Abstract
MFI-type zeolitic membranes were prepared on the porous α-A2O3 support to investigate the separation properties of dichlorobenzene isomers. The pervaporation tests were performed with unary and binary isomer mixtures at 333 K. The results indicate that the silicalite membranes, irrespective of being synthesized by the templated or template-free method, are permeable for all dichlorobenzene isomers. The pervaporation fluxes of the pure dichlorobenzene isomers decrease in the order p-DCB > o-DCB > m-DCB. For the binary pervaporation system, the dichlorobenzene fluxes are all less than those with a single component due to the binary interactions between DCB isomers and between the DCB isomer and the zeolite membrane. Comparatively, the template-free MFI-type zeolite exhibits higher selectivity for dichlorobenzene isomers due to less inter-crystalline gaps. The separation factors for p-/o-DCB and p-/m-DCB can reach 16.7 and 22.0, respectively.
1. Introduction
Dichlorobenzene isomers, that is, p-dichlorobenzene (p-DCB), m-dichlorobenzene (m-DCB), and o-dichlorobenzene (o-DCB), are particularly useful as important intermediates for pesticides (agricultural chemicals), medicines, and dyestuffs.1−4 They are normally prepared from the chlorination of benzene or mono-chlorobenzene, which always produces a mixture of the three isomeric dichlorobenzenes. The sequential isolation of the individual dichlorobenzene isomers is extremely complicated. The similar physicochemical properties of these isomers make the traditional separation method, which is distillation, a time- and energy-consuming process. However, only o-isomers could be separated through distillation. Additional fractional crystallization must be used for the preliminary separation of most of the p-dichlorobenzene from the m- and p-dichlorobenzene mixture because of their identical boiling points. Finally, through the selective adsorption on the zeolite adsorbent, the separation of dichlorobenzene isomers could be achieved.4−8 Up to date, the economical and efficient separation of dichlorobenzene isomers is still a challenge.
As a new alternative separation technology, membrane separation takes advantage of controlling the permeation rate of different species through the membrane.9,10 In many cases, it is faster, more economical, and more efficient than conventional separation techniques, which makes it attractive and competitive in mixture separation, purification, and enrichment, especially for isomeric, close-boiling, azeotropic, or heat-sensitive liquid mixtures.7−19 Among them, zeolite membranes present exceptionally high permeability and selectivity combined with thermal and chemical stabilities. In the past decade, various research groups have reported the synthesis of polycrystalline zeolite membranes with minimized gaps between the zeolite crystallites for vapor and liquid separation, and they found that the differences in the chemical affinities between the permeating molecule and the zeolite pore, rather than in the shape or size, often determine the separation properties of the zeolite membranes.14 Although good progress has been made and membrane separation has been extensively used in the dairy process industry for selective separation of different species, most of the zeolite membrane separation for isomers focuses on xylene and alkane.13−24 Little research has been done for the separation of dichlorobenzenes isomers. It is well known that dichlorobenzene isomers have a similar molecular shape and size as those of xylenes (molecule size: p-isomer ∼0.58 nm, m- and o-isomers ∼0.68 nm). Different chemical affinities and adsorption properties between each dichlorobenzene isomer and the MFI-type zeolite pore have also been proved by Guo and Long4 Therefore, if the appropriate zeolite membrane and separation conditions were selected, the dichlorobenzene isomers could be easily separated through membrane separation technology theoretically.
In this paper, the MFI-type zeolite silicalite membrane was developed for pervaporation separation of dichlorobenzene isomers. Although silicalite membranes prepared with a template have been well reported to show excellent performance, the potential risks of cracks and gaps, caused by the difference in thermal expansion between the support and membrane and the shrinkage of the lattice parameters of zeolite crystals during calcination, will significantly deteriorate the separation performance.14 Thus, the template-free protocol was adopted in the experiment to synthesize the silicalite membrane, and both single-isomer pervaporation measurement and mixed-isomer separation of dichlorobenzene were carried out. The results are also compared with those obtained with the traditional template method.
2. Results and Discussion
2.1. Preparation and Characterization of the MFI Zeolite Membrane
The formation of a zeolite membrane for pervaporation requires the development of a continuously two-dimensional and defect-free layer of zeolite crystals, so that only permeation through the zeolite pores may take place. In achieving the intrinsic selectivity of the selective membrane layer, a suitable support with the appropriate pore structure plays an important role. The pores must be small enough to support the thin zeolite layer under high pressure and must also be close together, so that it does not take a long tortuous path for the permeating components to reach the pore. In the experiment, mercury porosimetry measurement was used to quantitatively evaluate the pore structure of the α-Al2O3 support. Figure 1 depicts the total pore volume and the pore size distribution of the Al2O3 support. It clearly shows an average pore size of 280 nm and a very narrow pore distribution. In the meantime, the calculated porosity of the material is about 40% and the surface area is about 3.2 m2/g. Generally, it is easy to form a continuous zeolite membrane when the pore size of the support is less than 1 μm. Thus, in our experiment, the porous Al2O3 disk was directly used as the support to fabricate the MFI zeolite membrane without any intermediate layer.
Figure 1.
Pore distribution of the α-Al2O3 support.
After polishing and washing, the α-Al2O3 support was coated with MFI zeolite seeds for secondary growth of the MFI zeolite membrane. Figure 2 shows the SEM image of the seeded α-Al2O3 support. Through three times of seed coating, drying, and calcination processes, the surface of the Al2O3 support was fully covered with MFI zeolite particles with special morphology. The average particle size is about 300 nm. It should be noted that enough seed particles could ensure the sequential formation of continuous MFI zeolite membranes.
Figure 2.
SEM image of the MFI seeds on the surface of the α-Al2O3 support.
A continuous MFI zeolite membrane was formed on the surface of the α-Al2O3 support through the secondary growth method. Figure 3 presents the top and cross-sectional view of the MFI zeolite membrane with and without a template. From the top-view images, it can be seen that both membranes are continuing without any obvious defect on the surface, and the MFI membrane prepared by the template-free method shows relatively larger crystal size and better inter-growth with much higher integrity when compared with that from the template method. As to their cross-sectional analysis, both of the zeolite layer and Al2O3 support layer can be clearly distinguished in the images. Although they are synthesized under different hydrothermal conditions, the two membranes have similar thicknesses, 5.3 μm for the templated method and 5.6 μm for the template-free method. Besides, the zeolite crystal boundary of the membrane fabricated with the templated method is much clearer than that from the template-free method. This may be caused by the shrinkage of the lattice parameters during the template elimination process. It should be noted that the resulting shrinkage of the crystal size may generate some non-zeolite micropores within the membrane structure, which will inevitably affect the selectivity during the pervaporation process.
Figure 3.
SEM images of the top view (A,C) and cross-sectional view (B,D) for the MFI zeolite membrane synthesized by the templated (A,B) and template-free (C,D) μm method.
The processes for the growth of the MFI zeolite membrane on the α-Al2O3 support with the templated and template-free method were also monitored by XRD analysis, as shown in Figure 4. The original α-Al2O3 support only presents three characteristic diffraction peaks as labeled by the circle in the pattern. After three times of dip coating, drying, and calcination processes, four small diffraction peaks located at the 2 theta value of 8.0, 8.8, 23.1, and 23.8 emerged. They are corresponding well with (101), (200), (501), and (422) diffraction peaks of MFI silicate zeolite, respectively, indicating the formation of the MFI seed layer on the surface of the α-Al2O3 support. Sequential secondary growth under the hydrothermal conditions with or without a template generates the continuous silicalite membranes. The diffraction patterns (Figure 4c for template-free method and d for the templated method) are all corresponding well with those of the standard MFI crystal structure. Besides, all the membranes have a random orientation, irrespective of they being prepared by the templated or template-free method.
Figure 4.
XRD patterns of the α-Al2O3 support (a), MFI zeolite seed-coated support (b), and MFI zeolite membrane synthesized by the template-free method (c) and by the templated method (d).
2.2. Pervaporation Properties
Both MFI silicalite membranes, synthesized by templated and template-free methods, were first subjected to the quality test using 1,3,5-triisopropylbenzene (TIPB) (kinetic diameter: 0.85 nm) as a pervaporation agent. In general, MFI-type zeolite has an interconnected pore system with zig–zag channels in the a-direction with a diameter of 0.51 × 0.57 nm and 0.54 nm straight channels along the b-direction. Although it may distort under some reaction conditions, a molecule as large as TIPB still cannot permeate through these pore channels. Thus, any TIPB pervaporation flux detected means the presence of defects in the MFI zeolite membrane. Fortunately, both membranes in the experiment showed negligible flux of TIPB (lower than the detection limit of the pervaporation equipment), indicating the presence of a minimum number of defects and inter-crystalline gaps.
Figure 5A shows the pervaporation flux of p-dichlorobenzene at 60 °C through the MFI-type zeolite membranes synthesized by templated and template-free methods. As we know, the driving force of pervaporation is the chemical potential difference between the two sides of the membrane. Theoretically, the size selectivity of the zeolitic layer plays a key role in determining the membrane’s permeance and selectivity. Thus, the pervaporation profile should start with a gradual increase and then reach a constant value. However, the pervaporation profile of p-DCB (Figure 5A) presents an increase to a maximum flux first and then a gradual decrease to a relatively constant value. It clearly indicates that some permeation channels in the zeolite membrane are gradually blocked during the pervaporation process. Considering the high affinity between the p-DCB and zeolite membrane,4 the chemically absorbed p-DCB molecule in the zeolite channels may be responsible for this phenomenon. A similar phenomenon also has been reported during the pervaporation of xylene isomers.14 After 48 h, the constant permeation flux is about 0.52 kg/(h·m2) for the templated membrane and 0.19 kg/(h·m2) for the template-free membrane. The almost three times difference of the flux indicates that the template-free membrane is more perfect than the templated one. Generally, there are three types of pores in the zeolite membrane for flux to pass through: large pores (defects), non-zeolite micropore, and zeolite channel. Although the quality test through 1,3,5-triisopropylbenzene pervaporation had already excluded the possibility of the large pores, the non-zeolite micropore between crystallines of the zeolite, mainly generated during the template elimination process, may also act as a channel for dichlorobenzene molecule permeation. It should be noted that this kind of non-zeolite micropore is non-selective for isomer molecule separation. Therefore, the existence of non-zeolite micropore may be responsible for the relatively large permeation flux for the templated membrane. In the meantime, it will inevitably decrease the separation factor of the zeolite membrane for the different isomers.
Figure 5.
P-dichlorobenzene (A), o-dichlorobenzene (B), and m-dichlorobenzene (C) flux through the MFI zeolite membrane synthesized by the templated (a) and template-free method (b).
As shown in Figure 5B,C, o- and m-dichlorobenzene present a similar pervaporation profile with as the p-isomer, for membranes synthesized by templated and template-free methods. The only difference is their fluxes. Table 1 lists the pervaporation fluxes of the dichlorobenzene isomers through the templated and template-free membranes. The relatively stable pervaporation fluxes after 48 h of performance of the three isomers are all in the order of p-DCB > o-DCB > m-DCB. This may be concerned with their molecular shape and size. When using the zeolite membrane for pervaporation, a separation could be achieved when one of the permeants is excluded (filtered) from some of the pores in the membrane through which other permeants move. Theoretically, the molecule larger than the pore size cannot pass through the zeolite membranes. As reported in the literature,19 the kinetic diameter of p-DCB is similar to the pore channels of MFI zeolite; it is easy to be absorbed and transported through the zeolite channel. Meanwhile, the neighboring chloro-groups of the o-DCB could distort; thus, the molecule can squeeze into the pore channel to permeate. As for the m-DCB (kinetic diameter: 0.68 nm), it cannot enter the silicate zeolite pore theoretically due to size exclusion. The pore channel of the MFI zeolite usually decreases with an increase in the Si/Al ratio of the framework. Although the synthesis solution for our membranes did not contain any source of Al, several research studies have already proved the inevitable presence of Al in the silicate membrane when Al2O3 was used as a support during synthesis.17 Thus, some of the m-DCB molecules also could enter the MFI zeolite pore channel to transport. The ideal separation factors calculated from the pervaporation result for a single-isomer system are listed in Table 2. Obviously, the template-free membrane has a relatively high separation factor due to less defects within the membrane structure. For the template-free membrane, the ideal separation factor p-/o-DCB and p-/m-DCB could reach 19.0 and 31.7, respectively.
Table 1. Flux of Dichlorobenzene Isomers through MFI-Type Zeolite Membranes Synthesized by Templated and Template-free Methods at 60 °C.
| templated | DCB isomers system | component | flux (kg/h·m2) |
|---|---|---|---|
| yes | single | p-DCB | 0.52 |
| yes | single | o-DCB | 0.21 |
| yes | single | m-DCB | 0.10 |
| no | single | p-DCB | 0.19 |
| no | single | o-DCB | 0.01 |
| no | single | m-DCB | 0.006 |
| yes | p-/o-DCB | p-DCB | 0.28 |
| o-DCB | 0.12 | ||
| yes | p-/m-DCB | p-DCB | 0.25 |
| m-DCB | 0.08 | ||
| no | p-/o-DCB | p-DCB | 0.10 |
| o-DCB | 0.006 | ||
| no | p-/m-DCB | p-DCB | 0.11 |
| m-DCB | 0.005 |
Table 2. Separation Factor of Dichlorobenzene Isomers through MFI-Type Zeolite Membranes Synthesized by Templated and Template-free Methods at 60 °C.
| α (p-/o-DCB) |
α (p-/m-DCB) |
|||
|---|---|---|---|---|
| templated | ideal | binary | ideal | binary |
| yes | 2.5 | 2.3 | 5.2 | 3.1 |
| no | 19.0 | 16.7 | 31.7 | 22 |
To further evaluate the dichlorobenzene isomer separation properties, pervaporation of binary dichlorobenzene isomers through templated and template-free membranes was also conducted in the experiment. It should be noted that the DCB isomers have different affinities with MFI type zeolite.4 Thus, the binary interactions between the components and the membrane also should be considered in binary systems. Figure 6A depicts the pervaporation results of the p-/o-DCB binary system. The flux of p-DCB presents a similar profile as that of the single system. However, the flux of o-DCB shows a gradual increase and then approaches a relatively constant value, which is quite different from that in the single-pervaporation process. MFI-type zeolite channels prefer to absorb p-DCB first due to the relative strong affinity with each other, and p-DCB molecules may gradually be chemically absorbed and then block some zeolite channels during pervaporation. Due to the relatively small amount of absorbed o-DCB molecules within membranes in the first stage, the blocked channels of the membrane may have little effect on the permeation of the o-DCB isomers. This phenomenon clearly suggests that p- and o-DCB have competitive adsorption on the MFI-type zeolite membrane under the binary pervaporation conditions.21Figure 6B shows the pervaporation results of the p-/m-DCB binary system. Obviously, the results are similar to those of the p-/o-DCB binary system. The separation factors calculated from the pervaporation results of the binary system are listed in Table 2. For the template-free MFI membrane, the separation factors for p-/o-DCB and p-/m-DCB can reach 16.7 and 22.0, respectively. Collectively, the template-free MFI membrane has a much higher separation factor for DCB isomer separation and exhibits great potential application in this field. However, to realize the industrial application for DCB isomer pervaporation, it must realize large-scale membrane preparation and reduce the cost of the pervaporation plant.
Figure 6.
Pervaporation results for the mixture of equal molar binary p-/o- (A) and p-/m-dichlorobenzene (B) through MFI zeolite membranes synthesized by templated and template-free methods.
3. Conclusions
MFI-type zeolitic membranes were prepared on the porous α-alumina support by templated and template-free methods. The pervaporation results clearly show that both kinds of membranes are permeable for all dichlorobenzene isomers. The pervaporation fluxes of the pure dichlorobenzene isomers decrease in the order of p-DCB > o-DCB > m-DCB. For the binary pervaporation system, the dichlorobenzene fluxes are all less than those with a single component due to the binary interactions between DCB isomers and between the DCB isomer and the membrane. Comparatively, the template-free MFI-type zeolite membrane exhibits higher selectivity for dichlorobenzene isomers due to less inter-crystalline gaps. The separation factors for p-/o-DCB and p-/m-DCB can reach 16.7 and 22.0, respectively.
4. Experimental Section
4.1. Chemicals and Materials
All the chemicals were of analytical grade and used as received without any further purification.
o-Dichlorobenzene (o-DCB) (99%), m-dichlorobenzene (m-DCB) (98%), and p-dichlorobenzene (p-DCB) (99%) were purchased from Sigma-Aldrich. Other chemicals used in the experiment were all purchased from Shanghai Chemical Reagent Corporation. Porous α-Al2O3 disks (20 mm diameter; 2.0 mm thickness) were purchased from Shenzheng Yuli Electronic Ceramic Company and were used as the support in the experiment.
4.2. Preparation of the ZSM-5 Zeolite Membrane
The silicalite seeds were prepared by the hydrothermal method. In the typical synthesis, TEOS and TPAOH and EtOH were dissolved in deionized water at 60 °C under stirring. The prepared solution with a mole composition of 1 SiO2: 0.27 TPAOH: 54 H2O: 4 EtOH was hydrothermally treated at 175 °C for 24 h. The resulted silicalite seeds were then centrifuged and purified by repeated washing with deionized water. After that, the silicalite seed suspension was prepared by evenly mixing 1 g of silicalite seeds with 0.14 g of hydroxy propyl cellulose (HPC) and 94 mL of deionized water.
The seed layer was coated on the surface of the porous α-Al2O3 support by a dip coating method27,28 with abovementioned silicalite seed suspension. The dip coating procedure was repeated three times to ensure enough coverage of the seed layer. After each dip coating, the seed layer was calcined at 450 °C for 30 min to remove the template.
The templated silicalite membrane was prepared as follows: the abovementioned seed-coated α-Al2O3 support was placed in the silicalite synthesis solution (mole composition, 1 SiO2: 0.3 TPAOH: 300 H2O: 4 EtOH) and hydrothermally treated at 175 °C for 24 h. The resulting membrane was washed, dried, and then calcined at 500 °C for 10 h to remove the template.25,26
The template-free silicalite secondary growth solution was prepared according to the literature by mixing the desired amount of fumed silica powder to the NaOH solution at 80 °C under stirring.29 After aging for another 2 h, the solution with a mole composition of 1 SiO2: 0.16 NaOH: 10.5 H2O was used for template-free silicalite secondary growth under hydrothermal conditions at 180 °C for 4 h. The resulting membrane was washed and dried to get the template-free silicalite membrane. The average pore diameter of the template-free membrane is 0.532 nm.
4.3. Material Characterization
Powder X-ray diffraction (XRD) data were collected using a Bruker D8 advance X-ray spectrometer with graphite monochromatized Cu Kα radiation (λ = 0.15405 nm) operated at 40 kV and 40 mA. Scanning electron micrographs were obtained using a JSM-7001F field emission scanning electron microscope equipped with an energy-dispersive spectroscopic (EDS) detector. The porosity and average pore diameter of the support were determined by mercury porosimetry.
4.4. Pervaporation Test
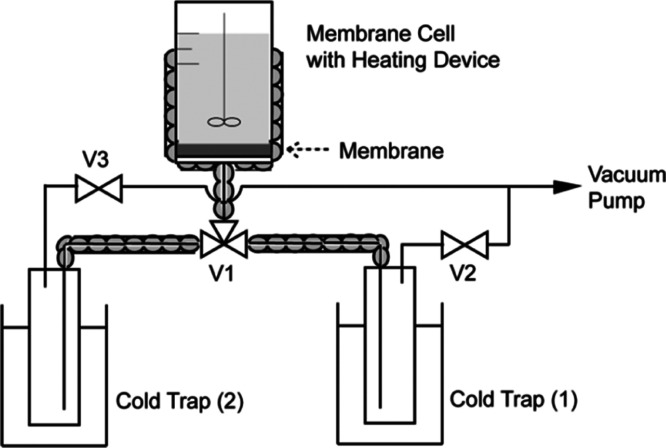
The pervaporation test was performed on the setup, as schematically depicted in Figure 7. The membrane was attached at the end of the cell with the membrane facing the organic liquid. The permeating side was kept in vacuum, and the permeated vapors were collected in the cold trap. The weight of the cold trap was measured before and after each run for flux calculation. In the binary cases, the compositions of the permeated mixture were determined using a gas chromatograph equipped with a flame ionization detector. The ideal separation factor was calculated by taking the ratio of each flux during the single-pervaporation process. For the equimolar binary mixture, the separation factor (βpervap) is defined as follows30
where Xa, Xb, Ya, and Yb are molar fractions of a and b isomers in the feed and permeate stream.
Figure 7.
Schematic diagram of the separation system for pervaporation of dichlorobenzene isomers.
All the silicalite membranes were subjected to the quality test first using 1,3,5-triisopropylbenzene (TIPB) as a pervaporation agent according to the literature16 (kinetic diameter 0.85 nm, larger than pore dimensions of MFI, 0.53 × 0.56 nm and 0.51 × 0.57 nm). The quality test (pervaporation with TIPB) measurements were performed at 25 °C for 6 h. After that, the membranes were calcined at 350 °C in the air to remove the absorbed TIPB.
The dichlorobenzene isomer pervaporation experiment was conducted with pure p-, o-, and m-dichlorobenzene or a mixture of equal molar binary o-/p- and m-/p-dichlorobenzene as the feed at 1 atm and 60 °C. Before each pervaporation test, the residual absorbed component in the membrane was removed by calcination at 673 K for 4 h.
Acknowledgments
We wish to thank the Shanghai Science and Technology Talent Project (No. 15XD1522300) for financial support through a strategic grant.
The authors declare no competing financial interest.
References
- Wu R.; Wang S.; Wang L. Atmospheric oxidation mechanism of chlorobenzene. Chemosphere 2014, 111, 537–544. 10.1016/j.chemosphere.2014.04.067. [DOI] [PubMed] [Google Scholar]
- Netskina O. V.; Tayban E. S.; Moiseenko A. P.; Komova O. V.; Mukha S. A.; Simagina V. I. Removal of 1,2-dichlorobenzene from water emulsion using adsorbent catalysts and its regeneration. J. Hazard. Mater. 2015, 285, 84–93. 10.1016/j.jhazmat.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Peng X.; Li Y.; Luan Z.; Di Z.; Wang H.; Tian B.; Jia Z. Adsorption of 1,2-dichlorobenzene from water to carbon nanotubes. Chem. Phys. Lett. 2003, 376, 154–158. 10.1016/s0009-2614(03)00960-6. [DOI] [Google Scholar]
- Guo G.; Long Y. Static equilibrium studies on separation of dichlorobenzene isomers on binder-free hydrophobic adsorbent of MFI type zeolite. Sep. Purif. Technol. 2001, 24, 507–518. 10.1016/s1383-5866(01)00150-2. [DOI] [Google Scholar]
- Siegbert R.; Adolf S.; Rudolf S.; Leonhard U.. Separation of m-and p-Dichlorobenzene[P]. U.S. Patent 5,152,875 A, 1992-10-06.
- Pentling U.; Buysch H. J.; Puppe L.; Roehlk K.; Grosser R.; Paul H. I.. Process for Separating Mixtures of m- and p-Dichlorobenzene[P]. U.S. Patent 5,386,067 A, 1995-01-31.
- Pies M.; Rohlk K.; Lahr H.; Fiege H.. Process for Isolating M-Dichlorobenzene from Mixtures of Dichlorobenzene Isomers[P]. U.S. Patent 5,436,377 A, 1995-07-25.
- Erdem G.; Leckebusch M.; Olf G.; Rinck K. J.; Zühlke G.. Process for the Separation of Mixtures Containing m- and p-Dichlorobenzene[P]. U.S. Patent 7,311,807 B2, 2007-12-25.
- Banihashemi F.; Ibrahim A. F. M.; Babaluo A. A.; Lin J. Y. S. Template-Free Synthesis of Highly b-Oriented MFI-Type Zeolite Thin Films by Seeded Secondary Growth. Angew. Chem., Int. Ed. 2019, 58, 2519–2523. 10.1002/anie.201814248. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Xiao Q.; Guo X.; Li N.; Kumar P.; Rangnekar N.; Jeon M. Y.; Al-Thabaiti S.; Narasimharao K.; Basahel S. N.; et al. Open-Pore Two-Dimensional MFI Zeolite Nanosheets for the Fabrication of Hydrocarbon-Isomer-Selective Membranes on Porous Polymer Supports. Angew. Chem., Int. Ed. 2016, 55, 7184–7187. 10.1002/anie.201601135. [DOI] [PubMed] [Google Scholar]
- Kim D.; Jeon M. Y.; Stottrup B. L.; Tsapatsis M. para -Xylene Ultra-selective Zeolite MFI Membranes Fabricated from Nanosheet Monolayers at the Air-Water Interface. Angew. Chem., Int. Ed. 2018, 57, 480–485. 10.1002/anie.201708835. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Qiang W.; Ji T.; Zhang M.; Li M.; Lu J.; Liu Y. Uniform hierarchical MFI nanosheets prepared via anisotropic etching for solution-based sub-100-nm-thick oriented MFI layer fabrication. Sci. Adv. 2020, 6, eaay5993 10.1126/sciadv.aay5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X.; Dong J.; Nenoff T. M.; Ozokwelu D. E. Separation of p-xylene from multicomponent vapor mixtures using tubular MFI zeolite mmbranes. J. Membr. Sci. 2006, 280, 624–633. 10.1016/j.memsci.2006.02.020. [DOI] [Google Scholar]
- Banihashemi F.; Meng L.; Babaluo A. A.; Lin Y. S. Xylene Vapor Permeation in MFI Zeolite Membranes Made by Templated and Template-Free Secondary Growth of Randomly Oriented Seeds: Effects of Xylene Activity and Microstructure. Ind. Eng. Chem. Res. 2018, 57, 16059–16068. 10.1021/acs.iecr.8b01373. [DOI] [Google Scholar]
- Solanki V. A.; Borah B. Exploring the Potentials of Metal-Organic Frameworks as Adsorbents and Membranes for Separation of Hexane Isomers. J. Phys. Chem. C 2019, 123, 17808–17822. 10.1021/acs.jpcc.9b03240. [DOI] [Google Scholar]
- Yuan W.; Lin Y. S.; Yang W. Molecular Sieving MFI-Type Zeolite Membranes for Pervaporation Separation of Xylene Isomers. J. Am. Chem. Soc. 2004, 126, 4776–4777. 10.1021/ja031653t. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Wu A.; Zhong S.; Wang B.; Zhou R. Highly (h0h)-oriented silicalite-1 membranes for butane isomer separation. J. Membr. Sci. 2017, 540, 50–59. 10.1016/j.memsci.2017.06.009. [DOI] [Google Scholar]
- Matsufuji T.; Nishiyama N.; Matsukata M.; Ueyama K. Separation of butane and xylene isomers with MFI-type zeolitic membrane synthesized by a vapor-phase transport method. J. Membr. Sci. 2000, 178, 25–34. 10.1016/s0376-7388(00)00462-2. [DOI] [Google Scholar]
- Wegner K.; Dong J.; Lin Y. S. Polycrystalline MFI zeolite membranes: Xylene pervaporation and its implication on membrane microstructure. J. Membr. Sci. 1999, 158, 17–27. 10.1016/s0376-7388(98)00339-1. [DOI] [Google Scholar]
- Daramola M. O.; Burger A. J.; pera-Titus M.; Giroir-Fendler A.; Miachon S.; Dalmon J.-A.; Lorenzen L. Separation and isomerization of xylenes using zeolite membranes: a short overview. J. Chem. Eng. 2010, 5, 815–837. 10.1002/apj.414. [DOI] [Google Scholar]
- Pham T. C. T.; Kim H. S.; Yoon K. B. Growth of Uniformly Oriented Silica MFI and BEA Zeolite Films on Substrates. Science 2011, 334, 1533–1538. 10.1126/science.1212472. [DOI] [PubMed] [Google Scholar]
- Dyer A.; Emms T. I. Liquid phase separations of organic isomers on high silica zeolites. Microporous Mesoporous Mater. 2007, 104, 137–144. 10.1016/j.micromeso.2007.01.020. [DOI] [Google Scholar]
- Mori N.; Tomita T. Separation of n-butane/iso-butane by self-supporting MFI membranes with various SiO2/Al2O3. Microporous Mesoporous Mater. 2008, 112, 88–96. 10.1016/j.micromeso.2007.09.014. [DOI] [Google Scholar]
- Yu L.; Grahn M.; Hedlund J. Ultra-thin MFI membranes for removal of C3+ hydrocarbons from methane. J. Membr. Sci. 2018, 551, 254–260. 10.1016/j.memsci.2018.01.054. [DOI] [Google Scholar]
- Xia S.; Peng Y.; Lu H.; Wang Z. The influence of nanoseeds on the pervaporation performance of MFI-type zeolite membranes on hollow fibers. Microporous Mesoporous Mater. 2016, 222, 128–137. 10.1016/j.micromeso.2015.10.010. [DOI] [Google Scholar]
- Kanezashi M.; O’Brien J.; Lin Y. S. Template-free synthesis of MFI-type zeolite membranes: Permeation characteristics and thermal stability improvement of membrane structure. J. Membr. Sci. 2006, 286, 213–222. 10.1016/j.memsci.2006.09.038. [DOI] [Google Scholar]
- Pan M.; Lin Y. S. Template-free secondary growth synthesis of MFI type zeolite membranes. Microporous Mesoporous Mater. 2001, 43, 319–327. 10.1016/s1387-1811(01)00212-8. [DOI] [Google Scholar]
- Ong Y. K.; Shi G. M.; Le N. L.; Tang Y. P.; Zuo J.; Nunes S. P.; Chung T.-S. Recent membrane development for pervaporation processes. Prog. Polym. Sci. 2016, 57, 1–31. 10.1016/j.progpolymsci.2016.02.003. [DOI] [Google Scholar]
- Zhou J.; Zhou C.; Xu K.; Caro J.; Huang A. Seeding-free synthesis of large tubular zeolite FAU membranes for dewatering of dimethyl carbonate by pervaporation. Microporous Mesoporous Mater. 2020, 292, 109713. 10.1016/j.micromeso.2019.109713. [DOI] [Google Scholar]
- Yeong Y. F.; Abdullah A. Z.; Ahmad A. L.; Bhatia S. Separation of p-xylene from binary xylene mixture over silicalite-1 membrane: Experimental and modeling studies. Chem. Eng. Sci. 2011, 66, 897–906. 10.1016/j.ces.2010.11.035. [DOI] [Google Scholar]