In this RCT, we report 1-year outcomes of clinical remission, weight restoration, and medical rehospitalization of HCR in hospitalized adolescents with AN.
Abstract
BACKGROUND AND OBJECTIVES:
We recently reported the short-term results of this trial revealing that higher-calorie refeeding (HCR) restored medical stability earlier, with no increase in safety events and significant savings associated with shorter length of stay, in comparison with lower-calorie refeeding (LCR) in hospitalized adolescents with anorexia nervosa. Here, we report the 1-year outcomes, including rates of clinical remission and rehospitalizations.
METHODS:
In this multicenter, randomized controlled trial, eligible patients admitted for medical instability to 2 tertiary care eating disorder programs were randomly assigned to HCR (2000 kcals per day, increasing by 200 kcals per day) or LCR (1400 kcals per day, increasing by 200 kcals every other day) within 24 hours of admission and followed-up at 10 days and 1, 3, 6, and 12 months post discharge. Clinical remission at 12 months post discharge was defined as weight restoration (≥95% median BMI) plus psychological recovery. With generalized linear mixed effect models, we examined differences in clinical remission over time.
RESULTS:
Of 120 enrollees, 111 were included in modified intent-to-treat analyses, 60 received HCR, and 51 received LCR. Clinical remission rates changed over time in both groups, with no evidence of significant group differences (P = .42). Medical rehospitalization rates within 1-year post discharge (32.8% [19 of 58] vs 35.4% [17 of 48], P = .84), number of rehospitalizations (2.4 [SD: 2.2] vs 2.0 [SD: 1.6]; P = .52), and total number of days rehospitalized (6.0 [SD: 14.8] vs 5.1 [SD: 10.3] days; P = .81) did not differ by HCR versus LCR.
CONCLUSIONS:
The finding that clinical remission and medical rehospitalization did not differ over 1-year, in conjunction with the end-of-treatment outcomes, support the superior efficacy of HCR as compared with LCR.
What’s Known on This Subject:
Lower-calorie refeeding is the standard of care in anorexia nervosa because of concerns about refeeding syndrome. Higher-calorie refeeding is associated with greater weight gain and shortened hospital stay, but rates of clinical remission and medical rehospitalization are unknown.
What This Study Adds:
We found no evidence of differences between higher-calorie refeeding and lower-calorie refeeding in rates of clinical remission, medical rehospitalization, number of medical readmissions, and number of days medically hospitalized 1-year post discharge.
Anorexia nervosa (AN) is associated with high medical and psychiatric morbidity and mortality.1 Both in the inpatient and outpatient settings, early weight gain is an important predictor of outcomes at 1-year follow-up.2,3 Although outpatient family-based treatment is currently regarded as the gold standard,4 up to 45% of low weight patients with restrictive eating disorders will require inpatient medical stabilization at some time during the course of treatment.5 Inpatient medical treatment is the most costly form of treatment.6–10
Nutritional rehabilitation is central to achieving medical stabilization.11 Historically, recommendations for nutritional rehabilitation in inpatient settings have been conservative,12–16 for fear of precipitating the refeeding syndrome, a constellation of electrolyte disturbances and multiorgan dysfunction that can develop early in the refeeding course of patients who are malnourished.17–21 Not surprisingly, the “start low, go slow” approach is associated with slow weight gain and long hospitalization.22,23
In comparison with lower-calorie refeeding (LCR), studies from our group have revealed that higher-calorie refeeding (HCR) is associated with faster weight gain and a shortened length of stay without an increased risk of electrolyte disturbances or the refeeding syndrome.24,25 Numerous other studies have demonstrated the feasiblity of HCR in hospital. 26–35 However, most of these published studies were retrospective or observational and limited to the period of hospitalization.24,25,27,30,33–36
The Study of Refeeding to Optimize Inpatient Gains (StRONG) is the largest randomized controlled trial (RCT) comparing refeeding approaches to date. We recently reported our end-of-treatment findings, demonstrating shorter time to medical stabilization with HCR, with no increased incidence of the electrolyte disturbances associated with refeeding syndrome and significant cost savings associated with a shorter length of stay.37 However, it is not known whether these short-term benefits of HCR are sustained over time. Furthermore, one of the criticisms of HCR is that participants might have difficulty sustaining progress after discharge from the hospital because they may feel overwhelmed with a higher caloric intake, more rapid advancement, and fewer days in the structured hospital environment to acclimate to these caloric changes, which might place them at greater risk for rehospitalization. Herein, we report 1-year outcomes of StRONG, including clinical remission and medical readmission rates.
The primary end point of this trial was clinical remission, defined as both weight restoration (≥95% median BMI [mBMI]), in which percentage mBMI equals BMI divided by 50th percentile BMI × 100, by using the 2000 Centers for Disease Control and Prevention growth charts,38 and psychological recovery, measured by an Eating Disorder Examination Questionnaire (EDE-Q) global score within 1 SD of community norms. We hypothesized that rates of achievement and maintenance of clinical remission would differ between the HCR and LCR groups. We also examined longitudinal trajectories of percentage mBMI and EDE-Q scores by treatment group. Other outcomes were readmission rates, number of readmissions, and total number of hospital days after the initial admission. We hypothesized that there would be no differences in readmission rates, number of admissions, or total number of hospital days after the initial admission between groups.
Methods
Design
StRONG (ClinicalTrials.gov identifier NCT02488109) was a multicenter RCT with 12 months follow-up after discharge. The clinical sites were the inpatient medical eating disorder units of Lucile Packard Children’s Hospital at Stanford and the University of California, San Francisco Benioff Children’s Hospital, with a separate data coordination center (DCC). Randomization was stratified by site in random blocks of 2 to 6 generated by the DCC. Written informed consent was obtained from each participant; parental consent and assent were obtained from minors. A written release of information was obtained to collect future follow-up data from an outside medical provider or electronic medical record, for any participant for whom we were unable to collect in-person data at follow-up. Participants received a $50 gift card as an incentive for keeping follow-up appointments and completing the questionnaires. The DCC ensured the safety of the participants and validity of the trial, with oversight from a data and safety monitoring board composed of experts in the field.
Participants
The StRONG study population has been previously described.37,39 Briefly, eligible patients with AN or atypical anorexia nervosa (AAN) (patients with all the features of AN, except that, despite significant weight loss, their weight remains in the normal or above normal range)40 who were admitted for medical instability between February 8, 2016, and March 7, 2019, were approached within 24 hours of admission for possible enrollment in the study. The inclusion criteria were diagnosis of AN or AAN confirmed by conducting a standardized psychiatric patient and parent interview, age 12 to 24 years, and no hospitalization within the previous 6 months. The exclusion criteria were those <60% mBMI, participants with bulimia nervosa or avoidant restrictive food intake disorder, those experiencing acute suicidality, and those with a comorbid chronic medical illness (eg, inflammatory bowel disease, diabetes, or celiac disease).
Intervention
As previously described,37 within 24 hours of admission, enrolled participants were randomly assigned to HCR (2000 kcals per day, increasing by 200 kcals per day) or LCR (1400 kcals per day, increasing by 200 kcals every other day). Calories were provided by meals, with oral liquid replacement for food refusal. Caloric advances during the hospitalization were performed by using an electronic order set within the electronic medical record. Patients were discharged from the hospital when their vital signs were stable for ≥24 hours and their weight was ≥75% mBMI for age and sex. Stabilization of vital signs was defined as achieving a lowest nighttime heart rate of ≥45 beats per minute on continuous cardiac monitoring, blood pressure of ≥90/45 mm Hg, and absence of orthostatic pulse or blood pressure changes (orthostasis defined as an increase in heart rate >35 beats per minute or drop of systolic blood pressure of >20 mm Hg on standing).
Follow-up
This was an open follow-up trial, with study visits at 10 days and 1, 3, 6, and 12 months after discharge. At each visit, height, weight, and vital signs were recorded. Height was measured with a wall-mounted stadiometer, and weight was measured with a digital scale, postvoiding wearing only a hospital gown. When the participant did not return for in-person study visits, medical information was obtained from an outside medical provider or from the electronic medical record, after previous authorization to do so was provided. In rare instances, home visits were performed by the research coordinator by using a medical grade digital scale and stadiometer (Seca 869 and Seca 437; Seca North America, Chino, CA). Participants completed the health care use questionnaire, which specifically asked about any medical hospitalizations since the last visit and the number of days of inpatient treatment received. At each visit (except the 10-day visit), participants completed the EDE-Q.41 The global score (range of 0–6), calculated from the average of 4 subscales, is well validated against the clinician-administered Eating Disorder Examination interview, considered the gold standard measure of eating disorder psychopathology.42,43
Outcome Measures
The primary prespecified outcome of this trial was clinical remission at 1-year follow-up, defined as achievement of a weight ≥95% mBMI plus an EDE-Q global score within 1 SD of community norms (age-matched mean: 1.59; SD: 1.32).41 The secondary outcomes were weight restoration (achievement of a weight ≥95% mBMI) and psychological recovery (EDE-Q global score within 1 SD of community norms), separately. Additional outcomes were rates of medical readmission, number of readmissions, and total number of hospital days after the initial admission.
Statistical Methods
The study was powered to detect a difference in 12-months clinical remission rates between groups. On the basis of studies of remission rates in AN,44,45 n = 60 per arm with 85% retention would provide 80% power on a 2-sided 0.05-level test to detect a 20% difference between groups in 12-months clinical remission rates.
In a modified intent-to-treat (mITT) approach, we included all the randomly assigned participants who received treatment of at least 1 day in the analyses, including those who withdrew during the refeeding intervention. Patients who were found ineligible post randomization provided no assent after parent’s consent, or withdrew before receiving any treatment, were not included in the mITT analysis for reasons of ethics and clinical relevance. Analyses were conducted to confirm that those patients were not different from patients included in the mITT analysis. In the primary analysis, we used a generalized linear mixed-effects regression model to compare study arms with respect to achievement and maintenance of clinical remission. Clinical remission was defined as the combination of percentage mBMI and EDE-Q score at 1, 3, 6, and 12 months. Instead of assuming missing data at random in the generalized linear mixed-effects regression model, clinical remission was modeled as a nominal multinomial outcome (yes, no, or missing), with time (1, 3, 6, or 12 months after discharge), treatment group, and time*treatment group interaction as fixed effects, whereas sites and patients were included as random effects to account for the correlation because of clustering. The time*treatment group interaction provides the mITT effect of HCR, compared with LCR, on clinical remission over time. The average remission rates and scores and their 95% confidence intervals were estimated from the model.
In secondary analyses, we included separate linear mixed-effects models to analyze continuous versions of percentage mBMI and EDE-Q to describe longitudinal trajectories. The 2 linear mixed-effects models included a quadratic term for time (time2) as a fixed effect, in addition to baseline value, time, group, and time*group interaction, while including random intercept for site and patients. The time*group interaction was of main interest.
We used Fisher’s exact test to compare the rate of medical rehospitalization between groups. The number of medical readmissions and total number of hospital days after discharge from the initial admission would be skewed and zero-inflated, given that many patients would not be readmitted, therefore, we used the Wilcoxon rank test to compare groups. Data are presented as mean (SD), frequency, and percentage. Data were analyzed by using SPSS for Windows (version 25.0; IBM SPSS Statistics, IBM Corporation) and SAS for Windows (version 9.4; SAS Institute, Inc, Cary, NC).
Results
Participant enrollment and follow-up data are shown in Fig 1. A total of 120 participants were recruited from a pool of 301 eligible patients admitted between February 2016 and March 2019. A total of 60 were randomly assigned to HCR, and 60 were randomly assigned to LCR. A total of 9 participants withdrew from the LCR group: 6 of these withdrew before receiving treatment, and 3 were found to be ineligible post randomization; 111 participants were included in the mITT analysis.
FIGURE 1.
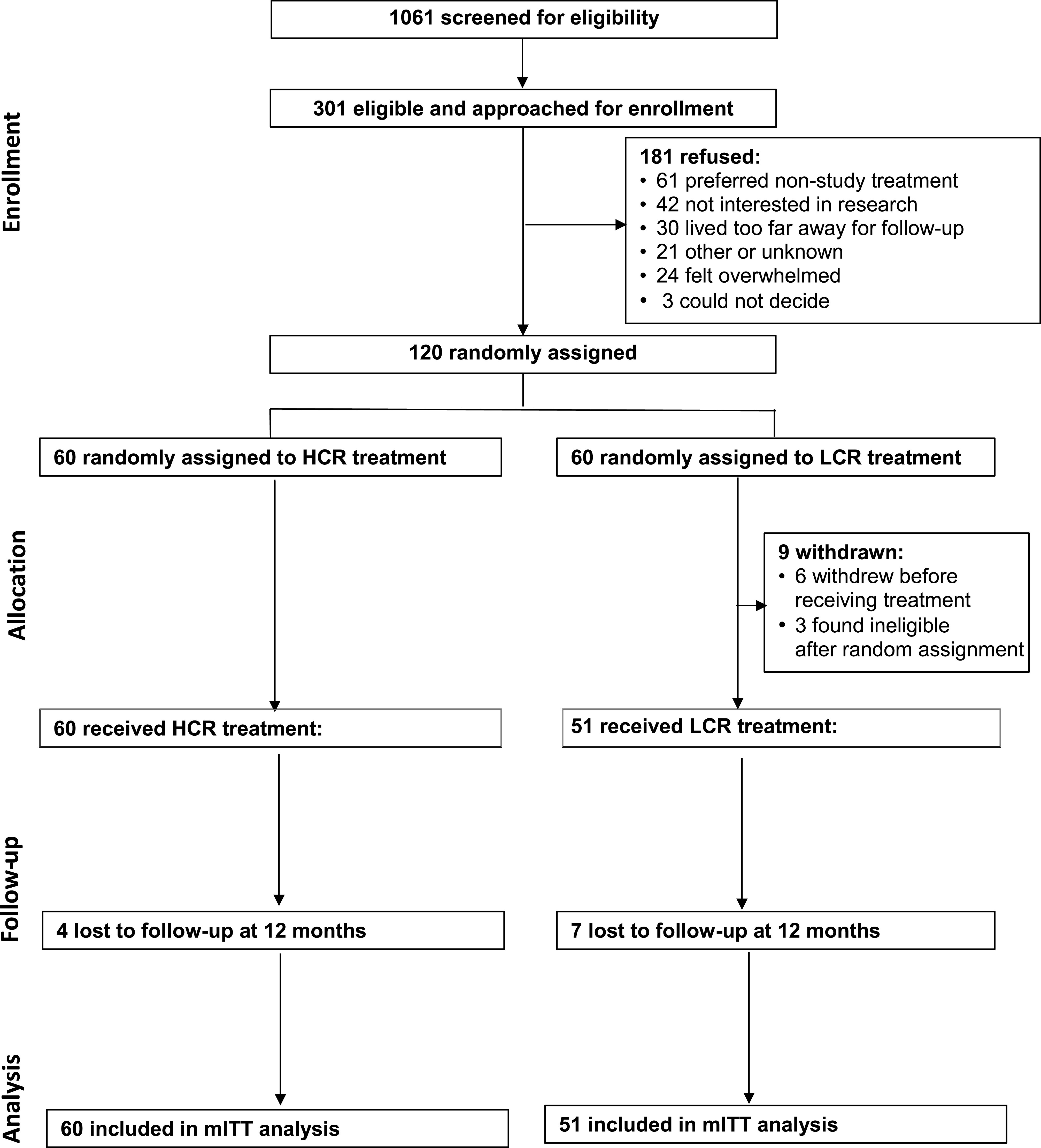
Consolidated Standards of Reporting Trials diagram: RCT of HCR, compared with LCR, in hospitalized adolescents with AN.
Demographic data and clinical variables by group on initial hospital admission have been previously reported37 and shown to be balanced on key characteristics, including sex, race and ethnicity, amount and duration of weight loss, degree of malnutrition, and EDE-Q scores. Participants were predominantly female (91%) and non-Hispanic white (65%), with a mean age of 16.4 (SD: 2.5) years. The mean percentage mBMI was 84.9% (SD: 11.7), with a range of 64.1% to 122.9%. Clinical data by group at discharge, 10 days, and 1, 3, 6, and 12 months are shown in Table 1.
TABLE 1.
Percentage mBMI and EDE-Q Scores by Group
| LCR (n = 51), Mean (SD) | HCR (n = 60), Mean (SD) | P (95% Confidence Interval) | |
|---|---|---|---|
| Discharge % mBMI | 89.8 (10.1) | 88.7 (11.3) | .61 (−.05 to .03) |
| 10-d % mBMI | 90.7 (12.2) | 90.4 (10.8) | .89 (−.49 to .04) |
| 1-mo follow-up % mBMI | 93.2 (12.1) | 91.6 (10.8) | .48 (−.06 to .03) |
| 3-mo follow-up % mBMI | 96.9 (11.7) | 94.5 (11.2) | .29 (−.07 to .02) |
| 6-mo follow-up % mBMI | 98.3 (13.2) | 95.6 (12.2) | .30 (−.08 to .02) |
| 12-mo follow-up % mBMI | 97.6 (12.2) | 96.4 (12.4) | .64 (−.06 to .04) |
| Baseline EDE-Q global score | 3.5 (1.7) | 3.3 (1.7) | .72 (−.80 to .56) |
| 1-mo EDE-Q global score | 2.7 (1.5) | 2.5 (1.5) | .53 (−.91 to .47) |
| 3-mo EDE-Q global score | 2.4 (1.6) | 2.2 (1.7) | .59 (−.99 to .57) |
| 6-mo EDE-Q global score | 2.4 (1.6) | 1.6 (1.5) | .05 (−1.6 to −.02) |
| 12-mo EDE-Q global score | 2.3 (1.6) | 2.4 (1.6) | .75 (−.62 to .87) |
HCR versus LCR. —, not applicable.
Height and weight data were available for 99 of 111 participants (89%) at 10 days, 105 of 111 participants (95%) at 1 month, 98 of 111 (88%) at 3 months, 91 of 111 (82%) at 6 months, and 98 of 111 (88%) at 12 months. As seen in Table 2, at each time point, the majority of the clinical data were obtained in person, in the outpatient clinics of each institution. EDE-Q data were available for 75 of 111 (67.6%) of participants at 1 month, 77 of 111 (69%) at 3 months, 67 of 111 (60%) at 6 months, and 77 of 111 (69%) at 12 months.
TABLE 2.
Source of Clinical Data for Follow-up Visits
| 10 d, n (%) | 1 mo, n (%) | 3 mo, n (%) | 6 mo, n (%) | 12 mo, n (%) | |
|---|---|---|---|---|---|
| In-house clinical visit | 86 (77) | 88 (79) | 80 (72) | 68 (61) | 64 (58) |
| Outside physician clinical visit | 9 (8) | 12 (11) | 9 (8) | 19 (17) | 30 (27) |
| Outside eating disorder program | 4 (4) | 5 (5) | 8 (7) | 2 (2) | 2 (2) |
| Home visit | 0 (0) | 0 (0) | 1 (1) | 2 (2) | 2 (2) |
| Missed visit | 10 (9) | 4 (4) | 11 (10) | 18 (16) | 11 (10) |
| Total weights for primary analysis | 99 (89) | 105 (95) | 98 (88) | 91 (82) | 98 (88) |
Percentages reflect the percentage of total visits possible (111 at each planned visit).
Primary Outcome Measure
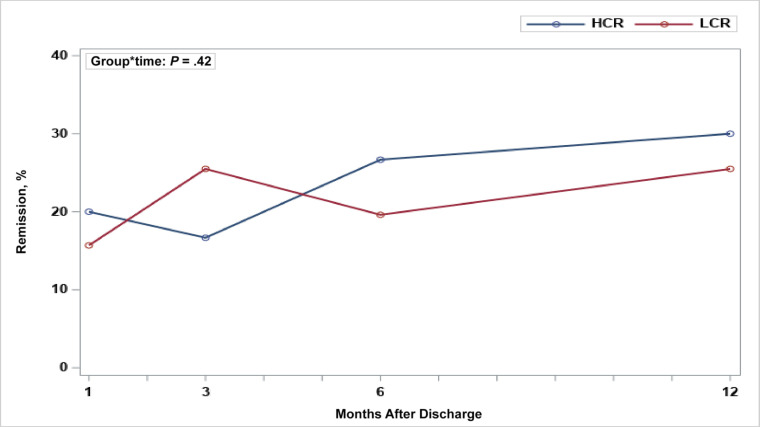
Changes in rates of clinical remission over time in the mITT model are shown in Fig 2 and reveal no evidence of a significant group difference in the change of remission over time (P = .42). The rates of clinical remission for LCR and HCR for participants with complete percentage mBMI and EDE-Q data at each time point were as follows: 1 month (LCR: 8 of 32 [25.0%]; HCR: 12 of 40 [29.3%]), 3 months (LCR: 13 of 34 [38.2%]; HCR: 10 of 39 [25.6%]), 6 months (LCR: 10 of 26 [38.5%]; HCR: 16 of 39 [41.0%]), and 12 months after discharge (LCR: 13 of 27 [48.1%]; HCR: 18 of 44 [40.9%]).
FIGURE 2.
Changes in rates of clinical remission over time: HCR versus LCR by using an mITT analysis.
Secondary Outcome Measures
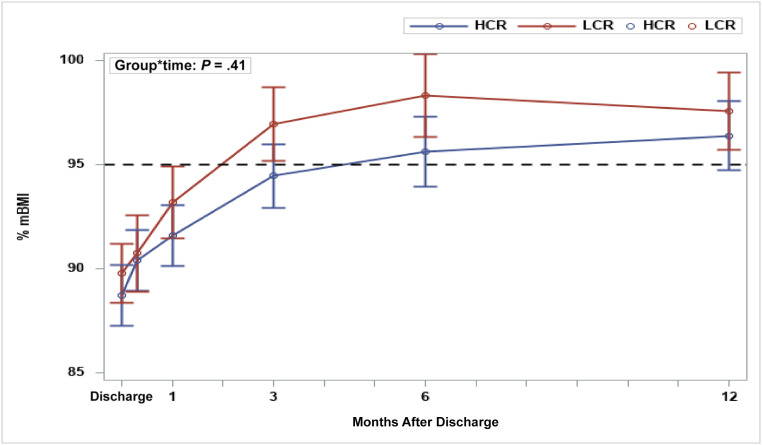
As shown in Table 1, both groups gained weight over time: 10 days (LCR: 90.7% mBMI; HCR: 90.4% mBMI), 1 month (LCR: 93.2% mBMI; HCR: 91.6% mBMI), 3 months (LCR: 96.9% mBMI; HCR: 94.5% mBMI), 6 months (LCR: 98.3% mBMI; HCR: 95.6% mBMI), and 12 months (LCR: 97.6% mBMI; HCR: 96.4% mBMI), but there was no evidence of a significant group difference in change of percentage mBMI over time (P = .41; Fig 3). In both groups, the EDE-Q global scores decreased over time: 1 month (LCR: 2.7; HCR: 2.5), 3 months (LCR: 2.4; HCR: 2.2), 6 months (LCR: 2.4; HCR: 1.6), and 12 months after discharge (LCR: 2.3; HCR: 2.4). However, there was no evidence of a significant group difference in change of EDE-Q global score over time (P = .32).
FIGURE 3.
Changes in percentage mBMI (mean ± SD) by treatment group over time: HCR versus LCR.
Follow-up data were available for 106 participants regarding medical rehospitalization. Of the 106 participants, 36 (34%) were rehospitalized to a medical unit within 1-year after discharge (Table 3). Groups did not differ by the proportion rehospitalized, the number of times rehospitalized, or days spent rehospitalized.
TABLE 3.
Medical Rehospitalization by Group
| LCR (n = 51) | HCR (n = 60) | P | |
|---|---|---|---|
| Readmission, n (%) | .84 | ||
| Yes | 17 of 48 (35.4) | 19 of 58 (32.8) | |
| No | 31 of 48 (64.6) | 39 of 58 (67.2) | |
| Missing | 3 of 51 (5.9) | 2 of 60 (3.3) | |
| No. readmissions, mean (SD) | 2.0 (1.6) | 2.4 (2.2) | .52 |
| 0, n (%) | 31 of 48 (64.6) | 39 of 58 (67.2) | |
| 1, n (%) | 10 of 48 (20.8) | 10 of 58 (17.2) | |
| 2, n (%) | 3 of 48 (6.3) | 3 of 58 (5.2) | |
| >3, n (%) | 4 of 48 (8.3) | 6 of 58 (10.3) | |
| Missing, n (%) | 3 of 51 (5.9) | 2 of 60 (3.3) | |
| No. readmission days | 5.10 (10.3) | 6.03 (14.8) | .81 |
| Median (Q1–Q3) | 0 (0–6) | 0 (0–5) | |
| Maximum | 40 | 85 |
HCR versus LCR. Q, quartile.
Discussion
In this multicenter RCT, we found that rates of clinical remission did not differ between HCR and LCR, which is the current standard of care. Importantly, HCR was not associated with higher rates of medical readmission, number of readmissions, or an increase in the total number of hospital days after the initial admission. These results, taken together with our end-of-treatment findings,37 support the efficacy of HCR. Specifically, the lack of difference in rehospitalization rates between groups maintains the initial cost savings associated with shorter length of stay during the first admission.
StRONG is the only RCT to date in which HCR and LCR were compared. In a previous RCT from the United Kingdom, researchers compared weight gain and cardiovascular outcomes in 36 adolescents with AN assigned to receive either 1200 or 500 kcal/day and found that the higher calorie intake was associated with a greater weight gain, with no increase in adverse cardiovascular outcomes.28 However, the higher calorie intake in that study (1200 kcals/day) was lower than the lower calorie intake in most studies conducted in the United States, Canada, or Australia. In two previous retrospective studies from our programs,24,25 we compared HCR with LCR and provided the impetus for this RCT. These two studies revealed an increased rate of weight gain with a shortened length of hospital stay and no increased risk of electrolyte disturbances with HCR. Since our initial publications, multiple observational studies, most without comparison groups, have revealed that a more accelerated approach to oral nutritional rehabilitation in the hospital is feasible, provided there is close monitoring and correction of electrolytes.30–33,35,36 Other studies from Australia and Canada, demonstrated similar outcomes with more aggressive continuous nasogastric refeeding protocols.26,27,34,45 In the Unites States, oral meal-based refeeding is preferred. To date, the longest follow-up period has been 4 weeks after discharge.35 Consensus-based recommendations for LCR have endured for decades because of concerns for refeeding syndrome and a lack of well-designed RCTs to assess efficacy and inform clinical guidelines. With the findings reported here, we provide the evidence to inform updated treatment guidelines for adolescents and young adults with restrictive eating disorders who are >60% mBMI.
Concerns have been raised that more rapid inpatient weight gain in AN would be associated with rehospitalization.46 We found that 34.0% of participants were rehospitalized to a medical unit during the 12 months of follow-up and rates of rehospitalization did not differ by treatment group. This readmission rate is consistent with the literature, in which rates of rehospitalization to specialized eating disorder units within the first year of follow-up for adolescents and young adults with restrictive eating disorders are 30% to 45%.47–50 Readmission during the first year after initial hospitalization does not necessarily portend a poor long-term prognosis,47 and, in general, long-term outcomes for children and adolescents are better than for adults.51 The finding of no increased rates of medical readmission with HCR is important, revealing that more rapid refeeding with an earlier discharge from the hospital does not lead to a revolving door of rehospitalization. For adolescents with restricting eating disorders, the treatment of choice is family-based treatment, a manualized outpatient therapy.44 An earlier discharge from the hospital after medical stabilization may allow for earlier initiation of family-based treatment.
In our study, the majority of participants were weight restored to ≥95% mBMI by 3 months, but, at 1-year, less than one-half of all participants met the full criteria for clinical remission. Rates of recovery in AN depend on the definitions used to define recovery.52,53 Our findings are consistent with other clinical trials, revealing that clinical remission is 18% to 55% at 1-year.54 Our finding that rates of weight restoration were higher than rates of clinical remission at each time point is consistent with other studies revealing that psychological recovery lags behind weight recovery in AN.52
Strengths of our study include the rigorous prospective randomized controlled design, moderately large sample size, use of follow-up data that included psychological variables, in addition to weight to define clinical remission, and mITT approach. In addition, the retention rate was relatively high. This study is limited by its exclusion of participants <60% mBMI, the population most vulnerable to complications related to refeeding, so our results are not generalizable to patients with extreme malnutrition. In addition, despite good participant retention, we were able to obtain measures of height and weight from outside providers but had more difficulty obtaining follow-up EDE-Q scores for participants who moved out of the area. Further studies should be conducted in patients with eating disorders who are malnourished to determine efficacy and safety in this population.
Conclusions
In this RCT, we did not find evidence of a difference between HCR and LCR in rates of clinical remission, medical rehospitalization, number of readmissions, or number of days hospitalized 1-year post discharge in hospitalized adolescents and young adults with AN or AAN. With the results of this study, together with our earlier findings of shorter time to medical stabilization, reduced length of stay, and significant cost savings, we provide compelling data supporting the need to change existing treatment guidelines for inpatient refeeding practices in adolescent and young adults with AN and AAN.
Acknowledgments
We thank Kristina Saffran, BA, for assistance with patient enrollment and data collection. We also thank the patients who agreed to participate in this study.
Glossary
- AAN
atypical anorexia nervosa
- AN
anorexia nervosa
- DCC
data coordination center
- EDE-Q
Eating Disorder Examination Questionnaire
- HCR
higher-calorie refeeding
- LCR
lower-calorie refeeding
- mBMI
median BMI
- mITT
modified intent-to-treat
- RCT
randomized controlled trial
- StRONG
Study of Refeeding to Optimize Inpatient Gains
Footnotes
Dr Golden is a coprincipal investigator on this project, developed the original idea, conceptualized and designed the project, contributed to data analysis, enrolled participants and advised on clinical care, and drafted the initial manuscript; Dr Garber is a coprincipal investigator on this project, developed the original idea, conceptualized and designed the project, and assisted with data analysis and drafting of the initial manuscript; Dr Cheng is the faculty biostatistician who performed the data analysis and oversaw the data coordination center staffed by Dr Adams, the data analyst, and Ms Machen, the senior research clinical research coordinator; Dr Kreiter, the research coordinator at Stanford, collected data and reported to the data coordination center; Ms Sy supervised the in-hospital refeeding protocol at Stanford, contributed to the design of the order sets, and collected and analyzed the nutrition data; Dr Buckelew enrolled participants and advised on clinical care; Dr Kapphahn enrolled participants and advised on clinical care and helped develop protocols for managing electrolyte abnormalities; Dr Moscicki led the data safety managing board and contributed to study design; Drs Accurso and Le Grange oversaw the psychometric data collection and critically reviewed the manuscript for psychological content; Dr Wilson contributed to questionnaire design and implementation; and all authors reviewed, revised and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Deidentified individual participant data will not be made available.
This trial has been registered at www.clinicaltrials.gov (identifier NCT02488109).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by National Institutes of Health grant R01HD082166. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Le Grange receives royalties from Guilford Press and Routledge and is codirector of the Training Institute for Child and Adolescent Eating Disorders; the other authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2020-043737.
References
- 1.Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch Gen Psychiatry. 2011;68(7):724–731 [DOI] [PubMed] [Google Scholar]
- 2.Lund BC, Hernandez ER, Yates WR, Mitchell JR, McKee PA, Johnson CL. Rate of inpatient weight restoration predicts outcome in anorexia nervosa. Int J Eat Disord. 2009;42(4):301–305 [DOI] [PubMed] [Google Scholar]
- 3.Madden S, Miskovic-Wheatley J, Wallis A, Kohn M, Hay P, Touyz S. Early weight gain in family-based treatment predicts greater weight gain and remission at the end of treatment and remission at 12-month follow-up in adolescent anorexia nervosa. Int J Eat Disord. 2015;48(7):919–922 [DOI] [PubMed] [Google Scholar]
- 4.Golden NH, Katzman DK, Sawyer SM, et al.; Society for Adolescent Health and Medicine . Position Paper of the Society for Adolescent Health and Medicine: medical management of restrictive eating disorders in adolescents and young adults. J Adolesc Health. 2015;56(1):121–125 [DOI] [PubMed] [Google Scholar]
- 5.Kapphahn CJ, Graham DA, Woods ER, et al. Effect of hospitalization on percent median body mass index at one year, in underweight youth with restrictive eating disorders. J Adolesc Health. 2017;61(3):310–316 [DOI] [PubMed] [Google Scholar]
- 6.Lock J, Couturier J, Agras WS. Costs of remission and recovery using family therapy for adolescent anorexia nervosa: a descriptive report. Eat Disord. 2008;16(4):322–330 [DOI] [PubMed] [Google Scholar]
- 7.Ágh T, Kovács G, Supina D, et al. A systematic review of the health-related quality of life and economic burdens of anorexia nervosa, bulimia nervosa, and binge eating disorder. Eat Weight Disord. 2016;21(3):353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Striegel-Moore RH, Leslie D, Petrill SA, Garvin V, Rosenheck RA. One-year use and cost of inpatient and outpatient services among female and male patients with an eating disorder: evidence from a national database of health insurance claims. Int J Eat Disord. 2000;27(4):381–389 [DOI] [PubMed] [Google Scholar]
- 9.Samnaliev M, Noh HL, Sonneville KR, Austin SB. The economic burden of eating disorders and related mental health comorbidities: an exploratory analysis using the U.S. Medical Expenditures Panel Survey. Prev Med Rep. 2014;2:32–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le LK-D, Hay P, Mihalopoulos C. A systematic review of cost-effectiveness studies of prevention and treatment for eating disorders. Aust N Z J Psychiatry. 2018;52(4):328–338 [DOI] [PubMed] [Google Scholar]
- 11.Shamim T, Golden NH, Arden M, Filiberto L, Shenker IR. Resolution of vital sign instability: an objective measure of medical stability in anorexia nervosa. J Adolesc Health. 2003;32(1):73–77 [DOI] [PubMed] [Google Scholar]
- 12.American Psychiatric Association . Treatment of patients with eating disorders, third edition. American Psychiatric Association. Am J Psychiatry. 2006;163(suppl 7):4–54 [PubMed] [Google Scholar]
- 13.American Dietetic Association . Position of the American Dietetic Association: nutrition intervention in the treatment of anorexia nervosa, bulimia nervosa, and other eating disorders. J Am Diet Assoc. 2006;106(12):2073–2082 [DOI] [PubMed] [Google Scholar]
- 14.Mehler PS, Winkelman AB, Andersen DM, Gaudiani JL. Nutritional rehabilitation: practical guidelines for refeeding the anorectic patient. J Nutr Metab. 2010;2010:625782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentile MG, Pastorelli P, Ciceri R, Manna GM, Collimedaglia S. Specialized refeeding treatment for anorexia nervosa patients suffering from extreme undernutrition. Clin Nutr. 2010;29(5):627–632 [DOI] [PubMed] [Google Scholar]
- 16.National Collaborating Centre for Mental Health (UK) . Eating Disorders: Core Interventions in the Treatment and Management of Anorexia Nervosa, Bulimia Nervosa and Related Eating Disorders. Leicester, United Kingdom: British Psychological Society; 2004 [PubMed] [Google Scholar]
- 17.Kohn MR, Golden NH, Shenker IR. Cardiac arrest and delirium: presentations of the refeeding syndrome in severely malnourished adolescents with anorexia nervosa. J Adolesc Health. 1998;22(3):239–243 [DOI] [PubMed] [Google Scholar]
- 18.Beumont PJ, Large M. Hypophosphataemia, delirium and cardiac arrhythmia in anorexia nervosa. Med J Aust. 1991;155(8):519–522 [DOI] [PubMed] [Google Scholar]
- 19.Hall DE, Kahan B, Snitzer J. Delirium associated with hypophosphatemia in a patient with anorexia nervosa. J Adolesc Health. 1994;15(2):176–178 [DOI] [PubMed] [Google Scholar]
- 20.Fisher M, Simpser E, Schneider M. Hypophosphatemia secondary to oral refeeding in anorexia nervosa. Int J Eat Disord. 2000;28(2):181–187 [DOI] [PubMed] [Google Scholar]
- 21.Norris ML, Pinhas L, Nadeau P-O, Katzman DK. Delirium and refeeding syndrome in anorexia nervosa. Int J Eat Disord. 2012;45(3):439–442 [DOI] [PubMed] [Google Scholar]
- 22.Garber AK, Michihata N, Hetnal K, Shafer M-A, Moscicki A-B. A prospective examination of weight gain in hospitalized adolescents with anorexia nervosa on a recommended refeeding protocol. J Adolesc Health. 2012;50(1):24–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MARSIPAN : Management of really sick patients with anorexia nervosa, college report CR189 from the Royal College of Psychiatrists, Oct 2014. Available at: https://www.rcpsych.ac.uk/docs/default-source/improving-care/better-mh-policy/college-reports/college-report-cr189.pdf?sfvrsn=6c2e7ada_2. Accessed February 12, 2021
- 24.Golden NH, Keane-Miller C, Sainani KL, Kapphahn CJ. Higher caloric intake in hospitalized adolescents with anorexia nervosa is associated with reduced length of stay and no increased rate of refeeding syndrome. J Adolesc Health. 2013;53(5):573–578 [DOI] [PubMed] [Google Scholar]
- 25.Garber AK, Mauldin K, Michihata N, Buckelew SM, Shafer M-A, Moscicki A-B. Higher calorie diets increase rate of weight gain and shorten hospital stay in hospitalized adolescents with anorexia nervosa. J Adolesc Health. 2013;53(5):579–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madden S, Miskovic-Wheatley J, Clarke S, Touyz S, Hay P, Kohn MR. Outcomes of a rapid refeeding protocol in adolescent anorexia nervosa. J Eat Disord. 2015;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker EK, Faruquie SS, Anderson G, et al. Higher caloric refeeding is safe in hospitalised adolescent patients with restrictive eating disorders. J Nutr Metab. 2016;2016:5168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Connor G, Nicholls D, Hudson L, Singhal A. Refeeding low weight hospitalized adolescents with anorexia nervosa: a multicenter randomized controlled trial. Nutr Clin Pract. 2016;31(5):681–689 [DOI] [PubMed] [Google Scholar]
- 29.Garber AK, Sawyer SM, Golden NH, et al. A systematic review of approaches to refeeding in patients with anorexia nervosa. Int J Eat Disord. 2016;49(3):293–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peebles R, Lesser A, Park CC, et al. Outcomes of an inpatient medical nutritional rehabilitation protocol in children and adolescents with eating disorders. J Eat Disord. 2017;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redgrave GW, Coughlin JW, Schreyer CC, et al. Refeeding and weight restoration outcomes in anorexia nervosa: challenging current guidelines. Int J Eat Disord. 2015;48(7):866–873 [DOI] [PubMed] [Google Scholar]
- 32.Whitelaw M, Gilbertson H, Lam P-Y, Sawyer SM. Does aggressive refeeding in hospitalized adolescents with anorexia nervosa result in increased hypophosphatemia? J Adolesc Health. 2010;46(6):577–582 [DOI] [PubMed] [Google Scholar]
- 33.Leclerc A, Turrini T, Sherwood K, Katzman DK. Evaluation of a nutrition rehabilitation protocol in hospitalized adolescents with restrictive eating disorders. J Adolesc Health. 2013;53(5):585–589 [DOI] [PubMed] [Google Scholar]
- 34.Agostino H, Erdstein J, Di Meglio G. Shifting paradigms: continuous nasogastric feeding with high caloric intakes in anorexia nervosa. J Adolesc Health. 2013;53(5):590–594 [DOI] [PubMed] [Google Scholar]
- 35.Smith K, Lesser J, Brandenburg B, et al. Outcomes of an inpatient refeeding protocol in youth with anorexia nervosa and atypical anorexia nervosa at Children’s Hospitals and Clinics of Minnesota. J Eat Disord. 2016;4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maginot TR, Kumar MM, Shiels J, Kaye W, Rhee KE. Outcomes of an inpatient refeeding protocol in youth with anorexia nervosa: Rady Children’s Hospital San Diego/University of California, San Diego. J Eat Disord. 2017;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garber AK, Cheng J, Accurso EC, et al. Short-term outcomes of the study of refeeding to optimize inpatient gains for patients with anorexia nervosa: a multicenter randomized clinical trial [published online ahead of print October 19, 2020]. JAMA Pediatr. doi: 10.1001/jamapediatrics.2020.3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention . National Center for Health Statistics growth charts. Available at: https://www.cdc.gov/growthcharts/. Accessed February 12, 2021
- 39.Garber AK, Cheng J, Accurso EC, et al. Weight loss and illness severity in adolescents with atypical anorexia nervosa. Pediatrics. 2019;144(6):e20192339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013 [Google Scholar]
- 41.Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire? Int J Eat Disord. 1994;16(4):363–370 [PubMed] [Google Scholar]
- 42.Cooper Z, Cooper PJ, Fairburn CG. The validity of the eating disorder examination and its subscales. Br J Psychiatry. 1989;154:807–812 [DOI] [PubMed] [Google Scholar]
- 43.Berg KC, Peterson CB, Frazier P, Crow SJ. Psychometric evaluation of the eating disorder examination and eating disorder examination-questionnaire: a systematic review of the literature. Int J Eat Disord. 2012;45(3):428–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lock J, Le Grange D, Agras WS, Moye A, Bryson SW, Jo B. Randomized clinical trial comparing family-based treatment with adolescent-focused individual therapy for adolescents with anorexia nervosa. Arch Gen Psychiatry. 2010;67(10):1025–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madden S, Miskovic-Wheatley J, Wallis A, et al. A randomized controlled trial of in-patient treatment for anorexia nervosa in medically unstable adolescents. Psychol Med. 2015;45(2):415–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willer MG, Thuras P, Crow SJ. Implications of the changing use of hospitalization to treat anorexia nervosa. Am J Psychiatry. 2005;162(12):2374–2376 [DOI] [PubMed] [Google Scholar]
- 47.Strober M, Freeman R, Morrell W. The long-term course of severe anorexia nervosa in adolescents: survival analysis of recovery, relapse, and outcome predictors over 10-15 years in a prospective study. Int J Eat Disord. 1997;22(4):339–360 [DOI] [PubMed] [Google Scholar]
- 48.Eckert ED, Halmi KA, Marchi P, Grove W, Crosby R. Ten-year follow-up of anorexia nervosa: clinical course and outcome. Psychol Med. 1995;25(1):143–156 [DOI] [PubMed] [Google Scholar]
- 49.Carter JC, Blackmore E, Sutandar-Pinnock K, Woodside DB. Relapse in anorexia nervosa: a survival analysis. Psychol Med. 2004;34(4):671–679 [DOI] [PubMed] [Google Scholar]
- 50.Steinhausen H-C, Grigoroiu-Serbanescu M, Boyadjieva S, Neumärker K-J, Winkler Metzke C. Course and predictors of rehospitalization in adolescent anorexia nervosa in a multisite study. Int J Eat Disord. 2008;41(1):29–36 [DOI] [PubMed] [Google Scholar]
- 51.Steinhausen H-C. The outcome of anorexia nervosa in the 20th century. Am J Psychiatry. 2002;159(8):1284–1293 [DOI] [PubMed] [Google Scholar]
- 52.Couturier J, Lock J. What is recovery in adolescent anorexia nervosa? Int J Eat Disord. 2006;39(7):550–555 [DOI] [PubMed] [Google Scholar]
- 53.Couturier J, Lock J. What is remission in adolescent anorexia nervosa? A review of various conceptualizations and quantitative analysis. Int J Eat Disord. 2006;39(3):175–183 [DOI] [PubMed] [Google Scholar]
- 54.Le Grange D, Lock J, Accurso EC, et al. Relapse from remission at two- to four-year follow-up in two treatments for adolescent anorexia nervosa. J Am Acad Child Adolesc Psychiatry. 2014;53(11):1162–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]