In this retrospective cohort study, we assessed whether the diagnosis of severe IVH was independently associated with all-cause in-hospital mortality among mechanically ventilated extremely premature neonates.
Abstract
BACKGROUND:
Severe intraventricular hemorrhage (IVH) is a leading mortality risk factor among extremely premature neonates. Because other life-threatening conditions also occur in this population, it is unclear whether severe IVH is independently associated with death. The existence and potential implications of regional variation in severe IVH–associated mortality are unknown.
METHODS:
We performed a retrospective cohort study of mechanically ventilated neonates born at 22 to 29 weeks’ gestation who received care in 242 American NICUs between 2000 and 2014. After building groups composed of propensity score–matched and center-matched pairs, we used the Cox proportional hazards analysis to test our hypothesis that severe IVH would be associated with greater all-cause in-hospital mortality, defined as death before transfer or discharge. We also performed propensity score–matched subgroup analyses, comparing severe IVH–associated mortality among 4 geographic regions of the United States.
RESULTS:
In our analysis cohort, we identified 4679 patients with severe IVH. Among 2848 matched pairs, those with severe IVH were more likely to die compared with those without severe IVH (hazard ratio 2.79; 95% confidence interval 2.49–3.11). Among 1527 matched pairs still hospitalized at 30 days, severe IVH was associated with greater risk of death (hazard ratio 2.03; 95% confidence interval 1.47–2.80). Mortality associated with severe IVH varied substantially between geographic regions.
CONCLUSIONS:
The early diagnosis of severe IVH is independently associated with all-cause in-hospital mortality in extremely premature neonates. Regional variation in severe IVH–associated mortality suggests that shared decision-making between parents and neonatologists is strongly influenced by ultrasound-based IVH assessment and classification.
In critically ill neonates, severe intraventricular hemorrhage (IVH) consists of bleeding that distends the lateral ventricle (grade 3) and periventricular hemorrhagic infarction (grade 4), as detected on cranial ultrasonography.1–3 Severe IVH occurs most commonly among neonates born at <29 weeks’ gestation,4,5 among whom it is thought to be an important factor associated with death.6,7 Because overall severity of illness is associated with the development of IVH and also predisposes to death,8,9 it remains unclear whether severe IVH itself is a truly independent risk factor associated with mortality.
When death does occur in neonates with severe IVH, it often follows a shared decision to provide comfort care and discontinue life-sustaining medical treatment (LSMT).7,8,10,11 To the extent that cranial ultrasonography informs this decision-making, it is troubling that interrater variability in the ultrasound assessment of IVH12–15 and classification of severe IVH according to a 4-stage scale14,16,17 limits the prognostic value of this screening test. Studies reveal a wide range of low positive predictive values for ultrasound-diagnosed severe IVH regarding neurodevelopmental impairment (17%–61%) and cerebral palsy (11%–49%).4,7,9,10,14,16 Regional variation in end-of-life care intensity suggests that provider beliefs and patient and family characteristics influence shared decision-making in adult patients.18–20 There is little regional variation data to explain what impacts such decisions for neonates.21 Thus, it remains unclear what influences parents and neonatologists as they decide whether to continue LSMT in the setting of severe IVH.22–27
To resolve these knowledge gaps, we studied a large cohort of mechanically ventilated neonates aged <29 weeks’ gestation, hypothesizing that the presence of grade 3 or grade 4 IVH on the early bedside ultrasound would be associated with greater all-cause in-hospital mortality, irrespective of overall illness severity. To ascertain whether provider and/or parental decision-making might underlie any observed difference in survival between neonates with and without severe IVH, we conducted subgroup survival analyses of patients from 4 geographic regions of the United States.
Methods
Study Design
We performed a retrospective cohort study using data from the Clinical Data Warehouse (CDW). The CDW contains the demographic and clinical information of >1 million neonates who were hospitalized in a Pediatrix Medical Group (PMG) NICU.28 The Mayo Clinic Institutional Review Board (Rochester, MN) deemed this study exempt because the data set provided was deidentified.
Study Setting and Population
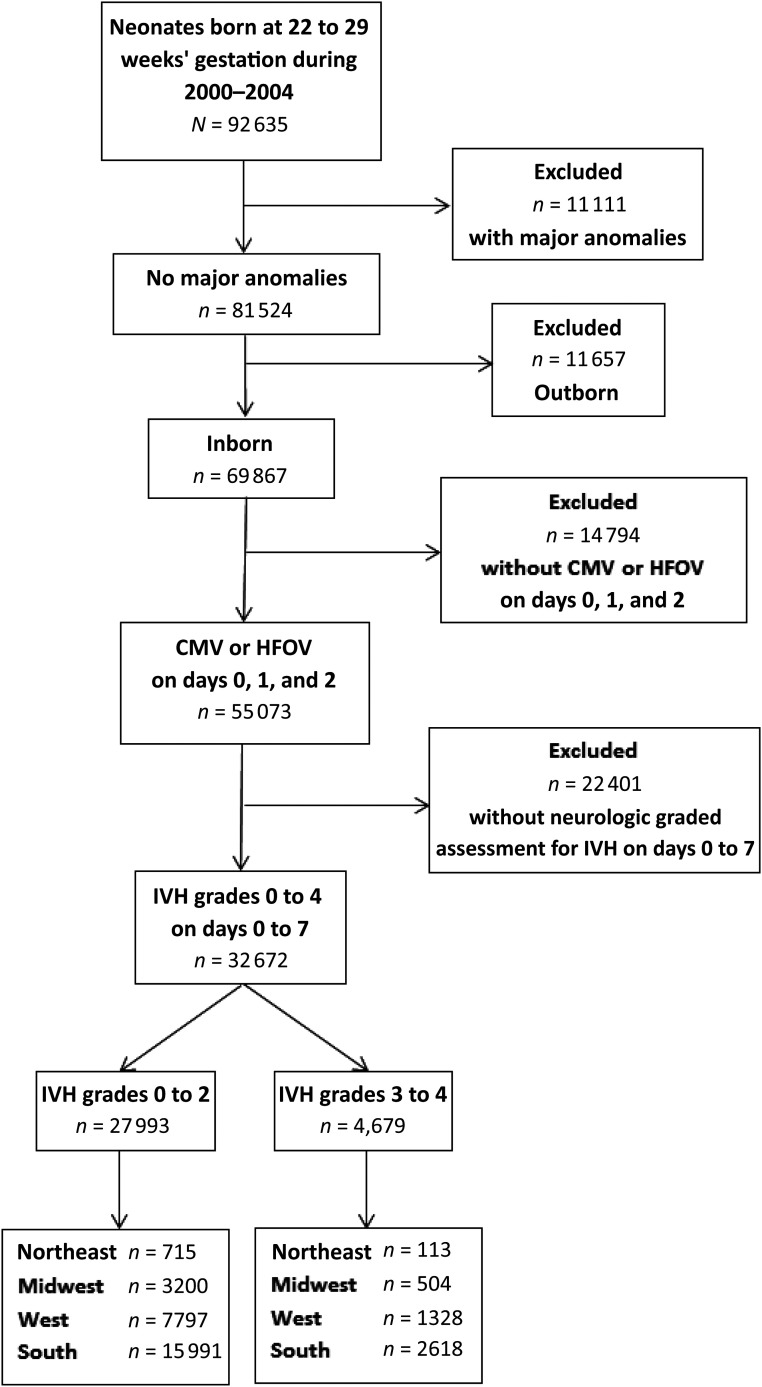
We queried CDW data tables to identify neonates who had been admitted to a PMG NICU for intensive care; thus we excluded those who died in the delivery room or who had been admitted for comfort measures only. Among these patients, we included in our study neonates who were born at 22 to 29 weeks’ gestation, diagnosed with at least 1 form of respiratory distress, and discharged between January 1, 2000, and December 31, 2014. We then excluded neonates with major congenital anomalies, those who were outborn, those who required neither conventional nor high-frequency ventilation on days of life 0 to 2, and those without documented neurologic imaging or a documented grade of IVH on days of life 0 to 7.
Patient Characteristics and Outcomes
We obtained a variety of maternal and neonatal characteristics (Table 1) for each patient, including age and IVH grade at the time of the patient’s maximum IVH grade within the first 7 days of life. We defined severe IVH as a maximum grade 3 or 4 hemorrhage; patients with no hemorrhage (grade 0) or with a maximum grade 1 or 2 hemorrhage were considered not to have severe IVH. The primary outcome was all-cause in-hospital mortality (hereafter mortality), defined as death before transfer or discharge; clinician-documented cause of death was not included in the CDW data set. Secondary outcomes were necrotizing enterocolitis (NEC); retinopathy of prematurity that required treatment during the index hospitalization (tROP); chronic lung disease (CLD), defined as a supplemental oxygen requirement or pressure-supported ventilation at 36 weeks’ corrected gestational age (CGA); and periventricular leukomalacia (PVL).
TABLE 1.
Baseline Characteristics of Full and Matched Cohorts by Severity of IVH
| Characteristic | Full Cohort | Matched Cohort | ||||
|---|---|---|---|---|---|---|
| Severe IVH (n = 4679) | Nonsevere IVH (n = 27 993) | Standardized Difference | Severe IVH (n = 2848) | Nonsevere IVH (n = 2848) | Standardized Difference | |
| Maternal characteristics, n (%) | ||||||
| Prolonged ROM | 613 (13.1) | 5008 (17.9) | 0.133 | 442 (15.5) | 426 (15.0) | 0.016 |
| Oligohydramnios | 60 (1.3) | 467 (1.7) | 0.032 | 48 (1.7) | 47 (1.7) | 0.003 |
| Antenatal steroids given | 3136 (67.0) | 23 010 (82.2) | 0.354 | 2092 (73.5) | 2120 (74.4) | 0.022 |
| Infant characteristics | ||||||
| Age at maximum IVH grade, d, n (%) | ||||||
| 0 | 1651 (35.3) | 9103 (32.5) | — | 1305 (45.8) | 1305 (45.8) | — |
| 1 | 496 (10.6) | 2475 (8.8) | — | 249 (8.7) | 249 (8.7) | — |
| 2 | 704 (15.0) | 2339 (8.4) | — | 313 (11.0) | 313 (11.0) | — |
| 3 | 554 (11.8) | 2699 (9.6) | — | 255 (9.0) | 255 (9.0) | — |
| 4 | 358 (7.7) | 1802 (6.4) | — | 166 (5.8) | 166 (5.8) | — |
| 5 | 241 (5.2) | 1673 (6.0) | — | 110 (3.9) | 110 (3.9) | — |
| 6 | 253 (5.4) | 2319 (8.3) | — | 149 (5.2) | 149 (5.2) | — |
| 7 | 422 (9.0) | 5583 (19.9) | — | 301 (10.6) | 301 (10.6) | — |
| Respiratory diagnosis group, n (%) | ||||||
| Pneumonia | 214 (4.6) | 1301 (4.6) | 0.004 | 145 (5.1) | 178 (6.3) | 0.050 |
| Pneumonia and PPHN | 24 (0.5) | 57 (0.2) | 0.052 | 11 (0.4) | 8 (0.3) | 0.018 |
| PH | 41 (0.9) | 140 (0.5) | 0.046 | 28 (1.0) | 22 (0.8) | 0.023 |
| PH and PPHN | 28 (0.6) | 101 (0.4) | 0.034 | 16 (0.6) | 14 (0.5) | 0.010 |
| RDS | 3981 (85.1) | 25 597 (91.4) | 0.199 | 2496 (87.6) | 2473 (86.8) | 0.024 |
| RDS and PPHN | 391 (8.4) | 797 (2.8) | 0.241 | 152 (5.3) | 153 (5.4) | 0.002 |
| Gestational age, mean (SD), wk | 25.2 (1.8) | 26.7 (1.8) | 0.836 | 25.5 (1.7) | 25.5 (1.7) | 0.019 |
| Gestational age, wk, n (%) | ||||||
| 22 | 81 (1.7) | 87 (0.3) | — | 14 (0.5) | 17 (0.6) | — |
| 23 | 763 (16.3) | 1143 (4.1) | — | 296 (10.4) | 307 (10.8) | — |
| 24 | 1123 (24.0) | 2863 (10.2) | — | 624 (21.9) | 602 (21.1) | — |
| 25 | 873 (18.7) | 3739 (13.4) | — | 559 (19.6) | 595 (20.9) | — |
| 26 | 725 (15.5) | 4387 (15.7) | — | 520 (18.3) | 515 (18.1) | — |
| 27 | 536 (11.5) | 4960 (17.7) | — | 401 (14.1) | 392 (13.8) | — |
| 28 | 350 (7.5) | 5603 (20.0) | — | 263 (9.2) | 269 (9.4) | — |
| 29 | 228 (4.9) | 5211 (18.6) | — | 171 (6.0) | 151 (5.3) | — |
| Birth wt, mean (SD), kg | 0.79 (0.24) | 0.93 (0.27) | 0.543 | 0.83 (0.24) | 0.82 (0.24) | 0.042 |
| Birth size assessment, n (%) | ||||||
| SGA | 489 (10.5) | 3943 (14.1) | 0.111 | 312 (11.0) | 373 (13.1) | 0.066 |
| AGA | 3815 (81.5) | 21 999 (78.6) | 0.074 | 2296 (80.6) | 2257 (79.2) | 0.034 |
| LGA | 375 (8.0) | 2051 (7.3) | 0.026 | 240 (8.4) | 218 (7.7) | 0.028 |
| Sex, n (%) | 0.151 | 0.001 | ||||
| Female | 1865 (39.9) | 13 254 (47.3) | — | 1207 (42.4) | 1209 (42.5) | — |
| Male | 2812 (60.1) | 14 734 (52.6) | — | 1640 (57.6) | 1638 (57.5) | — |
| Unknown | 2 (0.0) | 5 (0.0) | — | 1 (0.0) | 1 (0.0) | — |
| Race, n (%) | ||||||
| White | 2073 (44.3) | 12 912 (46.1) | 0.037 | 1296 (45.5) | 1271 (44.6) | 0.018 |
| Asian American | 106 (2.3) | 773 (2.8) | 0.032 | 62 (2.2) | 44 (1.5) | 0.047 |
| Black | 1276 (27.3) | 7380 (26.4) | 0.020 | 763 (26.8) | 803 (28.2) | 0.031 |
| Hispanic | 983 (21.0) | 5351 (19.1) | 0.047 | 580 (20.4) | 583 (20.5) | 0.003 |
| Other | 241 (5.2) | 1577 (5.6) | 0.021 | 147 (5.2) | 147 (5.2) | 0.000 |
| Calendar year, n (%) | 0.129 | 0.026 | ||||
| 2000–2004 | 1448 (30.0) | 7083 (25.3) | — | 1037 (36.4) | 972 (34.1) | — |
| 2005–2009 | 1695 (36.2) | 10 528 (37.6) | — | 1013 (35.6) | 1071 (37.6) | — |
| 2010–2014 | 1536 (32.8) | 10 382 (37.1) | — | 798 (28.0) | 805 (28.3) | — |
| Birth number, n (%) | 0.035 | 0.000 | ||||
| Singleton | 3387 (72.4) | 20 695 (73.9) | — | 2225 (78.1) | 2225 (78.1) | — |
| 2+ | 1292 (27.6) | 7298 (26.1) | — | 623 (21.9) | 623 (21.9) | — |
| Surfactant given, n (%) | 4132 (88.3) | 24 372 (87.1) | 0.038 | 2487 (87.3) | 2516 (88.3) | 0.031 |
| CMV or HFOV, n (%) | 0.564 | 0.006 | ||||
| CMV | 2028 (43.3) | 19 665 (70.2) | — | 1453 (51.0) | 1462 (51.3) | — |
| HFOV | 2651 (56.7) | 8328 (29.8) | — | 1395 (49.0) | 1386 (48.7) | — |
| At least 1 vasopressor reported on days 0–3, n (%) | 2591 (55.4) | 7499 (26.8) | 0.607 | 1362 (47.8) | 1383 (48.6) | 0.015 |
AGA, appropriate for gestational age; CMV, conventional mechanical ventilation; HFOV, high-frequency oscillatory ventilation; LGA, large for gestational age; PH, pulmonary hypoplasia; PPHN, persistent pulmonary hypertension; RDS, respiratory distress syndrome; ROM, rupture of membranes; SGA, small for gestational age; —, not applicable.
Data Analysis
We used propensity score (PS) matching to reduce the imbalance of measured baseline characteristics between patients with and without severe IVH during the first 7 days of life.29 The PS was defined as the probability of a patient having severe IVH, given a set of 25 baseline covariates, as estimated by using a multivariable logistic regression model (Table 1, Supplemental Fig 4). For each patient with severe IVH, 1 patient was randomly selected from the pool of patients with a grade 0 to 2 hemorrhage who met the matching criteria. Patients were matched on (1) the logit of the PS by using calipers of width equal to 0.2 SD of the logit of the PS, (2) the day of life (0–7) on which their maximum IVH status was diagnosed, (3) the gestation status (singleton or multiple-gestation pregnancy), and (4) the center. We assessed covariate imbalance between those with and without severe IVH by evaluating the standardized difference for each baseline covariate. The standardized difference for a continuous covariate was defined as the absolute difference in group means divided by an estimate of the pooled SD. The derivation was similar for nominal covariates. A standardized difference <0.1 denoted negligible covariate imbalance between the 2 groups.30
We used a Cox proportional hazards model to assess the association between IVH severity status and mortality. We used age as the time scale, with patients entering the risk set at their age at the index date,31 which we defined as the day of life (0–7) on which maximum IVH status was diagnosed. We used the counting process formulation of a Cox model; patients entered the analysis at their index age (left truncation) and exited at their death, transfer, or discharge age. We summarized associations using the hazard ratio (HR) and corresponding 95% confidence interval (CI).
We employed the same time-to-event analysis methods to assess NEC as a secondary outcome. For the other secondary outcomes, we identified separate PS-matched subcohorts of patients who were eligible for assessment of these conditions. For tROP, we restricted the starting cohort to patients with retinopathy of prematurity (ROP) evaluated; for CLD, we restricted the starting cohort to patients still in the hospital at 36 weeks’ CGA; and for PVL, we restricted the starting cohort to patients with documented brain imaging. We evaluated the association between IVH severity and each of these 3 secondary outcomes using logistic regression. We summarized associations using the odds ratio (OR) and corresponding 95% CI.
All calculated P values were 2 sided, and P values <.05 were considered statistically significant. Statistical analyses were performed by using SAS version 9.4 software (SAS Institute, Inc, Cary, NC) and R version 3.4.2 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria).
Regional Subgroup Analyses
Regional differences in provider beliefs and practices and in patient and family characteristics underlie regional variation in end-of-life decision-making.18–20 We therefore stratified patients into 4 geographic regions, as defined in the National (Nationwide) Inpatient Sample (NIS).32 Within each region, we used the same strategy as our primary analysis to create a matched cohort of patients with and without severe IVH. Given the smaller sample sizes, a standardized difference threshold <0.25 denoted acceptable covariate imbalance.30 For each region, we used a Cox proportional hazards model to assess the association between IVH severity status and mortality. We then compared the 2 regions to the highest and lowest disparity in mortality risk by evaluating the interaction between region and IVH status in a Cox model. We also performed a sensitivity analysis in which we created 2 PS-matched subsets, (1) with severe IVH and (2) without severe IVH, from the same 2 regions. For each subset, the association between region and mortality was evaluated by fitting a Cox model.
Results
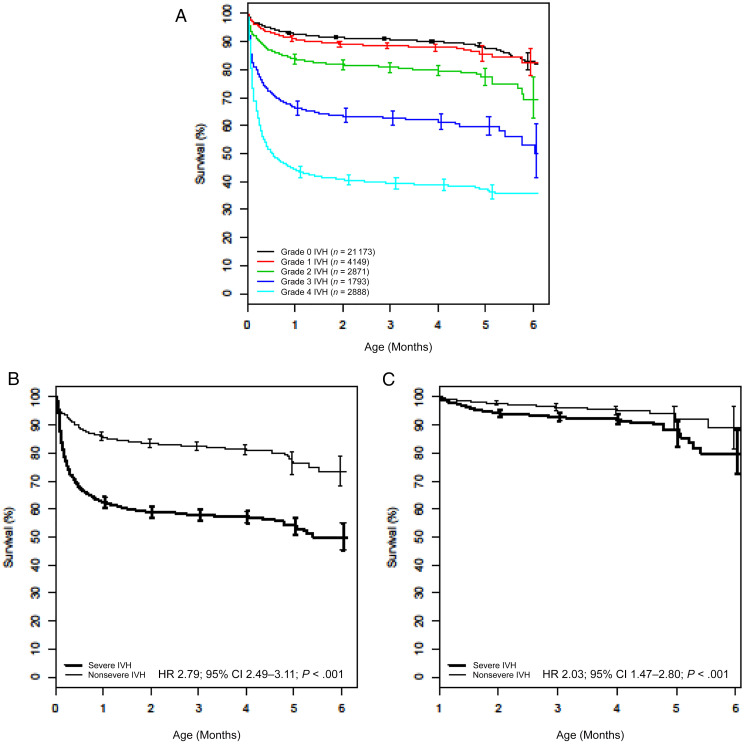
There were 92 635 neonates born at 22 to 29 weeks’ gestation who were diagnosed with at least 1 form of respiratory distress and discharged from PMG NICUs between 2000 and 2014. As shown in Fig 1, patients were sequentially excluded because of the presence of major congenital anomalies; outborn status; no requirement for mechanical ventilation on days of life 0 to 2; and no documentation of neurologic imaging, grade of IVH, age at maximum IVH grade on days of life 0 to 7, or age >7 days at maximum IVH grade. The resulting analysis cohort thus was composed of 32 672 neonates from 242 PMG NICUs; baseline characteristics are shown in Table 1. Neonates with severe IVH were less likely to have been exposed to antenatal steroids, were born more premature and at a lower weight, and were more likely to require high-frequency ventilation and receive at least 1 vasopressor medication in the first days of life. As shown in Fig 2A, incremental increases in IVH grade were associated with an increased risk of mortality in the unmatched cohort.
FIGURE 1.
Consolidated Standards of Reporting Trials flow diagram. CMV, continuous mandatory ventilation; HFOV, high-frequency oscillatory ventilation.
FIGURE 2.
A–C, Overall survival by severity of IVH for patients in the full cohort (N = 32 672 patients) (A), matched cohort (n = 2848 matched pairs) (B), and landmark cohort, which was restricted to 1527 matched pairs in which both patients were still alive and in the hospital at 30 days (C).
Primary Analysis: Outcomes of Severe IVH
From the analysis cohort of 32 672 neonates, we created matched groups that each contained 2848 patients and were balanced for all measured covariates (Table 1, Supplemental Fig 4A). Nearly half of the patients included in our analysis required high-frequency ventilation or vasopressor support. Among patients in the severe IVH group, 1030 died at a median age of 7.5 days (interquartile range: 3.5–17.5 days), whereas 440 of those without severe IVH died at a median age of 13.5 days (interquartile range: 5.5–28.5 days). Neonates with severe IVH were significantly more likely to die compared with those without severe IVH (Fig 2B). A landmark analysis restricted to the 1527 matched pairs in which both patients in the pair were alive and still in the hospital at 30 days revealed that severe IVH also was associated with greater mortality beyond 30 days of life (Fig 2C).
To determine if survival might vary depending on the grade of severe IVH, we separated patients with severe IVH from the matched cohort into those with grade 3 (n = 1169) or grade 4 (n = 1679) hemorrhages. Neonates with either grade 3 or grade 4 hemorrhages were more likely to die than were matched neonates without severe IVH (grade 3 IVH: HR 1.76 [95% CI 1.45–2.14]; grade 4 IVH: HR 3.54 [95% CI 3.09–4.06]). The landmark analysis revealed that this survival disparity persisted after 30 days of life only for those with grade 4 hemorrhages (grade 3 IVH [n = 760 matched pairs]: HR 1.40 [95% CI 0.86–2.28]; grade 4 IVH [n = 767 matched pairs]: HR 2.68 [95% CI 1.72–4.17]).
Severe IVH was not associated with an increased risk of NEC (HR 1.09; 95% CI 0.94–1.26). Among the secondary outcomes shown in Table 2, severe IVH was associated only with a higher rate of PVL (OR 4.52; 95% CI 3.53–5.80). Please refer to Supplemental Fig 4 B–D to see the standardized differences in baseline characteristics for the tROP, CLD, and PVL analysis groups (all below the 0.1 threshold).
TABLE 2.
Comparison of Secondary Outcomes by Severity of IVH in the Matched Cohort
| Severe IVH | Nonsevere IVH | OR (95% CI) | P | |
|---|---|---|---|---|
| ROP treated | ||||
| No. neonates with ROP evaluated | 2250 | 23 077 | — | — |
| No. matched pairs used in analysis | 1470 | 1470 | — | — |
| No | 1274 (86.7%) | 1294 (88.0%) | — | — |
| Yes | 196 (13.3%) | 176 (12.0%) | 1.13 (0.91–1.41) | .27 |
| CLD | ||||
| No. neonates still in hospital at ≥36 wk CGA | 1880 | 18 174 | — | — |
| No. matched pairs used in analysis | 1168 | 1168 | — | — |
| No | 493 (42.2%) | 500 (42.8%) | — | — |
| Yes | 675 (57.8%) | 668 (57.2%) | 1.03 (0.87–1.21) | .77 |
| PVL | ||||
| No. neonates with imaging | 4510 | 30 135 | — | — |
| No. matched pairs used in analysis | 2841 | 2841 | — | — |
| No | 2508 (88.3%) | 2760 (97.2%) | — | — |
| Yes | 333 (11.7%) | 81 (2.9%) | 4.52 (3.53–5.80) | <.001 |
—, not applicable.
Secondary Analysis: Regional Survival in Severe IVH
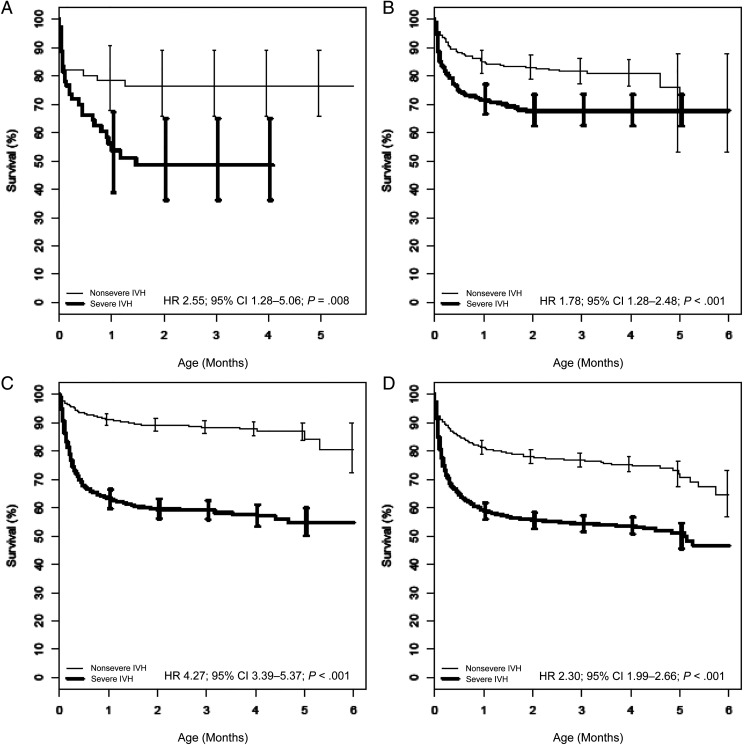
From the analysis cohort of 32 672 patients, we created matched groups of patients with and without severe IVH for each of 4 NIS regions: Northeast (n = 60 each), Midwest (n = 332 each), West (n = 854 each), and South (n = 1467 each). As was the case in our primary survival analysis, these region-specific groups were balanced for all measured covariates (Supplemental Table 3) and matched for center. In all 4 regions, neonates with severe IVH were more likely to die than those without severe IVH (Fig 3). Among paired regional comparisons, the greatest difference in severe IVH–associated mortality risk was observed between the West and the Midwest (West: HR 4.27 [95% CI 3.39–5.37]; Midwest: HR 1.78 [95% CI 1.28–2.48]; P < .001).
FIGURE 3.
A–D, Overall survival by severe IVH of patients in the matched cohort for the Northeast (A), the Midwest (B), the West (C), and the South (D). The survival estimates were derived from a Cox model, with patients entering the risk set at their age at the index date.
To further investigate the above difference between the West and Midwest regions, we conducted a sensitivity analysis in which we directly compared the survival of patients from these 2 regions according to IVH status. To do so, we created 2 matched subsets with severe IVH (n = 465 each from the West and Midwest) and without severe IVH (n = 3112 each from the West and Midwest) that were balanced for all measured covariates (Supplemental Table 4). Patients with severe IVH in the West were more likely to die than those in the Midwest (HR 1.52; 95% CI 1.25–1.85), whereas survival among those without severe IVH did not vary between the regions (HR 0.87; 95% CI 0.72–1.04).
Discussion
We conducted this study to determine the extent to which severe IVH is associated with mortality in extremely premature neonates. To begin, we impaneled the largest known cohort of neonates <29 weeks’ gestation whose natural, all-cause mortality was described according to IVH status. In this overall cohort, the prevalence of severe IVH (14.3%) was similar to that of other cohorts in large retrospective cohort studies4,5 and suggests an annual incidence of ∼5000 cases in the United States.33 Given that 50% of the patients in the matched cohort had severe IVH and 1470 patients died, we had 80% power to detect a HR ≥1.16. Having controlled for a variety of maternal and neonatal characteristics, including markers of overall illness severity, we found that severe IVH was independently associated with an increased risk of mortality (HR 2.79). Among all extremely premature neonates who died, patients with severe IVH died earlier than those without severe IVH.
Both grade 3 and grade 4 IVH were independently associated with mortality (HR 1.76 and 3.54, respectively). To put this in context with other morbidities affecting extremely premature neonates, these mortality HRs are similar to those seen in patients with early evidence of pulmonary hypertension (HR 2.0) and those with NEC (HR 2.67).34,35 We also found that grade 4 IVH was associated with greater mortality among patients who survived the first 30 days of life. This increased risk could be due to complications of posthemorrhagic hydrocephalus or ventriculoperitoneal shunt placement,7 although we do not know the prevalence of these variables in our cohort. We also compared the risks of 4 secondary outcomes associated with long-term neurodevelopmental compromise (NEC, CLD, tROP, and PVL).36–40 Consistent with previous observations, severe IVH was associated with an increased risk of PVL.37 The similar risks of NEC, CLD, and tROP observed among patients with and without severe IVH likely resulted from survivorship bias.
Although we could not directly assess cause or mode of death in this study, 3 pieces of evidence suggest that severe IVH–associated mortality in our cohort was due to withdrawal of LSMT, as others have established.7,8,11 First, in the overall cohort, approximately half of the 14.3% of patients diagnosed with severe IVH died before transfer or discharge (Fig 2A), similar to the proportion of deaths caused by IVH in a subset of 641 patients included in our analyses (9.4%).6 Second, the median age of death for patients with severe IVH was 7.5 days, nearly 1 week earlier than patients without severe IVH and within days of maximum-grade IVH diagnosis. Last, we observed regional differences in mortality, suggesting that provider and/or parental decision-making may have informed the decision to withdraw LSMT for patients with severe IVH. Providers’ beliefs and practices are known to underlie regional variation in the intensity of care at the end of life,18–20 and there is evidence that rates of LSMT discontinuation differ among the NIS regions.41 Although these regional comparisons have important implications for future study of neonatal outcomes and decision-making in the NICU, we must acknowledge that the geographic distribution of PMG NICUs influenced the outcomes of the primary analyses (eg, overrepresentation of hospitals in the South and underrepresentation of hospitals in the Northeast).
In this study, we only included patients who required mechanical ventilation on days of life 0 to 2. Although this limits the patient population to which our findings may be generalized, a more rigorous matching strategy (eg, requiring the same mode of ventilation on the day the maximum IVH grade was diagnosed) would have prevented us from creating cohorts balanced for other important covariates. It is also important to note that uninformative patient characteristics were excluded from the PS model. For example, some variables were highly collinear (eg, maximum fraction of inspired oxygen and mode of ventilation) and others were common to patients with and without severe IVH (echocardiographic assessment of PDA: 49.9% and 49.5%, respectively).
The maximum IVH grade and diagnosis of PVL were derived from all clinical reports generated during routine clinical care for each PMG NICU patient. We were unable to know whether a neonatologist or pediatric radiologist provided a given grade or diagnosis or whether independent readings were performed to yield final consensus interpretations. Likewise, we were unable to know why some patients did not receive a graded neurologic assessment during the first 7 days of life. It is conceivable that some providers or parents elected to forgo a cranial ultrasound because the result would not influence their decision-making. Interestingly, in the overall cohort, we found that 13.3% of patients in the Midwest had no IVH grade assigned. In a post hoc analysis in which we considered all Midwest patients without neurologic imaging to have severe IVH, we observed an increased risk differential between those with severe IVH and those without, further suggesting that a cranial ultrasound was unlikely to influence decision-making in this region.
Last, we did not have access to patient-level data regarding IVH laterality or the size and location of hemorrhages. A post hoc review of all CDW patients <29 weeks’ gestation revealed bilateral severe IVH in 68% of patients with maximum grade 3 IVH and in 59% of patients with maximum grade 4 IVH. Determining if laterality influences survival would be particularly important for patients with grade 4 IVH because the neurodevelopmental consequences of unilateral and bilateral hemorrhage are drastically different.14
We strongly encourage the validation of more refined ultrasound classification systems for IVH and periventricular hemorrhagic infarction made possible with advances in ultrasound technologies.42,43 By improving our understanding of prognosis in severe IVH, we can better support neonatologists and their patients’ parents when they engage in difficult, but indicated, discussions about limiting LSMT.44–46
Conclusions
The early diagnosis of severe IVH is independently associated with mortality in extremely premature neonates. Regional differences in survival suggest that neonatologists and/or families emphasize the presence of severe IVH when deciding whether to continue LSMT, irrespective of overall severity of illness. Given the apparent importance of severe IVH in end-of-life decision-making, there is a need for tools that improve the predictive value of cranial ultrasound results. Such tools, coupled with a provider’s clinical expertise and parental perspectives on their child’s outcomes,47,48 could better inform goals of care and help navigate shared decision-making for extremely premature neonates.49,50
Glossary
- CDW
Clinical Data Warehouse
- CGA
corrected gestational age
- CI
confidence interval
- CLD
chronic lung disease
- HR
hazard ratio
- IVH
intraventricular hemorrhage
- LSMT
life-sustaining medical treatment
- NEC
necrotizing enterocolitis
- NIS
National (Nationwide) Inpatient Sample
- OR
odds ratio
- PMG
Pediatrix Medical Group
- PS
propensity score
- PVL
periventricular leukomalacia
- ROP
retinopathy of prematurity
- tROP
retinopathy of prematurity that required treatment during the index hospitalization
Footnotes
Drs McCauley, E.C. Carey, W.A. Carey, and Collura conceptualized and designed the study, interpreted the data, and drafted and revised the manuscript; Ms Weaver and Ms Mara designed and conducted the statistical analyses, interpreted the data, and drafted the manuscript; Dr Clark conceptualized the study, acquired the data, interpreted the data, and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and are accountable for all aspects of the work and the accuracy and integrity of all data as presented.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The data analysis was funded by the Mayo Clinic Children’s Research Center. This publication was made possible by Clinical and Translational Science Awards grant UL1 TR002377 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Inder TE, Perlman JM, Volpe JJ. Preterm Intraventricular Hemorrhage/Posthemorrhagic Hydrocephalus. In: Volpe JJ, Inder TE, Darras BT, eds., et al. Volpe’s Neurology of the Newborn, 6th ed. Philadelphia, PA: Elsevier; 2018:637–698.e21 [Google Scholar]
- 2.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534 [DOI] [PubMed] [Google Scholar]
- 3.Bassan H, Benson CB, Limperopoulos C, et al. Ultrasonographic features and severity scoring of periventricular hemorrhagic infarction in relation to risk factors and outcome. Pediatrics. 2006;117(6):2111–2118 [DOI] [PubMed] [Google Scholar]
- 4.Bolisetty S, Dhawan A, Abdel-Latif M, Bajuk B, Stack J, Lui K; New South Wales and Australian Capital Territory Neonatal Intensive Care Units’ Data Collection . Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics. 2014;133(1):55–62 [DOI] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Bell EF, et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacob J, Kamitsuka M, Clark RH, Kelleher AS, Spitzer AR. Etiologies of NICU deaths. Pediatrics. 2015;135(1). Available at: www.pediatrics.org/cgi/content/full/135/1/e59 [DOI] [PubMed] [Google Scholar]
- 7.Davis AS, Hintz SR, Goldstein RF, et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Outcomes of extremely preterm infants following severe intracranial hemorrhage. J Perinatol. 2014;34(3):203–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheehan JW, Pritchard M, Heyne RJ, et al. Severe intraventricular hemorrhage and withdrawal of support in preterm infants. J Perinatol. 2017;37(4):441–447 [DOI] [PubMed] [Google Scholar]
- 9.Roze E, Kerstjens JM, Maathuis CG, ter Horst HJ, Bos AF. Risk factors for adverse outcome in preterm infants with periventricular hemorrhagic infarction. Pediatrics. 2008;122(1). Available at: www.pediatrics.org/cgi/content/full/122/1/e46 [DOI] [PubMed] [Google Scholar]
- 10.Brouwer A, Groenendaal F, van Haastert IL, Rademaker K, Hanlo P, de Vries L. Neurodevelopmental outcome of preterm infants with severe intraventricular hemorrhage and therapy for post-hemorrhagic ventricular dilatation. J Pediatr. 2008;152(5):648–654 [DOI] [PubMed] [Google Scholar]
- 11.Bassan H, Feldman HA, Limperopoulos C, et al. Periventricular hemorrhagic infarction: risk factors and neonatal outcome. Pediatr Neurol. 2006;35(2):85–92 [DOI] [PubMed] [Google Scholar]
- 12.Hintz SR, Slovis T, Bulas D, et al. ; NICHD Neonatal Research Network. Interobserver reliability and accuracy of cranial ultrasound scanning interpretation in premature infants. J Pediatr. 2007;150(6):592–596, 596.e1–596.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Vries LS, Dubowitz LM, Dubowitz V, et al. Predictive value of cranial ultrasound in the newborn baby: a reappraisal. Lancet. 1985;2(8447):137–140 [DOI] [PubMed] [Google Scholar]
- 14.Maitre NL, Marshall DD, Price WA, et al. Neurodevelopmental outcome of infants with unilateral or bilateral periventricular hemorrhagic infarction. Pediatrics. 2009;124(6). Available at: www.pediatrics.org/cgi/content/full/124/6/e1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Shea TM, Kuban KCK, Allred EN, et al.; Extremely Low Gestational Age Newborns Study Investigators . Neonatal cranial ultrasound lesions and developmental delays at 2 years of age among extremely low gestational age children. Pediatrics. 2008;122(3). Available at: www.pediatrics.org/cgi/content/full/122/3/e662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broitman E, Ambalavanan N, Higgins RD, et al.; National Institute of Child Health and Human Development Neonatal Research Network . Clinical data predict neurodevelopmental outcome better than head ultrasound in extremely low birth weight infants. J Pediatr. 2007;151(5):500–505, 505.e1–505.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuban KCK, Allred EN, O’Shea TM, et al.; ELGAN study investigators . Cranial ultrasound lesions in the NICU predict cerebral palsy at age 2 years in children born at extremely low gestational age. J Child Neurol. 2009;24(1):63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keating NL, Huskamp HA, Kouri E, et al. Factors contributing to geographic variation in end-of-life expenditures for cancer patients. Health Aff (Millwood). 2018;37(7):1136–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Hare AM, Rodriguez RA, Hailpern SM, Larson EB, Kurella Tamura M. Regional variation in health care intensity and treatment practices for end-stage renal disease in older adults. JAMA. 2010;304(2):180–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwok AC, Semel ME, Lipsitz SR, et al. The intensity and variation of surgical care at the end of life: a retrospective cohort study. Lancet. 2011;378(9800):1408–1413 [DOI] [PubMed] [Google Scholar]
- 21.Fry JT, Matoba N, Datta A, et al.; Children’s Hospital Neonatal Consortium (CHNC) . Center, gestational age, and race impact end-of-life care practices at regional neonatal intensive care units. J Pediatr. 2020;217:86–91.e1 [DOI] [PubMed] [Google Scholar]
- 22.Hintz SR, Kendrick DE, Vohr BR, Poole WK, Higgins RD; National Institute of Child Health and Human Development Neonatal Research Network . Changes in neurodevelopmental outcomes at 18 to 22 months’ corrected age among infants of less than 25 weeks’ gestational age born in 1993-1999. Pediatrics. 2005;115(6):1645–1651 [DOI] [PubMed] [Google Scholar]
- 23.Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR; EPICure Study Group . The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2005;90(2):F134–F140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R; NICHD Research Network . Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics. 2008;121(5). Available at: www.pediatrics.org/cgi/content/full/121/5/e1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hellmann J, Knighton R, Lee SK, Shah PS; Canadian Neonatal Network End of Life Study Group . Neonatal deaths: prospective exploration of the causes and process of end-of-life decisions. Arch Dis Child Fetal Neonatal Ed. 2016;101(2):F102–F107 [DOI] [PubMed] [Google Scholar]
- 26.Lam V, Kain N, Joynt C, van Manen MA. A descriptive report of end-of-life care practices occurring in two neonatal intensive care units. Palliat Med. 2016;30(10):971–978 [DOI] [PubMed] [Google Scholar]
- 27.Singh J, Lantos J, Meadow W. End-of-life after birth: death and dying in a neonatal intensive care unit. Pediatrics. 2004;114(6):1620–1626 [DOI] [PubMed] [Google Scholar]
- 28.Ellsworth MA, Harris MN, Carey WA, Spitzer AR, Clark RH. Off-label use of inhaled nitric oxide after release of NIH consensus statement. Pediatrics. 2015;135(4):643–648 [DOI] [PubMed] [Google Scholar]
- 29.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281 [DOI] [PubMed] [Google Scholar]
- 30.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Spriger-Verlag; 2000 [Google Scholar]
- 32.Agency for Healthcare Research and Quality. NIS description of data elements. Available at: https://www.hcup-us.ahrq.gov/db/vars/hosp_region/nisnote.jsp. Accessed March 15, 2020
- 33.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2016. Natl Vital Stat Rep. 2018;67(1):1–55 [PubMed] [Google Scholar]
- 34.Berenz A, Vergales JE, Swanson JR, Sinkin RA. Evidence of early pulmonary hypertension is associated with increased mortality in very low birth weight infants. Am J Perinatol. 2017;34(8):801–807 [DOI] [PubMed] [Google Scholar]
- 35.Juhl SM, Gregersen R, Lange T, Greisen G. Incidence and risk of necrotizing enterocolitis in Denmark from 1994-2014. PLoS One. 2019;14(7):e0219268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamrick SEG, Miller SP, Leonard C, et al. Trends in severe brain injury and neurodevelopmental outcome in premature newborn infants: the role of cystic periventricular leukomalacia. J Pediatr. 2004;145(5):593–599 [DOI] [PubMed] [Google Scholar]
- 37.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50(5):553–562 [DOI] [PubMed] [Google Scholar]
- 38.Anderson PJ, Doyle LW. Neurodevelopmental outcome of bronchopulmonary dysplasia. Semin Perinatol. 2006;30(4):227–232 [DOI] [PubMed] [Google Scholar]
- 39.Stoll BJ, Hansen NI, Adams-Chapman I, et al.; National Institute of Child Health and Human Development Neonatal Research Network . Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365 [DOI] [PubMed] [Google Scholar]
- 40.Beligere N, Perumalswamy V, Tandon M, et al. Retinopathy of prematurity and neurodevelopmental disabilities in premature infants. Semin Fetal Neonatal Med. 2015;20(5):346–353 [DOI] [PubMed] [Google Scholar]
- 41.Qureshi AI, Adil MM, Suri MFK. Rate of use and determinants of withdrawal of care among patients with subarachnoid hemorrhage in the United States. World Neurosurg. 2014;82(5):e579–e584 [DOI] [PubMed] [Google Scholar]
- 42.Parodi A, Govaert P, Horsch S, Bravo MC, Ramenghi LA; eurUS.brain Group . Cranial ultrasound findings in preterm germinal matrix haemorrhage, sequelae and outcome. Pediatr Res. 2020;87(suppl 1):13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dudink J, Lequin M, Weisglas-Kuperus N, Conneman N, van Goudoever JB, Govaert P. Venous subtypes of preterm periventricular haemorrhagic infarction. Arch Dis Child Fetal Neonatal Ed. 2008;93(3):F201–F206 [DOI] [PubMed] [Google Scholar]
- 44.Verhagen AA, Janvier A, Leuthner SR, et al. Categorizing neonatal deaths: a cross-cultural study in the United States, Canada, and the Netherlands. J Pediatr. 2010;156(1):33–37 [DOI] [PubMed] [Google Scholar]
- 45.Brecht M, Wilkinson DJC. The outcome of treatment limitation discussions in newborns with brain injury. Arch Dis Child Fetal Neonatal Ed. 2015;100(2):F155–F160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lantos JD. Ethical problems in decision making in the neonatal ICU. N Engl J Med. 2018;379(19):1851–1860 [DOI] [PubMed] [Google Scholar]
- 47.Sharman M, Meert KL, Sarnaik AP. What influences parents’ decisions to limit or withdraw life support? Pediatr Crit Care Med. 2005;6(5):513–518 [DOI] [PubMed] [Google Scholar]
- 48.Meyer EC, Burns JP, Griffith JL, Truog RD. Parental perspectives on end-of-life care in the pediatric intensive care unit. Crit Care Med. 2002;30(1):226–231 [DOI] [PubMed] [Google Scholar]
- 49.Knaus WA, Harrell FE Jr., Lynn J, et al. The SUPPORT prognostic model. Objective estimates of survival for seriously ill hospitalized adults. Study to understand prognoses and preferences for outcomes and risks of treatments. Ann Intern Med. 1995;122(3):191–203 [DOI] [PubMed] [Google Scholar]
- 50.Charlson ME, Hollenberg JP, Hou J, Cooper M, Pochapin M, Pecker M. Realizing the potential of clinical judgment: a real-time strategy for predicting outcomes and cost for medical inpatients. Am J Med. 2000;109(3):189–195 [DOI] [PubMed] [Google Scholar]