Abstract
Herein, we report a concise and stereoselective approach for the asymmetric total synthesis of hetiamacins A–F on the basis of the total synthesis of amicoumacin C, which could be synthesized from a known l-aspartic acid derivative. The synthesis of hetiamacin A was accomplished by an 11-step sequence that featured 1,3-oxazinane ring formation of amicoumacin B followed by amidation in one pot. Hetiamacins B–F were synthesized from amicoumacin A in only one step.
Introduction
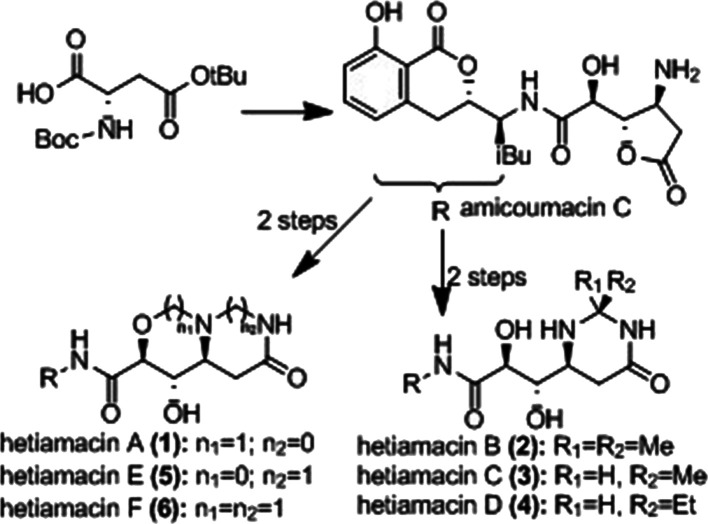
Hetiamacins A–F are new members of amicoumacin group antibiotics isolated from the cultured broth of Bacillus subtilis PJS by our group recently.1 Amicoumacins are known to exhibit various biological properties such as antimicrobial, anticancer, antiulcer, and herbicidal activities.2 This class of secondary metabolites produced structurally features a dihydroisocoumarin unit (left-hand amine segment) linked through an amide bond to an unusual amino acid of considerable structural diversity (right-hand acid segment), as exemplified by amicoumacins A–C.3 The planar structures (Figure 1) of hetiamacins were elucidated by spectroscopic methods; however, the stereochemistry of all chiral centers on hetiamacins A–D was not originally determined. The structure of hetiamacin A was revised through the total synthesis of the initially proposed structure and the newly assigned one.4 Also, for the other additional amicoumacin-type natural products hetiamacins B–D, their absolute configurations were unambiguously determined by Kuwahara and co-workers through their synthetic study.5 Recently, full stereochemical assignments for hetiamacins E and F were determined by spectroscopic methods.1c
Figure 1.
Structure of hetiamacins A–F
The synthesis of hetiamacins and other amicoumacin family members has attracted much attention due to their interesting biological activities.6 An additional synthetic challenge involves controlling the configuration of the amino acid segment, which is susceptible to stereoisomerization. In our previous work, we succeeded in the first total synthesis of hetiamacin A, and then Kuwahara et al. accomplished the synthesis of hetiamacins A–D from a known l-aspartic acid derivative via Sharpless asymmetric dihydroxylation and Wittig reactions. Our ongoing research program that is focused on a more practical and shorter synthetic route, which allows to obtain scalable hetiamacins.
Results and Discussion
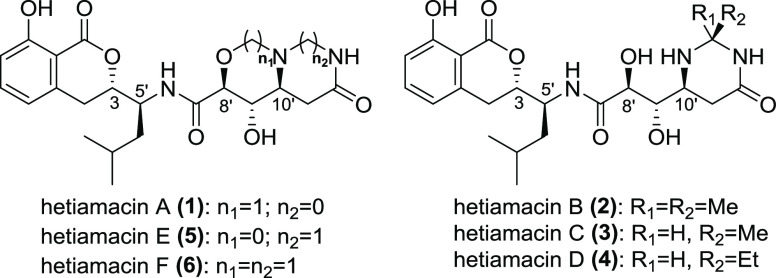
Our retrosynthetic analysis of hetiamacins is outlined in Scheme 1. The hetiamacins A–F could be synthesized from amicoumacin C (7) as the common intermediate. The compound 7 could be obtainable via condensation of the amine segment 8 and the acid segment that would be derived from hydrolysis of N-protected lactam 9. The lactam 9 was considered to be readily accessible by stereoselective dihydroxylation of unsaturated lactam 10 followed by protecting dihydroxy. The unsaturated lactam 10 would be traced back to N-Boc-protected aspartic acid derivative 11 by a route featuring condensation, reduction, and elimination.
Scheme 1. Retrosynthetic Analysis of Compounds 1–6.
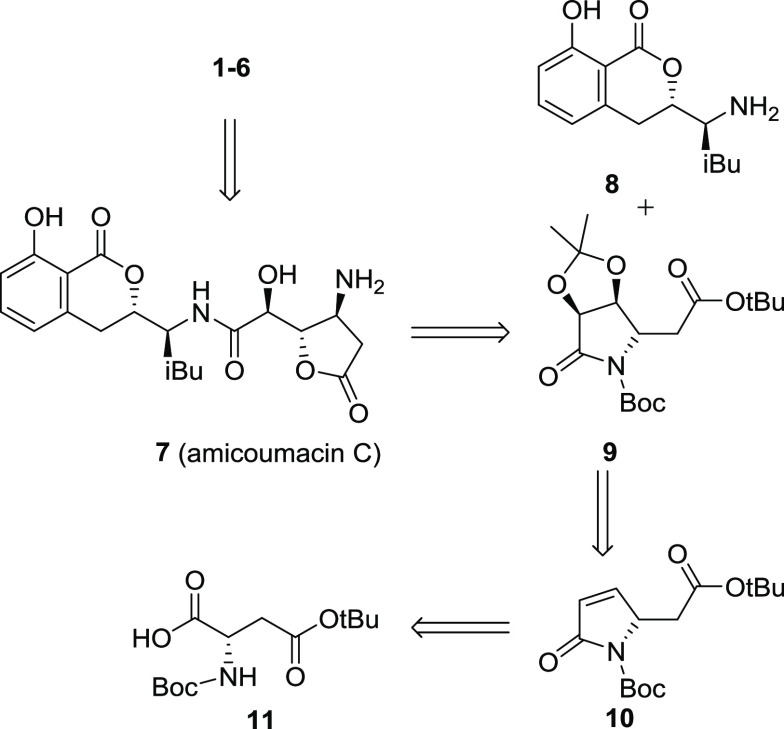
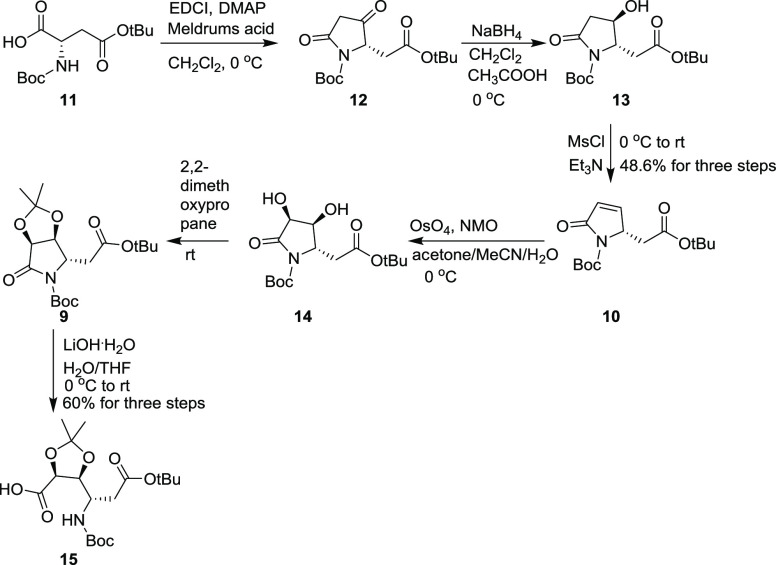
Based on the synthetic plan described above, our synthesis of hetiamacins A–F began with the conversion of 11 into β-hydroxy lactam 13 in 50% yield by involving the acylation of Meldrum’s acid with 11 followed by deprotection and decarboxylation to produce tetramic acid 12 and then reduction by NaBH4 to give β-hydroxy lactam 13.7 Subjection of 13 to a one-pot mesylation/elimination protocol quantitatively provided dehydration product 10, which was then exposed to dihydroxylation conditions to give 14 with litter diastereomer, albeit in a moderate isolated yield of 58%. After protection of the diol 14 as its acetonide, the product 9 was hydrolyzed with LiOH·H2O in the presence of a catalytic amount of H2O2 to obtain the acid segment 15 (Scheme 2).6j,6h,8
Scheme 2. Synthesis of Compound 15.
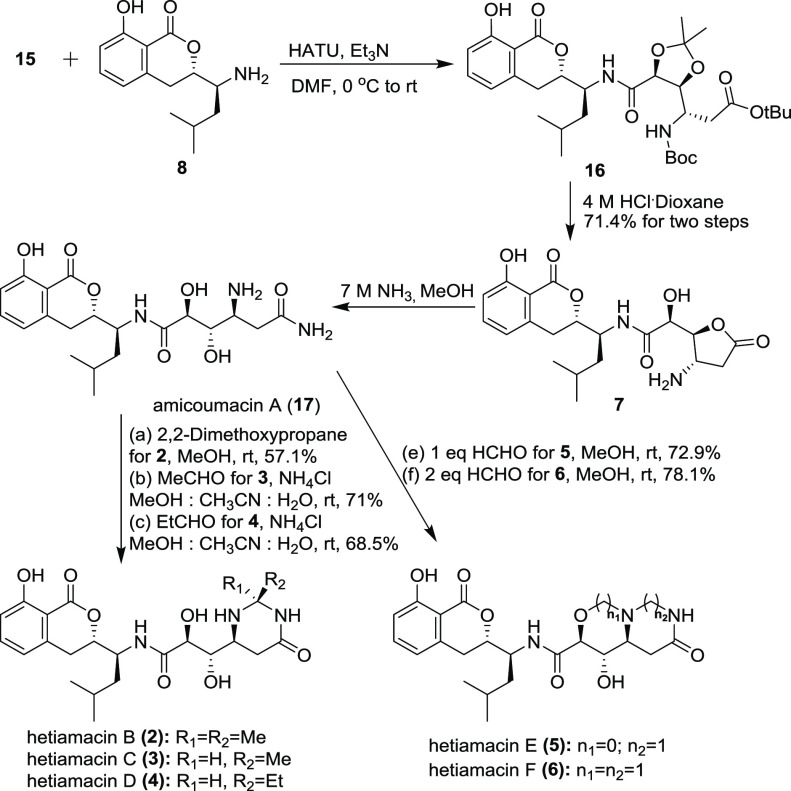
The synthesis of hetiamacins B–F was performed as shown in Scheme 3. With the key intermediate 15 in hand, we proceeded to the final stage of the synthesis of compounds 1–6. Coupling of the acid segment 15 with the known compound 8, prepared according to a reported protocol,6j in the presence of HATU and DIEA gave compound 16 in 92% yield. Exposure of 16 to HCl in dioxane brought about deprotection of all of its three protecting groups to furnish the hydrochloride of amicoumacin C (7) [α]D26 = −62.5 (c = 0.024, MeOH); lit.6c [α]D = −71.1 (c = 0.34, MeOH)). Ammonolytic opening of the γ-lactone moiety of compound 7 afforded amide 17 in equivalent yield, then treated with 2,2-dimethoxypropane to furnish hetiamacin B (2) in 57.1% yield ([α]D25 = −120 (c = 0.01, CHCl3); lit.5 [α]D = −115 (c = 0.08, CHCl3)).9 Application of the same step (N,N-acetal ring formation) to amide 17 (amicoumacin A) using acetaldehyde or propionaldehyde instead of 2,2-dimethoxypropane afforded compounds 3 and 4 and their epimers (14′-epi-hetiamacins C: 45%, 14′-epi-hetiamacins D: 43%) as byproducts. Therefore, we varied several factors in the reactions including temperature, additives, and solvents.6g,10 Among the reaction conditions attempted, treatment of compound 17 with NH4Cl and aldehyde in MeOH:CH3CN:H2O (1:1:1) gave the best result (hetiamacin C: ([α]D24 = −100 (c = 0.02, CHCl3); lit.5 [α]D = −99 (c = 0.115, CHCl3)) and hetiamacin D: ([α]D27= −90 (c = 0.02, CHCl3); lit.5 [α]D = −97 (c = 0.155, CHCl3))) and furnished the desired cyclization product up to 70% yield. The 1H and 13C NMR spectra of compounds 2–4 were also in good agreement with those of natural hetiamacins B–D.
Scheme 3. Synthesis of Hetiamacins B–F.
The intermediate amicoumacin A (17) reacted with 1 equiv formaldehyde to form the N,N-acetal unit, providing hetiamacin E (5) in 72.9% yield and less isomer hetiamacin A with an N,O-acetal ring as products. Also, in the same process, reaction with 2 equiv formaldehyde to form the N,N-acetal unit and N,O-acetal ring gave hetiamacin F (6) in 78.1% yield. Based on the excellent agreement of the 1H and 13C NMR spectra of 5 and 6 with those of hetiamacins E and F from natural origin, respectively, we concluded that the relative stereostructures of hetiamacins E and F should be represented by 5 and 6 (see the Supporting Information for comparison of their 13C NMR data with authentic data). The absolute configurations of hetiamacins E and F would be confirmed to be 3S,5′S,8′S,9′S,10′S.
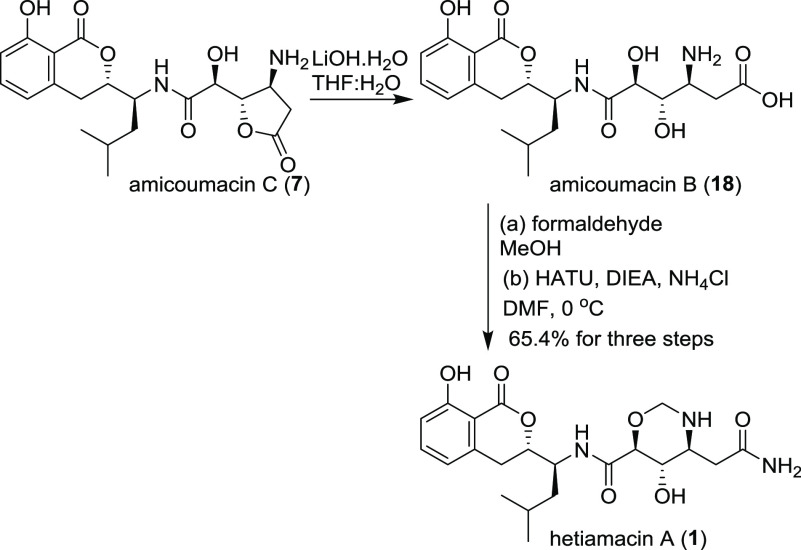
The synthesis of hetiamacin A (1) was from the intermediate amicoumacin B (17), which hydrolyzed from amicoumacin C (7), reacted with formaldehyde to form the N,O-acetal unit, providing the 1,3-oxazinane derivative, and then coupled with NH4Cl in the presence of HATU and DIEA in 92% yield for three steps ([α]D26 = −103.3 (c = 0.03, MeOH); lit.5 [α]D = −106 (c = 0.26, MeOH)) (Scheme 4).
Scheme 4. Synthesis of Hetiamacin A.
Conclusions
In conclusion, the enantioselective total synthesis of hetiamacins A–F was achieved with the common intermediate amicoumacin C (7), which was accomplished from N-Boc-l-aspartic by the concise eight-step sequence of reactions. Based on the excellent agreement of the NMR spectra of the synthetic compounds with those of respective natural samples, the absolute configurations of hetiamacins E and F were concluded to be represented by structures 5 and 6. Our efficient approach to hetiamacins would readily be applicable and economical to the synthesis of other members of amicoumacin-type natural products. Especially, the synthetic method will also facilitate the preparation of analogues for structure–activity relationship studies to develop new types of antibiotics.
Experimental Section
All reactions were carried out in oven-dried glassware under a nitrogen atmosphere employing standard techniques in handling air-sensitive materials. Chemicals were either used as received or purified according to the procedures outlined in Purification of Common Laboratory Chemicals. Glassware was dried in an oven at 160 °C or flame-dried under vacuum and cooled under an inert atmosphere before use. Unless otherwise noted, reactions were magnetically stirred and monitored by thin-layer chromatography with Sorbent Technologies 0.20 mm silica gel 60 plates. Column chromatography was performed with silica gel 300–400 supplied by Sorbent Technologies. The preparative HPLC separations were performed on a prominence system (LC–20AT, Shimadzu) instrument equipped with a binary pump and a UV–visible diode array detector (190–800 nm) using a ZORBAX SB–C18 column (9.4 × 250 mm, i.d. of 5 μm; Agilent). Yields refer to chromatographically and spectroscopically pure compounds, unless otherwise noted. 1H and 13C NMR spectra were recorded using an internal deuterium lock on Bruker 500 spectrometers or Bruker 600 spectrometers. All signals were reported in ppm with the internal references of 7.26 ppm or 77.0 ppm for chloroform, 2.50 ppm or 39.5 ppm for dimethyl sulfoxide, and 3.30 ppm or 49.1 ppm for methanol as standards. Data is presented as follows: multiplicity (s = singlet, d = doublet, t = triplet, m = mutiplet, br = broad, ABq = AB quartet, dd = doublet of doublet, and dt = doublet of triplet), coupling constant (J/Hz), and integration. Optical rotations were recorded on an Autopol IV digital polarimeter at 589 nm and reported as follows: [α]DT concentration (g/100 mL) and solvent. High-resolution mass spectra were obtained on a Waters Xevo G2-XS QTof with an ESI-QTof high-resolution mass spectrometer.
tert-Butyl (S)-2-(2-(tert-Butoxy)-2-oxoethyl)-3,5-dioxopyrrolidine-1-carboxylate (12)
A solution of ester 11 (5 g, 17.28 mmol), 2,2-dimethyl-1,3-dioxane-4,6-dione (3.74 g, 25.92 mmol, 1.5 equiv), and DMAP (3.17 g, 25.92 mmol, 1.5 equiv) in CH2Cl2 (150 mL) was treated with EDCI (4.97 g, 25.92 mmol, 1.5 equiv) at 0 °C, then warmed to room temperature, and stirred for 2 h. The reaction mixture was cooled to 0 °C, diluted with EtOAc (300 mL), quenched with brine (10 mL), and then washed with 5% KHSO4 (100 mL × 3) and brine (100 mL × 3). The organic layer was dried over Na2SO4, filtered, and concentrated in vacuum. The residue was added to EtOAc (200 mL), refluxed for 0.5 h, and concentrated to give compound 12 as a yellow oil for the next step without being purified. [α]D26 = −42.9 (c = 0.212, CHCl3); 1H NMR (500 MHz, methanol-d4) δ 4.93 (s, 2H), 4.68 (dd, J = 6.7, 2.7 Hz, 1H), 2.97 (dd, J = 14.4, 6.7 Hz, 1H), 2.87 (dd, J = 14.4, 2.7 Hz, 1H), 1.57 (s, 9H), 1.42 (s, 9H). 13C NMR (125 MHz, methanol-d4) δ 178.31, 173.21, 169.83, 150.45, 83.87, 82.45, 58.85, 37.24, 28.39, 28.21. HRMS (ESI-TOF): m/z calcd. for C15H23NO6Na ([M + Na]+) 336.1423, found 336.1441.
tert-Butyl (S)-2-(2-(tert-Butoxy)-2-oxoethyl)-5-oxo-2,5-dihydro-1H-pyrrole-1-carboxylate (10)
To a solution of tetramic acid 12 (5 g) in CH2Cl2:CH3COOH (10:1, 99 mL) was added portionwise NaBH4 (1.21 g, 31.91 mmol) over 1 h at 0 °C. The reaction was quenched with water after 10 h and then extracted with CH2Cl2 (100 mL × 3). The combined organic layer was washed with brine (300 mL), dried over Na2SO4, filtered, and concentrated in vacuum. The crude residue was then finally purified by flash chromatography on a silica gel (hexane:EtOAc = 1:1) to afford alcohol 13 (3 g, 60%). To a solution of alcohol 13 (2 g, 6.34 mmol) and Et3N (1.76 mL, 12.68 mmol) in CH2Cl2 (50 mL) was added methanesulfonyl chloride (1.21 g, 31.91 mmol) at 0 °C. The solution was warmed to room temperature, stirred for 2 h, then added with DBU, quenched with NH4Cl after 30 min, and extracted with EtOAc (100 mL × 3). The combined organic layer was washed with water (100 mL × 3) and brine (100 mL × 3), dried over Na2SO4, filtered, and concentrated in vacuum. The crude residue was then finally purified by flash chromatography on a silica gel (hexane:EtOAc = 85:15) to afford compound 10 (1.7 g, 90%) as a white solid. [α]D24 = +158.5 (c = 0.26, CHCl3); 1H NMR (500 MHz, chloroform-d) δ 7.30 (dd, J = 6.1, 2.0 Hz, 1H), 6.10 (dd, J = 6.1, 1.7 Hz, 1H), 4.81 (ddt, J = 9.7, 3.7, 1.8 Hz, 1H), 3.13 (dd, J = 15.6, 3.7 Hz, 1H), 2.41 (dd, J = 15.6, 9.7 Hz, 1H), 1.55 (s, 9H), 1.43 (s, 9H); 13C NMR (125 MHz, chloroform-d) δ 169.08, 168.80, 150.17, 149.38, 126.95, 83.45, 81.87, 59.06, 37.85, 28.23, 28.15. HRMS (ESI-TOF): m/z calcd. for C15H23NO5Na ([M + Na]+) 320.1474, found 320.1471.
tert-Butyl (2S,3S,4S)-2-(2-(tert-Butoxy)-2-oxoethyl)-3,4-dihydroxy-5-oxopyrrolidine-1-carboxylate (14)
To a solution of compound 10 (1 g, 3.36 mmol) in acetone:acetonitrile:H2O (1:1:1, 90 mL) were added OsO4 (13.6 mg, 0.054 mmol) and NMO (473 mg, 4.04 mmol). After stirring at room temperature for 48 h, the reaction was quenched by adding saturated Na2SO3 (100 mL) and the resultant mixture was stirred for 1 h at room temperature. The mixture was then diluted with EtOAc (300 mL). The organic layer was separated, and the aqueous phase was further extracted with EtOAc (300 mL). The combined organic layer was washed with brine (300 mL), dried over Na2SO4, filtered, and concentrated in vacuum. The crude residue was then finally purified by flash chromatography on a silica gel (hexane:EtOAc = 1:1) to afford diol 14 (0.7 g, 63.1%) as a white solid. [α]D26 = +2.7 (c = 0.26, CHCl3); 1H NMR (500 MHz, chloroform-d) δ 4.43 (d, J = 4.8 Hz, 1H), 4.38 (dd, J = 10.6, 3.3 Hz, 1H), 4.31 (d, J = 4.8 Hz, 1H), 3.30–3.04 (m, 1H), 2.89 (s, 1H), 2.80 (dd, J = 15.7, 3.3 Hz, 1H), 2.42 (dd, J = 15.4, 10.4 Hz, 1H), 1.54 (s, 9H), 1.46 (s, 9H); 13C NMR (125 MHz, chloroform-d) δ 172.82, 169.16, 149.37, 84.30, 82.12, 70.87, 69.16, 59.79, 36.82, 28.16, 28.10. HRMS (ESI-TOF): m/z calcd. for C15H25NO7Na ([M + Na]+) 354.1529, found 354.1542.
tert-Butyl (3aS,4S,6aS)-4-(2-(tert-Butoxy)-2-oxoethyl)-2,2-dimethyl-6-oxotetrahydro-5H-[1,3]dioxolo[4,5-c]pyrrole-5-carboxylate (9)
To a solution of the diol 14 (200 mg, 0.6 mmol) in 2,2-dimethoxypropane (5 mL) was added P-toluene sulfonic acid and stirred at room temperature for 30 min. The solution mixture was poured into saturated NaHCO3 (100 mL) and extracted with EtOAc (100 mL × 2). The organic layer was washed with water (100 mL × 2) and brine (100 mL × 2), dried over Na2SO4, and concentrated in vacuum to give the crude compound, and the residue was purified by flash column chromatography on a silica gel (hexane:EtOAc =85:15) to give compound 9 as a colorless oil (220 mg, 97.9%). [α]D26 = +6.5 (c = 0.184, CHCl3); 1H NMR (500 MHz, chloroform-d) δ 4.81 (d, J = 5.7 Hz, 1H), 4.58 (d, J = 5.7 Hz, 1H), 4.34 (dd, J = 7.3, 2.8 Hz, 1H), 2.79 (dd, J = 16.1, 7.3 Hz, 1H), 2.71 (dd, J = 16.1, 2.9 Hz, 1H), 1.54 (s, 9H), 1.45 (s, 3H), 1.43 (s, 9H), 1.37 (s, 3H); 13C NMR (125 MHz, chloroform-d) δ 170.95, 169.57, 149.72, 112.63, 84.00, 82.23, 77.75, 76.47, 58.17, 37.38, 28.14, 28.12, 27.10, 25.61. HRMS (ESI-TOF): m/z calcd. for C18H29NO7Na ([M + Na]+) 394.1842, found 394.1841.
tert-Butyl (S)-3-((tert-Butoxycarbonyl)amino)-3-((4S,5S)-5-(((S)-1-((S)-8-hydroxy-1-oxoisochroman-3-yl)-3-methylbutyl)carbamoyl)-2,2-dimethyl-1,3-dioxolan-4-yl)propanoate (16)
To a solution of the compound 9 (120 mg, 0.32 mmol) in THF:H2O (1:1, 5 mL) was added LiOH·H2O (15 mg, 0.35 mmol) and stirred at 0 °C for 1 h. The solution was acidified and extracted with EtOAc (100 mL × 3). The combined organic layer was washed with brine (100 mL × 2), dried over Na2SO4, and then concentrated in vacuum to give the compound 15 as a yellow oil for the next step without being further purified. To a solution of compound 15 (120 mg, 0.31 mmol), dihydroisocoumarin 8 (87 mg, 0.31 mmol), and DIEA (0.06 mL, 0.34 mmol) in CH2Cl2 (20 mL) was added HATU (117 mg, 0.31 mmol) at 0 °C. The solution was warmed to room temperature and stirred for 2 h. Then, the solution was added to CH2Cl2 (50 mL), washed with HCl (1 mol/L, 50 mL) and brine (50 mL), dried over Na2SO4, and concentrated in vacuum. The crude residue was purified by flash column chromatography on a silica gel (hexane:EtOAc = 85:15) to give compound 16 as a white solid (150 mg, 78.4%). [α]D20 = −28.6 (c = 0.184, CHCl3); 1H NMR (500 MHz, chloroform-d) δ 10.84 (s, 1H), 7.43 (t, J = 7.9 Hz, 1H), 6.89 (dd, J = 14.2, 9.2 Hz, 2H), 6.72 (d, J = 7.4 Hz, 1H), 5.55 (s, 1H), 5.32 (s, 1H), 4.72–4.60 (m, 2H), 4.56 (d, J = 7.4 Hz, 1H), 4.43–4.35 (m, 1H), 4.25–4.16 (m, 1H), 3.05 (dd, J = 16.5, 13.0 Hz, 1H), 2.85 (dd, J = 16.6, 2.9 Hz, 1H), 2.63–2.53 (m, 1H), 1.80 (ddd, J = 13.5, 9.3, 5.9 Hz, 1H), 1.74–1.65 (m, 1H), 1.56 (s, 4H), 1.45 (d, J = 6.7 Hz, 18H), 1.38 (s, 3H), 1.00 (s, 3H), 0.99 (s, 3H); 13C NMR (125 MHz, chloroform-d) δ 170.94, 170.03, 169.82, 155.31, 139.63, 136.73, 118.46, 116.41, 110.02, 108.26, 80.92, 80.78, 76.16, 48.86, 40.55, 30.64, 28.60, 28.24, 27.05, 24.91, 24.74, 23.03, 22.36. HRMS (ESI-TOF): m/z calcd. for C32H49N2O10 ([M + H]+) 621.3382, found 621.3376.
(S)-2-((2S,3S)-3-Amino-5-oxotetrahydrofuran-2-yl)-2-hydroxy-N-((S)-1-((S)-8-hydroxy-1-oxoisochroman-3-yl)-3-methylbutyl)acetamide hydrochloride (Amicoumacin C·HCl) (7)
The amide 16 (100 mg, 0.161 mmol) was added to a solution of HCl in dioxane (4 M, 2 mL) at room temperature. After stirring for 4 h, the mixture was concentrated in vacuo to give the crude compound 7 as a yellow oil. The crude compound was purified by flash column chromatography on a silica gel (CH2Cl2:MeOH = 40:1) to give 7 as a white solid (65 mg, 91.1%). [α]D26 = −62.5 (c = 0.024, MeOH); 1H NMR (600 MHz, methanol-d4) δ 7.46–7.41 (m, 1H), 6.81 (d, J = 8.4 Hz, 1H), 6.77 (d, J = 7.4 Hz, 1H), 4.68 (ddt, J = 9.9, 6.2, 3.6 Hz, 1H), 4.42 (q, J = 2.1, 1.6 Hz, 1H), 4.19–4.12 (m, 2H), 3.17 (dd, J = 18.7, 8.8 Hz, 1H), 3.00 (qd, J = 16.5, 7.3 Hz, 2H), 2.52 (dq, J = 18.8, 2.6 Hz, 1H), 1.76 (dddd, J = 13.2, 11.0, 4.3, 1.6 Hz, 1H), 1.69–1.60 (m, 1H), 1.38 (ddd, J = 13.7, 9.8, 4.0 Hz, 1H), 0.94 (d, J = 6.6 Hz, 3H), 0.87 (d, J = 6.5 Hz, 3H); 13C NMR (150 MHz, methanol-d4) δ 174.95, 172.46, 171.08, 163.19, 141.03, 137.75, 119.65, 116.89, 109.38, 84.43, 82.19, 73.01, 51.27, 49.35, 40.00, 34.69, 30.92, 25.92, 23.80, 21.81. HRMS (ESI-TOF): m/z calcd. for C20H27O7N2 ([M + H]+) 407.1813, found 407.1841.
(4S,5S,6S)-4-(2-Amino-2-oxoethyl)-5-hydroxy-N-((S)-1-((S)-8-hydroxy-1-oxoisochroman-3-yl)-3-methylbutyl)-1,3-oxazinane-6-carboxamide (Hetiamacin A) (1)
To a solution of the compound 7 (10 mg, 25 μmol) in THF:H2O (1:1, 5 mL) was added LiOH·H2O (2.1 mg, 25 μmol) and stirred at 0 °C for 1 h. The solution was acidified and extracted with EtOAc (100 mL × 3). The combined organic layer was washed with brine (100 mL × 2), dried over Na2SO4, and concentrated in vacuum to give the oil compound 18. Then, the crude residue 18 was dissolved in methanol (5 mL) before the formaldehyde (4 mg, 50 μmol, 37%) was added. After stirring for 12 h, the solution was concentrated in vacuo to give the crude compound. To a solution of the crude compound in DMF (2 mL) were added NH4Cl (1.9 mg, 35 μmol), DIEA (10.5 μL, 59 μmol), and HATU (12.5 mg, 35 μmol). After stirring at rt for 2 h, the solution was filtered and the filtrate was purified by prepared HPLC (MeOH:H2O = 60:40) to give 1 (7 mg, 65.4%) as a white solid. [α]D26 = −113.3 (c = 0.03, MeOH); 1H NMR (600 MHz, methanol-d4) δ 7.38 (dd, J = 8.4, 7.2 Hz, 1H), 6.77 (d, J = 8.4 Hz, 1H), 6.72 (d, J = 7.2 Hz, 1H), 4.60 (dt, J = 12.5, 3.0 Hz, 1H), 4.50 (d, J = 10.4 Hz, 1H), 4.29 (dt, J = 10.9, 4.0, 2.8 Hz, 1H), 4.15 (d, J = 10.5 Hz, 1H), 3.75 (d, J = 9.3 Hz, 1H), 3.22 (dd, J = 9.3, 9.3 Hz, 1H), 3.02 (dd, J = 16.5, 12.5 Hz, 1H), 2.89 (dd, J = 9.3, 3.6 Hz, 1H), 2.84 (dd, J = 16.6, 3.1 Hz, 1H), 2.62 (dd, J = 15.3, 3.6 Hz, 1H), 2.22 (dd, J = 15.3, 8.8 Hz, 1H), 1.80–1.73 (m, 1H), 1.67–1.59 (m, 1H), 1.42–1.36 (m, 1H), 0.92 (d, J = 6.7 Hz, 3H), 0.88 (d, J = 6.5 Hz, 3H); 13C NMR (150 MHz, methanol-d4) δ 176.8, 173.0, 171.0, 163.2, 141.6, 137.5, 119.5, 116.7, 109.4, 82.9, 81.4, 79.4, 70.4, 58.8, 50.3, 40.8, 38.2, 30.9, 25.9, 23.7, 22.0; HRMS (ESI-TOF): m/z calcd. for C21H30O7N3 ([M + H]+) 436.2078, found 436.2088.
(2S,3S,4S)-4-Amino-2,3-dihydroxy-N1-((S)-1-((S)-8-hydroxy-1-oxoisochroman-3-yl)-3-methylbutyl)hexanediamide (Amicoumacin A) (17)
The compound 7 (10 mg, 25 μmol) was dissolved in a solution of NH3 in methanol (7 M, 3 mL). After stirring for 12 h, the mixture was concentrated in vacuo to give the crude compound 17 as a yellow oil for the next step without being further purified. [α]D23 = −104 (c = 0.022, CHCl3); 1H NMR (600 MHz, chloroform-d) δ 7.39 (dd, J = 17.5, 9.6 Hz, 2H), 6.87 (d, J = 8.4 Hz, 1H), 6.69 (t, J = 6.8 Hz, 1H), 6.08 (s, 1H), 5.58 (s, 1H), 5.30 (s, 1H), 4.62 (dd, J = 11.4, 7.5 Hz, 1H), 4.32 (q, J = 9.6, 9.1 Hz, 1H), 4.04 (d, J = 8.7 Hz, 1H), 3.77–3.70 (m, 1H), 3.69–3.63 (m, 1H), 3.60 (dt, J = 8.5, 4.2 Hz, 1H), 3.43 (s, 1H), 3.30 (s, 1H), 3.05 (t, J = 14.7 Hz, 1H), 2.86–2.77 (m, 1H), 2.59 (dd, J = 24.7, 11.4 Hz, 2H), 1.84 (d, J = 13.5 Hz, 1H), 1.64 (dd, J = 14.5, 7.5 Hz, 1H), 1.54–1.43 (m, 1H), 0.96 (t, J = 7.0 Hz, 3H), 0.93 (d, J = 6.3 Hz, 3H); 13C NMR (150 MHz, chloroform-d) δ 175.30, 174.06, 169.62, 162.26, 139.46, 136.59, 118.35, 116.36, 108.24, 81.00, 74.05, 72.56, 71.49, 53.80, 48.83, 40.42, 30.39, 24.93, 23.19, 22.01. HRMS (ESI-TOF): m/z calcd. for C20H30O7N3 ([M + H]+) 424.2078, found 424.2098.
(2S,3S)-3-((S)-2,2-Dimethyl-6-oxohexahydropyrimidin-4-yl)-2,3-dihydroxy-N-((S)-1-((S)-8-hydroxy-1-oxoisochroman-3-yl)-3-methylbutyl)propanamide (Hetiamacin B) (2)
A solution of compound 17 (8 mg, 19 μmol) in MeOH (5 mL) was added 2,2-dimethoxypropane (5.2 μL, 38 μmol) at room temperature. After stirring for 4 h, the mixture was concentrated in vacuo to give the crude product as a yellow oil. The crude product was purified by prepared HPLC (MeOH:H2O = 60:40) to give 2 (5 mg, 57.1%) as a white solid. [α]D25 = −120 (c = 0.01, CHCl3); 1H NMR (600 MHz, DMSO-d6) δ 7.74 (s, 1H), 7.64 (d, J = 9.6 Hz, 1H), 7.48 (t, J = 7.9 Hz, 1H), 6.84 (dd, J = 16.1, 7.9 Hz, 2H), 5.59 (d, J = 6.0 Hz, 1H), 4.97 (d, J = 5.4 Hz, 1H), 4.70 (dt, J = 12.7, 3.0 Hz, 1H), 4.20 (ddd, J = 13.7, 10.0, 3.5 Hz, 1H), 3.91 (t, J = 6.4 Hz, 1H), 3.68–3.61 (m, 1H), 3.25–3.17 (m, 1H), 3.04 (dd, J = 16.6, 12.6 Hz, 1H), 2.86 (dd, J = 16.7, 3.0 Hz, 1H), 2.06–1.97 (m, 2H), 1.92 (d, J = 13.0 Hz, 1H), 1.67 (tdd, J = 15.9, 8.8, 4.5 Hz, 2H), 1.33 (ddd, J = 13.0, 9.0, 3.8 Hz, 2H), 1.24 (s, 3H), 1.19 (s, 3H), 0.89 (d, J = 6.5 Hz, 4H), 0.85 (d, J = 6.5 Hz, 4H); 13C NMR (150 MHz, DMSO-d6) δ 172.70, 169.03, 160.80, 140.65, 136.24, 118.49, 115.16, 108.29, 81.09, 73.46, 72.43, 66.80, 48.18, 47.86, 40.02, 31.71, 30.60, 29.09, 28.44, 23.96, 23.28, 21.53. HRMS (ESI-TOF): m/z calcd. for C23H34O7N3 ([M + H]+) 464.2391, found 464.2403.
(2S,3S)-2,3-Dihydroxy-N-((S)-1-((S)-8-hydroxy-1-oxoisochroman-3-yl)-3-methylbutyl)-3-((2R,4S)-2-methyl-6-oxohexahydropyrimidin-4-yl)propanamide (Hetiamacin C) (3)
A solution of compound 17 (8 mg, 19 μmol) in MeOH:CH3CN:H2O (1:1:1 3 mL) were added acetaldehyde (2.1 μL, 38 μmol) and NH4Cl (5 mg) at room temperature. After stirring for 30 h, the mixture was filtered and the filtrate was concentrated in vacuo to give the crude product as a yellow oil. The crude product was purified by prepared HPLC (MeOH:H2O = 60:40) to give 3 (6 mg, 71%) as a white solid. [α]D24 = −100 (c = 0.02, CHCl3); 1H NMR (600 MHz, chloroform-d) δ 10.77 (s, 1H), 7.40 (t, J = 7.9 Hz, 1H), 6.87 (d, J = 8.4 Hz, 1H), 6.69 (d, J = 7.4 Hz, 1H), 6.32 (s, 1H), 6.19 (s, 1H), 4.84 (s, 1H), 4.61 (d, J = 12.9 Hz, 1H), 4.45 (q, J = 6.1 Hz, 1H), 4.34 (td, J = 10.1, 4.4 Hz, 1H), 4.02 (d, J = 9.5 Hz, 1H), 3.56 (m, J = 8.1, 6.9 Hz, 1H), 3.25 (p, J = 5.1 Hz, 1H), 3.06 (dd, J = 16.4, 13.0 Hz, 1H), 2.82 (dd, J = 16.7, 2.9 Hz, 1H), 2.64 (dd, J = 17.9, 4.2 Hz, 1H), 2.25 (dd, J = 17.8, 11.6 Hz, 1H), 1.83 (m, 2H), 1.62 (m, J = 6.4 Hz, 2H), 1.47 (m, 1H), 1.33 (d, J = 6.0 Hz, 2H), 0.94 (dd, J = 11.8, 6.5 Hz, 7H); 13C NMR (150 MHz, chloroform-d) δ 174.42, 170.54, 169.50, 162.09, 139.28, 136.54, 118.25, 116.22, 108.02, 81.04, 73.25, 72.62, 63.88, 55.86, 48.75, 40.54, 33.14, 30.32, 24.81, 23.07, 22.59, 21.78. HRMS (ESI-TOF): m/z calcd. for C22H32O7N3 ([M + H]+) 450.2235, found 450.2243.
(2S,3S)-3-((2R,4S)-2-Ethyl-6-oxohexahydropyrimidin-4-yl)-2,3-dihydroxy-N-((S)-1-((S)-8-hydroxy-1-oxoisochroman-3-yl)-3-methylbutyl)propanamide (Hetiamacin D) (4)
A solution of compound 17 (8 mg, 19 μmol) in MeOH:CH3CN:H2O (1:1:1, 3 mL) were added propionaldehyde (2.7 μL, 38 μmol) and NH4Cl (5 mg) at room temperature. After stirring for 30 h, the mixture was filtered and the filtrate was concentrated in vacuo to give the crude product as a yellow oil. The crude product was purified by prepared HPLC (MeOH:H2O = 60:40) to give 4 (6 mg, 68.5%) as a white solid. [α]D27 = −90 (c = 0.02, CHCl3); 1H NMR (600 MHz, chloroform-d) δ 10.78 (s, 1H), 7.46–7.38 (m, 1H), 7.22 (d, J = 10.0 Hz, 1H), 6.87 (d, J = 8.4 Hz, 1H), 6.69 (d, J = 7.4 Hz, 1H), 6.15 (s, 1H), 4.88 (s, 1H), 4.61 (ddd, J = 12.9, 2.9, 1.7 Hz, 1H), 4.34 (tdd, J = 10.1, 4.9, 1.7 Hz, 1H), 4.25 (t, J = 6.1 Hz, 1H), 4.06 (d, J = 9.0 Hz, 1H), 3.53 (m, 1H), 3.22 (ddd, J = 11.9, 8.2, 4.3 Hz, 1H), 3.05 (dd, J = 16.4, 13.0 Hz, 1H), 2.82 (dd, J = 16.5, 3.0 Hz, 1H), 2.76 (dd, J = 18.0, 4.2 Hz, 1H), 2.24 (dd, J = 17.9, 11.6 Hz, 1H), 1.84 (ddd, J = 13.9, 10.3, 5.2 Hz, 1H), 1.61 (ddd, J = 16.4, 13.2, 7.2 Hz, 4H), 1.48 (ddd, J = 13.9, 9.0, 4.8 Hz, 1H), 1.00 (t, J = 7.4 Hz, 3H), 0.96 (d, J = 6.7 Hz, 3H), 0.94 (d, J = 6.5 Hz, 3H); 13C NMR (150 MHz, chloroform-d) δ 174.39, 169.50, 162.10, 139.27, 136.54, 118.26, 116.23, 108.00, 81.01, 68.77, 48.69, 40.54, 30.32, 29.33, 24.80, 23.06, 21.80, 8.65. HRMS (ESI-TOF): m/z calcd. for C23H34O7N3 ([M + H]+) 464.2391, found 464.2384.
(2S,3S)-2,3-Dihydroxy-N-((S)-1-((S)-8-hydroxy-1-oxoisochroman-3-yl)-3-methylbutyl)-3-((S)-6-oxohexahydropyrimidin-4-yl)propanamide (Hetiamacin E) (5)
A solution of compound 17 (8 mg, 0.19 μmol) in MeOH (5 mL) was added formaldehyde (1.5 μL, 0.19 μmol) at room temperature. After stirring for 4 h, the mixture was concentrated in vacuo to give the crude product as a yellow oil. The crude product was purified by prepared HPLC (MeOH:H2O = 60:40) to give 5 (6 mg, 72.9%) as a white solid. [α]D26 = −153.7 (c = 0.08, CHCl3); 1H NMR (600 MHz, DMSO-d6) δ 10.84 (s, 1H), 7.64 (d, J = 9.5 Hz, 1H), 7.60 (d, J = 2.3 Hz, 1H), 7.50 (dd, J = 8.4, 7.4 Hz, 1H), 6.85 (dd, J = 14.9, 7.9 Hz, 2H), 5.57 (s, 1H), 4.98 (d, J = 5.6 Hz, 1H), 4.71 (dt, J = 12.7, 3.0 Hz, 1H), 4.24–4.17 (m, 1H), 4.05–3.97 (m, 2H), 3.92 (dd, J = 6.5, 2.9 Hz, 1H), 3.69 (dt, J = 6.6, 5.0 Hz, 1H), 3.08–2.98 (m, 2H), 2.87 (dd, J = 16.7, 3.0 Hz, 1H), 2.14–2.09 (m, 2H), 1.76 (s, 1H), 1.68 (dddd, J = 25.6, 13.1, 10.9, 5.6 Hz, 2H), 1.34 (td, J = 9.2, 4.4 Hz, 1H), 0.91 (d, J = 6.5 Hz, 3H), 0.86 (d, J = 6.4 Hz, 3H); 13C NMR (150 MHz, DMSO-d6) δ 172.65, 169.36, 169.03, 160.79, 140.64, 136.24, 118.49, 115.16, 108.28, 81.12, 73.66, 72.28, 56.71, 52.56, 47.87, 40.02, 32.77, 29.10, 23.90, 23.31, 21.50. HRMS (ESI-TOF): m/z calcd. for C21H30O7N3 ([M + H]+) 436.2078, found 436.2087.
(3S,4S,4aS)-4-Hydroxy-N-((S)-1-((S)-8-hydroxy-1-oxoisochroman-3-yl)-3-methylbutyl)-6-oxohexahydro-1H,3H-pyrimido[1,6-c][1,3]oxazine-3-carboxamide (Hetiamacin F) (6)
A solution of compound 17 (10 mg, 0.24 μmol) in MeOH (5 mL) was added formaldehyde (3.8 μL, 0.48 μmol) at room temperature. After stirring for 4 h, the mixture was concentrated in vacuo to give the crude product as a yellow oil. The crude product was purified by prepared HPLC (MeOH:H2O = 60:40) to give 6 (8 mg, 78.1%) as a white solid. [α]D24.5 = −105 (c = 0.02, CHCl3); 1H NMR (600 MHz, DMSO-d6) δ 10.79 (s, 1H), 8.10 (d, J = 9.2 Hz, 1H), 7.89 (d, J = 2.4 Hz, 1H), 7.46 (t, J = 7.9 Hz, 1H), 6.82 (d, J = 8.4 Hz, 1H), 6.79 (d, J = 7.5 Hz, 1H), 5.28 (d, J = 6.2 Hz, 1H), 4.66 (dt, J = 12.7, 2.8 Hz, 1H), 4.48 (d, J = 9.2 Hz, 1H), 4.18 (tt, J = 11.2, 3.5 Hz, 1H), 4.14 (dd, J = 9.1, 2.7 Hz, 1H), 3.92 (d, J = 9.2 Hz, 1H), 3.69 (d, J = 9.3 Hz, 1H), 3.63 (dd, J = 9.0, 2.2 Hz, 1H), 3.44 (td, J = 9.4, 6.2 Hz, 1H), 3.14 (d, J = 4.9 Hz, 1H), 2.97 (dd, J = 16.6, 12.8 Hz, 1H), 2.79 (dd, J = 16.7, 2.9 Hz, 1H), 2.57 (dt, J = 9.5, 6.3 Hz, 1H), 2.40 (dd, J = 17.7, 6.3 Hz, 1H), 2.18 (dd, J = 17.6, 6.3 Hz, 1H), 1.68 (ddd, J = 13.5, 10.8, 4.6 Hz, 1H), 1.62–1.55 (m, 1H), 1.31 (ddd, J = 13.5, 9.4, 4.0 Hz, 1H), 0.88 (d, J = 6.6 Hz, 3H), 0.83 (d, J = 6.5 Hz, 3H); 13C NMR (150 MHz, DMSO-d6) δ 169.14, 168.87, 167.68, 160.88, 140.71, 136.35, 118.59, 115.26, 108.28, 81.49, 81.03, 65.89, 59.17, 57.61, 48.18, 40.04, 32.94, 29.23, 24.13, 23.31, 21.54. HRMS (ESI-TOF): m/z calcd. for C22H30O7N3 ([M + H]+) 448.2078, found 448.2077.
Acknowledgments
The research work was supported by the National Natural Science Foundation of China (NSFC, grant nos. 81803411 and 82011530051) and CAMS Innovation Fund for Medical Sciences (grant nos. CAMS 2017-I2M-B&R-08 and 2017-I2M-1-012).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c06267.
NMR spectra of all new compounds and comparative 13C NMR data (PDF)
Author Contributions
† G.W. and T.W. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- a Liu S.-W.; Jin J.; Chen C.; Liu J.-M.; Li J.-M.; Wang F.-F.; Jiang Z.-K.; Hu J.-H.; Gao Z.-X.; Yao F.; You X.-F.; Si S.-Y.; Sun C.-H. PJS, a novel isocoumarin with hexahydropyrimidine ring from Bacillus subtilis PJS. J. Antibiot. 2013, 66, 281–284. 10.1038/ja.2012.118. [DOI] [PubMed] [Google Scholar]; b Liu S.; Han X.; Jiang Z.; Wu G.; Hu X.; You X.; Jiang J.; Zhang Y.; Sun C. Hetiamacin B-D, new members of amicoumacin group antibiotics isolated from Bacillus subtilis PJS. J. Antibiot. 2016, 69, 769–772. 10.1038/ja.2016.3. [DOI] [PubMed] [Google Scholar]; c Wang T.; Lu Q.; Sun C.; Lukianov D.; Osterman I. A.; Sergiev P. V.; Dontsova O. A.; Hu X.; You X.; Liu S.; Wu G. Hetiamacin E and F, new amicoumacin antibiotics from Bacillus subtilis PJS using MS/MS-Based molecular networking. Molecules 2020, 25, 4446. 10.3390/molecules25194446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Shimojima Y.; Hayashi H.; Ooka T.; Shibukawa M.; Iitaka Y. Studies on AI-77s, microbial products with gastroprotective activity. Structures and the chemical nature of AI-77s. Tetrahedron 1984, 40, 2519–2527. 10.1016/S0040-4020(01)83504-3. [DOI] [Google Scholar]; b Shimojima Y.; Hayashi H.; Ooka T.; Shibukawa M.; Iitaka Y. (Studies on AI-77s, microbial products with pharmacological activity) structures and the chemical nature of AI-77s. Tetrahedron Lett. 1982, 23, 5435–5438. 10.1016/0040-4039(82)80150-0. [DOI] [Google Scholar]
- a McInerney B. V.; Taylor W. C.; Lacey M. J.; Akhurst R. J.; Gregson R. P. Biologically active metabolites from Xenorhabdus spp., Part 2. benzopyran-1-one derivatives with gastroprotective activity. J. Nat. Prod. 1991, 54, 785–795. 10.1021/np50075a006. [DOI] [PubMed] [Google Scholar]; b Cañedo L. M.; Puentes J. L. F.; Baz J. P.; Acebal C.; de la Calle F.; Grábalos D. G.; De Quesada T. G. PM-94128, a new isocoumarin antitumor agent produced by a marine bacterium. J. Antibiot. 1997, 50, 175–176. 10.7164/antibiotics.50.175. [DOI] [PubMed] [Google Scholar]; c Itoh J.; Omoto S.; Shomura T.; Nishizawa N.; Miyado S.; Yuda Y.; Shibata U.; Inouye S. Amicoumacin-A, a new antibiotic with strong antiinflammatory and antiulcer activity. J. Antibiot. (Tokyo) 1981, 34, 611–613. 10.7164/antibiotics.34.611. [DOI] [PubMed] [Google Scholar]
- Wu G.; Liu S.; Wang T.; Jiang Z.; Lv K.; Wang Y.; Sun C. Total synthesis of originally proposed and revised structure of hetiamacin A. Org. Lett. 2018, 20, 3566–3569. 10.1021/acs.orglett.8b01350. [DOI] [PubMed] [Google Scholar]
- Tsukaguchi S.; Enomoto M.; Towada R.; Ogura Y.; Kuwahara S. Unified total synthesis of hetiamacins A–D. European J. Org. Chem. 2019, 2019, 6110–6116. 10.1002/ejoc.201901114. [DOI] [Google Scholar]
- a Hamada Y.; Kawai A.; Kohno Y.; Hara O.; Shioiri T. New methods and reagents in organic synthesis. 83. Stereoselective total synthesis of AI-77-B, a gastroprotective substance from bacillus pumilus AI-77. J. Am. Chem. Soc. 1989, 111, 1524–1525. 10.1021/ja00186a071. [DOI] [Google Scholar]; b Durgant J.-M.; Vogel P. Total synthesis of the gastroprotective substance AI-77-B and of analogues. Helv. Chim. Acta 1993, 76, 222–240. 10.1002/hlca.19930760116. [DOI] [Google Scholar]; c Ward R. A.; Procter G. A total synthesis of the natural enantiomer of the gastroprotective natural products AI-77-B and amicoumacin C hydrochloride. Tetrahedron 1995, 51, 12301–12318. 10.1016/0040-4020(95)00776-5. [DOI] [Google Scholar]; d Kotsuki H.; Araki T.; Miyazaki A.; Iwasaki M.; Datta P. K. A new enantioselective total synthesis of AI-77-B. Org. Lett. 1999, 1, 499–502. 10.1021/ol990102y. [DOI] [Google Scholar]; e Broady S. D.; Rexhausen J. E.; Thomas E. J. Total synthesis of AI-77-B: stereoselective hydroxylation of 4-alkenylazetidinones. J. Chem. Soc., Perkin Trans. 1 1999, 1, 1083–1094. 10.1039/A900334G. [DOI] [Google Scholar]; f Ghosh A. K.; Bischoff A.; Cappiello J. Asymmetric total synthesis of the gastroprotective microbial agent AI-77-B. Eur. J. Org. Chem. 2003, 821–832. 10.1002/ejoc.200390125. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Enomoto M.; Kuwahara S. Total synthesis of bacilosarcins A and B. Angew. Chem., Int. Ed. 2009, 48, 1144–1148. 10.1002/anie.200804571. [DOI] [PubMed] [Google Scholar]; h Enomoto M.; Kuwahara S. Concise synthesis of PM-94128 and Y-05460M-A. J. Org. Chem. 2009, 74, 7566–7569. 10.1021/jo9014968. [DOI] [PubMed] [Google Scholar]; i Rao M. V.; Rao B. V.; Ramesh B. Total synthesis of AI-77-B. Tetrahedron Lett. 2014, 55, 5921–5924. 10.1016/j.tetlet.2014.09.009. [DOI] [Google Scholar]; j Suzuki T.; Nagasawa T.; Enomoto M. Stereoselective total synthesis of amicoumacin C. Tetrahedron 1992–1997, 71, 71. 10.1016/j.tet.2015.02.014. [DOI] [Google Scholar]; h Kurasawa K.; Kuwahara S.; Enomoto M. Synthesis of bacilosarcins B and C. Tetrahedron Lett. 2016, 57, 4997–4999. 10.1016/j.tetlet.2016.09.090. [DOI] [Google Scholar]
- a Jouin P.; Castro B.; Nisato D. Stereospecific synthesis of N-protected statine and its analogues via chiral tetramic acid. J. Chem. Soc., Perkin Trans. 1 1987, 1, 1177–1182. 10.1039/P19870001177. [DOI] [Google Scholar]; b Ma D.; Ma J.; Ding W.; Dai L. An improved procedure to homochiral cyclic statines. Tetrahedron: Asymmetry 1996, 7, 2365–2370. 10.1016/0957-4166(96)00291-1. [DOI] [Google Scholar]
- Muramatsu T.; Yamashita S.; Nakamura Y.; Suzuki M.; Mase N.; Yoda H.; Takabe K. Total synthesis of (−)-2-epi-lentiginosine by use of chiral 5-hydroxy-1,5-dihydropyrrol-2-one as a building. Tetrahedron Lett. 2007, 48, 8956–8959. 10.1016/j.tetlet.2007.10.125. [DOI] [Google Scholar]
- Chu K. S.; Negrete G. R.; Konopelski J. P.; Lakner F. J.; Woo N.-T.; Olmstead M. M. Enantiomerically pure dihydropyrimidinones as reagents and auxiliaries for asymmetric synthesis. J. Am. Chem. Soc. 1992, 114, 1800–1812. 10.1021/ja00031a039. [DOI] [Google Scholar]
- Castellanos E.; Reyes-Rangel G.; Juaristi E. Diastereoselective electrophilic amination of chiral 1-benzoyl-2,3,5,6-tetrahydro-3-methyl-2-(1-methylethyl)pyrimidin-4(1H)-one for the asymmetric syntheses of α-substituted α,β-diaminopropanoic acids. Helv. Chim. Acta 2004, 87, 1016–1024. 10.1002/hlca.200490073. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.