Abstract
Background
Socioeconomic factors may be involved in risk of multiple sclerosis (MS), either indirectly or as confounding factors. In this study two comprehensive indicators reflecting socioeconomic differences, including the Human Development Index (HDI) and Prosperity Index (PI), were used to assess the impact of these factors on the worldwide distribution of MS.
Methods
The data for this global ecological study were obtained from three comprehensive databases including the Global Burden of Disease (as the source of MS indices), United Nations Development Programme (source for HDI) and the Legatum Institute Database for PI. MS indices (including prevalence, incidence, mortality, and disability-adjusted life years) were all analyzed in the form of age- and sex-standardized. Correlation and regression analyses were used to investigate the relationship between HDI and PI and their subsets with MS indices.
Results
All MS indices were correlated with HDI and PI. It was also found that developed countries had significantly higher prevalence and incidence rates of MS than developing countries. Education and governance from the PI, and gross national income and expected years of schooling from the HDI were more associated with MS. Education was significantly related to MS indices (p < 0.01) in both developed and developing countries.
Conclusion
In general, the difference in income and the socioeconomic development globally have created a landscape for MS that should be studied in more detail in future studies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12883-021-02170-3.
Keywords: Multiple sclerosis, Human development index, Prosperity index, Socioeconomic factors, Ecology study
Background
Multiple sclerosis (MS) is a chronic inflammatory, demyelinating and neurodegenerative disease of the central nervous system (CNS) that usually starts in the third or fourth decades of life [1–3]. MS has a complex etiology and its causes are currently not fully understood, but it is known that it is one of the leading reasons of non-traumatic neurological disability in young adults, leading to remarkable socioeconomic impacts and the need for lifetime support and management [4–6]. It is estimated that about 2.2 million people are suffering from MS worldwide [7]. There is a broad variation in the prevalence and incidence of MS in different areas of the world [8, 9], supporting the hypothesis that environmental and genetic interaction may play a role in the etiology of MS [10–12]. Some possible risk factors include residential latitude, ultraviolet radiation, intake of vitamin D, Epstein–Barr virus and infectious mononucleosis, and some other non-infectious factors [13].
On the other hand, the rapid economic growth of countries causes changes in lifestyle, hygienic and psychosocial conditions [14]. Researches have demonstrated that countries with better social and economic situations have higher MS prevalence [15–17]. Socioeconomic factors such as education level, life expectancy, and life course socioeconomic position, may be linked to MS incidence and its subsequent progression [18]. Moreover, reported MS incidence is higher in high-income countries [19, 20]. For instance, recently published results from the Global Burden of Disease (GBD) 2016 study showed substantial associations between some neurological disorders, such as MS, and socio-demographic index (SDI) [7], whilst other studies found no significant social gradient or inverse results [21, 22]. In addition, adverse socioeconomic position in childhood has been linked with a proinflammatory phenotype [23], and may be an important factor to consider for complex neuroinflammation and neurological diseases such as MS [23–25]. Therefore, it is of critical importance to comprehend and develop disease-modifying strategies.
The Human Development Index (HDI) and Prosperity Index (PI) are two factors of the socioeconomic situations in countries, and have been previously utilized to study associations between socioeconomic factors and with various diseases, such as diabetes and cancer [26, 27]. However, to the best of our knowledge, these measures have not yet been used in MS studies. HDI is a comprehensive indicator of socioeconomic differences between countries, and PI is an integrated indicator consisted of community-level social well-being based on the state of health services, environmental conditions, and governmental power. Taken together, these two indices represent the extent of countries’ development and, given the importance of these indices in the distribution of other diseases, this study was designed to evaluate their impact on the global distribution of MS.
Methods
The present study is a global ecological study to analyze the correlation between PI, HDI and their components, and MS prevalence, incidence, mortality, and disability-adjusted life years (DALYs).
MS data
MS data for all countries in 2017 was acquired from Institute for Health Metrics and Evaluation Global Health Data Exchange (http://ghdx.healthdata.org/). All data analyses were performed with regard to age-standardized rates of MS in for both sexes and each country. The GBD database consists of the data from national and international registries, along with estimates burden of disease for hundreds of health outcomes, and is freely available for researchers [28].
Prosperity and human development indices
PI is a complex index measuring prosperity of countries not only by one parameter such as economic growth, but also by use of nine components (i.e. business environment, education, economic quality, governance, health, natural environment, personal freedom, safety and security, and social capital. The definitions of each term are listed in The Legatum Prosperity Index™ 2018 [29].
The PI values and rankings data from 149 countries in 2017 were downloaded from the Legatum Institute website (https://www.prosperity.com/). In this report, PI is classified into four categories: low (PI< 50.543), medium (50.543 ≤ PI< 57.570), high (50.570 ≤ HDI < 63.912), and very high (HDI ≥ 63.912) (Supplementary Figure S1 and S2).
HDI scores measuring development of countries were acquired from the United Nations Development Programme (UNDP) database (http://hdr.undp.org/en/data) [30]. HDI ranges from 0 to 1, and components include mean and expected years of schooling, gross national income per capita, and life expectancy at birth (LE) (See the definition of parameters in the supplementary file). In this database, HDI is classified into four categories: low (HDI < 0.556), medium (0.556 ≤ HDI < 0.700), high (0.700 ≤ HDI < 0.800), and very high (HDI ≥ 0.800) [30]. The United Nations considers countries with HDI ≥0.788 as “developed”, and any score below that as “developing” [31]. While HDI has improved in all groups and regions, more rapid increase has been observed in low and medium HDI countries, resulting in less inequitable health systems in certain countries. However, reported national averages may conceal remarkable variations and disparities within countries of both northern and southern hemispheres, as well as increase in income inequality [32–34].
Statistical analysis
Age-standardized rates of MS indices (incidence, prevalence, mortality, DALY) were stratified by global region. Mean (95% CI) incidence, prevalence, mortality, and DALY of MS was also calculated stratified by HDI categories. Maps of age-standardized incidence rates of MS, PI, and HDI were also created using ArcGIS 10.3 mapping software. We also assessed correlation between MS indices and HDI and PI and their components using Pearson correlation coefficients. In addition, the statistical significance of differences in MS indices among in developing and developed countries was assessed using independent-sample t-tests. On the other hand we used of One-Way ANOVA test to compare the means of more than two groups. We used multivariable linear regression to mutually adjust for HDI and PI components in relation to MS indices.
Results
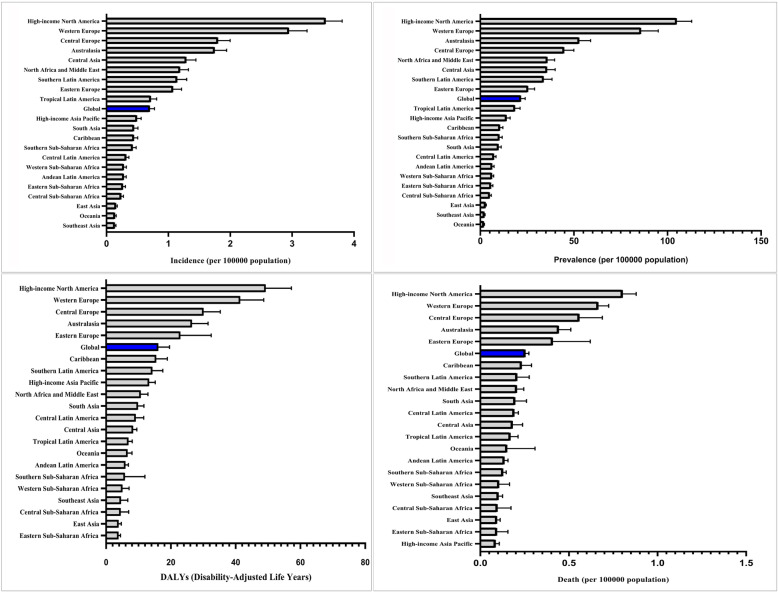
Estimates on the frequencies of MS for both sexes were available in GBD for 195 countries. In 2017, an estimated number of 1,761,078 (95% uncertainty interval (UI), 1,598,225–1,947,909) people worldwide had MS. Global MS prevalence was 21.70 (95% UI, 19.69–23.98) per 100,000 persons according to age-standardized rate data (29.34 (95% UI, 26.57–32.43 for female and 13.77 (95% UI, 12.42–15.32). Global MS incidence was 0.70 cases (95% UI, 0.64–0.78) per 100,000 persons (0.90 (95% UI, 0.82–1.00 for female and 0.77 (95% UI, 0.42–0.32). Age-standardized female/male prevalence ratio (F/M) was 2.13. Age-standardized F/M ratio of mortality was 1.32. Global MS mortality was 0.25 cases (95% UI, 0.22–0.27) per 100,000 persons (Fig. 1).
Fig. 1.
Global and regional Age-standardized of MS indices in 2017 obtained from the Institute for Health Metrics and Evaluation Global Health Data Exchange
Canada had the highest prevalence (168 cases (95% CI 142.22–197.95) per 100,000) and incidence rates (5.63 cases (95% CI 4.84–6.53) per 100,000). On the other hand, Maldives had the lowest prevalence (1.52 cases (95%CI 1.29–1.80) per 100,000) and incidence (0.09 cases (95% CI 0.08–0.1) per 100,000). UK had the highest age-standardized mortality rate of MS (1.21 (95% CI 0.83–1.31) per 100,000) with a number of 1294 MS mortality cases but USA had highest mortality of with 4019 MS-attributed deaths in 2017.
Among all the countries with available PI, Norway and Yemen had the highest and lowest PIs with 79.85 and 36.36 in 2017, respectively (Supplementary Figure S1, S2). Figure S1 in the supplementary shows PI values with the components in 2017. Also, the highest and lowest HDI were observed in Norway (0.953) and Niger (0.354), respectively (Supplementary Figure S2).
The mean values of MS indices in our study based on HDI categories are presented in Table 1. With the increasing in HDI category, MS indices also has increased. The rate ratio of incidence, prevalence, DALY and mortality in countries with overall high HDI category to those with overall low HDI were 5.38, 6.58, 4.57, and 3.86, respectively.
Table 1.
Mean (95%CI) MS indices in countries within different HDI categories
| MS indices | Low HDI | Medium HDI | High HDI | Very High HDI |
|---|---|---|---|---|
| Incidence a | 0.36 (0.28–0.43) | 0.40 (0.31–0.50) | 0.67 (0.50–0.83) | 1.92 (1.56–2.28) |
| Prevalence a | 8.25 (6.13–10.36) | 9.52 (6.76–12.28) | 17.08 (12.48–21.69) | 54.27 (43.44–65.11) |
| Mortality a | 0.13 (0.11–0.14) | 0.14 (0.13–0.16) | 0.22 (0.18–0.27) | 0.48 (0.41–0.56) |
| DALY a | 6.34 (5.27–7.42) | 7.22 (6.23–8.22) | 11.83 (9.40–14.25) | 28.96 (24.36–33.56) |
a unit of measure is per 100,000 persons-years
It can be observed from Figure S2 in the supplementary appendix that more developed countries (with higher overall HDI and PI) are facing higher rates of MS prevalence and incidence; however, in countries with HDI < 0.5 and PI< 50.5, lower rates of prevalence and incidence have been recorded. The PI and HDI distribution GIS map (Fig. 2) illustrates that the countries located in Northern America and Western Europe have the highest prevalence and incidence of MS.
Fig. 2.
Distribution map of the age-standardized prevalence and incidence of MS, PI and HDI in 2017 obtained from the Institute for Health Metrics and Evaluation Global Health Data Exchange
The results of latitude classification in the northern and southern hemispheres showed that countries with higher latitudes have higher MS indices (Table 2). There was also a significant difference between overall PI and HDI in low latitudes (< 20 degrees) and high latitudes (> 40 degrees) in the northern hemisphere (p < 0.01).
Table 2.
Mean (95%CI) MS indices in countries within different latitude categories
| Hemisphere | Latitude category | Variables | Mean | 95%CI | P | |
|---|---|---|---|---|---|---|
| North hemisphere | < 20 | Incidence | 0.34 | 0.26 | 0.42 | < 0.001 |
| Prevalence | 7.85 | 5.88 | 9.81 | |||
| DALY | 7.37 | 5.98 | 8.76 | |||
| Mortality | 0.16 | 0.13 | 0.18 | |||
| HDI | 0.62 | 0.58 | 0.67 | |||
| PI | 54.14 | 51.57 | 56.72 | |||
| 20–40 | Incidence | 0.88 | 0.69 | 1.06 | ||
| Prevalence | 23.96 | 18.36 | 29.57 | |||
| DALY | 13.46 | 11.02 | 15.90 | |||
| Mortality | 0.22 | 0.18 | 0.26 | |||
| HDI | 0.69 | 0.78 | 0.69 | |||
| PI | 53.00 | 58.93 | 53.00 | |||
| ≥40 | Incidence | 2.11 | 1.71 | 2.50 | ||
| Prevalence | 58.94 | 46.81 | 71.07 | |||
| DALY | 31.56 | 26.55 | 36.56 | |||
| Mortality | 0.53 | 0.44 | 0.61 | |||
| HDI | 0.82 | 0.87 | 0.82 | |||
| PI | 63.88 | 69.54 | 63.88 | |||
| South hemisphere | < 20 | Incidence | 0.26 | 0.21 | 0.31 | < 0.01a |
| Prevalence | 5.57 | 4.24 | 6.90 | |||
| DALY | 5.61 | 5.01 | 6.21 | |||
| Mortality | 0.13 | 0.11 | 0.14 | |||
| HDI | 0.52 | 0.64 | 0.52 | |||
| PI | 49.16 | 54.71 | 49.16 | |||
| 20–40 | Incidence | 0.81 | 0.43 | 1.20 | ||
| Prevalence | 22.87 | 10.50 | 35.25 | |||
| DALY | 12.26 | 6.92 | 17.59 | |||
| Mortality | 0.20 | 0.12 | 0.28 | |||
| HDI | 0.68 | 0.85 | 0.68 | |||
| PI | 57.55 | 68.91 | 57.55 | |||
All of country in south hemisphere was between ranges of 0–40 degree
a not significant for mortality
Association of MS indices with PI and HDI
The prevalence, incidence, DALY and mortality rates due to MS were positively and significantly correlated with overall PI and HDI (p < 0.01), with slightly higher correlation of MS and DALY (Table 3).
Table 3.
Correlation between PI, HDI and MS indices in 2017
| MS indices | Incidence a | Prevalence a | Mortality a | DALY a |
|---|---|---|---|---|
| HDI | 0.62** | 0.62** | 0.62** | 0.65** |
| PI | 0.68** | 0.68** | 0.66** | 0.69** |
PI prosperity index, HDI human development index
**P < 0.01
a unit of measure is per 100,000 persons-years
In subgroup analysis, the results demonstrated that the MS incidence and prevalence were significantly and positively correlated with all components of PI and HDI (p < 0.01) (Table 4).
Table 4.
Correlation between PI and HDI components and MS variables in 2017
| MS variables | Incidence a | Prevalence a | DALY a | Mortality a |
|---|---|---|---|---|
| r | r | r | r | |
| Expected Years of Schooling | 0.599** | 0.600** | 0.629** | 0.603** |
| Life Expectancy | 0.548** | 0.552** | 0.564** | 0.527** |
| Gross National Income | 0.585** | 0.595** | 0.576** | 0.526** |
| Mean Years of Schooling | 0.388** | 0.386** | 0.403** | 0.376** |
| Economic Quality | 0.593** | 0.596** | 0.595** | 0.563** |
| Business Environment | 0.600** | 0.605** | 0.597** | 0.566** |
| Governance | 0.665** | 0.672** | 0.660** | 0.628** |
| Education | 0.628** | 0.625** | 0.646** | 0.618** |
| Health | 0.544** | 0.550** | 0.538** | 0.502** |
| Safety and Security | 0.591** | 0.586** | 0.601** | 0.580** |
| Personal Freedom | 0.562** | 0.564** | 0.581** | 0.581** |
| Social Capital | 0.497** | 0.513** | 0.464** | 0.422** |
| Natural Environment | 0.471** | 0.473** | 0.494** | 0.490** |
** P < 0.01, a: unit of measure is per 100,000 persons-years
Table 5 presents the results of regression analyses of overall HDI and PI on MS incidence, prevalence, DALY, and mortality.
Table 5.
Regression coefficients for mutually adjusted associations between MS indices and PI and HDI (PI, HDI and latitude adjusted with together)
| MS indices | Independent variables | B | P | 95% CI |
|---|---|---|---|---|
| Incidence a | HDI | −0.39 | 0.58 | − 1.79, 0.99 |
| PI | 0.05 | < 0.001 | 0.03, 0.07 | |
| Prevalence a | HDI | −12.45 | 0.56 | −54.69, 29.78 |
| PI | 1.45 | < 0.001 | 0.81, 2.10 | |
| DALY | HDI | 0.13 | 0.99 | −18.11, 18.37 |
| PI | 0.56 | < 0.001 | 0.40, 0.60 | |
| Mortality a | HDI | −0.23 | 0.89 | −0.36, 0.31 |
| PI | 0.01 | < 0.001 | 0.004, 0.015 |
a: unit of measure is per 100,000 persons-years
The results demonstrated a positive association of overall HDI (adjusted for PI) on DALY (B (SE) = 25.90, p = 0.02). There were no statistically significant associations for overall HDI and other variables. In case of PI (adjusted for HDI), significant associations were found for all MS indices (p < 0.01). In addition, regression models performed on HDI subgroups showed the positive associations of expected years of schooling and gross national income on all MS indices (Supplementary Table S1). On the other hand, the results for PI subgroups demonstrated that education and governance were positively associated with MS prevalence. Education level was also associated with all MS indices (Supplementary Table S2).
One-way ANOVA tests demonstrated that all MS indices differed significantly among countries in different HDI levels (p < 0.01). The results of post hoc tests demonstrated that the difference between the averages of MS indices in the countries with very high HDI was significantly lower than other categories (p < 0.01). However, no significant associations were found for high, medium, and low HDI countries (p > 0.05) (Supplementary Figure S3).
MS and countries classification (developed vs. developing)
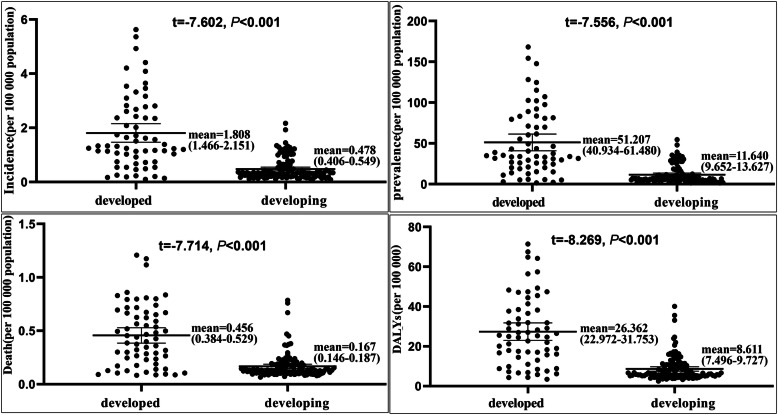
Additional analyses were performed for country categories (developed and developing) in order to investigate the impact of independent variables on MS indices. There were significant differences between MS indices in developed and developing countries (p < 0.01) (Fig. 3). Among developing countries, the average of prevalence was 11.64 (CI: 9.65–13.63), which increased with increasing national HDI (r = 0.351, p < 0.01). Also, MS prevalence in developed countries was 51.21 (CI: 40.93–61.48), and increased with the increase in national HDI (r = 0.706, p < 0.001).
Fig. 3.
Differences of MS indices in developed and developing countries by independent sample t-test. MS indices in developed countries was significantly higher than those in developing countries. Horizontal lines represent group means with 95% CI
Our results revealed that the correlations between all MS indices and HDI in developing countries were significant (Supplementary Table S3). Also, regression models demonstrated a significantly positive association with HDI and a negative association with PI for all MS indices (Supplementary Table S4). In developed countries, positive associations of HDI with MS incidence and prevalence were found, but there were no statistically significant relationships between DALY and mortality rate of MS with PI and HDI. In analysis of PI subgroups, education variable showed positive effect on all MS indices in developed and developing countries (Supplementary Table S5 and Table S6).
Discussion
This study aimed to investigate the association of HDI and PI with MS indices including incidence, prevalence, mortality rate and DALY globally. In the case of MS indices, we found some differences in different parts of world, indicating different patterns of incidence, prevalence and mortality due to MS. The highest rates of MS mortality were observed in developed countries (high-income North American and West European countries). This is possibly because of higher incidence and prevalence in these countries, leading to more deaths and greater DALY. On the other hand, the lowest mortality rates were not observed in areas with low incidence and prevalence. For instance, although the lowest incidence and prevalence are seen in Oceania and Southeast Asia, mortality rates in these areas were higher than those in Asia-Pacific high-income countries. This could be attributed to the differences in healthcare systems in these countries, including quality of care and access to healthcare [35].
Generally, in the last two decades, the prevalence of MS has significantly increased throughout the world [36, 37], along with the incidence and prevalence of disease at the community level [7, 38–40]. It could be related to improvements in the economic level and fast-changing lifestyle of communities. Research has suggested that long-term population growth can increase prevalence and incidence of other chronic diseases [41, 42]. This can vary in different parts of the world due to socioeconomic and cultural factors accompanied with governmental policies for population planning and control.
Figure 2, shows that countries with higher PI and HDI indexes generally have higher prevalence and incidence of MS. In case of other non-communicable diseases such as different types of cancer, higher HDI is related to better accessibility to diagnostic facilities, likewise, higher prevalence of MS could be linked with more updated and available health care facilities in developing countries.
There are also significant differences in prevalence and incidence and subsequent mortality between developed and developing countries, taking into account HDI in different regions and countries. The average prevalence of MS in developed and developing countries were 54.21 and 11.64 per 100,000 population. The average incidence rate in developed countries was about 5.5 times that of developing countries. There are several potential reasons for this observed difference. First, the high incidence of the disease and concurrent quality of healthcare can be one of the main reasons for higher prevalence in developed countries. In more developed areas, factors such as better access to diagnostic facilities and subsequent earlier diagnosis, treatment, and a higher surveillance may be major contributors to high prevalence [43, 44]. Additionally, easy access to better healthcare and diagnostics as well as greater awareness about the disease in more developed areas may increase the number of accurately ascertained MS cases [2]. On the other hand, better socioeconomic development is associated with factors such as obesity, higher smoking rates, more physical activity, etc., which have been suggested as potential risk factors of MS [45]. However, in areas with better socioeconomic status, as evident by higher HDI and PI, there appear to be factors that make people more susceptible to MS [35].
It is important to note, however, that these socioeconomic factors and subsequent access to care can also differ between urban and rural areas within the same country. However, studies have shown higher MS incidence and prevalence subjects from rural vs urban areas of Germany [46, 47], Moldova [48], and Norway [49]. These prior studies suggest that the differences observed are more likely due to country specific resources.
A closer look at the information and reports of GBD indicates that in developing countries, infant and child mortality rates are much higher than those in developed countries. In these areas, people with susceptible immune system may not reach adolescence or adulthood. In contrast, in developed countries, since child mortality and infections are much lower, all people have a higher chance of reaching older ages (i.e. they have higher life expectancy, which is one of the main components of HDI). In other words, people with weaker immune systems are more likely to develop autoimmune diseases such as MS at older ages. This hypothesis, which in fact describes the role of welfare and socioeconomic status driven natural selection, is substantially supported by the hygiene hypothesis of increased likelihood of later life disease [50], but should be further studied.
Regarding the relationship between PI and MS indices, it is worth noting that there is a great deal of similarity between HDI and PI, with PI providing more detailed and comprehensive information, and may be a more suitable measurement in future studies (Table 5). Also, the relationship between PI and MS indicators supports the role of economic stability in access to healthcare and subsequent longevity. Among PI subgroups, factors of health status, state stability, and higher education were more significantly associated with MS indices. These results suggest that countries with greater access to health services, information, education, and subsequent awareness have higher incidence, prevalence, and mortality of MS [35]. Additionally, after controlling for the role of latitude, PI as an index of welfare was the most important statistically effective indicator in the distribution of MS. The role of socioeconomic factors and other concurrent risk factors warrants more detailed studies.
We acknowledge that there are several limitations to the results presented here. As an ecological study, there is the inherent issue of no assumption of temporality. However, as we expect the PI and HDI components vary only slightly from year to year, and rankings of economic stability of countries have remained relatively consistent during the last decade, indices of prosperity and human development may provide more temporally relevant information than expected for many ecological studies. As country-level data, the data used in this study do not account for variability across the latitudinal range in lager countries or differences by urbanity and ethnic distributions in different cities within countries. The data used in this study also do not account for individual characteristics that may influence disease risk or subsequent mortality. Thus, it should be noted that individual genetic, lifestyle, and environmental factors could affect distribution patterns of MS. Nevertheless, studies of individual economic factors may also overlook important group-level determinants of disease. Although the present study provides a promising perspective on MS disease and used incidence data, it should be noted that there may be some measurement error due to differences in how data were collected for different countries and may underestimate or overestimate the actual surveillance reports. Furthermore, data used for this study were previously collected, and we have no information on differences in provision of medical care (i.e. private versus public insurance), which may influence the timing of diagnosis and mortality rate and result in residual confounding.
Although this global analysis provides substantial information on hypothesized factors influencing MS incidence, prevalence, quality of life, and mortality by country, future analyses at the smaller geographic levels – city, province, parish, etc.- could utilize other local data sources and area-based resources. These data sources also do not account for the influence of migration of MS cases to other geographic areas to attain better access to care, which may impact reported MS incidence, prevalence, and mortality rates, changing the risk profile in less developed countries. Future studies should also use data on lifestyle and environmental factors such as air pollution along with other psychosocial stressors, including hostility, violence, food availability, and employment. Despite these limitations, we believe that our use of reliable and validated surveillance data obtained from various government and community sources strengthens the results of this global study and generates several noteworthy findings contributing to the body of knowledge on factors that influence MS epidemiology and surveillance, especially for frequently understudied populations.
Conclusions
The present study showed that the prevalence of MS is increasing worldwide and developed regions and countries are facing this issue at a higher magnitude. Socioeconomic factors also appear to strongly correlate with the development of the disease, although the exact mechanism is still unclear. It also appears that socioeconomic factors have created a global perspective and a model of MS based on socioeconomic development and it seems that socioeconomic factors have an important role in multiple sclerosis distribution, globally. Many factors can be involved in this regard. This can be the subject of future studies.
Supplementary Information
Acknowledgments
The authors would like to thank Student Research Committee of Torbat Heydariyeh University of Medical Sciences for their financial support for performing this research.
Authors ‘contributions
VKM: Conceptualization, Methodology, Validation, Writing- Original draft, Writing - Review & Editing, Supervision. A S.D: Methodology, Validation, Writing- Original draft preparation, Supervision, Writing - Review & Editing. EB: Conceptualization, Writing - Review & Editing, Data Curation. SNS: Conceptualization, Validation, Writing - Review & Editing. FN: Formal analysis, Methodology, Validation, Writing - Review & Editing. MH: Validation, Writing- Reviewing and Editing. JM: Investigation, Conceptualization, Writing - Review & Editing. GM: Investigation, Conceptualization, Writing - Review & Editing. MS: Conceptualization, Methodology, Validation, Funding acquisition, Supervision, Writing - Original Draft, Writing - Review & Editing, Project administration. All authors read and approved the manuscript.
Abbreviations
- ANOVA
Analysis of variance
- CNS
Central nervous system
- DALY
Disability-adjusted life year
- GBD
Global Burden of Disease
- HDI
Human Development Index
- LE
Life expectancy at birth
- MS
Multiple sclerosis
- UNDP
United Nations Development Programme
- PI
Prosperity Index
- SDI
Socio-demographic index
Funding
This work was supported by the Student Research Committee of Torbat Heydariyeh University of Medical Sciences [grant numbers: SRC-97-114]. The funders had no role in study design, data collection and analysis, interpretation, and preparation of the manuscript.
Availability of data and materials
All data generated or analysed during this study are included publicly available dataset. MS data analyzed in this study are available in the GBD data tool: http://ghdx.healthdata.org/gbd-results-tool. The main source of Prosperity (PI) and human development indices (HDI) data in the world was the Legatum Prosperity Index™ 2017 and UNITED NATIONS DEVELOPMENT PROGRAMME websites: https://prosperitysite.s3-accelerate.amazonaws.com/3515/1187/1128/Legatum_Prosperity_Index_2017.pdf and http://hdr.undp.org/en/composite/HDI.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Torbat Heydariyeh University of Medical Sciences (Ethical code: IR.THUMS.REC.1397.032).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Vahid Kazemi Moghaddam and Aisha S. Dickerson contributed equally to this work.
References
- 1.Albor C, du Sautoy T, Kali Vanan N, Turner BP, Boomla K, Schmierer K. Ethnicity and prevalence of multiple sclerosis in East London. Mult Scler J. 2017;23(1):36–42. doi: 10.1177/1352458516638746. [DOI] [PubMed] [Google Scholar]
- 2.Kingwell E, Marriott JJ, Jetté N, Pringsheim T, Makhani N, Morrow SA, Fisk JD, Evans C, Béland SG, Kulaga S, Dykeman J, Wolfson C, Koch MW, Marrie RA. Incidence and prevalence of multiple sclerosis in Europe: a systematic review. BMC Neurol. 2013;13(1):128. doi: 10.1186/1471-2377-13-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AlJumah M, Bunyan R, Al Otaibi H, Al Towaijri G, Karim A, Al Malik Y, et al. Rising prevalence of multiple sclerosis in Saudi Arabia, a descriptive study. BMC Neurol. 2020;20(1):49. doi: 10.1186/s12883-020-1629-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eskandarieh S, Heydarpour P, Minagar A, Pourmand S, Sahraian MA. Multiple sclerosis epidemiology in East Asia, south East Asia and South Asia: a systematic review. Neuroepidemiology. 2016;46(3):209–221. doi: 10.1159/000444019. [DOI] [PubMed] [Google Scholar]
- 5.Benamer HT, Ahmed ES, Al-Din AS, Grosset DG. Frequency and clinical patterns of multiple sclerosis in Arab countries: a systematic review. J Neurol Sci. 2009;278(1–2):1–4. doi: 10.1016/j.jns.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Cristiano E, Patrucco L, Rojas J. A systematic review of the epidemiology of multiple sclerosis in South America. Eur J Neurol. 2008;15(12):1273–1278. doi: 10.1111/j.1468-1331.2008.02330.x. [DOI] [PubMed] [Google Scholar]
- 7.Wallin MT, Culpepper WJ, Nichols E, Bhutta ZA, Gebrehiwot TT, Hay SI, Khalil IA, Krohn KJ, Liang X, Naghavi M, Mokdad AH, Nixon MR, Reiner RC, Sartorius B, Smith M, Topor-Madry R, Werdecker A, Vos T, Feigin VL, Murray CJL. Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(3):269–285. doi: 10.1016/S1474-4422(18)30443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simpson S, Jr, Pittas F, van der Mei I, Blizzard L, Ponsonby AL, Taylor B. Trends in the epidemiology of multiple sclerosis in greater Hobart, Tasmania: 1951 to 2009. J Neurol Neurosurg Psychiatry. 2011;82(2):180–187. doi: 10.1136/jnnp.2010.215186. [DOI] [PubMed] [Google Scholar]
- 9.Alonso A, Hernan MA. Temporal trends in the incidence of multiple sclerosis: a systematic review. Neurology. 2008;71(2):129–135. doi: 10.1212/01.wnl.0000316802.35974.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol. 2007;61(4):288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- 11.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: noninfectious factors. Ann Neurol. 2007;61(6):504–513. doi: 10.1002/ana.21141. [DOI] [PubMed] [Google Scholar]
- 12.El-Muzaini H, Akhtar S, Alroughani R. A matched case-control study of risk factors associated with multiple sclerosis in Kuwait. BMC Neurol. 2020;20(1):64. doi: 10.1186/s12883-020-01635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev. 2010;9(5):A387–AA94. doi: 10.1016/j.autrev.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Pan H-Y, Dai Y-N, Zheng J-N, Shi K-Q, Van Poucke S, Zou H, et al. National incidence of autoimmune liver diseases and its relationship with the human development index. Oncotarget. 2016;7(29):46273–46282. doi: 10.18632/oncotarget.10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO . Atlas: multiple sclerosis resources in the world 2008. 2008. [Google Scholar]
- 16.Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14(3):263–273. doi: 10.1016/S1474-4422(14)70267-4. [DOI] [PubMed] [Google Scholar]
- 17.Shabas D, Heffner M. Multiple sclerosis management for low-income minorities. Mult Scler J. 2005;11(6):635–640. doi: 10.1191/1352458505ms1215oa. [DOI] [PubMed] [Google Scholar]
- 18.Hammond SR, McLeod JG, Macaskill P, English DR. Multiple sclerosis in Australia: socioeconomic factors. J Neurol Neurosurg Psychiatry. 1996;61(3):311–313. doi: 10.1136/jnnp.61.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goulden R, Ibrahim T, Wolfson C. Is high socioeconomic status a risk factor for multiple sclerosis? A systematic review. Eur J Neurol. 2015;22(6):899–911. doi: 10.1111/ene.12586. [DOI] [PubMed] [Google Scholar]
- 20.Mahdavifar N, Towhidi F, Makhsosi BR, Pakzad R, Moini A, Ahmadi A, Lotfi S, Salehiniya H. Incidence and mortality of nasopharynx cancer and its relationship with human development index in the world in 2012. World J Oncol. 2016;7(5–6):109–118. doi: 10.14740/wjon980w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riise T, Kirkeleit J, Harald Aarseth J, Farbu E, Midgard R, Mygland Å, Eikeland R, Jørgen Mørland T, Telstad W, Tore Førland P, Kjell-Morten Myhr Risk of MS is not associated with exposure to crude oil, but increases with low level of education. Mult Scler J. 2011;17(7):780–787. doi: 10.1177/1352458510397686. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen NM, Jørgensen KT, Bager P, Stenager E, Pedersen BV, Hjalgrim H, et al. Socioeconomic factors in childhood and the risk of multiple sclerosis. Am J Epidemiol. 2013;177(11):1289–1295. doi: 10.1093/aje/kws350. [DOI] [PubMed] [Google Scholar]
- 23.Briggs FB, Acuña BS, Shen L, Bellesis KH, Ramsay PP, Quach H, et al. Adverse socioeconomic position during the life course is associated with multiple sclerosis. J Epidemiol Community Health. 2014;68(7):622–629. doi: 10.1136/jech-2013-203184. [DOI] [PubMed] [Google Scholar]
- 24.Stringhini S, Sabia S, Shipley M, Brunner E, Nabi H, Kivimaki M, Singh-Manoux A. Association of socioeconomic position with health behaviors and mortality. Jama. 2010;303(12):1159–1166. doi: 10.1001/jama.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagel G, Linseisen J, Boshuizen HC, Pera G, Del Giudice G, Westert GP, et al. Socioeconomic position and the risk of gastric and oesophageal cancer in the European prospective investigation into cancer and nutrition (EPIC-EURGAST) Int J Epidemiol. 2007;36(1):66–76. doi: 10.1093/ije/dyl275. [DOI] [PubMed] [Google Scholar]
- 26.Hassanipour-Azgomi S, Mohammadian-Hafshejani A, Ghoncheh M, Towhidi F, Jamehshorani S, Salehiniya H. Incidence and mortality of prostate cancer and their relationship with the human development index worldwide. Prostate Int. 2016;4(3):118–124. doi: 10.1016/j.prnil.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Acheson E, Bachrach C, Wright F. Some comments on the relationship of the distribution of multiple sclerosis to latitude, solar radiation, and other variables. Acta Psychiatr Scand. 1960;35(S147):132–147. doi: 10.1111/j.1600-0447.1960.tb08674.x. [DOI] [PubMed] [Google Scholar]
- 28.Global Burden of Disease Collaborative Network . Global Burden of Disease Study 2017 (GBD 2017) results. Seattle: Institute for Health Metrics and Evaluation (IHME) GBD; 2018. [Google Scholar]
- 29.Stroud BP. The Legatum Prosperity IndexTM 2017. London: Legatum Institute; 2017. [Google Scholar]
- 30.UNDP . Human Development Reports: United Nations Development Programme. 2018. [Google Scholar]
- 31.Xu Z, Yu D, Yin X, Zheng F, Li H. Socioeconomic status is associated with global diabetes prevalence. Oncotarget. 2017;8(27):44434–44439. doi: 10.18632/oncotarget.17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malik K. Human development report 2013. The rise of the south: human progress in a diverse world. The rise of the south: human Progress in a diverse world (March 15, 2013) UNDP-HDRO Human Development Reports. 2013. [Google Scholar]
- 33.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13(8):790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 34.Ghoncheh M, Mirzaei M, Salehiniya H. Incidence and mortality of breast cancer and their relationship with the human development index (HDI) in the world in 2012. Asian Pac J Cancer Prev. 2015;16(18):8439–8443. doi: 10.7314/apjcp.2015.16.18.8439. [DOI] [PubMed] [Google Scholar]
- 35.Pakdel M, Karin Hedström A, Bidkhori M, Hadei M, Kazemi Moghaddam V, Sarmadi M, et al. Do socioeconomic factors affect the prevalence of multiple sclerosis in Iran? Acta Neurol Scand. 2019;140(5):328–335. doi: 10.1111/ane.13148. [DOI] [PubMed] [Google Scholar]
- 36.Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, Robertson N, la Rocca N, Uitdehaag B, van der Mei I, Wallin M, Helme A, Angood Napier C, Rijke N, Baneke P. Rising prevalence of multiple sclerosis worldwide: insights from the atlas of MS. Mult Scler J. 2020;26(14):1816–1821. doi: 10.1177/1352458520970841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melcon MO, Correale J, Melcon CM. Is it time for a new global classification of multiple sclerosis? J Neurol Sci. 2014;344(1):171–181. doi: 10.1016/j.jns.2014.06.051. [DOI] [PubMed] [Google Scholar]
- 38.Ribbons K, Lea R, Tiedeman C, Mackenzie L, Lechner-Scott J. Ongoing increase in incidence and prevalence of multiple sclerosis in Newcastle, Australia: a 50-year study. Mult Scler J. 2016;23(8):1063–1071. doi: 10.1177/1352458516671819. [DOI] [PubMed] [Google Scholar]
- 39.Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, Adebayo OM, Afarideh M, Agarwal SK, Agudelo-Botero M, Ahmadian E, al-Aly Z, Alipour V, Almasi-Hashiani A, al-Raddadi RM, Alvis-Guzman N, Amini S, Andrei T, Andrei CL, Andualem Z, Anjomshoa M, Arabloo J, Ashagre AF, Asmelash D, Ataro Z, Atout MM'W, Ayanore MA, Badawi A, Bakhtiari A, Ballew SH, Balouchi A, Banach M, Barquera S, Basu S, Bayih MT, Bedi N, Bello AK, Bensenor IM, Bijani A, Boloor A, Borzì AM, Cámera LA, Carrero JJ, Carvalho F, Castro F, Catalá-López F, Chang AR, Chin KL, Chung SC, Cirillo M, Cousin E, Dandona L, Dandona R, Daryani A, Das Gupta R, Demeke FM, Demoz GT, Desta DM, Do HP, Duncan BB, Eftekhari A, Esteghamati A, Fatima SS, Fernandes JC, Fernandes E, Fischer F, Freitas M, Gad MM, Gebremeskel GG, Gebresillassie BM, Geta B, Ghafourifard M, Ghajar A, Ghith N, Gill PS, Ginawi IA, Gupta R, Hafezi-Nejad N, Haj-Mirzaian A, Haj-Mirzaian A, Hariyani N, Hasan M, Hasankhani M, Hasanzadeh A, Hassen HY, Hay SI, Heidari B, Herteliu C, Hoang CL, Hosseini M, Hostiuc M, Irvani SSN, Islam SMS, Jafari Balalami N, James SL, Jassal SK, Jha V, Jonas JB, Joukar F, Jozwiak JJ, Kabir A, Kahsay A, Kasaeian A, Kassa TD, Kassaye HG, Khader YS, Khalilov R, Khan EA, Khan MS, Khang YH, Kisa A, Kovesdy CP, Kuate Defo B, Kumar GA, Larsson AO, Lim LL, Lopez AD, Lotufo PA, Majeed A, Malekzadeh R, März W, Masaka A, Meheretu HAA, Miazgowski T, Mirica A, Mirrakhimov EM, Mithra P, Moazen B, Mohammad DK, Mohammadpourhodki R, Mohammed S, Mokdad AH, Morales L, Moreno Velasquez I, Mousavi SM, Mukhopadhyay S, Nachega JB, Nadkarni GN, Nansseu JR, Natarajan G, Nazari J, Neal B, Negoi RI, Nguyen CT, Nikbakhsh R, Noubiap JJ, Nowak C, Olagunju AT, Ortiz A, Owolabi MO, Palladino R, Pathak M, Poustchi H, Prakash S, Prasad N, Rafiei A, Raju SB, Ramezanzadeh K, Rawaf S, Rawaf DL, Rawal L, Reiner RC, Jr, Rezapour A, Ribeiro DC, Roever L, Rothenbacher D, Rwegerera GM, Saadatagah S, Safari S, Sahle BW, Salem H, Sanabria J, Santos IS, Sarveazad A, Sawhney M, Schaeffner E, Schmidt MI, Schutte AE, Sepanlou SG, Shaikh MA, Sharafi Z, Sharif M, Sharifi A, Silva DAS, Singh JA, Singh NP, Sisay MMM, Soheili A, Sutradhar I, Teklehaimanot BF, Tesfay B, Teshome GF, Thakur JS, Tonelli M, Tran KB, Tran BX, Tran Ngoc C, Ullah I, Valdez PR, Varughese S, Vos T, Vu LG, Waheed Y, Werdecker A, Wolde HF, Wondmieneh AB, Wulf Hanson S, Yamada T, Yeshaw Y, Yonemoto N, Yusefzadeh H, Zaidi Z, Zaki L, Zaman SB, Zamora N, Zarghi A, Zewdie KA, Ärnlöv J, Coresh J, Perico N, Remuzzi G, Murray CJL, Vos T. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, Culpepper WJ, Dorsey ER, Elbaz A, Ellenbogen RG, Fisher JL, Fitzmaurice C, Giussani G, Glennie L, James SL, Johnson CO, Kassebaum NJ, Logroscino G, Marin B, Mountjoy-Venning WC, Nguyen M, Ofori-Asenso R, Patel AP, Piccininni M, Roth GA, Steiner TJ, Stovner LJ, Szoeke CEI, Theadom A, Vollset SE, Wallin MT, Wright C, Zunt JR, Abbasi N, Abd-Allah F, Abdelalim A, Abdollahpour I, Aboyans V, Abraha HN, Acharya D, Adamu AA, Adebayo OM, Adeoye AM, Adsuar JC, Afarideh M, Agrawal S, Ahmadi A, Ahmed MB, Aichour AN, Aichour I, Aichour MTE, Akinyemi RO, Akseer N, al-Eyadhy A, al-Shahi Salman R, Alahdab F, Alene KA, Aljunid SM, Altirkawi K, Alvis-Guzman N, Anber NH, Antonio CAT, Arabloo J, Aremu O, Ärnlöv J, Asayesh H, Asghar RJ, Atalay HT, Awasthi A, Ayala Quintanilla BP, Ayuk TB, Badawi A, Banach M, Banoub JAM, Barboza MA, Barker-Collo SL, Bärnighausen TW, Baune BT, Bedi N, Behzadifar M, Behzadifar M, Béjot Y, Bekele BB, Belachew AB, Bennett DA, Bensenor IM, Berhane A, Beuran M, Bhattacharyya K, Bhutta ZA, Biadgo B, Bijani A, Bililign N, Bin Sayeed MS, Blazes CK, Brayne C, Butt ZA, Campos-Nonato IR, Cantu-Brito C, Car M, Cárdenas R, Carrero JJ, Carvalho F, Castañeda-Orjuela CA, Castro F, Catalá-López F, Cerin E, Chaiah Y, Chang JC, Chatziralli I, Chiang PPC, Christensen H, Christopher DJ, Cooper C, Cortesi PA, Costa VM, Criqui MH, Crowe CS, Damasceno AAM, Daryani A, de la Cruz-Góngora V, de la Hoz FP, de Leo D, Demoz GT, Deribe K, Dharmaratne SD, Diaz D, Dinberu MT, Djalalinia S, Doku DT, Dubey M, Dubljanin E, Duken EE, Edvardsson D, el-Khatib Z, Endres M, Endries AY, Eskandarieh S, Esteghamati A, Esteghamati S, Farhadi F, Faro A, Farzadfar F, Farzaei MH, Fatima B, Fereshtehnejad SM, Fernandes E, Feyissa GT, Filip I, Fischer F, Fukumoto T, Ganji M, Gankpe FG, Garcia-Gordillo MA, Gebre AK, Gebremichael TG, Gelaw BK, Geleijnse JM, Geremew D, Gezae KE, Ghasemi-Kasman M, Gidey MY, Gill PS, Gill TK, Girma ET, Gnedovskaya EV, Goulart AC, Grada A, Grosso G, Guo Y, Gupta R, Gupta R, Haagsma JA, Hagos TB, Haj-Mirzaian A, Haj-Mirzaian A, Hamadeh RR, Hamidi S, Hankey GJ, Hao Y, Haro JM, Hassankhani H, Hassen HY, Havmoeller R, Hay SI, Hegazy MI, Heidari B, Henok A, Heydarpour F, Hoang CL, Hole MK, Homaie Rad E, Hosseini SM, Hu G, Igumbor EU, Ilesanmi OS, Irvani SSN, Islam SMS, Jakovljevic M, Javanbakht M, Jha RP, Jobanputra YB, Jonas JB, Jozwiak JJ, Jürisson M, Kahsay A, Kalani R, Kalkonde Y, Kamil TA, Kanchan T, Karami M, Karch A, Karimi N, Kasaeian A, Kassa TD, Kassa ZY, Kaul A, Kefale AT, Keiyoro PN, Khader YS, Khafaie MA, Khalil IA, Khan EA, Khang YH, Khazaie H, Kiadaliri AA, Kiirithio DN, Kim AS, Kim D, Kim YE, Kim YJ, Kisa A, Kokubo Y, Koyanagi A, Krishnamurthi RV, Kuate Defo B, Kucuk Bicer B, Kumar M, Lacey B, Lafranconi A, Lansingh VC, Latifi A, Leshargie CT, Li S, Liao Y, Linn S, Lo WD, Lopez JCF, Lorkowski S, Lotufo PA, Lucas RM, Lunevicius R, Mackay MT, Mahotra NB, Majdan M, Majdzadeh R, Majeed A, Malekzadeh R, Malta DC, Manafi N, Mansournia MA, Mantovani LG, März W, Mashamba-Thompson TP, Massenburg BB, Mate KKV, McAlinden C, McGrath JJ, Mehta V, Meier T, Meles HG, Melese A, Memiah PTN, Memish ZA, Mendoza W, Mengistu DT, Mengistu G, Meretoja A, Meretoja TJ, Mestrovic T, Miazgowski B, Miazgowski T, Miller TR, Mini GK, Mirrakhimov EM, Moazen B, Mohajer B, Mohammad Gholi Mezerji N, Mohammadi M, Mohammadi-Khanaposhtani M, Mohammadibakhsh R, Mohammadnia-Afrouzi M, Mohammed S, Mohebi F, Mokdad AH, Monasta L, Mondello S, Moodley Y, Moosazadeh M, Moradi G, Moradi-Lakeh M, Moradinazar M, Moraga P, Moreno Velásquez I, Morrison SD, Mousavi SM, Muhammed OS, Muruet W, Musa KI, Mustafa G, Naderi M, Nagel G, Naheed A, Naik G, Najafi F, Nangia V, Negoi I, Negoi RI, Newton CRJ, Ngunjiri JW, Nguyen CT, Nguyen LH, Ningrum DNA, Nirayo YL, Nixon MR, Norrving B, Noubiap JJ, Nourollahpour Shiadeh M, Nyasulu PS, Ogah OS, Oh IH, Olagunju AT, Olagunju TO, Olivares PR, Onwujekwe OE, Oren E, Owolabi MO, PA M, Pakpour AH, Pan WH, Panda-Jonas S, Pandian JD, Patel SK, Pereira DM, Petzold M, Pillay JD, Piradov MA, Polanczyk GV, Polinder S, Postma MJ, Poulton R, Poustchi H, Prakash S, Prakash V, Qorbani M, Radfar A, Rafay A, Rafiei A, Rahim F, Rahimi-Movaghar V, Rahman M, Rahman MHU, Rahman MA, Rajati F, Ram U, Ranta A, Rawaf DL, Rawaf S, Reinig N, Reis C, Renzaho AMN, Resnikoff S, Rezaeian S, Rezai MS, Rios González CM, Roberts NLS, Roever L, Ronfani L, Roro EM, Roshandel G, Rostami A, Sabbagh P, Sacco RL, Sachdev PS, Saddik B, Safari H, Safari-Faramani R, Safi S, Safiri S, Sagar R, Sahathevan R, Sahebkar A, Sahraian MA, Salamati P, Salehi Zahabi S, Salimi Y, Samy AM, Sanabria J, Santos IS, Santric Milicevic MM, Sarrafzadegan N, Sartorius B, Sarvi S, Sathian B, Satpathy M, Sawant AR, Sawhney M, Schneider IJC, Schöttker B, Schwebel DC, Seedat S, Sepanlou SG, Shabaninejad H, Shafieesabet A, Shaikh MA, Shakir RA, Shams-Beyranvand M, Shamsizadeh M, Sharif M, Sharif-Alhoseini M, She J, Sheikh A, Sheth KN, Shigematsu M, Shiri R, Shirkoohi R, Shiue I, Siabani S, Siddiqi TJ, Sigfusdottir ID, Sigurvinsdottir R, Silberberg DH, Silva JP, Silveira DGA, Singh JA, Sinha DN, Skiadaresi E, Smith M, Sobaih BH, Sobhani S, Soofi M, Soyiri IN, Sposato LA, Stein DJ, Stein MB, Stokes MA, Sufiyan M'B, Sykes BL, Sylaja PN, Tabarés-Seisdedos R, te Ao BJ, Tehrani-Banihashemi A, Temsah MH, Temsah O, Thakur JS, Thrift AG, Topor-Madry R, Tortajada-Girbés M, Tovani-Palone MR, Tran BX, Tran KB, Truelsen TC, Tsadik AG, Tudor Car L, Ukwaja KN, Ullah I, Usman MS, Uthman OA, Valdez PR, Vasankari TJ, Vasanthan R, Veisani Y, Venketasubramanian N, Violante FS, Vlassov V, Vosoughi K, Vu GT, Vujcic IS, Wagnew FS, Waheed Y, Wang YP, Weiderpass E, Weiss J, Whiteford HA, Wijeratne T, Winkler AS, Wiysonge CS, Wolfe CDA, Xu G, Yadollahpour A, Yamada T, Yano Y, Yaseri M, Yatsuya H, Yimer EM, Yip P, Yisma E, Yonemoto N, Yousefifard M, Yu C, Zaidi Z, Zaman SB, Zamani M, Zandian H, Zare Z, Zhang Y, Zodpey S, Naghavi M, Murray CJL, Vos T. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes care. 2004;27(5):1047–53. [DOI] [PubMed]
- 42.Liu Z, Suo C, Mao X, Jiang Y, Jin L, Zhang T, Chen X. Global incidence trends in primary liver cancer by age at diagnosis, sex, region, and etiology, 1990-2017. Cancer. 2020;126(10):2267–2278. doi: 10.1002/cncr.32789. [DOI] [PubMed] [Google Scholar]
- 43.Romanelli RJ, Huang Q, Lacy J, Wong A, Hashemi L, Smith A. Multiple sclerosis prevalence rates within a healthcare delivery system in Northern California: a retrospective, electronic health records-based study from 2010 to 2016 (P4. 2-062). Stockholm/Sweden: AAN Enterprises; 2019. https://n.neurology.org/content/92/15_Supplement/P4.2-062.
- 44.Marrie RA, O’Mahony J, Maxwell CJ, Ling V, Yeh EA, Arnold DL, Bar-Or A, Banwell B, for the Canadian Pediatric Demyelinating Disease Network High rates of health care utilization in pediatric multiple sclerosis: a Canadian population-based study. PLoS One. 2019;14(6):e0218215. doi: 10.1371/journal.pone.0218215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ascherio A, Munger KL. Epidemiology of Multiple Sclerosis: From Risk Factors to Prevention—An Update. Semin Neurol, 2016;36(02):103–14. [DOI] [PubMed]
- 46.Conradi S, Malzahn U, Schröter F, Paul F, Quill S, Spruth E, Harms L, Then Bergh F, Ditzenbach A, Georgi T, Heuschmann P, Rosche B. Environmental factors in early childhood are associated with multiple sclerosis: a case-control study. BMC Neurol. 2011;11(1):123. doi: 10.1186/1471-2377-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daltrozzo T, Hapfelmeier A, Donnachie E, Schneider A, Hemmer B. A systematic assessment of prevalence, incidence and regional distribution of multiple sclerosis in Bavaria from 2006 to 2015. Front Neurol. 2018;9:871. doi: 10.3389/fneur.2018.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marcoci C, Lisnic V, Gavriliuc M, Odainic O, Sangheli M, Belenciuc A, Leone MA, Casetta I, Pugliatti M. Prevalence of multiple sclerosis in the Republic of Moldova. Neuroepidemiology. 2016;46(3):166–172. doi: 10.1159/000443931. [DOI] [PubMed] [Google Scholar]
- 49.Flemmen HØ, Simonsen CS, Berg-Hansen P, Moen SM, Kersten H, Heldal K, Celius EG. Prevalence of multiple sclerosis in rural and urban districts in Telemark county, Norway. Mult Scler Relat Disord. 2020;45:102352. doi: 10.1016/j.msard.2020.102352. [DOI] [PubMed] [Google Scholar]
- 50.Fleming JO, Cook TD. Multiple sclerosis and the hygiene hypothesis. Neurology. 2006;67(11):2085–2086. doi: 10.1212/01.wnl.0000247663.40297.2d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included publicly available dataset. MS data analyzed in this study are available in the GBD data tool: http://ghdx.healthdata.org/gbd-results-tool. The main source of Prosperity (PI) and human development indices (HDI) data in the world was the Legatum Prosperity Index™ 2017 and UNITED NATIONS DEVELOPMENT PROGRAMME websites: https://prosperitysite.s3-accelerate.amazonaws.com/3515/1187/1128/Legatum_Prosperity_Index_2017.pdf and http://hdr.undp.org/en/composite/HDI.