Abstract
Clinicians fear pediatric advance care planning (pACP) for adolescents is too distressing for families. Multisite longitudinal randomized controlled trial of adolescents with HIV tested the effect of FAmily-CEntered (FACE®) pACP intervention on families’ anxiety and depression. One hundred five adolescent/family dyads were randomized to FACE® (n = 54 dyads) or control (n = 51 dyads). Families were 90% African American, 37% HIV-positive, and 22% less than high school educated. Families reported lower anxiety 3 months post-FACE® intervention than control (β = −4.71, 95% confidence interval [CI] = [−8.20, −1.23], p = .008). Male family members were less anxious than female family members (β = −4.55, 95% CI = [−6.96, −2.138], p ≤ .001). Family members living with HIV reported greater depressive symptoms than HIV-uninfected families (β = 3.32, 95% CI = [0.254, 6.38], p = .034). Clinicians can be assured this structured, facilitated FACE® pACP model minimized family anxiety without increasing depressive symptoms. Adolescent/family dyads should be invited to have access to, and provision of, evidence-based pACP as part of patient-centered/family-supported care in the HIV continuum of care.
Keywords: pediatrics, family intervention, advance care planning, HIV
Life-threatening and chronic illnesses in adolescence can drastically alter the lives of youth and their families. Not only does serious illness affect the daily functioning, activities, and role of the adolescent, but also serious illness affects family caregiver adjustment, including adapting to changing caregiver responsibilities, expectations, and decision making. One particular area of concern is pediatric human immunodeficiency virus (HIV). Despite treatment advances and ongoing prevention efforts, HIV remains a prevalent public health concern for adolescents. Among persons with HIV, adolescents and young adults living with HIV have consistently poorer outcomes than older age groups (Centers for Disease Control and Prevention, 2019). In the United States, new HIV diagnoses among those aged 13 to 29 years increased by 6% from 2012 to 2016 (Guilamo-Ramos et al., 2019). In 2017, rates of new HIV diagnoses were highest among racial and ethnic minority youth and young men who have sex with men, accounting for 63% of new HIV diagnoses among adolescents and young adults (Guilamo-Ramos et al., 2019). Geographically, new infections are still highly concentrated in the Southern United States, encompassing the region where our study took place (UNAIDS, 2015; Zanoni & Mayer, 2014). Globally, in 2015, HIV-associated mortality was the eighth leading cause of adolescent death and the fourth leading cause of death in African low- and middle-income countries (Slogrove & Sohn, 2018). Although treatment for HIV has become increasingly more effective, HIV remains a serious illness with life-limiting sequelae, appropriate for advance care planning (ACP).
ACP provides a means for patients to discuss their goals and wishes regarding medical decisions while medically stable (Rietjens et al., 2017). Despite advances in antiretroviral therapy to treat HIV, stringent adherence of at least 90% is required to suppress the virus. In Africa and Asia, more than 70% of adolescents and young adults with HIV receiving antiretroviral therapy are adherent, whereas only 50% to 60% are adherent in Europe and North America (Kim et al., 2014). Suboptimal antiretroviral adherence can lead to a compromised immune system, treatment resistance, and development of life-threatening sequelae. Although it is difficult to predict when a medical emergency will occur, risk of medical complications in youth with HIV is further compounded by suboptimal medication adherence and treatment resistance. By planning ahead, the adolescent’s medical preferences can be known, even if unable to speak for himself or herself at the time of need.
As an ongoing process, ACP requires continual communication between patients and their surrogate decision maker(s) (hereafter referred to as “families”), with their physicians, to make medical decisions based on the patient’s preferences, which can then be documented in an advance directive (Rietjens et al., 2017). Among adult patients, particularly the elderly, there is evidence demonstrating that ACP positively impacts the quality of end-of-life care (Bischoff et al., 2013; Brinkman-Stoppelenburg et al., 2014; Teno et al., 2007). This may be due to better communication, compliance with the patient’s preferences, and the family knowing what to expect during and following a medical emergency. Not only is ACP patient centered, it reduced anxiety and depression in surviving family decision makers by reducing the decisional burden placed upon families at the time of need for elderly patients (Detering et al., 2010). Although there is growing research interest advocating for pediatric ACP interventions, it remains in its infancy (Hinds et al., 2007; Lotz et al., 2013), and the effect of pediatric advance care planning (pACP) on family decision makers’ anxiety and depression is unknown.
Adolescents and their families may find themselves in a situation where they are forced to make difficult medical decisions, including future care and end-of-life decisions. In these situations, families often make choices they believe are in the best interest of the adolescent, who may not necessarily have been included in the discussion. Without a conversation with the adolescent, families would not know whether the choices they make are indeed what their adolescent wants (Lyon et al., 2018), which can be emotionally distressing for both the adolescent patient and their family decision makers. A prior study of pediatric patients near the end of life, aged 10 years and older, not only demonstrated the reasoning ability of children to make important decisions about their health care but also confirmed they preferred to be involved (Hinds et al., 2005). Although the distress of ACP can put family caregivers at risk of poor mental and physical health outcomes (Schulz & Beach, 1999), recent research has demonstrated that both preparedness and anxiety among family caregivers can be appropriately addressed, resulting in better overall outcomes for family caregivers (Henriksson et al., 2015). As a result, guidelines for shared decision making in pediatrics, which include involving the young patient, have recently been published (Madrigal & Kelly, 2018).
Pediatric ACP is a form of caregiving in which families engage in conversations with their adolescent or young adult living with a serious illness about the adolescent’s goals for future medical care, if there were to be a serious complication whereby the adolescent could not speak for himself or herself, for the family to know their adolescent’s preferences and represent their adolescent if a decision was needed. However, despite the recent availability of guidelines for shared decision making, concerns persist among clinicians that introducing pACP for minor children and adolescents will be too anxiety provoking or uncomfortable for families (Durall et al., 2012; Sanderson et al., 2016), creating a service barrier in offering pACP.
Being a surrogate decision maker can be stressful and anxiety-provoking (Meeker & Jezewski, 2005; Wendler & Rid, 2011). Although much of the work related to ACP has been to investigate its efficacy and effect on patients, relatively few have examined the effect on family decision makers (Wendler & Rid, 2011). The closest identified study is Kreicbergs et al.’s (2004) study of decisional regret among bereaved parents, which reported that among parents who talked with their child about death, none regretted doing so; however, 27% of parents who avoided these conversations with their child reported decisional regret (Kreicbergs et al., 2004). Family decision makers for adult loved ones at end of life are more likely to experience stress and negative emotions, such as helplessness, anxiety, and depression, than families who made decisions for those who had an advance care plan in place (Song et al., 2012). In a systematic review of the effect on surrogates of making treatment decisions for others, knowing which treatment is consistent with the patient’s preferences was frequently cited as reducing the negative effect on surrogates (Wendler & Rid, 2011). One aim of pACP is to increase families’ understanding of their adolescent’s treatment preferences (Lyon et al., 2018; Sanderson et al., 2016). Not only does pACP improve communication between the patient and the family decision maker by providing timely discussions about treatment options and decisions, it has been found to decrease patient’s disease-specific symptoms (Lyon et al., 2018).
The primary aim of the parent study from which these data were derived was to test the efficacy of a pACP intervention on treatment congruence and to evaluate the sustainability of postintervention congruence in end-of-life treatment preferences among intervention adolescent/family dyads compared with control dyads (Lyon et al., 2018). As hypothesized, there was increased congruence in end-of-life treatment preferences between adolescents and their families, and the pACP intervention engaged them in beginning these conversations early before a medical crisis. It also increased support for the patient and family, respecting patients’ autonomy and the role of the family in decision making, decreasing the likelihood for suffering by discussing these difficult issues while medically stable.
A planned secondary aim of this trial was to test whether this pACP intervention increased symptoms of anxiety or depression among families in the adolescent/family dyad, 3 months postintervention, compared with an active control group. We hypothesized that pACP intervention would reduce symptoms of anxiety and depression among family decision makers compared with controls. Findings from this trial will significantly extend what is known about the emotional impact of pACP on this study population of family surrogate decision makers for adolescents living with HIV.
Method
Participants
This pACP study is a longitudinal, single-blinded randomized controlled clinical trial of adolescents with HIV and their family decision makers, recruited from six hospital-based outpatient HIV specialty care clinics in the United States. One hundred five adolescent/family dyads were enrolled and randomized from July 2011 to June 2014. See the flow of participants through the trial in Figure 1.
Figure 1. Consort FAmily-CEntered (FACE®) participant flow diagram.
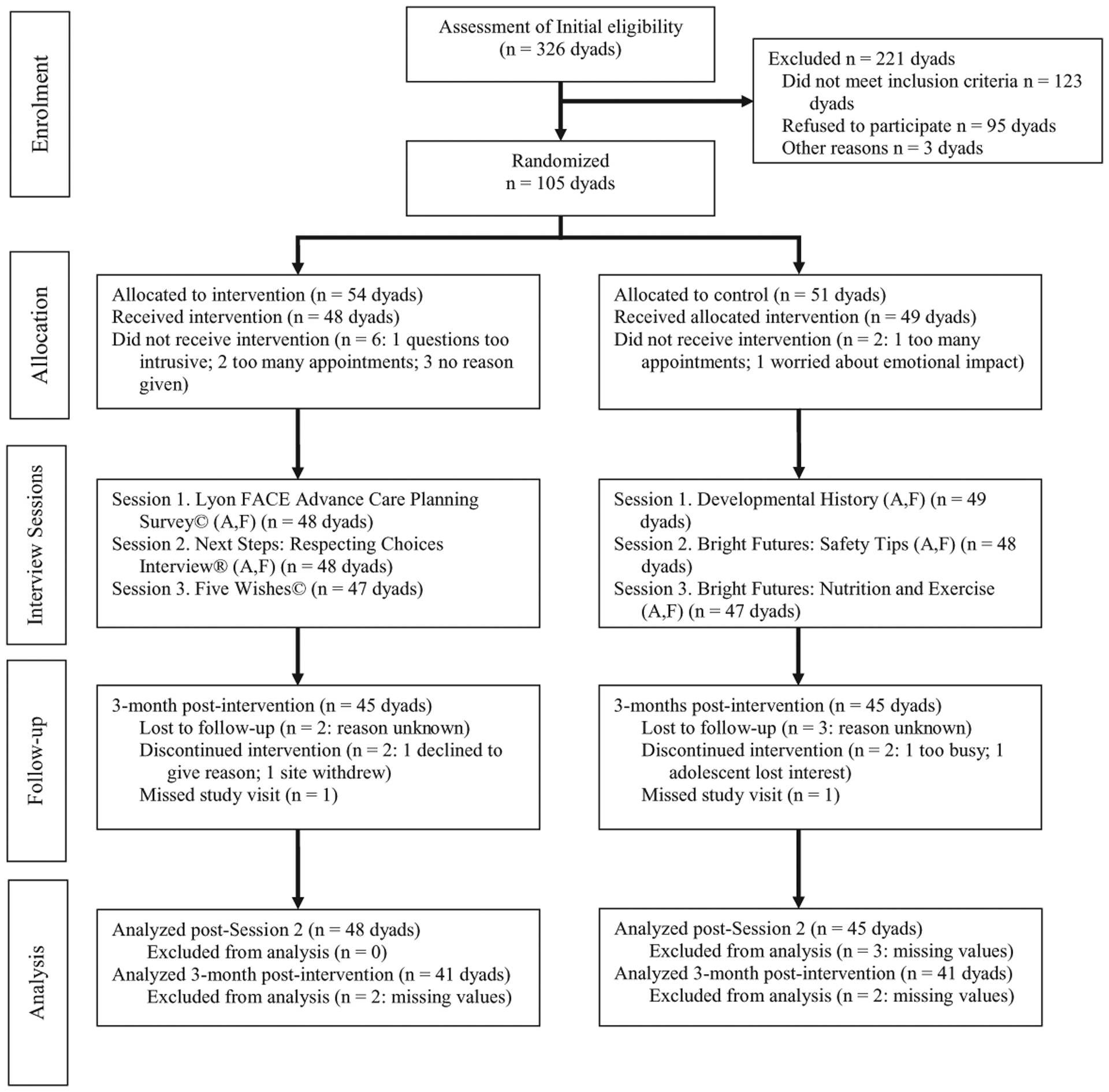
Source. Reproduced from Lyon et al. (2017).
Note. A = adolescent; F = family.
Using recommended guidelines for selecting a surrogate decision maker (Briggs & Hammes, 2010), adolescents ≥18 years were asked to choose a family decision maker, who also was ≥18 years, to participate in the study. The adolescent’s family participant could be a parent, a partner, or other chosen legal adult who knew the youth’s HIV status. Otherwise, due to current legal standards regarding the age of majority in the United States, participants aged 14 through 17 years required their legal guardian to participate as the family decision maker. This study was institutional review board approved at all participating sites. All participants provided written informed consent, or assent if below age 18 with parental consent to participate per institutional guidelines. The study was routinely monitored by a safety monitoring committee.
Families’ inclusion criteria required knowledge of the adolescent’s HIV diagnosis and being proficient in speaking and understanding English. Exclusion criteria for decision makers were having a known cognitive delay; upon screening, being identified as severely depressed, suicidal, homicidal, or psychotic; and/or screening positive for HIV dementia. Full details of the study protocol are published elsewhere (Dallas et al., 2012). Although families participated with their adolescent as a dyad in this study, the present analyses examine the impact of the dyadic intervention on surrogate emotional health outcomes, specifically anxiety and depression. Thus, only surrogate data are presented herein. Adolescent data have been published elsewhere (Lyon et al., 2018).
Measures
Demographic Questionnaire.
This study-specific interview was conducted by trained research assistants (who did not administer either the pACP intervention or control condition) with the surrogate separately to gather information, which included self-reported gender, race/ethnicity, primary language, education, family income, housing status, household density, sexual orientation, marital status, and HIV status.
Beck Depression Inventory–II (BDI-II).
The BDI-II is a published and validated 21-item self-report measure to assess presence of depressive symptoms and severity of symptoms endorsed (Beck et al., 1996). Items range from appetite and sleep disturbance to irritability and suicidal ideation, rated on a 0 to 3, 4-point Likert-type scale and summed for a total score (range = 0–63) indicating severity of depressive symptoms as follows: 0 to 13, minimal; 14 to 19, mild; 20 to 28, moderate; ≥29, severe. This instrument has shown a high content, construct, and factorial validity and acceptable internal consistency and reproducibility (Beck et al., 1996).
Beck Anxiety Inventory (BAI).
The BAI is a published and validated 21-item measure assessing the severity of subjective, somatic, and panic-related symptoms of anxiety (Beck & Steer, 1993). Participants rate the degree of discomfort experienced by each symptom over the past week on a 0 to 3, 4-point Likert-type scale. Item scores are summed to derive a total score (range = 0–63) reflecting anxiety symptom severity as follows: 0 to 9, minimal; 10 to 16, mild; 17 to 29, moderate; ≥30 severe. Test–retest reliability for this measure is acceptable (r = .75) as is internal consistency reliability (α = .92). The BAI has demonstrated adequate content, concurrent, construct, discriminant, and factorial validity (Beck & Steer, 1993).
Procedure
The study implemented three 60-min sessions, 1 week apart, to examine the efficacy of the pACP intervention, using a sample of HIV adolescent/family dyads (n = 105 dyads). Randomization of dyads was triggered by scanning completed baseline assessment documents into a computerized database, which was programmed to block by study site at a 1:1 ratio to either the pACP intervention (n = 54) or healthy living control (n = 51; Lyon et al., 2018). Randomization was generated by computer using a permuted block design, centrally located at the coordinating center. Randomization was blocked by study site and mode of HIV transmission. Study coordinators were notified of randomization, and weekly intervention or control sessions 1 to 3 were scheduled. Sessions were facilitated using a structured guide. The site facilitators scheduled all study visits to keep research assistants who collected follow-up assessments blinded to random assignment. Both the intervention and control sessions were delivered by trained facilitators at participating sites, supervised by the site investigator and monthly conference calls with the principal investigator. Certified facilitators ranged from health care professionals in nursing and psychology to graduate students in psychology, counseling, and public health. The second session of both intervention and control conditions was video or audio recorded for fidelity purposes and to monitor for potential contamination between the intervention and control arms.
Three 60-min pACP intervention sessions involved completion of (a) an ACP survey, (b) Respecting Choices: Next Steps Interview, and (c) the completion of an Advance Directive, the Five Wishes. These sessions provided patients and their families the opportunity to review and discuss together the difficult issue of ACP and to consider the youth’s preferences for quality of life near end of life. A summary of the pACP intervention sessions is shown in Table 1.
Three 60-min healthy living control sessions involved completion of (a) the Barkley Developmental History, with all medical questions removed to prevent any risk of contamination with the experimental condition; (b) Bright Futures: Safety Tips; and (c) Bright Futures: Nutrition and Exercise. During the nutrition session, nutritional status was assessed, and advice was provided for maintaining nutrition to boost immune functioning. A summary of healthy living control sessions is shown in Table 2.
Table 1.
Pediatric Advance Care Planning Intervention.
| Sessions | Foundation | Goals | Processa |
|---|---|---|---|
| Session 1 | Lyon Family Centered Advance Care Planning (FACE® ACP) Survey—Adolescent and Surrogate Versions©, which engages the participant in end-of-life questions |
|
|
| Session 2 | Next steps: Respecting Choices Advance Care Planning Conversation® (Briggs & Hammes) |
|
Stage 1 assesses the adolescent’s understanding of current medical condition, prognosis, complications Stage 2 explores adolescent’s philosophy regarding end-of-life decision making and their understanding of the facts Stage 3 reviews rationale for future medical decisions the adolescent would want the surrogate to understand/act on Stage 4 uses the Statement of Treatment Preferences to describe clinical situations common to HIV and related treatment choices Stage 5 summarizes the discussion/need for future discussions as situations/preferences change Gaps in information are identified and referrals are made |
| Session 3 | The Five Wishes© is a legal document that helps a person express how they want to be treated if they are seriously ill and unable to speak for himself or herself Unique among living will and health agent forms—it looks to all of a person’s needs: medical, personal, emotional, spiritual |
|
For adolescents below the age of 18, the Five Wishes© must be signed by their legal guardian Processes, such as labeling feelings and concerns, as well as finding solutions to any identified problem, are facilitated. Appropriate referrals are made to help resolve disagreements over decision making (e.g., a hospital ethicist or their doctor) or spiritual issues (e.g., a hospital chaplain or their clergy) |
Note. RA = research assistant.
These sessions may include other family members or loved ones.
Table 2.
Healthy Living Control Condition.
| Sessions | Foundation | Goals | Process |
|---|---|---|---|
| Session 1 | Developmental history The Barkley Developmental History form will be administered, with all medical questions removed from the developmental history as well as the information on mother’s pregnancy and the birth, to prevent any risk of contamination with the experimental condition |
|
|
| Session 2 | Safety tips Participants will be asked questions about seat belt use, oral health, etc. Safety information will be provided following the questionnaire administration |
|
These structured questionnaires/information were administered by a trained RA to prevent contamination with the pACP condition, as content in this review is not related to pACP |
| Session 3 | Nutrition and exercise Participants will be asked questions about nutrition and exercise |
|
Nutrition and exercise information will be provided following the questionnaire administration. These structured questionnaires/information were administered by the trained RA to prevent contamination with the pACP condition, as content in this review is not related to ACP. |
Note. pACP = pediatric advance care planning; RA = research assistant.
Sample size was estimated using a formula (Diggle et al., 1998) widely used for longitudinal studies and generalized estimating equations (GEE) models. This formula was applied to estimate the sample size that would provide a statistical power no less than .80 in detecting a small effect size Δ—a small meaningful difference of the average response measure in standard deviation units between two groups of interest (e.g., intervention vs. control). For a modest Δ= 0.20 and a moderate effect size 0.35, the estimated sample size to achieve a power of 0.80 at α = .05 level is N = 72 for analyzing numeric outcomes. The sample size estimate here is relatively conservative, as the results of our pilot study show that outcome differences between the intervention groups at 3-month follow-up were large (e.g., effect size was greater than 0.45 for psychological adjustment and quality of life; Lyon et al., 2010).
Data Analysis
Outcome assessments were conducted at baseline and 3 months postintervention. Teleforms (forms that can be scanned and data abstracted to an electronic database) were used to record and enter data and were stored at the coordinating site.
Means, standard deviations, frequencies, and percent were reported for social demographics and outcomes of interest. GEE model was used to assess the effect of the pACP intervention on change in families’ anxiety and depression, from baseline to 3-month follow-up, compared with the healthy living control, controlling for covariates, such as gender, education, income, and HIV status (Liang & Zeger, 1986). GEE model performs well under the assumption of ignorable missing data and does not require the outcome measure to be normally distributed. All analyses used SAS 9.2 (SAS Institute Inc., SAS® 9.2 Enhanced Logging Facilities, Cary, NC; SAS Institute Inc., 2008).
Results
A total of 105 adolescent/family dyads were enrolled to participate during the study period. The retention rate was 80% (n = 84 dyads) at 3-month postintervention. The baseline demographic characteristics of the participating families are shown in Table 3. Family participants were primarily African American (90%) and female (82%). Half of household incomes were at or below the federal poverty level (50%); and, approximately one fourth had less than a high school education or General Education Diploma (GED) equivalency (22%). Approximately one third of family participants (36%) were also HIV-positive.
Table 3.
Family Participants Demographics (N = 105).
| Characteristics | N (%) |
|---|---|
| Age (years) | |
| M (SD) | 44.9 (13.5) |
| Range | 20–77 |
| Gender | |
| Male | 19 (18.1) |
| Female | 86 (81.9) |
| Race | |
| American Indian/Alaska Native | 2 (1.9) |
| Black/African American | 94 (89.5) |
| White/Caucasian | 8 (7.6) |
| Declined | 1 (1.0) |
| Ethnicity | |
| Not Hispanic/Latino | 99 (94.3) |
| Hispanic or Latino | 5 (4.8) |
| Declined | 1 (1.0) |
| Education | |
| No high school diploma or GED equivalency | 23 (21.9) |
| High school or GED equivalency | 42 (40.0) |
| Some college | 33 (31.4) |
| Bachelor/master/doctorate degree | 8 (6.7) |
| Household income | |
| Equal, below federal poverty line | 52 (49.5) |
| Higher than federal poverty line | 41 (39.1) |
| Unknown/unreported | 12 (11.4) |
| HIV-positive | |
| No | 57 (54.3) |
| Yes | 38 (36.2) |
| Do not know | 10 (9.5) |
Note. GED = General Education Diploma.
By 3-month postintervention, pACP families endorsed lower anxiety (BAI M = 5.45 and 5.25, respectively; Table 4), whereas healthy living control families’ anxiety levels nearly doubled (BAI M = 5.32 and 10.02, respectively; Table 4). pACP family anxiety levels remained at the minimal clinical range, but healthy living control family anxiety levels rose from the minimal to the mild clinical range (Table 4).
Table 4.
Descriptive Statistics of Family Participants’ Anxiety by Visit and Intervention.a
| Visit | Intervention group | M (SD) | Range |
|---|---|---|---|
| Baseline | Control (N = 51) | 5.32 (6.92) | (0, 28) |
| Intervention (N = 54) | 5.45 (7.30) | (0, 39) | |
| 3 months follow-up | Control (N = 43) | 10.02 (10.63) | (0, 39) |
| Intervention (N = 41) | 5.25 (5.05) | (0, 18) |
Beck Anxiety Inventory is a 21-item self-report on a 4-point Likert-type scale 0 to 3. Total score range is 0 to 63. Interpretation: 0 to 9, minimal; 10 to 16, mild; 17 to 29, moderate; 30 to 63, severe.
The results of the GEE model for surrogate anxiety are shown in Table 5. The main effect of intervention is not statistically significant (β = −0.01, p = .994), indicating no significant difference in anxiety between intervention and control groups across two time points. The main effect of time is positive and statistically significant (β = 4.46, p = .005), indicating an overall increase in anxiety from baseline to 3-month follow-up across two intervention groups. However, the interaction between intervention and time is negative and statistically significant (β = −4.71, p = .008; Table 5). This indicates families randomized to the intervention group had significantly lower anxiety at 3-month postintervention compared with the control group controlling for covariates.
Table 5.
Selected Results of GEE Model for Family Participants’ Anxiety.
| Predictor | Estimate for β | 95% confidence interval | P |
|---|---|---|---|
| Time | 4.46 | [1.32, 7.60] | .005 |
| Intervention | −0.01 | [−2.66, 2.64] | .994 |
| Interaction of time and intervention | −4.71 | [−8.20, −1.23] | .008 |
| Age (years) | −0.04 | [−0.12, 0.05] | .417 |
| Gender | |||
| Male | −4.55 | [−6.96, −2.14] | <.001 |
| Femalea | — | — | — |
| Education | |||
| Below high school | 3.53 | [1.67, 0.26] | .0341 |
| High school or GED equivalency | 2.36 | [1.43, −0.45] | .1 |
| Some college or higher educationa | — | — | — |
| Income | |||
| Equal, below federal poverty linea | — | — | — |
| Higher than federal poverty line | 0.76 | [−2.20, 3.72] | .616 |
| Unknown/unreported | −2.49 | [−5.22, 0.25] | .075 |
| HIV-positive | |||
| Yes | 1.56 | [−0.92, 4.05] | .217 |
| No/do not knowa | — | — | — |
Note. Significance set at p < .05. GEE = generalized estimating equations; GED = General Education Diploma.
Used as the reference group for a categorical predictor.
Significantly lower anxiety was found in male family participants (β = −4.55, p < .001). Significantly higher anxiety was found in family participants with less than a high school education (β = 3.53, p = .034) compared with families in some college or higher education in Table 5.
pACP families experienced fewer depressive symptoms (BDI-II M = 6.39) than healthy living control families (M = 8.86) at baseline. However, both groups demonstrated an increase in reported depressive symptoms by 3 months postintervention (BDI-II M = 7.25 and M = 11.58, respectively; Table 6), although mean scores remained in the minimal clinical range. The intervention did not show a statistically significant effect on family depression (β = −1.38, p = .425 for interaction of time and intervention; Table 7), controlling for the effect of time and covariates. The results indicate that at 3 months postintervention, family decision makers with less than a high school education (β = 5.82, p = .001; Table 7) or equal to a high school education (β = 5.28, p = .001; Table 7) reported significantly higher depression, compared with families with higher education levels, similar results as family anxiety. Significantly higher depression also was observed in family participants who were themselves HIV-positive (β = 3.32, p = .034; Table 7).
Table 6.
Descriptive Statistics of Family Participants’ Depression by Visit and Intervention.a
| Visit | Intervention group | M (SD) | Range |
|---|---|---|---|
| Baseline | Control (N = 51) | 8.86 (8.52) | (0, 33) |
| Intervention (N = 54) | 6.39 (6.26) | (0, 27) | |
| 3 months follow-up | Control (N = 43) | 11.58 (10.94) | (0, 41) |
| Intervention (N = 41) | 7.25 (7.21) | (0, 27) |
Beck Depression Inventory–II is a 21-item self-report on a 4-point Likert-type scale 0 to 3. Total score range is 0 to 63. Interpretation: 0 to 13, minimal; 14 to 19, mild; 20 to 28, moderate; 29 to 63, severe.
Table 7.
GEE Selected Model Results for Family Participants’ Depression.
| Predictor | Estimate for β | 95% confidence interval | P |
|---|---|---|---|
| Time | 2.69 | [−0.05, 5.42] | .055 |
| Intervention | −2.44 | [−5.10, 0.21] | .071 |
| Interaction of time and intervention | −1.38 | [−4.78, 2.02] | .425 |
| Age (years) | −0.08 | [−0.17, 0.01] | .09 |
| Gender | |||
| Male | −2.65 | [−6.0, 0.70] | .121 |
| Femalea | — | — | — |
| Education | |||
| Below high school | 5.82 | [2.31, 9.34] | .001 |
| High school or GED equivalency | 5.28 | [2.04, 8.53] | .001 |
| Some college or higher educationa | — | — | — |
| Income | |||
| Equal, below federal poverty linea | — | — | — |
| Higher than federal poverty line | 0.85 | [−2.18, 3.88] | .584 |
| Unknown/unreported | −0.17 | [−3.46, 3.13] | .921 |
| HIV-positive | |||
| Yes | 3.32 | [0.25, 6.38] | .034 |
| No/do not knowa | — | — | — |
Note. Significance set at p < .05. GEE = generalized estimating equations; GED = General Education Diploma.
Used as the reference group for a categorical predictor.
Discussion
This study is the first fully powered randomized clinical trial to test the hypotheses that an adolescent-centered/family-supported dyadic pACP intervention will decrease symptoms of anxiety or depression for families, compared with control families. Key barriers for pACP are clinician barriers related to their perspective on parental factors, that is, their belief that parents are not ready for facing the future, because it is too emotional, too distressing, or too challenging for them (Durall et al., 2012; Lotz et al., 2015; Needle et al., 2019). This trial moves the field of pACP forward by demonstrating that these fears are misplaced in this population of families. Contrary to clinician fears, this randomized clinical trial demonstrated that participation in pACP alleviated families’ anxiety at 3 months postintervention, consistent with the study’s theoretical foundation, transaction stress and coping theory, which posits that increasing control in a low control situation decreases anxiety (Lazarus & Folkman, 1984). Families were willing and able to engage in emotional conversations (Dallas et al., 2012), so they could learn their adolescent’s preferences. Trial results are consistent with recent findings that one coping strategy for caring for a child receiving palliative care is to take control (Verberne et al., 2019), in this instance, problem-solve with their child in a respectful and authentic conversation about their child’s goals and values in the context of the “what ifs” if the worse were to happen, creating an action plan through pACP. Although anxiety decreased for pACP families over time, the doubling of anxiety for control families is also consistent with this theory. Being in the control group created an avoidance situation in the face of the threat of end-of-life decisions and uncertainty about the future, that is, these families and their children did not have the experience of pACP families in which they created an advance care plan through a process of conversations over time. The lack of observed intervention effect on depression is likely due to the exclusion of families who presented with severe depression or suicidal ideation at screening, restricting the baseline depressive symptom range, thus producing a floor effect. Nonetheless, families with high school education or less, and families who also have HIV, endorsed greater depressive symptoms. The significant confounding variables identified in the GEE models are consistent with evidence-based research. Males are less likely to have anxiety compared with females (Altemus et al., 2014). Individuals with less education endorse more depressive symptoms, on average, than those with higher education (Bauldry, 2015). Adults living with HIV are more likely to have depressive symptoms than uninfected adults (Eller et al., 2010). Study findings are noteworthy, supporting the hypothesis that family participation in pACP discussions with their adolescent does not cause undo distress (i.e., increased anxiety and/or depression). Rather, these pACP trial results may prove critical for improving the continuum of HIV care services for youth, assisting providers and families to become better informed on how to provide treatment aligned with the adolescents’ preferences. Findings from this trial also support the practical implication that patient-centered pACP conversations between adolescents living with HIV and their families can be successfully conducted with the assistance of certified facilitators, without unduly distressing families in the process.
Thus, trial findings should move the field forward by allaying previously reported clinician concerns that pACP is too uncomfortable for the family (Sanderson et al., 2016), removing this barrier to offering pACP in the first place. Families know whether they are ready or not, but should be provided with the invitation, rather than have the clinician avoid the subject completely. In this trial, 47% (108/203) of eligible dyads declined participation, of whom 35 stated they were interested but not ready. Some families cope by suppressing their emotions about the potential loss of their child, which can help them to maintain a degree of optimism to carry on in daily life (Verberne et al., 2019) and this should be respected. This is especially sensitive for parents who are HIV-positive themselves. Part of patient-centered/family-supported care is to respect these choices. Trial findings support an integrated approach to pACP by respecting patients’ autonomy in decision-making and the role of the family in engaging in, understanding, and supporting the youth’s decision for future care at a time when the adolescent is clinically stable. Adolescent/family dyads should be invited to have access to, and provision of, evidence-based pACP as part of patient-centered/family-supported care within the HIV continuum of care.
Our study has several strengths. Strengths include multisite recruitment from hospital-based outpatient adolescent HIV clinics, with high retention rate and randomized control trial research design. This is a single-blinded, intent-to-treat, longitudinal, randomized clinical trial with an active control for time and attention. Study measures are established, published, reliable, and valid instruments, increasing the opportunity for replicability of study findings. The associated strength of this sample is that the intervention was designed to be culturally sensitive for adolescents living with HIV, extending its generalizability to those primarily living in urban areas, who are African American, female, with low income and low education. We are encouraged by these trial results, as it demonstrates the health equity of this pACP model, which successfully engaged an underserved and vulnerable group of African American youth and families with low income and less education. The retention rate of 80% at 3-month follow-up suggests families were highly engaged and motivated to improve long-term care.
These strengths notwithstanding, the study had a number of limitations that should be addressed in future research. First, data on the relationship of the patient to the surrogate decision maker were not collected for this study. This error occurred when transitioning institutionally supported databases to REDCap. Unfortunately, this oversight was not caught until the study was completed. Based on colloquial facilitator observations and the findings from the pilot study, surrogate decision makers were primarily biological relatives, but friends, partners, and adoptive parents also participated. Second, selection bias may limit the generalizability of study findings, in that, those who declined to participate may not have been interested in talking about ACP, participating in research, or otherwise were unable to identify a suitable decision maker due to nondisclosure of HIV status. About half of the adolescents approached reported difficulty identifying a trusted adult decision maker to participate with them (Lee et al., 2017). However, the predetermined enrollment benchmark was met, enrolling more than 50% of patient/family dyads approached (Lyon et al., 2018). Randomization controlled for other potential forms of selection bias (Lyon et al., 2018).
Conclusion
Families are placed in a stressful and unenviable position when making end-of-life decisions for their children and adolescents, potentially leading to negative family outcomes. Compounded by the high prevalence of suboptimal antiretroviral adherence in youth with HIV, unexpected complications can arise when surrogate decision makers are unprepared to make these difficult decisions. Trial findings could potentially improve the family’s response in these situations as well as mitigate the complications that further arise from being unprepared, thereby supporting family health and well-being, compared with current standard of care where these conversations are often avoided or simply not had. Findings are consistent with recommendations that ACP conversations continue over the course of illness, and with the desire that families of seriously ill children and adolescents be involved in pACP (Beecham et al., 2017). Although pACP conversations are potentially inherently emotional and stressful for adolescents living with HIV and their families, as they explore the “what ifs” should the worse happen with their HIV, through a positive process of facilitated conversations over 3 weeks, families learned about their adolescent’s goals of care, committed to honoring their wishes, and in this process, families were not unduly distressed by symptoms of anxiety or depression. This pACP model demonstrated that it can be administered by certified facilitators, including professionals and nonprofessionals alike. Evidence-based pACP should become a standard part of the continuum of HIV care for adolescents living with HIV and their families, regardless of prognosis. Nurses throughout the world are often the first or the primary providers of care for patients with HIV and their families. Training in this structured and facilitated pACP provides a context and process for interacting with patients and their families, which may be generalized to other families and young patient populations with serious illnesses and pediatric diseases. This evidence-based pACP model can improve the delivery of, and access to, high-quality pACP with the ultimate aim of reducing unnecessary suffering and enhancing the quality of life of both adolescent patients living with HIV and their families, optimizing adolescent-informed/family-centered care.
Acknowledgments
The authors would like to thank our adolescents and families for participation in this research. Special thanks also to the coinvestigators: Patricia M. Flynn and Ronald H. Dallas at St. Jude Children’s Research Hospital; Ana Garcia and Lawrence B. Friedman at University of Miami-Miller School of Medicine; Linda Briggs at Respecting Choices, A Division of C-TAC Innovations, Washington, D.C.; Megan L. Wilkins at Department of Infectious Diseases, St. Jude Children’s Research Hospital; and Sohail R. Rana and Patricia E. Houston at Howard University Hospital. Thanks also to our many clinical coordinators and research assistants.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The primary study (Lyon et al., 2018) was funded by an R01 grant from the National Institute of Nursing Research/National Institutes of Health and approved by the authors’ institutional review board and by each of the participating study sites’ respective institutional review board. This work was supported by the National Institute of Nursing Research of the National Institutes of Health (NINR; grant number 5R01 NR012711-05). This research has also been facilitated by the services and resources the NIH National Center for Advancing Translational Sciences-Children’s National (CTSI-CN) UL1RR031988.
Author Biographies
Christopher J. Lin, MA, is a doctoral student in the School-Clinical Child Psychology Program at Yeshiva University, a Fisher Landau Center for Treatment of Learning Disabilities extern, and a Leadership Education in Neurodevelopmental and Related Disabilities Program trainee in Rose F. Kennedy Children’s Evaluation and Rehabilitation Center, Bronx, New York. He served as a research intern in the Family Centered Advance Care Planning Team at Children’s National Hospital in Washington, D.C. He is broadly interested in pediatric health psychology, namely, familial and environmental factors impacting health outcomes in youth.
Yao I. Cheng, MS, is a statistical data analyst at Division of Biostatistics and Study Methodology at Children’s National Research Institute, Washington, D.C. She conducts statistical analyses according to the statistical plan or other hypotheses, prepares tables and figures for publications. Her recent publications include “The Role of Religiousness and Spirituality in Health-Related Quality of Life of Persons Living With HIV: A Latent Class Analysis” in Psychology of Religion and Spirituality (2019, with M. Lyon et al., http://doi.org/10.1037/rel0000301), and “Relationship of Spiritual Constructs to Mental Health PROs in Adolescents with Cancer” in Journal of Clinical Oncology (2019, with D. Grossoehme et al., http://doi.org/10.1200/JCO.2019.37.31_suppl.136).
Patricia A. Garvie, PhD, is a pediatric psychologist in the Research Department at Children’s Diagnostic & Treatment Center, Fort Lauderdale, Florida. Her scholarship focuses on addressing the cognitive, behavioral, mental health, advance care planning, and adherence issues in youth and young adults with HIV, as well as monitoring longitudinal neurodevelopmental sequelae. Her recent publications include “Advance Care Planning and HIV-Symptoms in Adolescence” in Pediatrics (2018, with M. Lyon et al., https://doi.org/10.1542/peds.2017-3869), and an editorial “Transitioning Youth With HIV to Adult HIV Care: Bridging the Gap With Adult Care Clinics for the Life Span” in Journal of Adolescent Health (2017, https://doi.org/10.1016/j.jadohealth.2017.07.013).
Lawrence J. D’Angelo, MD, MPH, is a clinician researcher with more than 30 years of experience in the care of HIV-infected and at-risk adolescents and young adults. He is a professor of pediatrics, medicine, and epidemiology at the George Washington University and former chief of the Division of Adolescent and Young Adult Medicine at Children’s National Hospital in Washington, D.C. His publications include “The Influence of Religiousness Beliefs and Practices on Health Care Decision-Making Among HIV Positive Adolescents” in AIDS Care (2019, with M. Lyon et al., http://doi.org/10.1080/09540121.2019.1668523), and “FAmily CEntered (FACE) Advance Care Planning Among African American and Non-African American Adults Living With HIV in Washington, DC: A Randomized Controlled Trial to Increase Documentation & Health Equity” in Journal of Pain and Symptom Management (2019, with M. Lyon et al., http://doi.org/10.1177/0825859719860129).
Jichuan Wang, PhD, is a biostatistician at Children’s National Hospital and professor of epidemiology and biostatistics at the George Washington University School of Medicine and Health Sciences, Washington, D.C. He has been working in the field of public health studies for more than two decades and collaborated on numerous National Institutes of Health (NIH)-funded behaviorally focused projects. His recent publications are books on multilevel modeling and structural equation modeling, including Multilevel Models: Applications Using SAS (2012, with H. Xie & J. Fisher) and Structural Equation Modeling: Applications Using Mplus (2020, with X. Wang).
Maureen E. Lyon, PhD, is a clinical health psychologist at Children’s National Hospital and professor of pediatrics at George Washington University School of Medicine and Health Sciences, Washington, D.C. Her research focuses on advance care planning with persons with serious illness and their families, particularly how spiritual beliefs influence well-being. Her publications include “The Role of Religiousness and Spirituality in Health-Related Quality of Life of Persons Living With HIV” in Psychology of Religion and Spirituality (2020, with K. B. Grill et al., http://doi.org/10.1037/rel0000301), and “Can You Tell Me Why You Made That Choice? A Qualitative Study of the Influences on Treatment Decisions in Advance Care Planning Among Adolescents and Young Adults Undergoing Bone Marrow Transplant” in Palliative Medicine (2019, with J. Needle et al., http://doi.org/10.1177/0269216319883977).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Research Ethics and Patient Consent
This research involves human subjects and was conducted according to the World Medical Association Declaration of Helsinki.
Data Availability
Deidentified data can be obtained by contacting Maureen E. Lyon at her email address: mlyon@childrensnational.org, or by her telephone number, 202-476-5442.
References
- Altemus M, Sarvaiya N, & Epperson CN (2014). Sex differences in anxiety and depression clinical perspectives. Frontiers in Neuroendocrinology, 35(3), 320–330. 10.1016/j.yfrne.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauldry S (2015). Variation in the protective effect of higher education against depression. Society and Mental Health, 5(2), 145–161. 10.1177/2156869314564399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, & Steer RA (1993). Beck Anxiety Inventory manual. Psychological Corporation. [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II. Psychological Corporation. [Google Scholar]
- Beecham E, Oostendorp L, Crocker J, Kelly P, Dinsdale A, Hemsley J, Russell J, Jones L, & Bluebond-Langner M (2017). Keeping all options open: Parents’ approaches to advance care planning. Health Expectations: An International Journal of Public Participation in Health Care and Health Policy, 20(4), 675–684. 10.1111/hex.12500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff KE, Sudore R, Miao Y, Boscardin WJ, & Smith AK (2013). Advance care planning and the quality of end-of-life care among older adults. Journal of the American Geriatrics Society, 61(2), 209–214. 10.1111/jgs.12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs L, & Hammes BJ (2010). Disease Specific-Advance Care Planning (DS-ACP): Facilitator certification training manual: A communication skills training program. Gundersen Lutheran Medical Foundation. [Google Scholar]
- Brinkman-Stoppelenburg A, Rietjens JA, & van der Heide A (2014). The effects of advance care planning on end-of-life care: A systematic review. Palliative Medicine, 28(8), 1000–1025. 10.1177/0269216314526272 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2019). HIV and youth. https://www.cdc.gov/hiv/group/age/youth/index.html
- Dallas RH, Wilkins ML, Wang J, Garcia A, & Lyon ME (2012). Longitudinal pediatric palliative care: Quality of life & spiritual struggle (FACE®): Design and methods. Contemporary Clinical Trials, 33(5), 1033–1043. 10.1016/j.cct.2012.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detering KM, Hancock AD, Reade MC, & Silvester W (2010). The impact of advance care planning on end of life care in elderly patients: Randomised controlled trial. The British Medical Journal, 340, Article c1345. 10.1136/bmj.c1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle PJ, Liang K-Y, & Zeger SL (1998). Analysis of longitudinal data. Oxford University Press. [Google Scholar]
- Durall A, Zurakowski D, & Wolfe J (2012). Barriers to conducting advance care discussions for children with life-threatening conditions. Pediatrics, 129(4), e975–e982. 10.1542/peds.2011-2695 [DOI] [PubMed] [Google Scholar]
- Eller LS, Bunch EH, Wantland DJ, Portillo CJ, Reynolds NR, Nokes KM, Coleman CL, Kemppainen JK, Kirksey KM, Corless IB, Hamilton MJ, Dole PJ, Nicholas PK, Holzemer WL, & Tsai Y-F (2010). Prevalence, correlates, and self-management of HIV-related depressive symptoms. AIDS Care, 22(9), 1159–1170. 10.1080/09540121.2010.498860 [DOI] [PubMed] [Google Scholar]
- Guilamo-Ramos V, Thimm-Kaiser M, Benzekri A, & Futterman D (2019). Youth at risk of HIV: The overlooked US HIV prevention crisis. The Lancet. HIV, 6(5), e275–e278. 10.1016/S2352-3018(19)30037-2 [DOI] [PubMed] [Google Scholar]
- Henriksson A, Carlander I, & Årestedt K (2015). Feelings of rewards among family caregivers during ongoing palliative care. Palliative & Supportive Care, 13(6), 1509–1517. 10.1017/S1478951513000540 [DOI] [PubMed] [Google Scholar]
- Hinds PS, Burghen EA, & Pritchard M (2007). Conducting end-of-life studies in pediatric oncology. Western Journal of Nursing Research, 29(4), 448–465. 10.1177/0193945906295533 [DOI] [PubMed] [Google Scholar]
- Hinds PS, Drew D, Oakes LL, Fouladi M, Spunt SL, Church C, & Furman WL (2005). End-of-life care preferences of pediatric patients with cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 23(36), 9146–9154. 10.1200/JCO.2005.10.538 [DOI] [PubMed] [Google Scholar]
- Kim S-H, Gerver SM, Fidler S, & Ward H (2014). Adherence to antiretroviral therapy in adolescents living with HIV: Systematic review and meta-analysis. AIDS (London, England), 28(13), 1945–1956. 10.1097/QAD.0000000000000316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreicbergs U, Valdimarsdóttir U, Onelöv E, Henter J-I, & Steineck G (2004). Talking about death with children who have severe malignant disease. The New England Journal of Medicine, 351(12), 1175–1186. 10.1056/NEJMoa040366 [DOI] [PubMed] [Google Scholar]
- Lazarus RS, & Folkman S (1984). Stress, appraisal, and coping. Springer. [Google Scholar]
- Lee BC, Houston PE, Rana SR, Kimmel AL, D’Angelo LJ, & Lyon ME (2017). Who will speak for me? Disparities in palliative care research with “unbefriended” adolescents living with HIV/AIDS. Journal of Palliative Medicine, 20(10), 1135–1138. 10.1089/jpm.2017.0053 [DOI] [Google Scholar]
- Liang K-Y, & Zeger SL (1986). Longitudinal data analysis using generalized linear models. Biometrika, 73(1), 13–22. 10.1093/biomet/73.1.13 [DOI] [Google Scholar]
- Lotz JD, Jox RJ, Borasio GD, & Führer M (2013). Pediatric advance care planning: A systematic review. Pediatrics, 131(3), e873–880. 10.1542/peds.2012-2394 [DOI] [PubMed] [Google Scholar]
- Lotz JD, Jox RJ, Borasio GD, & Führer M (2015). Pediatric advance care planning from the perspective of health care professionals: A qualitative interview study. Palliative Medicine, 29(3), 212–222. 10.1177/0269216314552091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon ME, D’Angelo LJ, Dallas RH, Hinds PS, Garvie PA, Wilkins ML, Garcia A, Briggs L, Flynn PM, Rana SR, Cheng YI, & Wang J (2017). A randomized clinical trial of adolescents with HIV/AIDS: Pediatric advance care planning. AIDS Care, 29(10), 1287–1296. 10.1080/09540121.2017.1308463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon ME, Garvie PA, Briggs L, He J, Malow R, D’Angelo LJ, & McCarter R (2010). Is it safe? Talking to teens with HIV/AIDS about death and dying: A 3-month evaluation of Family Centered Advance Care (FACE) planning—Anxiety, depression, quality of life. HIV/AIDS (Auckland, N.Z.), 2, 27–37. 10.2147/HIV.S7507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon ME, Garvie PA, D’Angelo LJ, Dallas RH, Briggs L, Flynn PM, Garcia A, Cheng YI, Wang J, & Adolescent Palliative Care Consortium. (2018). Advance care planning and HIV symptoms in adolescence. Pediatrics, 142(5), Article e20173869. 10.1542/peds.2017-3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigal VN, & Kelly KP (2018). Supporting family decision-making for a child who is seriously ill: Creating synchrony and connection. Pediatrics, 142(Suppl. 3), S170–S177. 10.1542/peds.2018-0516H [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker MA, & Jezewski MA (2005). Family decision making at end of life. Palliative & Supportive Care, 3(2), 131–142. 10.1017/S1478951505050212 [DOI] [PubMed] [Google Scholar]
- Needle JS, Peden-McAlpine C, & Liaschenko J (2019). Physicians’ perspectives on adolescent and young adult advance care planning the fallacy of informed decision making. The Journal of Clinical Ethics, 30(2), 131–142. [PubMed] [Google Scholar]
- Rietjens JAC, Sudore RL, Connolly M, van Delden JJ, Drickamer MA, Droger M, van der Heide A, Heyland DK, Houttekier D, Janssen DJA, Orsi L, Payne S, Seymour J, Jox RJ, & Korfage IJ, & European Association for Palliative Care. (2017). Definition and recommendations for advance care planning: An international consensus supported by the European Association for Palliative Care. The Lancet. Oncology, 18(9), e543–e551. 10.1016/S1470-2045(17)30582-X [DOI] [PubMed] [Google Scholar]
- Sanderson A, Hall AM, & Wolfe J (2016). Advance care discussions: Pediatric clinician preparedness and practices. Journal of Pain and Symptom Management, 51(3), 520–528. 10.1016/j.jpainsymman.2015.10.014 [DOI] [PubMed] [Google Scholar]
- Schulz R, & Beach SR (1999). Caregiving as a risk factor for mortality: The Caregiver Health Effects Study. Journal of the American Medical Association, 282(23), 2215–2219. 10.1001/jama.282.23.2215 [DOI] [PubMed] [Google Scholar]
- Slogrove AL, & Sohn AH (2018). The global epidemiology of adolescents living with HIV: Time for more granular data to improve adolescent health outcomes. Current Opinion in HIV and AIDS, 13(3), 170–178. 10.1097/COH.0000000000000449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M-K, Ward SE, Hanson LC, Lin F-C, Hamilton JB, Hladik G, Fine JP, Bridgman JC, Sun SK, & Miles MS (2012). Psychological symptoms and end-of-life decision making confidence in surrogate decision-makers of dialysis patients. The Journal of Nephrology Social Work/the Council of Nephrology Social Workers, National Kidney Foundation, 36, Article A3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4169048/ [PMC free article] [PubMed] [Google Scholar]
- Teno JM, Gruneir A, Schwartz Z, Nanda A, & Wetle T (2007). Association between advance directives and quality of end-of-life care: A national study. Journal of the American Geriatrics Society, 55(2), 189–194. 10.1111/j.1532-5415.2007.01045.x [DOI] [PubMed] [Google Scholar]
- UNAIDS. (2015). All in to #EndAdolescentAIDS. https://www.unaids.org/sites/default/files/media_asset/20150217_ALL_IN_brochure.pdf
- Verberne LM, Kars MC, Schouten-van Meeteren AYN, van den Bergh EMM, Bosman DK, Colenbrander DA, Grootenhuis MA, & van Delden JJM (2019). Parental experiences and coping strategies when caring for a child receiving paediatric palliative care: A qualitative study. European Journal of Pediatrics, 178(7), 1075–1085. 10.1007/s00431-019-03393-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler D, & Rid A (2011). Systematic review: The effect on surrogates of making treatment decisions for others. Annals of Internal Medicine, 154(5), 336–346. 10.7326/0003-4819-154-5-201103010-00008 [DOI] [PubMed] [Google Scholar]
- Zanoni BC, & Mayer KH (2014). The adolescent and young adult HIV cascade of care in the United States: Exaggerated health disparities. AIDS Patient Care and STDs, 28(3), 128–135. 10.1089/apc.2013.0345 [DOI] [PMC free article] [PubMed] [Google Scholar]


