation did not significantly improve insulin resistance, endothelial dysfunction, oxidative stress, inflammation or liver injury in patients with NAFLD.
Our patients with NAFLD have a higher mean baseline sVCAM-1 (714 ng/ml, range 277–1151 ng/ml) than healthy volunteers (531 ng/ml, range 341–897 ng/ml) suggesting they have underlying endothelial dysfunction. The mean baseline hsCRP (4.0 mg/l) was suggestive of a cohort at high cardiovascular risk [26]. Similar levels were reported in another study in which hsCRP was associated with a significantly higher relative risk of CVD in patients with NAFLD [13].
This is the first study to examine the impact of VSL#3® on ASQ in patients with NAFLD. ASQ has not been fully validated in patients with NAFLD and the threshold that defines the severity of fatty infiltration has not been determined. Toyoda et al. reported strong correlation between ASQ data and grades of liver fibrosis in 148 patients with histologically proven chronic hepatitis C [36]. The median values of mode ASQ (C2m) of patients in the study suggest that majority of them did not have significant fibrosis [placebo group: median value of mode C2m was 101.0 (range 77.0–114.0) before treatment and 93.0 (83.0–123.0) after treatment; VSL#3® group: 89.0 (73.0–110.0) before treatment and 96.0 (72.0–115.0) after treatment].
The majority of our patients did not have pre-existing liver biopsy to correlate ASQ data, hence it was not possible to quantify the severity of hepatic steatosis. Focal disturbance ratio (a statistical model on ASQ) can quantify liver fat in patients with NAFLD [37], however this analysis was not possible retrospectively as it required a different software version.
Several factors may have contributed to the lack of demonstrable effects of VSL#3® in our cohort with NAFLD. These include a relatively small sample, and the absence of age and sex-matched controls to determine whether our cohort was at high cardiovascular risk. The difference in baseline insulin resistance between the two treatment groups despite randomisation may be partly due to the sample size.
Studies on VSL#3® in patients with NAFLD are limited with the majority assessing liver-related endpoints in cirrhotic patients. Improvement in insulin sensitivity and metabolic parameters, and reduction in inflammation were demonstrated with 1 capsule of VSL#3® (112.5 billion bacteria) per day over 6 weeks in overweight/obese Indian patients [20]. Reduction in markers of oxidative stress was observed with 3 months of 1800 billion bacteria daily [21]. Ultrasonographic improvement in liver fat was seen in children with NAFLD after 4 months of VSL#3® [38]. The dose and duration of VSL#3® vary considerably (112.5 billion bacteria to 3600 billion bacteria per day; 6 weeks to 6 months) in reported studies [20, 21, 23, 38, 39], and a 10-week intervention may be considered too short to achieve clinically meaningful results.
There were no measurements on changes in gut microbiota or endotoxin levels, which may have provided supporting evidence in the pathogenesis of NAFLD should VSL#3® demonstrates modification of gut microbiota and reduction in gut-derived endotoxaemia.
Although HOMA is not validated for use in patients on insulin therapy, the use of HOMA-IR in such patients has been previously reported [40]. Re-analysis of the data excluding the 15% of patients on insulin therapy did not alter the study findings. Patients were less insulin-resistant than reported (HOMA-IR: 2.6 ± 1.8; or 2.2 ± 1.3 if those on insulin treatment were excluded) which may be related to co-existing metformin use [41].
The reproducibility of digital volume pulse as measured by digital photoplethysmography can be affected by the ambient temperature, the perfusion of patients’ index finger, patients’ position, and sudden movements (e.g. a sneeze or cough) when measurements were recorded. Maintaining similar conditions in all patients undergoing digital photoplethysmography can be challenging and considered a limitation of the study.
The moderately positive correlation between baseline hsCRP and sVCAM-1 supports an association between endothelial dysfunction and inflammation in patients with NAFLD, and this has been similarly reported in another study [42]. Paradoxically, there was a moderate positive correlation between hsCRP and blood glutathione ratio. Perhaps, there is an initial upregulation of antioxidant mechanisms to compensate for pathological states such as chronic inflammation and once these mechanisms are overwhelmed, glutathione ratio falls [43].
Insulin resistance and liver inflammation are known to be closely linked, and this has been demonstrated by the relationship between HOMA-IR and AST in our study. A similar relationship between insulin sensitivity and liver transaminases (AST and ALT) has been reported in the IRAS study [44].
The majority of VSL#3® studies are based on murine models and this study provides further insight into possible effects of probiotic supplementation cardiovascular risk and liver injury in patients with NAFLD. VSL#3® treatment has been shown to improve tight junction protein function [45] which is vital at maintaining intestinal epithelial barrier function and preventing translocation of gut-derived endotoxins. VSL#3® inhibited hepatic JNK and NF-κB activity suggesting that treatment improved hepatic insulin sensitivity and inhibited proinflammatory pathways [46]. VSL#3® also increased hepatic NKT cells which was associated with reduced TNFα expression and inhibition of IKK-β activity [47]. Decreased inflammatory mediators (TNFα, ICAM-1, RANTES and macrophage inflammatory protein-1α) within the liver was observed following treatment with VSL#3®, and resulted in histological improvement in liver inflammation and fibrosis [19].
Conclusion
This study does not support the hypothesis that probiotics improve biomarkers of cardiovascular risk and liver injury in patients with NAFLD. However, a number of limitations in particular the small sample size and short duration of VSL#3® treatment may have negatively influenced the study outcomes. Given modification of gut microbiota remains a potential therapeutic option in NAFLD, further studies are warranted to evaluate the benefits of probiotics in patients with NALFD.
CONSORT guidelines
The study adheres to the CONSORT guidelines and a completed CONSORT has been submitted separately.
Supplementary Information
Additional file 1. CONSORT 2010 checklist for the study.
Acknowledgements
The authors acknowledge the assistance of Reuben O’Gollah (Statistician at Research Office, QAH), Dr. Richard Beable (Radiologist, QAH), Research nurses (Diabetes Centre, QAH) and Scott Elliott (Biomedical Scientist, QAH). We also acknowledge the Diabetes, Hepatology and Radiology departments at QAH, and Primary Care in Portsmouth for their assistance with recruitment. The authors would like to thank all patients who volunteered for the study.
Abbreviations
- NAFLD
Non-alcoholic fatty liver disease
- sVCAM-1
Soluble vascular cell adhesion molecule-1
- cGMP
Cyclic guanosine monophosphate
- LHP
Lipid hydroperoxides
- hsCRP
High sensitivity C-reactive protein
- HOMA-IR
Homeostasis model assessment-insulin resistance
- ASQ
Acoustic structure quantification
- IQR
Interquartile range
- T2DM
Type 2 diabetes mellitus
- ROS
Reactive oxygen species
- SIBO
Small intestinal bowel overgrowth
- LPS
Lipopolysaccharide
- TLR 4
Toll-like receptor 4
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- LHBT
Lactulose hydrogen breath test
- RI
Reflective index
- GTN
Glycerol trinitrate
- GSH:GSSG
Glutathione ratio
- CUPRAC-BCS
Cupric ion reducing antioxidant capacity-bathocuproinedisulfonic acid disodium salt
- ANCOVA
Analysis of co-variance
- CVD
Cardiovascular disease
- JNK
C-Jun N-terminal kinase
- NF-κB
Nuclear factor kappa β
- IKKß
I-κB kinase β
- NKT
Natural killer T cells
- RANTES
Regulated on activation, normal T cell expressed and secreted
Authors’ contributions
LC was involved in the design and recruitment of the study, carrying out methodology, data analysis, and was the main author of the manuscript. DL was involved in the design of the study, supervision of laboratory analysis of biomarkers of cardiovascular risk, and review of the manuscript. RA was involved in the design and recruitment of the study particularly from a hepatology perspective, and review of the manuscript. AH was involved in the design of the study, performing acoustic structure quantification, and review of the manuscript. MC was responsible for the supervision of the study from its design to completion, and review of the manuscript. All Authors have read and approved the manuscript.
Funding
The salary of the first author (LC), consumables of laboratory investigations, medications for LHBT and digital photoplethysmography, and radiological imaging of the study were fully funded by Portsmouth Hospitals NHS Trust, UK. VSL#3® probiotic and its placebo equivalent were provided free of charge by Actial Farmaceutica, Italy.
Availability of data and materials
Relevant data have been presented in the main manuscript. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Ethical approval was granted by NRES Committee South Central—Hampshire B (Ref. 11/SC/0532) and written informed consent was obtained from all eligible patients.
Consent for publication
Not applicable.
Competing interests
The authors have nothing to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 3.Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50(8):1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 4.Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45(4):600–606. doi: 10.1016/j.jhep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30(5):1212–1218. doi: 10.2337/dc06-2247.Abbreviations. [DOI] [PubMed] [Google Scholar]
- 6.Day CP, James OFW. Steatohepatitis: A tale of two “hits”? Gastroenterology. 1998;114(4):842–845. doi: 10.1016/S0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 7.Mokhtari Z, Gibson DL, Hekmatdoost A. Nonalcoholic fatty liver disease, the gut microbiome, and diet. Adv Nutr. 2017;8(2):240–252. doi: 10.3945/an.116.013151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(July):1761–1772. doi: 10.2337/db06-1491.P.D.C. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Shanab A, Quigley EMM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7(12):691–701. doi: 10.1038/nrgastro.2010.172. [DOI] [PubMed] [Google Scholar]
- 10.Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49(6):1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 11.Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 12.Söderberg C, Stål P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51(2):595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 13.Lizardi-Cervera J, Chavez-Tapia NC, Pérez-Bautista O, Ramos MH, Uribe M. Association among C-reactive protein, Fatty liver disease, and cardiovascular risk. Dig Dis Sci. 2007;52(9):2375–2379. doi: 10.1007/s10620-006-9262-6. [DOI] [PubMed] [Google Scholar]
- 14.Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30(8):2119–2121. doi: 10.2337/dc07-0349.Abbreviations. [DOI] [PubMed] [Google Scholar]
- 15.Agerholm-Larsen L, Raben A, Haulrik N, Hansen A, Manders M, Astrup A. Effect of 8 week intake of probiotic milk products on risk factors for cardiovascular diseases. Eur J Clin Nutr. 2000;54(4):288–297. doi: 10.1038/sj.ejcn.1600937. [DOI] [PubMed] [Google Scholar]
- 16.Andreasen AS, Larsen N, Pedersen-Skovsgaard T, et al. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr. 2010;104(12):1831–1838. doi: 10.1017/S0007114510002874. [DOI] [PubMed] [Google Scholar]
- 17.Kullisaar T, Songisepp E, Mikelsaar M, Zilmer K, Vihalemm T, Zilmer M. Antioxidative probiotic fermented goats’ milk decreases oxidative stress-mediated atherogenicity in human subjects. Br J Nutr. 2003;90(02):449–456. doi: 10.1079/BJN2003896. [DOI] [PubMed] [Google Scholar]
- 18.Kekkonen R-A, Lummela N, Karjalainen H, et al. Probiotic intervention has strain-specific anti-inflammatory effects in healthy adults. World J Gastroenterol. 2008;14(13):2029–2036. doi: 10.3748/wjg.14.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mencarelli A, Cipriani S, Renga B, et al. VSL#3 resets insulin signaling and protects against NASH and atherosclerosis in a model of genetic dyslipidemia and intestinal inflammation. PLoS ONE. 2012;7(9):e45425. doi: 10.1371/journal.pone.0045425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajkumar H, Mahmood N, Kumar M, Varikuti SR, Challa HR, Myakala SP. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: a randomized, controlled trial. Mediators Inflamm. 2014;2014:348959. doi: 10.1155/2014/348959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loguercio C, Federico A, Tuccillo C, et al. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39(6):540–543. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 22.Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ. 2008;336(7659):1475–1482. doi: 10.1136/bmj.39609.449676.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tandon P, Moncrief K, Madsen K, et al. Effects of probiotic therapy on portal pressure in patients with cirrhosis: a pilot study. Liver Int. 2009;29(7):1110–1115. doi: 10.1111/j.1478-3231.2009.02020.x. [DOI] [PubMed] [Google Scholar]
- 24.Peralta S, Cottone C, Doveri T, Almasio PL, Craxi A. Small intestine bacterial overgrowth and irritable bowel syndrome-related symptoms: experience with Rifaximin. World J Gastroenterol. 2009;15(21):2628. doi: 10.3748/wjg.15.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.CIR.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 27.Chowienczyk PJ, Kelly RP, MacCallum H, et al. Photoplethysmographic assessment of pulse wave reflection. J Am Coll Cardiol. 1999;34(7):2007–2014. doi: 10.1016/S0735-1097(99)00441-6. [DOI] [PubMed] [Google Scholar]
- 28.Peter K, Nawroth P, Conradt C, et al. Circulating vascular cell adhesion molecule-1 correlates with the extent of human atherosclerosis in contrast to circulating intercellular adhesion molecule-1, E-selectin, P-selectin, and thrombomodulin. Arterioscler Thromb Vasc Biol. 1997;17(3):505–512. doi: 10.1161/01.ATV.17.3.505. [DOI] [PubMed] [Google Scholar]
- 29.Rapoport RM, Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983;52(3):352–357. doi: 10.1161/01.RES.52.3.352. [DOI] [PubMed] [Google Scholar]
- 30.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 31.Shaik IH, Mehvar R. Rapid determination of reduced and oxidized glutathione levels using a new thiol-masking reagent and the enzymatic recycling method: application to the rat liver and bile samples. Anal Bioanal Chem. 2006;385(1):105–113. doi: 10.1007/s00216-006-0375-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campos C, Guzmán R, López-Fernández E, Casado Á. Evaluation of the copper(II) reduction assay using bathocuproinedisulfonic acid disodium salt for the total antioxidant capacity assessment: the CUPRAC-BCS assay. Anal Biochem. 2009;392(1):37–44. doi: 10.1016/j.ab.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz C, Alegria A, Barbera R, Farre R, Lagarda MJ. Determination of plasma lipid hydroperoxides by an NADPH/NADP + coupled enzyme reaction system. Eval Method. 1997;35(12):893–898. doi: 10.1515/cclm.1997.35.12.893. [DOI] [PubMed] [Google Scholar]
- 34.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 35.Shah A, Lydecker A, Murray K, et al. Use of the FIB4 index for non-invasive evaluation of fibrosis in nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7(10):1104–1112. doi: 10.1016/j.cgh.2009.05.033.USE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toyoda H, Kumada T, Kamiyama N, et al. B-mode ultrasound with algorithm based on statistical analysis of signals: evaluation of liver fibrosis in patients with chronic hepatitis C. AJR Am J Roentgenol. 2009;193(4):1037–1043. doi: 10.2214/AJR.07.4047. [DOI] [PubMed] [Google Scholar]
- 37.Karlas T, Berger J, Garnov N, et al. Acoustic structure quantification (ASQ): a new ultra- sound-based approach for non-invasive quantification of liver fat content (AASLD Abstracts) Hepatology. 2014;60(Suppl 4):633A. [Google Scholar]
- 38.Alisi A, Bedogni G, Baviera G, et al. Randomised clinical trial: the beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39(11):1276–1285. doi: 10.1111/apt.12758.Randomised. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhiman RK, Rana B, Agrawal S, et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147(6):1327–1337. doi: 10.1053/j.gastro.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 40.Elkeles RS, Godsland IF, Feher MD, et al. Coronary calcium measurement improves prediction of cardiovascular events in asymptomatic patients with type 2 diabetes: the PREDICT study. Eur Heart J. 2008;29(18):2244–2251. doi: 10.1093/eurheartj/ehn279. [DOI] [PubMed] [Google Scholar]
- 41.Mather KJ, Verma S, Anderson TJ. Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol. 2001;37(5):1344–1350. doi: 10.1016/S0735-1097(01)01129-9. [DOI] [PubMed] [Google Scholar]
- 42.Lucero D, Zago V, López GI, et al. Pro-inflammatory and atherogenic circulating factors in non-alcoholic fatty liver disease associated to metabolic syndrome. Clin Chim Acta. 2011;412(1–2):143–147. doi: 10.1016/j.cca.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 43.Buckley BJ, Whorton AR. Adaptive responses to peroxynitrite: increased glutathione levels and cystine uptake in vascular cells. Am J Physiol Cell Physiol. 2000;279(4):C1168–C1176. doi: 10.1016/S0891-5849(99)90744-X. [DOI] [PubMed] [Google Scholar]
- 44.Hanley AJG, Williams K, Festa A, et al. Elevations in markers of liver injury and risk of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2004;53(10):2623–2632. doi: 10.2337/diabetes.53.10.2623. [DOI] [PubMed] [Google Scholar]
- 45.Krishnan M, Penrose HM, Shah NN, Marchelletta RR, McCole DF. VSL#3 probiotic stimulates T-cell protein tyrosine phosphatase-mediated recovery of IFN-γ-induced intestinal epithelial barrier defects. Inflamm Bowel Dis. 2016;22(12):2811–2823. doi: 10.1097/MIB.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Z, Yang S, Lin H, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37(2):343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 47.Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49(5):821–830. doi: 10.1016/j.jhep.2008.05.025.Probiotics. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. CONSORT 2010 checklist for the study.
Data Availability Statement
Relevant data have been presented in the main manuscript. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


