Abstract
Prior studies using behavioral tasks and neuroimaging have shown that children and adolescents with bipolar disorder (BD) have deficits in cognitive flexibility (CF)—defined as adaptation to changing rewards and punishments. However, no study, to our knowledge, has examined the white matter microstructural correlates of CF in youth with BD. To address this gap, we examined the relationship between CF assessed with the Cambridge Neuropsychological Testing Automated Battery (CANTAB)’s Intra-Extra Dimensional Set Shift task (ID/ED) and diffusion tensor imaging analyzed with FSL’s preprocessing tools and Tract-Based Spatial Statistics (TBSS). We found a significantly different relationship between microstructural integrity of multiple white matter regions and CF performance in BD (n=28) and age-matched typically developing control (TDC) youths (n=26). Evaluation of the slopes of linear regressions in BD vs. TDC (ID/ED Simple Reversal error rate vs. fractional anisotropy) revealed significantly different slopes across the groups, indicating an aberrant relationship between CF and underlying white matter microstructure in youth with BD. These results underscore the importance of examining specific CF-neuroimaging relationships in BD youth. Future longitudinal studies could seek to define the white matter microstructural trajectories in BD vs. TDC, and relative to CF deficits and BD illness course.
Keywords: Magnetic resonance imaging (MRI), Diffusion tensor imaging (DTI), Tract-based spatial statistics (TBSS), Reversal learning, lithium, Young mania rating scale (YMRS), Cambridge neuropsychological test automated battery (CANTAB)
1. Introduction
Cognitive flexibility (CF) is defined as the ability to adjust one’s cognitive and/or behavioral response based on shifting rewards and punishments. CF can be assessed using reversal learning tasks, whereby participants must first acquire the stimulus/reward association by choosing the correct (rewarded) stimulus from two simultaneously presented images, and then adapt when the stimulus/reward relationship reverses—i.e., the previously rewarded stimulus is now punished, and vice versa. CF deficits have been described in children, adolescents, and adults with bipolar disorder (BD) using tasks such as the Intra-Extra Dimensional (ID/ED) Set Shift task (a computerized analog of the Wisconsin Card Sorting Test), which is part of the Cambridge Neuropsychological Test Automated Battery (CANTAB) (Dickstein et al., 2004; Dickstein et al., 2007; Gorrindo et al., 2005; Wegbreit et al., 2016; Lee et al., 2014; O’Donnell et al., 2017). Moreover, CF deficits are clinically relevant in BD. For example, CF has been associated with occupational difficulties (e.g., attendance, performance at work) in a prospective longitudinal study of adults with BD (O’Donnell et al., 2017). Functional magnetic resonance imaging (fMRI) studies have shown that youth with BD have alterations during CF as indexed by reversal learning tasks incorporating probabilistic feedback to increase task difficulty. Compared to TDCs, youth with BD have shown greater activation in the reversal phase in fronto-parietal regions, suggesting inefficient recruitment of these regions in the CF task (Dickstein et al., 2010). Additionally, children and adolescents with BD had reduced functional activation compared to TDC youth in the right caudate in response to errors during a reversal learning paradigm (Adleman et al., 2011).
However, to the best of our knowledge, no study has evaluated the white matter (WM) microstructural alterations underlying these CF deficits in BD youth using diffusion tensor imaging (DTI). DTI assesses WM microstructural organization and integrity, via the fact that water diffuses anisotropically (asymmetrically) in axons (Beaulieu, 2002). DTI outcomes include: fractional anisotropy (FA; a composite measure that ranges between 0 and 1 and reflects the degree of diffusion anisotropy within a voxel); axial diffusivity (AD; diffusion along the principal axis of the diffusion ellipsoid); radial diffusivity (RD; average diffusivity in the plane, perpendicular to the principal axis); and mean diffusivity (MD; average water diffusion amount in a voxel) (Curran et al., 2016). In TDC youth, FA in the corpus callosum and superior corona radiata was found to correlate with CF as assessed with Stroop or Stroop-like tasks (Seghete et al., 2013; Treit et al., 2014). Interestingly, DTI studies have shown that BD youths have decreased FA compared to TDC in areas associated with attention and cognitive control, including the anterior corona radiata (Lagopoulos et al., 2013; Pavuluri et al., 2009) and WM areas adjacent to the cingulate cortex (Frazier et al., 2007; Gao et al., 2013; Gonenc et al., 2010) and prefrontal and orbitofrontal cortices (Kafantaris et al., 2009; Adler et al., 2006). BD youths also have decreased FA, compared to TDCs, in the corpus collosum, a tract essential for facilitating interhemispheric communication (Barnea-Goraly et al., 2009; James et al., 2011; Lagopoulos et al., 2013; Saxena et al., 2012).
We sought to address this gap in knowledge about WM alterations underlying CF deficits in BD vs. TDC youth. We hypothesized that the microstructural integrity (as measured by FA, AD and/or RD) of frontally-projecting WM regions (including fronto-striatal tracts) would correlate with performance on a CF task in TDC, such that higher FA would correlate with lower error rate on the CF task, and that the relationship between CF performance and WM integrity would be altered in youth with BD vs. TDC.
2. Methods
2.1. Participants
Twenty-eight children and adolescents with BD and twenty-six age-matched TDC youth participated in an IRB-approved study at Bradley Hospital and Brown University. Prior to study participation, child participants and their parent/guardian provided informed assent and informed consent, respectively. Participants were recruited from the local community through a variety of methods, including contact with local child health care providers and parent support groups, presentations to community groups, information on hospital and other web pages, and referral from other research studies.
BD participant inclusion criteria were: (1) age between 7–17 years, (2) English fluency, and (3) diagnosis of bipolar I disorder, confirmed by the Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime version (K-SADS-PL), a semi-structured psychiatric diagnostic interview (Kaufman et al., 1997). BD exclusion criteria were: (1) implanted metal due to MRI safety, (2) IQ of less than 70, (3) active psychosis, autism spectrum disorder, or substance abuse within the last 2 months, and (4) medical/neurological disorders that could mimic BD (e.g., thyroid disease). BD participants were not excluded if they had other comorbid psychiatric diagnoses (other than the conditions listed in the exclusion criteria above).
TDC participant inclusion criteria were: (1) age between 7–17 years, (2) English fluency, and (3) no lifetime diagnosis of a mental health disorder (including substance use disorder) in the participant or his/her first-degree relatives. TDC exclusion criteria were: (1) implanted metal due to MRI safety, (2) IQ of less than 70, and (3) psychiatric illness in the participant or their first-degree relatives (per the K-SADS-PL and parental report respectively).
BD participants continued their outpatient medication regimen, as it would be unethical to withdraw them from their effective medications for this non-treatment study. However, BD participants were asked (but not required) to withhold their ADHD stimulant medication for 4 drug half-lives prior to their MRI scan, since such ADHD stimulant medication holidays are common in clinical practice (e.g., on weekends and/or school vacations).
Five participants (3 with BD and 2 TDC) were excluded due to poor quality of the DTI data (including artifacts due to excessive motion), leaving a final sample of 28 BD and 26 TDC youths for the current report. The participants in the current analysis overlap partially with the participants included in a report by Cabeen et al., 2018; notably, the focus of their paper was the correlates of indices of WM integrity with age rather than with CF.
2.2. Assessment procedures
Psychiatric diagnoses for BD and TDC participants’ inclusion/exclusion criteria were established by the K-SADS-PL administered by licensed child psychologist and psychiatrist with inter-rater reliability (kappa >0.85) (Kaufman et al., 1997). To further characterize the sample, clinicians rated participants using the Young Mania Rating Scale (YMRS) (Young et al., 1978), Children’s Depressive Rating Scale-Revised (CDRS-R) (Poznanski EO and Mokros HB, 1996), and Children’s Global Assessment Scale (CGAS) (Shaffer et al., 1983). Full-scale intelligence quotient (FSIQ) was assessed using the Wechsler Abbreviated Scales of Intelligence (WASI) (Wechsler, 2005).
2.3. Cognitive flexibility (CF) testing
As described above, the CANTAB ID/ED Set Shift task is a computerized analog of the Wisconsin Card Sorting Test administered on a touch-screen laptop. Participants progress through nine stages whereby a series of stimuli pairs, consisting initially of purple shapes to which white line designs are added in stage 3. Participants must choose one of the two stimuli and receive feedback about whether or not their selection was correct. After six consecutive correct responses, the rule and/or the stimuli are changed, though this transition is not explicitly announced. The new rule could be based on the same stimuli (e.g., white lines) that guided the selection rule in the prior stage (intra-dimensional shift) or on the alternative stimuli (e.g., purple shapes) (extra-dimensional shift). Here, we focused our analyses on the Stage 2 Simple Reversal Stage Errors—as this is the first stage where the stimulus/reward relationship reverses. Prior studies have shown that participants with BD have higher error rates in this stage of the ID/ED task compared to TDC (Dickstein et al., 2004; Dickstein et al., 2007; Dickstein et al., 2016; Wegbreit et al., 2016).
2.4. Neuroimaging data acquisition
All participants completed neuroimaging data acquisition on a SIEMENS Tim Trio 3T scanner with a 12-channel head coil. Prior to MRI scanning, participants completed a training session in an MRI scan simulator. This allowed the participants to get accustomed with the scanner set up, and practice staying still in the scanner. Participants completed a DTI scan with the following acquisition parameters: 64 gradient directions, voxel size=1.8 × 1.8 × 1.8 mm, interleaved axial slice acquisition, number of slices=70, TR= 10,100 ms, TE=103 ms, b-value=1000 s/mm2, at least one B0 volume without diffusion weighting, and DTI scan duration of 11.27 min.
2.5. Neuroimaging data analyses
DTI data was preprocessed using FSL 6.0.0. (Smith et al., 2004). The preprocessing incorporated skull-stripping of the B0 volume, Eddy current, slice-to-volume motion correction and replacement of outlier slices (Andersson and Sotiropoulos, 2016; Andersson et al., 2016; Andersson et al., 2017). A tensor model was fit to the data, and FA, AD, RD, and MD maps were generated. Next, Tract-Based Spatial Statistics (TBSS) data analysis was conducted on the FA maps (Smith et al., 2006). Specifically, the FA maps for all participants were nonlinearly aligned to each other, and the most representative brain across all participants was selected to serve as the initial template for the current sample. The most representative brain was then affine-aligned to the MNI152 template (to resolution of 1 × 1 × 1 mm). The respective nonlinear and affine transformation were applied to each participant’s FA map. Next, the aligned FA maps for all participants were averaged to generate an averaged FA map. A WM skeleton (with FA threshold of 0.2) was created based on the averaged brain FA map. Further statistical analyses were conducted only within the WM skeleton. The coregistration parameters (nonlinear and affine transformations) derived from the FA analyses were also applied to the AD, MD, and RD maps, and averaged AD, MD, and RD maps were created.
FSL’s randomise algorithm (Winkler et al., 2014) was used to compare the FA, AD, RD, and MD maps between the BD and TDC groups, while controlling for Age (as a proxy for development). Next, FSL’s randomise was used to correlate ID/ED Stage 2 (simple reversal) error rate and FA, AD, RD, or MD, incorporating Diagnosis information in the model, and controlling for Age. As in prior studies (Kikinis et al., 2017), 5000 permutations were implemented for all analyses. To correct for multiple comparisons, threshold-free cluster enhancement (tfce) threshold was applied (p<0.05), a widely used technique for cluster-free thresholding (Smith and Nichols, 2009; Kikinis et al., 2017; Gao et al., 2013).
To learn more about the correlations within specific WM regions, the union between the Johns Hopkins WM atlas regions and statistically significant voxels (derived from the ID/ED analysis above) was obtained. Average FA within each TBSS-ROI-CF union region for each participant was extracted and correlated with ID/ED Stage 2 error rate as post-hoc analyses. SPSS was used for the majority of these follow-up analyses, except for (1) the comparison between regression line slopes (which was completed in GraphPad Prism 8); and (2) false discovery rate (FDR) multiple comparison corrections (completed in R software, version 3.6).
3. Results
3.1. Participant characteristics
Twenty-eight children and adolescents with BD, type I (12 girls and 16 boys, average age 13.9 ± 2.8 years), and twenty-six age-matched TDC: n=26 (16 girls and 10 boys, average age12.7 ± 3.4 years) were included in the current report (Table 1). The BD and TDC groups did not differ significantly on age, sex, and FSIQ (Table 1). Eight of the participants were prescribed stimulant ADHD medication. Per our protocol, the families were offered (but not required) to withhold stimulant medication prior to the scan, and for 6 (out of 8) participants the stimulant medication was held for 22–48 hours prior to the scan (Concerta was held for 48hrs; Ritalin for 22–48hrs; Adderall for 24–48hrs; Focalin for 24–48hrs); for the remaining 2 participants (out of 8) the stimulant medication was not held prior to the scan.
Table 1.
Demographics, clinical characteristics, and medication use
| BD (n=28) | TDC (n=26) | Statistical Comparison | |
|---|---|---|---|
| Female/Male | 12/16 | 16/10 | X2=1.885; p=0.170 |
| Age (Mean ± SD) | 13.9 (2.8) | 12.7 (3.4) | F(1,52) = 1.910; p = 0.173 |
| Intelligence Quotient and Clinical Scales (Mean ± SD) | |||
| Full-Scale Intelligence Quotient (FSIQ) | 105.2 ± 11.7 | 110.6 ± 8.4 | F(1,52) = 3.697; p = 0.060 |
| Young Mania Rating Scale (YMRS) | 7.2 ± 4.9 | - | |
| Children’s Depression Rating Scale (CDRS) | 29.3 ± 12.5 | - | |
| Children’s Global Assessment Scale (CGAS) | 63.8 ± 16.0 | - | |
| Comorbid Diagnoses (n (%)) | |||
| ADHD (all types) | 20 (71.4%) | - | |
| Oppositional Defiant Disorder (ODD) | 21 (75%) | - | |
| Generalized Anxiety Disorder (GAD) | 3 (10.7%) | - | |
| Social Phobia | 3 (10.7%) | - | |
| Specific Phobia | 5 (17.9%) | ||
| Obsessive-Compulsive Disorder (OCD) | 2 (7.1%) | ||
| Separation Anxiety | 2 (7.1%) | ||
| Panic Disorder | 1 (3.6%) | - | |
| Conduct Disorder | 1 (3.6%) | - | |
| Medications (n (%)) | |||
| Antipsychotic | 17 (61%) | - | |
| Lithium | 9 (32%) | - | |
| Stimulant* | 8 (29%) | - | |
| Anti-depressant | 7 (25%) | - | |
| Anti-epileptic | 5 (18%) | - | |
| Non-stimulant ADHD medication** | 4 (14%) | - | |
| Benzodiazepine | 2 (7%) | - | |
| Any psychoactive medication | 24 (86%) | - | |
Notes: Mean (SD) displayed for Age and Full-Scale IQ. Comorbid Current Diagnoses are based on the K-SADS Current diagnostic category (spanning the most recent 6 months relative to the time of the K-SADS interview)
Stimulant medication was held for 22–48hrs prior to the scan in 6 participants; for 2 participants, the stimulant medication was not held prior to the MRI scan.
Non-stimulant ADHD medication includes clonidine or atomoxetine.
Abbreviations: BD: bipolar disorder; TDC: typically developing controls
3.2. WM correlates of BD diagnosis in children and adolescents (main effect of diagnosis)
While age did not differ across the groups, all neuroimaging analyses included age in the models as a proxy for development. When controlling for age, FA was significantly lower in participants with BD than in TDC youth in two regions: WM in the anterior temporal lobe (including portions of the uncinate fasciculus) and WM underlying the parietal lobe (superior parietal lobule/TPJ) (Fig. 1). There were no significant differences between BD and TDC for AD, RD, and MD. ANOVAs with dependent variables FA in either the anterior temporal lobe or the parietal lobe region, and independent variable Group (BD vs. TDC) and covariate Age, showed the following: for the anterior temporal lobe region: significant main effect of Group (F=32.977, p<0.001; Average FABD 0.387 ± 0.007; Average FATDC 0.442 ± 0.007); and significant effect of Age (F(1,51)=5.696, p=0.021); for the parietal lobe region: Average FABD =0.458 ± 0.020; Average FATDC 0.569 ± 0.020 (F(1,51)=15.291, p<0.001); Age: N.S.
Fig. 1.
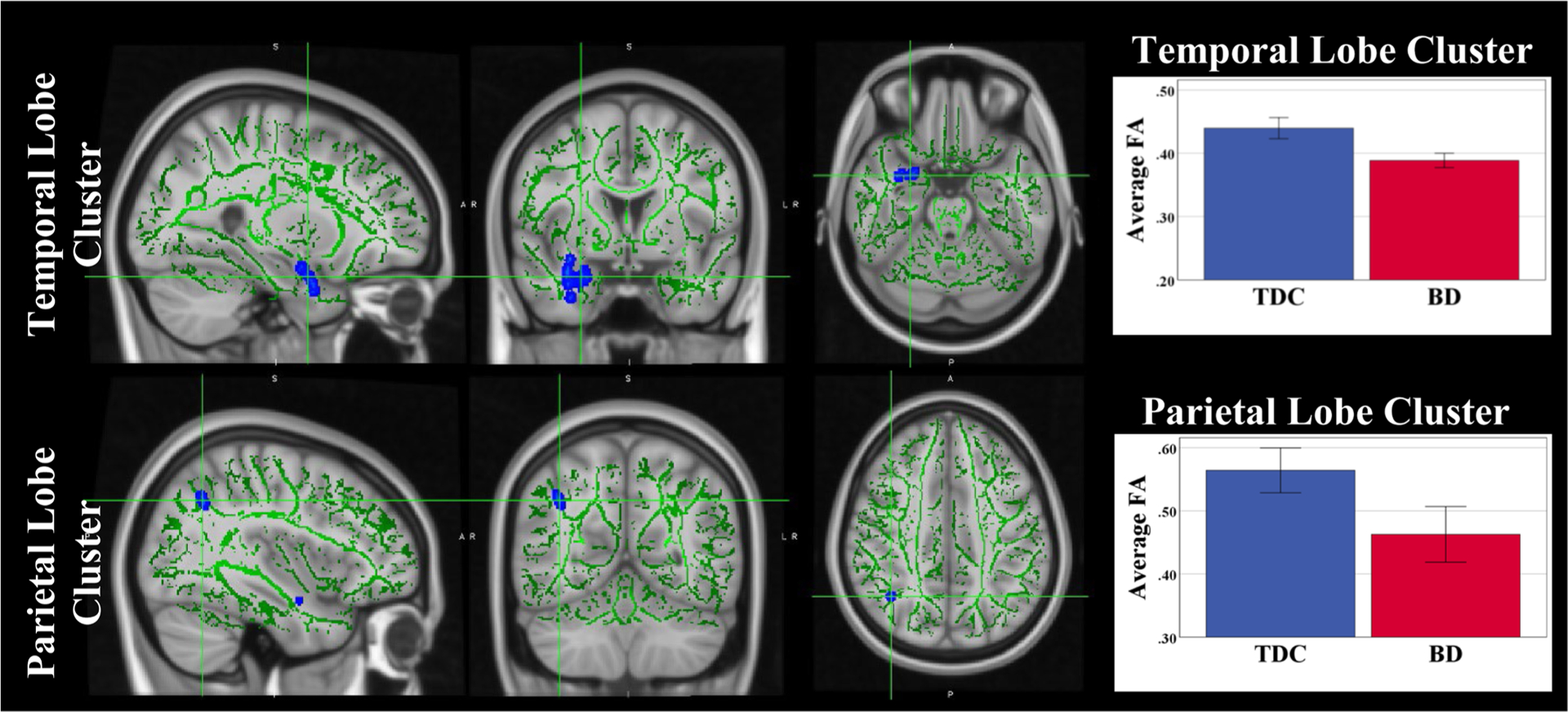
FA Differences in youth with Bipolar Disorder as compared to TDC. Voxels with significant p-values are displayed in blue/light blue) with thickened outline for better visualization. The remaining portions of the TBSS skeleton are shown in green. Error bars show ± 2SE.
3.3. Diagnosis X CF interaction
There was no significant main effect of Stage 2 Error Rate on any of the WM microstructural indices. There was a significant interaction between diagnosis and Stage 2 Error Rate on FA in multiple WM regions, including WM regions projecting to frontal lobes, such as the Anterior and Superior Corona Radiata, Genu of the Corpus Callosum and Superior Longitudinal Fasciculus (Fig. 2, Table 2, Supplementary Table 1). There were no significant Diagnosis X CF interactions for AD, RD or MD.
Fig. 2.
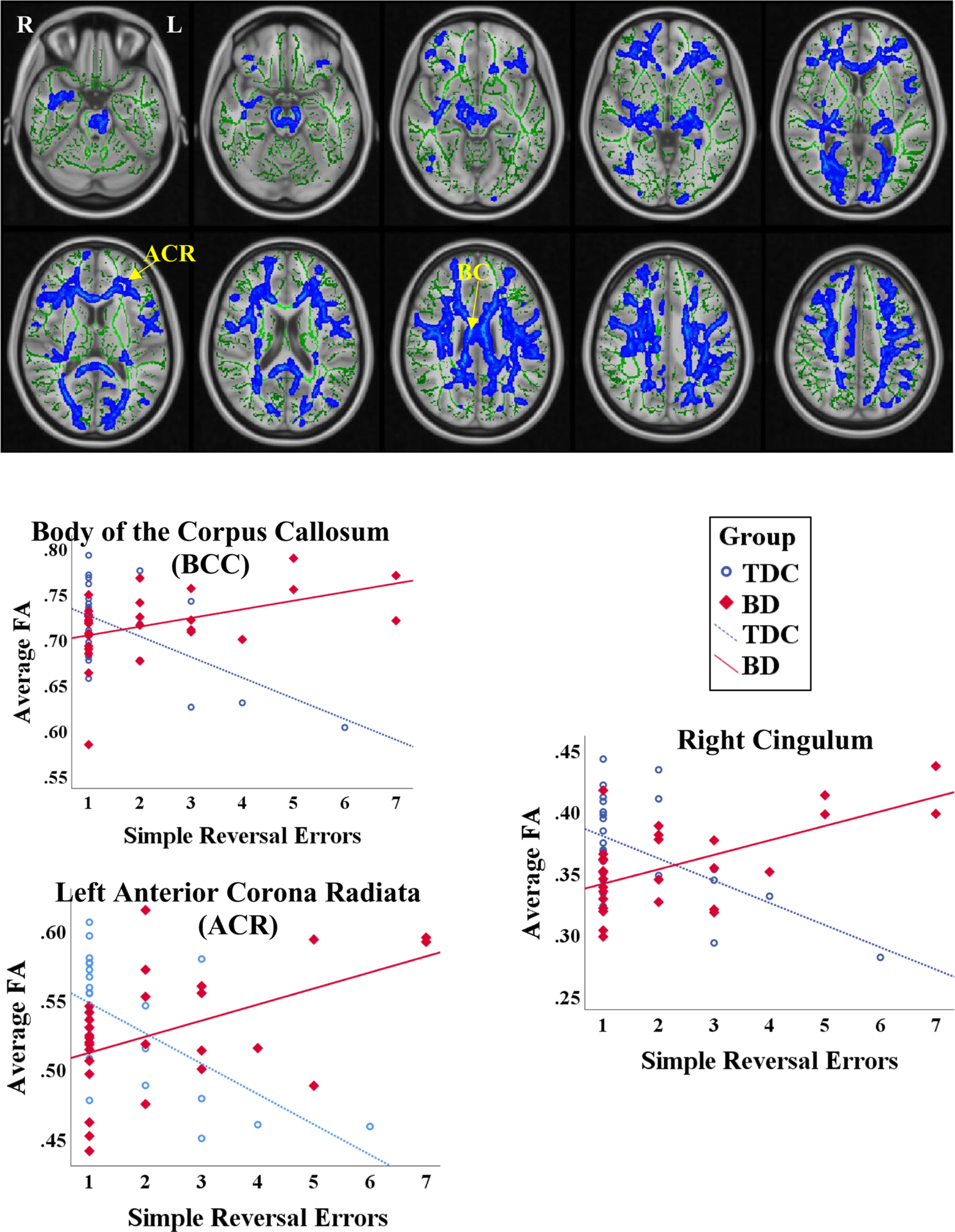
Interaction between Diagnosis and ID/ED Simple Reversal Error Rate: (A) Significant voxels across the TBSS skeleton (green) are displayed in blue/light blue, with thickened outline for better visualization; (B) Graphs representing the interaction in a subset of significant regions in the TBSS skeleton.
Table 2.
Regions in the TBSS skeleton, containing significant voxels (Diagnosis X ID/ED Simple Reversal Error rate interaction): Linear regression results (B: slope of the regression and StdE: standard error for B). (White Matter region list restricted to significant regions with FDR p-value<0.05)
| White Matter Region | Voxel Number | TDC (n = 26) | BD (n = 28) | Between-Group Slope | Difference | ||
|---|---|---|---|---|---|---|---|
| B | StdE (B) | B | StdE (B) | FDR | |||
| F-value | p-value | ||||||
| Middle cerebellar peduncle (MCP) | 73 | −0.032 | 0.008 | 0.008 | 0.008 | 10.4 | 0.003 |
| Pontine crossing tract (PCT) | 96 | −0.022 | 0.006 | 0.009 | 0.005 | 14.5 | 0.001 |
| Genu of corpus callosum (GCC) | 818 | −0.02 | 0.005 | 0.01 | 0.003 | 25.6 | <0.001 |
| Body of the Corpus Callosum (BBC) | 1281 | −0.023 | 0.006 | 0.009 | 0.004 | 19.3 | <0.001 |
| Splenium of corpus callosum (SCC) | 1045 | −0.02 | 0.005 | 0.007 | 0.003 | 19.8 | <0.001 |
| Corticospinal tract R (CST_R) | 1 | −0.042 | 0.018 | 0.009 | 0.01 | 6.8 | 0.014 |
| Corticospinal tract L (CST_L) | 114 | −0.031 | 0.006 | 0.004 | 0.005 | 17.9 | <0.001 |
| Medial lemniscus L (ML_L) | 21 | −0.02 | 0.009 | 0.009 | 0.006 | 7.1 | 0.012 |
| Superior cerebellar peduncle L (SCP_L) | 42 | −0.021 | 0.006 | 0.005 | 0.005 | 9.7 | 0.004 |
| Cerebral peduncle R (CP_R) | 228 | −0.025 | 0.006 | 0.005 | 0.004 | 16.1 | <0.001 |
| Cerebral peduncle L (CP_L) | 146 | −0.019 | 0.006 | 0.008 | 0.004 | 12.4 | 0.001 |
| Posterior limb of internal capsule R (PLIC_R) | 195 | −0.017 | 0.004 | 0.005 | 0.003 | 19.8 | <0.001 |
| Posterior limb of internal capsule L (PLIC_L) | 102 | −0.019 | 0.005 | 0.006 | 0.003 | 15.4 | <0.001 |
| Retrolenticular part of internal capsule R (RPIC_R) | 251 | −0.02 | 0.005 | 0.006 | 0.004 | 15.1 | 0.001 |
| Retrolenticular part of internal capsule L (RPIC_L) | 186 | −0.019 | 0.005 | 0.01 | 0.003 | 24.2 | <0.001 |
| Anterior corona radiata R (ACR_R) | 791 | −0.024 | 0.007 | 0.008 | 0.004 | 17.0 | <0.001 |
| Anterior corona radiata L (ACR_L) | 764 | −0.022 | 0.006 | 0.012 | 0.004 | 21.2 | <0.001 |
| Superior corona radiata R (SCR_R) | 678 | −0.016 | 0.006 | 0.012 | 0.004 | 14.5 | 0.001 |
| Superior corona radiata L (SCR_L) | 441 | −0.017 | 0.007 | 0.012 | 0.004 | 15.5 | <0.001 |
| Posterior corona radiata R (PCR_R) | 141 | −0.019 | 0.006 | 0.004 | 0.004 | 9.7 | 0.004 |
| Posterior corona radiata L (PCR_L) | 243 | −0.019 | 0.006 | 0.004 | 0.004 | 11.9 | 0.002 |
| Posterior thalamic radiation R (PTR_R) | 370 | −0.024 | 0.005 | 0.005 | 0.004 | 19.9 | <0.001 |
| Posterior thalamic radiation L (PTR_L) | 182 | −0.024 | 0.006 | 0.007 | 0.004 | 17.2 | <0.001 |
| Sagittal stratum (SS_R) | 121 | −0.028 | 0.006 | 0.003 | 0.004 | 18.3 | <0.001 |
| External capsule R (EC_R) | 235 | −0.021 | 0.004 | 0.006 | 0.004 | 18.0 | <0.001 |
| Cingulum (cingulate gyrus) R (Cingulum_R) | 87 | −0.018 | 0.006 | 0.012 | 0.003 | 22.9 | <0.001 |
| Cingulum (cingulate gyrus) L (Cingulum_L) | 43 | −0.026 | 0.012 | 0.003 | 0.004 | 6.6 | 0.015 |
| Fornix (cres) / Stria terminalis L (Fornix_cres_R) | 78 | −0.024 | 0.005 | 0.006 | 0.005 | 15.4 | <0.001 |
| Fornix (cres) / Stria terminalis L (Fornix_cres_L) | 64 | −0.019 | 0.007 | 0.007 | 0.004 | 9.5 | 0.004 |
| Superior longitudinal fasciculus R (SLF_R) | 827 | −0.027 | 0.006 | 0.008 | 0.004 | 21.1 | <0.001 |
| Superior longitudinal fasciculus L (SLF_L) | 854 | −0.022 | 0.006 | 0.009 | 0.003 | 22.3 | <0.001 |
| Uncinate fasciculus R (UF_R) | 3 | −0.015 | 0.013 | 0.016 | 0.006 | 5.4 | 0.027 |
| Tapetum R (Tapetum_R) | 37 | −0.014 | 0.009 | 0.008 | 0.005 | 4.9 | 0.034 |
Abbreviations: BD: bipolar disorder; TDC: typically developing controls; FDR: false discovery rate
To evaluate for differences in the nature of the relationship between FA and Stage 2 Error rate between BD and TDC participants, we assessed whether the slopes (in the Stage 2 Error rate vs. FA regressions) were significantly different in BD vs. TDC youth. We found that the slope was significantly different in the BD youth than in TDC youth across multiple WM regions (Table 2). While we found significant negative correlations between CF and FA for TDC in all regions listed in Table 2 (more errors on the CF task correlating with worse WM integrity via lower FA), this was not the case for BD youth. In fact, for some regions [genu of the corpus callosum (GCC), left superior corona radiata (SCR), left retrolenticular part of internal capsule (RPIC), and right cingulum], we found the opposite relationship: more errors/worse CF task performance correlating with greater FA (Supplementary Table 1, Fig. 2).
3.4. Post-hoc analyses of potential medication effects
To evaluate the potential role of medications in our primary findings, we conducted post-hoc analyses of the two most common antimanic agents that our BD sample was taking: lithium and second-generation antipsychotic agents (SGAs) (Table 1). We used independent samples t-tests with independent variable lithium use OR SGA use and dependent variable region-specific FA values (derived from the analyses described in section 3.3.). We focused only on WM regions that showed significant regressions both in the BD and TDC groups, with opposite signs of the regression slopes (original p-value<0.05 in Supplementary Table 1). Given the exploratory nature of our analyses, we present both the original and multiple comparison-corrected (FDR-corrected) p-values (Table 3a and 3b).
Table 3a.
Medication effects: Lithium: Post-hoc Independent Samples T Tests, comparing FA in White Matter regions in participants with BD taking Lithium (BD+Li) vs. participants with BD not taking SGAs (BD-Li)
| White Matter Region | BD + Li Mean ± SE n = 9 |
BD – Li Mean ± SE n = 19 |
Original p-value |
FDR p-value |
|---|---|---|---|---|
| Genu of corpus callosum (GCC) | 0.75 ± 0.010 | 0.71 ± 0.006 | 0.013 | 0.052 |
| Body of the Corpus Callosum (BBC) | 0.74 ± 0.009 | 0.70 ± 0.009 | 0.013 | 0.052 |
| Retrolenticular part of internal capsule L (RPIC_L) | 0.63 ± 0.006 | 0.60 ± 0.008 | 0.051 | 0.103 |
| Anterior corona radiata L (ACR_L) | 0.55 ± 0.012 | 0.52 ± 0.010 | 0.120 | 0.192 |
| Superior corona radiata R (SCR_R) | 0.54 ± 0.016 | 0.52 ± 0.010 | 0.283 | 0.323 |
| Superior corona radiata L (SCR_L) | 0.57 ± 0.011 | 0.54 ± 0.009 | 0.039 | 0.103 |
| Cingulum R (Cingulum_R) | 0.37 ± 0.011 | 0.35 ± 0.008 | 0.351 | 0.351 |
| Superior longitudinal fasciculus L (SLF_L) | 0.56 ± 0.011 | 0.54 ± 0.008 | 0.169 | 0.226 |
Abbreviations: BD: bipolar disorder; SE: standard error; FDR: false discovery rate; WM: white matter; Li: lithium
Table 3b.
Medication effects: Second-Generation Antipsychotic (SGA) Medications: Post-hoc Independent Samples T Tests, comparing FA in White Matter regions in participants with BD taking SGAs (BD+SGA) vs. participants with BD not taking SGAs (BD-SGA)
| White Matter Region | BD + SGA Mean ± SE n = 17 |
BD – SGA Mean ± SE n = 11 |
Original p-value |
FDR p-value |
|---|---|---|---|---|
| Genu of corpus callosum (GCC) | 0.73 ± 0.008 | 0.72 ± 0.01 | 0.351 | 0.863 |
| Body of the Corpus Callosum (BBC) | 0.72 ± 0.011 | 0.72 ± 0.009 | 0.933 | 0.933 |
| Retrolenticular part of internal capsule L (RPIC_L) | 0.61 ± 0.007 | 0.6 ± 0.011 | 0.280 | 0.863 |
| Anterior corona radiata L (ACR_L) | 0.52 ± 0.012 | 0.54 ± 0.009 | 0.188 | 0.863 |
| Superior corona radiata R (SCR_R) | 0.52 ± 0.009 | 0.54 ± 0.016 | 0.473 | 0.863 |
| Superior corona radiata L (SCR_L) | 0.54 ± 0.009 | 0.55 ± 0.013 | 0.818 | 0.933 |
| Cingulum R (Cingulum_R) | 0.35 ± 0.008 | 0.36 ± 0.013 | 0.558 | 0.863 |
| Superior longitudinal fasciculus L (SLF_L) | 0.55 ± 0.009 | 0.55 ± 0.009 | 0.648 | 0.863 |
Abbreviations: BD: bipolar disorder; FDR: false discovery rate; SGA: second-generation antipsychotic
BD participants taking lithium (BD+lithium) had greater FA vs. BD youth not taking lithium (BD-lithium) in the genu and body of the corpus callosum (GCC, BCC) and the left SCR (original p-value<0.05, pFDR: N.S.) (Table 3a). Supplementary Figure 1 visualizes the relationship between ID/ED Stage 2 error rate and FA in each WM region that showed significance in the independent samples t-test (lithium vs. no lithium use). Overall, the BD+lithium and BD-lithium groups had a similarly altered relationship between FA and CF as compared to TDC. We did not test for differences in the slopes or intercepts of the regressions within the subgroups given the small sample sizes. There were no significant differences in FA across the antipsychotic medication groups (BD+SGA vs. BD-SGA) (Table 3b).
3.5. Post-Hoc analyses of clinical features
We also conducted post-hoc correlations between clinical scales of depression (CDRS), mania (YMRS) and global functioning (CGAS) and our WM findings. YMRS mania scores were associated with FA in the Superior Corona Radiata bilaterally (pFDR<0.05) (Table 4, Supplementary Figure 1 and 2) and with ID/ED Stage 2 error rate (Supplementary Figure 2). CDRS and CGAS were not significantly associated with FA in any of the tested regions (Table 4).
Table 4.
Post-hoc Correlations between FA in WM regions and clinical scales
| YMRS | CDRS | CGAS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Corr. | Orig. | FDR | Corr. | Orig. | FDR | Corr. | Orig. | FDR | |
| White Matter Region | Coeff. | p | p | Coeff. | p | p | Coeff. | p | p |
| Genu of corpus callosum (GCC) | 0.189 | 0.334 | 0.382 | 0.087 | 0.661 | 0.83 | −0.061 | 0.760 | 0.832 |
| Body of the Corpus Callosum (BBC) | 0.115 | 0.560 | 0.56 | 0.053 | 0.787 | 0.83 | 0.075 | 0.705 | 0.832 |
| Retrolenticular part of internal capsule L (RPIC_L) | 0.368 | 0.054 | 0.118 | 0.059 | 0.765 | 0.83 | −0.073 | 0.714 | 0.832 |
| Anterior corona radiata L (ACR_L) | 0.301 | 0.120 | 0.191 | −0.106 | 0.593 | 0.83 | −0.111 | 0.574 | 0.832 |
| Superior corona radiata R (SCR_R) | 0.555 | 0.002 | 0.009 | 0.279 | 0.150 | 0.601 | −0.369 | 0.053 | 0.424 |
| Superior corona radiata L (SCR_L) | 0.572 | 0.001 | 0.009 | 0.172 | 0.383 | 0.83 | −0.283 | 0.145 | 0.579 |
| Cingulum R (Cingulum_R) | 0.223 | 0.253 | 0.338 | 0.314 | 0.103 | 0.601 | −0.042 | 0.832 | 0.832 |
| Superior longitudinal fasciculus L (SLF_L) | 0.361 | 0.059 | 0.118 | 0.042 | 0.830 | 0.83 | −0.128 | 0.517 | 0.832 |
Abbreviations: Correl. Coeff.: Correlation Coefficient; Orig.: original; CDRS: Children’s Depressive Rating Scale; CGAS: Children’s Global Assessment Scale; FDR: false discovery rate; YMRS: Young Mania Rating Scale
4. Discussion
Our current report is the first to examine the relationship between CF (ID/ED set-shifting task) and WM microstructural organization (as assessed with DTI) in children and adolescents with BD. We found an altered WM microstructural-CF relationship in youth with BD as compared to TDC across multiple WM regions, most notably in those projecting to the frontal lobes (such as the Anterior and Superior Corona Radiata, Genu of the Corpus Callosum and Superior Longitudinal Fasciculus) and other regions (Posterior Corona Radiata, Posterior Thalamic Radiation, Posterior Limb of the Internal Capsule). Post-hoc analyses suggested that some of the variability in WM microstructure appeared to also correlate with lithium use and mood state (mania ratings), though these findings did not seem to explain the altered WM microstructural-CF relationship in youth with BD vs. TDC. Our results point to the importance of examining specific neurocognitive-neuroimaging relationships in BD, and emphasize the need to consider additional potentially contributing variables in neuroimaging studies such as medication use, clinical features, and age.
The association between FA and CF performance (Simple Reversal Error Rate) had opposite direction as compared to TDC: namely, positive correlation was often observed in BD while negative correlation was seen in TDC. Our findings of higher error rate in the CF task in TDC correlating with lower FA in WM regions, including tracts that project to/from the frontal lobes (Table 2), are consistent with prior work in TDC (Treit et al., 2014; Seghete et al., 2013). Our findings in BD could be related to WM microstructural alterations in some participants with BD (e.g., aberrant/inefficient axonal transfer of information between regions involved in CF processing). Support for this idea comes from prior fMRI work by Dickstein and colleagues (2010) that demonstrated greater activation in youth with BD (than in TDC youth) in the reversal phase of a CF task in fronto-parietal regions, including the dorsomedial prefrontal cortex, anterior cingulate, middle and superior frontal gyri bilaterally, right inferior parietal lobule and left inferior frontal gyrus. This finding implicated inefficient recruitment of these regions during the CF task. Notably, we found abnormally higher FA associated with worse CF performance in areas projecting to frontal and parietal regions, including the anterior, superior and posterior corona radiata, superior longitudinal fasciculus, cingulum, and the genu and body of the corpus callosum. It is possible that aberrant axonal pruning may be associated with higher, but inefficient, axonal transfer along with higher activation in fronto-parietal regions in BD. Likewise, in schizophrenia, abnormally high FA in certain WM tracts has been postulated to correlate with aberrant axonal pruning and maintenance of inefficient neural networks (Alba-Ferrara and de Erausquin, 2013).
In a post-hoc analysis, we found a positive correlation between YMRS scores and portions of the SCR bilaterally. The association between high FA in the SCR and both higher mania ratings and deficits in CF may suggest that abnormal connectivity, perhaps hyperconnectivity, in the SCR may be a contributor to both of these intertwined/co-occurring clinical and neurocognitive deficits. Disrupted structural connectivity (underconnectivity) has been previously reported in mania in adults with BD, specifically in the cingulum; however, that study focused only on the cingulum in a region-of-interest analysis, and did not evaluate structural connectivity of other WM regions (Martino et al., 2016).
Through post-hoc testing, we also found that lithium use was associated with higher FA values in BD in portions of several WM regions, including the right GCC, BCC and the left SCR, although this finding did not pass FDR multiple comparison correction and thus should be interpreted with caution. Yet, prior literature has also described higher FA in participants with BD taking lithium, including: (1) an increase in left hippocampal cingulum FA at follow-up as compared to baseline in a prospective clinical trial of lithium in BD youth; and (2) higher global FA in adults with BD taking lithium as compared to BD adults not taking lithium (Gildengers et al., 2015; Kafantaris et al., 2017; Abramovic et al., 2018). It has been postulated that lithium may attenuate WM microstructural differences between participants with BD and controls (Abramovic et al., 2018), through re-myelination induced by lithium based on animal work (Chen et al., 2016). Our current report along with prior DTI studies in BD highlights the importance of taking medication use into consideration in neuroimaging studies of BD, especially as medication use may contribute to the heterogeneity of neuroimaging data.
Another important factor to consider in our study is age. Prior work has demonstrated that WM undergoes a maturational trajectory during childhood, adolescence, and into young and middle adulthood in TDC (Peters et al., 2014; Lebel et al., 2012). Alterations in WM maturational trajectories in youth with BD have been reported for multiple tracts [including in the corpus callosum, limbic structures (fornix, uncinate) and/or frontal-projecting tracts (inferior longitudinal fasciculus, anterior thalamic radiation, and inferior fronto-occipital fasciculus)] (Toteja et al., 2015; Cabeen et al., 2018). Given these prior findings, we ensured that our TDC and BD groups were well-matched on age, and we also incorporated age as a covariate in all of our neuroimaging analyses.
The current study has several limitations including sample size, limited behavioral score variability in the CF task, limited post-hoc examination of FA-clinical features/medication use, and comorbidity with other psychiatric disorders. Although our sample size is comparable to or larger than the sample sizes of the majority of prior DTI reports of youth with BD [n’s=10–26 youth with BD (Barnea-Goraly et al., 2009; Kafantaris et al., 2009; Pavuluri et al., 2009; Teixeira et al., 2014; Frazier et al., 2007; Saxena et al., 2012)], it should be noted that our sample size is still relatively small and is overall a limitation of our current report. Another potential limitation is the narrow range of the behavioral CF scores, i.e., error rates in the ID/ED Stage 2 ranging from 1 to 7. Future studies could incorporate additional paradigms to assess CF, and/or compute a composite CF score across several CF paradigms. Here, we also emphasize that we did not systematically assess correlations between WM microstructure and clinical variables, medication use (including whether a medication was held vs. not held prior to the scan—as was the case for the stimulant medication class), and all analyses were restricted to post-hoc follow-up analyses based on our main findings (CF-FA correlations in specific subregions of the TBSS skeleton). Thus, we did not evaluate the full TBSS-JHU atlas WM overlapping regions (e.g., the entire cingulum-TBSS or entire SCR-TBSS) but only focused on the subsections of these regions, in which FA was significantly associated with CF performance. Future larger and fully-powered studies could specifically focus on clinical features-medication use-WM microstructural correlates in youth with BD.
Another important consideration is that a relatively large number of our participants with BD had co-occurring psychiatric disorders, including attention-deficit/hyperactivity disorder (ADHD), oppositional defiant disorder (ODD) and anxiety disorders. The high rate of comorbid psychopathology observed in our study corresponds with other studies of participants with BD, and increases the generalizability of our findings to participants with BD. Yet, it is important to acknowledge that given the relatively small sample of participants within each subcategory, we lacked sufficient power to assess the potential individual contributions of psychiatric comorbidities to the altered CF-FA relationship in BD (e.g., BD without ADHD, BD without ODD, or BD with individual anxiety disorders). Future larger samples would be in a better position to differentiate whether some of the currently observed heterogeneity in the CF-FA relationship in BD may be partially related to underlying neural mechanisms of co-occurring psychiatric disorders. Moreover, longitudinal studies of BD may be best suited to carefully track the developmental trajectories in youth with BD (vs. TDC), evaluate for altered relationships between cognition and WM microstructural organization across development, and begin to disentangle potential contributions of medication use, mood states, and/or co-occurring psychiatric disorders.
In summary, we found an altered relationship between FA (an index of WM microstructural integrity) in multiple WM regions and CF performance in youth with BD vs. TDC children and adolescents. We also found that some of the variability of FA in youth with BD was associated with lithium use and mood state (mania scores). One intriguing future research direction for potential clinical intervention strategies/targets (raised by our results) is to explore whether youth with BD may benefit from concurrent treatment with lithium and targeted cognitive flexibility remediation/training: thus, potentially harnessing (or retraining) a normalized-by-lithium underlying WM circuit. In terms of broader implications, our results point to the importance of understanding the specific neurocognitive-neuroimaging relationships in BD, and emphasize the need to be mindful of additional variables (such as medications and clinical features).
Supplementary Material
Acknowledgement
Funding sources include the National Institute of Mental Health grants R01MH087513 and R21/R33MH096850 (Principal Investigator D.P.D). P.R. (a Child and Adolescent Psychiatry Fellow) would like to thank the Child and Adolescent Psychiatry Training Program at Brown Alpert Medical School for support for research activities. Preliminary analyses in the current report were presented in a poster at the 58th Annual Meeting of the American College of Neuropsychopharmacology in December 2019. We would like to thank the children and families who participated in the study.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.pscychresns.2020.111169.
Declaration of Competing Interest
The authors declare no known conflicts of interest.
References
- Abramovic L, Boks MPM, Vreeker A, Verkooijen S, van Bergen AH, Ophoff RA, Kahn RS, van Haren NEM, 2018. White matter disruptions in patients with bipolar disorder. Eur. Neuropsychopharmacol 28, 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adleman NE, Kayser R, Dickstein D, Blair RJ, Pine D, Leibenluft E, 2011. Neural correlates of reversal learning in severe mood dysregulation and pediatric bipolar disorder. J. Am. Acad. Child Adolesc. Psychiatry 50, 1173–1185 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler CM, Adams J, DelBello MP, Holland SK, Schmithorst V, Levine A, Jarvis K, Strakowski SM, 2006. Evidence of white matter pathology in bipolar disorder adolescents experiencing their first episode of mania: a diffusion tensor imaging study. Am. J. Psychiatry 163, 322–324. [DOI] [PubMed] [Google Scholar]
- Alba-Ferrara LM, de Erausquin GA, 2013. What does anisotropy measure? Insights from increased and decreased anisotropy in selective fiber tracts in schizophrenia. Front. Integr. Neurosci 7, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Graham MS, Drobnjak I, Zhang H, Filippini N, Bastiani M, 2017. Towards a comprehensive framework for movement and distortion correction of diffusion MR images: Within volume movement. Neuroimage 152, 450–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Graham MS, Zsoldos E, Sotiropoulos SN, 2016. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage 141, 556–572. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Sotiropoulos SN, 2016. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125, 1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Chang KD, Karchemskiy A, Howe ME, Reiss AL, 2009. Limbic and corpus callosum aberrations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biol. Psychiatry 66, 238–244. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, 2002. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 15, 435–455. [DOI] [PubMed] [Google Scholar]
- Cabeen RP, Laidlaw DH, Ruggieri A, Dickstein DP, 2018. Preliminary mapping of the structural effects of age in pediatric bipolar disorder with multimodal MR imaging. Psychiatry. Res. Neuroimage 273, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Weng J, Han D, Chen B, Ma M, Yu Y, Li M, Liu Z, Zhang P, Jiang B, 2016. GSK3beta inhibition accelerates axon debris clearance and new axon re-myelination. Am. J. Transl. Res 8, 5410–5420. [PMC free article] [PubMed] [Google Scholar]
- Curran KM, Emsell L, Leemans A, 2016. Quantitative DTI measures. In: Van Hecke W, Emsell L, Sunaert S (), Diffusion Tensor Imaging: A Practical Handbook, 1st ed. Springer, New York, pp. 65–87. [Google Scholar]
- Dickstein DP, Axelson D, Weissman AB, Yen S, Hunt JI, Goldstein BI, Goldstein TR, Liao F, Gill MK, Hower H, Frazier TW, Diler RS, Youngstrom EA, Fristad MA, Arnold LE, Findling RL, Horwitz SM, Kowatch RA, Ryan ND, Strober M, Birmaher B, Keller MB, 2016. Cognitive flexibility and performance in children and adolescents with threshold and sub-threshold bipolar disorder. Eur. Child Adolesc. Psychiatry 25, 625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Finger EC, Skup M, Pine DS, Blair JR, Leibenluft E, 2010. Altered neural function in pediatric bipolar disorder during reversal learning. Bipolar Disord 12, 707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Nelson EE, McClure EB, Grimley ME, Knopf L, Brotman MA, Rich BA, Pine DS, Leibenluft E, 2007. Cognitive flexibility in phenotypes of pediatric bipolar disorder. J. Am. Acad. Child Adolesc. Psychiatry 46, 341–355. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Treland JE, Snow J, McClure EB, Mehta MS, Towbin KE, Pine DS, Leibenluft E, 2004. Neuropsychological performance in pediatric bipolar disorder. Biol. Psychiatry 55, 32–39. [DOI] [PubMed] [Google Scholar]
- Frazier JA, Breeze JL, Papadimitriou G, Kennedy DN, Hodge SM, Moore CM, Howard JD, Rohan MP, Caviness VS, Makris N, 2007. White matter abnormalities in children with and at risk for bipolar disorder. Bipolar Disord 9, 799–809. [DOI] [PubMed] [Google Scholar]
- Gao W, Jiao Q, Qi R, Zhong Y, Lu D, Xiao Q, Lu S, Xu C, Zhang Y, Liu X, Yang F, Lu G, Su L, 2013. Combined analyses of gray matter voxel-based morphometry and white matter tract-based spatial statistics in pediatric bipolar mania. J. Affect. Disord 150, 70–76. [DOI] [PubMed] [Google Scholar]
- Gildengers AG, Butters MA, Aizenstein HJ, Marron MM, Emanuel J, Anderson SJ, Weissfeld LA, Becker JT, Lopez OL, Mulsant BH, Reynolds CF 3rd, 2015. Longer lithium exposure is associated with better white matter integrity in older adults with bipolar disorder. Bipolar Disord. 17, 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonenc A, Frazier JA, Crowley DJ, Moore CM, 2010. Combined diffusion tensor imaging and transverse relaxometry in early-onset bipolar disorder. J. Am. Acad. Child Adolesc. Psychiatry 49, 1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrindo T, Blair RJ, Budhani S, Dickstein DP, Pine DS, Leibenluft E, 2005. Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. Am. J. Psychiatry 162, 1975–1977. [DOI] [PubMed] [Google Scholar]
- James A, Hough M, James S, Burge L, Winmill L, Nijhawan S, Matthews PM, Zarei M, 2011. Structural brain and neuropsychometric changes associated with pediatric bipolar disorder with psychosis. Bipolar Disord. 13, 16–27. [DOI] [PubMed] [Google Scholar]
- Kafantaris V, Kingsley P, Ardekani B, Saito E, Lencz T, Lim K, Szeszko P, 2009. Lower orbital frontal white matter integrity in adolescents with bipolar I disorder. J. Am. Acad. Child Adolesc. Psychiatry 48, 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafantaris V, Spritzer L, Doshi V, Saito E, Szeszko PR, 2017. Changes in white matter microstructure predict lithium response in adolescents with bipolar disorder. Bipolar Disord. 19, 587–594. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N, 1997. Schedule for Affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Kikinis Z, Cho KIK, Coman IL, Radoeva PD, Bouix S, Tang Y, Eckbo R, Makris N, Kwon JS, Kubicki M, Antshel KM, Fremont W, Shenton ME, Kates WR, 2017. Abnormalities in brain white matter in adolescents with 22q11.2 deletion syndrome and psychotic symptoms. Brain Image. Behav 11, 1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagopoulos J, Hermens DF, Hatton SN, Tobias-Webb J, Griffiths K, Naismith SL, Scott EM, Hickie IB, 2013. Microstructural white matter changes in the corpus callosum of young people with Bipolar Disorder: a diffusion tensor imaging study. PLoS One 8, e59108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C, 2012. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage 60, 340–352. [DOI] [PubMed] [Google Scholar]
- Lee RS, Hermens DF, Scott J, Redoblado-Hodge MA, Naismith SL, Lagopoulos J, Griffiths KR, Porter MA, Hickie IB, 2014. A meta-analysis of neuropsychological functioning in first-episode bipolar disorders. J. Psychiatry. Res 57, 1–11. [DOI] [PubMed] [Google Scholar]
- Martino M, Magioncalda P, Saiote C, Conio B, Escelsior A, Rocchi G, Piaggio N, Marozzi V, Huang Z, Ferri F, Amore M, Inglese M, Northoff G, 2016. Abnormal functional-structural cingulum connectivity in mania: combined functional magnetic resonance imaging-diffusion tensor imaging investigation in different phases of bipolar disorder. Acta Psychiatry. Scand 134, 339–349. [DOI] [PubMed] [Google Scholar]
- O’Donnell LA, Deldin PJ, Grogan-Kaylor A, McInnis MG, Weintraub J, Ryan KA, Himle JA, 2017. Depression and executive functioning deficits predict poor occupational functioning in a large longitudinal sample with bipolar disorder. J. Affect. Disord 215, 135–142. [DOI] [PubMed] [Google Scholar]
- O’Donnell LA, Deldin PJ, Pester B, McInnis MG, Langenecker SA, Ryan KA, 2017. Cognitive flexibility: A trait of bipolar disorder that worsens with length of illness. J. Clin. Exp. Neuropsychol 39, 979–987. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Yang S, Kamineni K, Passarotti AM, Srinivasan G, Harral EM, Sweeney JA, Zhou XJ, 2009. Diffusion tensor imaging study of white matter fiber tracts in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Biol. Psychiatry 65, 586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BD, Ikuta T, DeRosse P, John M, Burdick KE, Gruner P, Prendergast DM, Szeszko PR, Malhotra AK, 2014. Age-related differences in white matter tract microstructure are associated with cognitive performance from childhood to adulthood. Biol. Psychiatry 75, 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski EO, Mokros HB, 1996. Children’s Depression Rating Scale-Revised (CDRS-R). WPS, Los Angeles. [Google Scholar]
- Saxena K, Tamm L, Walley A, Simmons A, Rollins N, Chia J, Soares JC, Emslie GJ, Fan X, Huang H, 2012. A preliminary investigation of corpus callosum and anterior commissure aberrations in aggressive youth with bipolar disorders. J. Child Adolesc. Psychopharmacol 22, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghete KL, Herting MM, Nagel BJ, 2013. White matter microstructure correlates of inhibition and task-switching in adolescents. Brain Res. 1527, 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S, 1983. A children’s global assessment scale (CGAS). Arch. Gen. Psychiatry 40, 1228–1231. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE, 2006. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM, 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 (Suppl 1), S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE, 2009. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44, 83–98. [DOI] [PubMed] [Google Scholar]
- Teixeira AM, Kleinman A, Zanetti M, Jackowski M, Duran F, Pereira F, Lafer B, Busatto GF, Caetano SC, 2014. Preserved white matter in unmedicated pediatric bipolar disorder. Neurosci. Lett 579, 41–45. [DOI] [PubMed] [Google Scholar]
- Toteja N, Guvenek-Cokol P, Ikuta T, Kafantaris V, Peters BD, Burdick KE, John M, Malhotra AK, Szeszko PR, 2015. Age-associated alterations in corpus callosum white matter integrity in bipolar disorder assessed using probabilistic tractography. Bipolar Disord. 17, 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit S, Chen Z, Rasmussen C, Beaulieu C, 2014. White matter correlates of cognitive inhibition during development: a diffusion tensor imaging study. Neuroscience 276, 87–97. [DOI] [PubMed] [Google Scholar]
- Wechsler D, 2005. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Wegbreit E, Cushman GK, Weissman AB, Bojanek E, Kim KL, Leibenluft E, Dickstein DP, 2016. Reversal-learning deficits in childhood-onset bipolar disorder across the transition from childhood to young adulthood. J. Affect. Disord 203, 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE, 2014. Permutation inference for the general linear model. Neuroimage 92, 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry 133, 429–435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


