ABSTRACT
Many animal species show aggression to gain mating partners and to protect territories and other resources from competitors. Both male and female fruit flies of the species Drosophila melanogaster exhibit aggression in same-sex pairings, but the strategies used are sexually dimorphic. We have begun to explore the biological basis for the differing aggression strategies, and the cues promoting one form of aggression over the other. Here, we describe a line of genetically masculinized females that switch between male and female aggression patterns based on the sexual identity of their opponents. When these masculinized females are paired with more aggressive opponents, they increase the amount of male-like aggression they use, but do not alter the level of female aggression. This suggests that male aggression may be more highly responsive to behavioral cues than female aggression. Although the masculinized females of this line show opponent-dependent changes in aggression and courtship behavior, locomotor activity and sleep are unaffected. Thus, the driver line used may specifically masculinize neurons involved in social behavior. A discussion of possible different roles of male and female aggression in fruit flies is included here. These results can serve as precursors to future experiments aimed at elucidating the circuitry and triggering cues underlying sexually dimorphic aggressive behavior.
KEY WORDS: Drosophila, Aggression, Sexual dimorphism
Summary: Genetically masculinized female Drosophila melanogaster change their fighting strategy depending on the sexual identity of their opponents. With more aggressive flies, these females adapt to higher intensity levels.
INTRODUCTION
Aggression is widespread in the animal kingdom, with animals fighting to obtain resources such as food and mating opportunities. Male and female fruit flies (Drosophila melanogaster) will fight over a small cup holding a desirable food source; in addition, males will fight for the opportunity to mate with females (Chen et al., 2002). Although male and female flies show aggression towards other flies of the same sex, the two sexes employ different behavioral patterns when fighting (Nilsen et al., 2004). Males display a mid-intensity behavior called a lunge, in which they rise up on their hind legs and snap down on their opponent (Chen et al., 2002). If one fly retreats after being lunged at, the other has a far greater likelihood of becoming the winner of the fight (Chen et al., 2002; Yurkovic et al., 2006). Females, by contrast, employ a mid-intensity behavior called a head butt, in which they move rapidly forward to strike their opponents, then recoil. Unlike the lunge, the head butt does not predict the outcome of the fight, and is strictly in the horizontal plane. Furthermore, while a lunge visibly destabilizes the fly receiving it, a head butt does not and thus appears to be less intense (Nilsen et al., 2004). In the fight chambers we use routinely, only males form dominance relationships (Nilsen et al., 2004). Recently, however, we found that activating a subset of the PC1 neurons causes female flies to fight at levels comparable to the high intensities that males display and establish hierarchical relationships (Palavicino-Maggio et al., 2019). The observation of these highly aggressive females suggests that female aggression can proceed to intensities beyond head butts. Indeed, higher intensity aggression has been occasionally observed in wild-type female fights (Nilsen et al., 2004). In typical fights with wild-type D. melanogaster, both male and female flies engage in low intensity behaviors such as low-posture fencing (opponents extend their legs to tap each other). This is usually followed by mid-intensity patterns such as lunges or head butts. After that, males progress to the highest intensity aggressive behaviors of fights such as boxing or high posture fencing (HPF). In both of these high intensity behaviors, male flies stand on their hind legs and either fence or lunge at each other (Chen et al., 2002; Dow and von Schilcher, 1975). However, such high intensity behaviors are rare even in male fights, indicating that not all fights are destined to progress to the highest levels. Among wild-type flies, male aggression against females has not been observed, although females can display a rejection behavior if they want to discourage pursuing males.
Why some fights progress to high intensity levels, while others do not, is still not fully understood. Genetics undoubtedly plays a role as multiple genes have been found to be associated with control of aggression (Chowdhury et al., 2017; Edwards et al., 2009; Shorter et al., 2015). Males also can be bred to be hyperaggressive ‘bullies’ that almost always win fights against genetic background controls, and that commonly display high intensity behaviors such as boxing (Dierick and Greenspan, 2006; Penn et al., 2010). We also know that aggression levels can be regulated by brain neurohormonal modulators, as activating serotonergic and tachykininergic-mediated circuitry increases aggression, and dopamine, octopamine, neuropeptide F and other substances also alter aggressive behavior (Alekseyenko et al., 2014, 2013, 2019; Asahina et al., 2014; Certel et al., 2007; Dierick and Greenspan, 2007; Hoyer et al., 2008). In addition, activation of specific cholinergic neurons stimulates high intensity aggressive behavior only in females (Palavicino-Maggio et al., 2019). While these findings are intriguing, none explain why some fights between presumptively genetically identical flies progress to higher intensity levels than others.
The transition between fighting using female or male patterns of aggression is also a switch to higher intensity aggression. Therefore, understanding what motivates this transition might give us insight into why and how flies choose their aggressive strategies. Because wild-type flies typically use fight patterns appropriate to their sex in agonistic encounters with conspecifics, it is difficult to determine what conditions stimulate the use of one aggression strategy over the other. Here, we have identified a line of masculinized female flies that can switch between female and male patterns of aggression depending on the sex of their opponent. Hence, these experiments have the potential to give us new insight into decision-making on this important behavior.
The flies used in these experiments were masculinized in neurons targeted by the 60IIA-Gal4 driver, which is broadly expressed in the brain (Chan and Kravitz, 2007). To masculinize the neurons, we took advantage of the Drosophila sex-determination pathway, in which the presence of Transformer (TRA) causes cells to produce a female-specific splicing variant of doublesex (dsx) (Burtis and Baker, 1989). In the absence of the TRA protein, cells produce male-specific transcripts of dsx and fruitless (fru), resulting in male-like behavior and morphology (Billeter et al., 2006; Burtis and Baker, 1989; Ryner et al., 1996; Villella and Hall, 1996). To masculinize select neurons in the female fly brain, we used RNAi against the transformer (tra) gene in the form of a UAS-traRNAi construct, and paired it with the 60IIA-Gal4 driver to create 60IIA>traRNAi masculinized females.
In this study, we sought to understand the conditions that induce male rather than female aggression. We found that 60IIA>traRNAi masculinized females, which were first identified in Chan and Kravitz (2007), change their aggressive behavior based on behavioral cues from their opponent. Specifically, 60IIA>traRNAi masculinized females use female aggression when paired with other females, but switch to male aggression when paired with males. This suggests that, in addition to the well-known pheromonal and visual identification cues used by fruit flies (Agrawal et al., 2014; Coyne and Oyama, 1995; Ferveur et al., 1997) to determine the sex of conspecifics, the behavior of opponents also can serve as important cues in determining aggressive strategy. The masculinization of the 60IIA>traRNAi females appears to be specific to social behavior, as sleep and activity were not affected by the silencing of tra. These results suggest that multiple cues, including the behavior of conspecifics, may be important for inducing flies to choose and possibly switch between behavioral strategies used in social interactions.
MATERIALS AND METHODS
Fly husbandry and stocks
Drosophila melanogaster Meigen 1830 stocks were raised on standard fly food medium containing cornmeal, sucrose, yeast and agar. All crosses used to produce experimental flies were maintained at 25°C on a 12 h:12 h light:dark (LD) cycle.
The following stocks were used to produce the flies used in the study: w*; 60IIA-Gal4;+ (obtained from Bloomington Stock Center, #7029), w*; elav-Gal4/Cyo;+ (obtained from Bloomington Stock Center, #8765) and w*; UAS-traRNAi;+ (obtained from Barry Dickson, Vienna Drosophila RNAi Center #2560, also referred to as traIR). Flies used for female–female aggression and sleep/circadian experiments were crossed into the Canton-S background to mitigate background-mediated behavioral effects.
Aggression experiments
Flies used for aggression assays were collected as dark pupae about 24 h before eclosion, and placed in individual glass tubes (47729-576, VWR International, Radnor, PA, USA). These tubes were incubated at 25°C on a 12 h:12 h LD cycle until the day of testing. For female–female parings, flies were anesthetized with carbon dioxide and painted with a dot of either white or blue acrylic paint at least 3 days before testing.
Testing was conducted 5–7 days after eclosion in a room kept at 24–26°C and 45–65% humidity. Twelve-well plates (353043, Corning, Corning, NY, USA) were used as chambers for testing pairs of flies. On the day of testing, a microfuge tube lid (SCO-C, Corning, Corning, NY, USA) was filled with standard fly food, topped with a dot of yeast paste, then placed in each chamber. Chambers were covered with glass slides, and fly pairs were introduced into chambers at the same time by gentle aspiration. Fights were then filmed with Sony Digital Handycam camcorders for 1–1.5 h.
Videos of fights were scored blinded to genotype and condition. Only behavior occurring on the food cup was scored, so videos in which flies did not encounter each other on the food cup within 50 min were not analyzed. A goal sample size of n=25–30 fighting pairs was determined before the start of the experiments. In fights where behaviors were counted, fights were scored for 10 min beginning with the first encounter on the food cup. A head butt was scored when a fly lurched horizontally forward and then visibly recoiled. A lunge was scored when a fly reared up on its hind legs and rapidly snapped downwards onto its opponent. A lunge-like behavior was scored when a fly moved forward with some vertical movement that did not achieve the height or speed of a lunge. Finally, a wing extension was scored when a fly extended a wing all the way out to the side and appeared to vibrate it. Statistical analysis was conducted using Prism (GraphPad Software, San Diego, CA, USA, versions 5.0b and 8), and statistical significance was determined using a Kruskal–Wallis test with Dunn's post hoc test, a Mann–Whitney test, a Fisher's exact two-tailed test, a chi-squared test or a Spearman's test as appropriate.
Circadian and sleep experiments
Locomotor activity, sleep and circadian rhythms of adult male and female flies were recorded using DAM2 Drosophila Activity Monitors (TriKinetics, Waltham, MA, USA). Three- to 5-day old flies were placed individually in Trikinetics capillary tubes with 2% agar–4% sucrose food and loaded onto the DAM2 monitors. For standard LD entrainment and transfer to free running conditions, flies were exposed to 12 h:12 h LD cycles for 5 days and then released into constant darkness and temperature for 8 days (constant darkness and a temperature of 25°C). For LD conditions, lights on [zeitgeber time 0 (ZT0)] was at 09:00 h and lights off (ZT12) at 21:00 h.
Activity counts were collected in 1-min bins that were summed into 30-min bins for the time-series analysis of locomotor activity. Averaged population activity profiles of specific genotypes in LD were generated in MATLAB (MathWorks, Natick, MA, USA). Activity levels were normalized for individual flies by setting the average activity level for all 30-min bins across the last 4 days in LD equal to 1.0. Population averages of this normalized activity were then determined for each 30-min bin over the last 3 days of entrainment (LD3–5). Finally, the population averages for these three LD cycles were averaged into a single representative 24-h day, which are displayed as either histograms (for activity) or line plots (for sleep).
The error bars displayed in all figures represent ±s.e.m. All datasets were tested for normality using a D'Agostino–Pearson normality test in GraphPad Prism 8.0. For parametric datasets, unpaired one-way ANOVAs with Tukey's multiple comparisons tests were used. For non-parametric datasets, Kruskal–Wallis tests with Dunn's multiple comparisons tests were used. Asterisks indicate statistical significance, where *P<0.05, **P<0.01 and ***P<0.001.
Immunohistochemistry and confocal microscopy
Immunostaining of whole-mount D. melanogaster adult brains was done as previously described (Fernandez et al., 2020). Flies were entrained to 12 h:12 h LD cycles at 25°C and heads from adult females were fixed in 4% formaldehyde for 1 h at 4°C, at ZT2. Brains were dissected in PBS and rinsed in PBS+0.3% Triton (PBS-TX) for 1 h (4×15 min). Then, brains were blocked with a 3% normal goat serum solution for 1 h at room temperature and incubated with primary antibodies at 4°C for two nights, and finally rinsed in PBS-TX. The following antibodies were used: rabbit anti-GFP (1:1000, Invitrogen A-6455, Carlsbad, CA, USA), rat anti-mCD8 (1:100, clone 5H10, MCD0800, Invitrogen) and mouse anti-nc82 (1:50, Developmental Studies Hybridoma Bank, Iowa City, IA, USA). The primary antibody was rinsed from brains five times in PBS-TX for 15 min or more with high agitation. The brains were then placed in the secondary antibody cocktail overnight at 4°C, after which they were rinsed in PBS-TX as with the primary antibody. Alexa Fluor conjugated secondary antibodies (Alexa Fluor 488 and 568 conjugated goat anti-mouse, Jackson Immuno Research Labs, West Grove, PA, USA) were diluted 1:1000. Brains were rinsed three times in PBS, mounted on a poly-l-lysine coated cover slip, dehydrated/cleared in a graded glycerol series (30%, 50% and 70% glycerol in PBS, 5 min each), and then mounted between coverslip bridges in HardSet Vectashield Mounting Medium (Vector Laboratories, Burlingame, CA, USA). Samples were viewed on an Olympus Fluoview 1000 or Olympus Fluoview 3000 laser-scanning confocal microscope using either a UplanSApo 20x/0.75 NA or a 60x/1.10 NA W, FUMFL N objective (Olympus, Center Valley, PA, USA).
RESULTS
Genetically masculinized females use female aggression and male courtship in same-genotype pairings
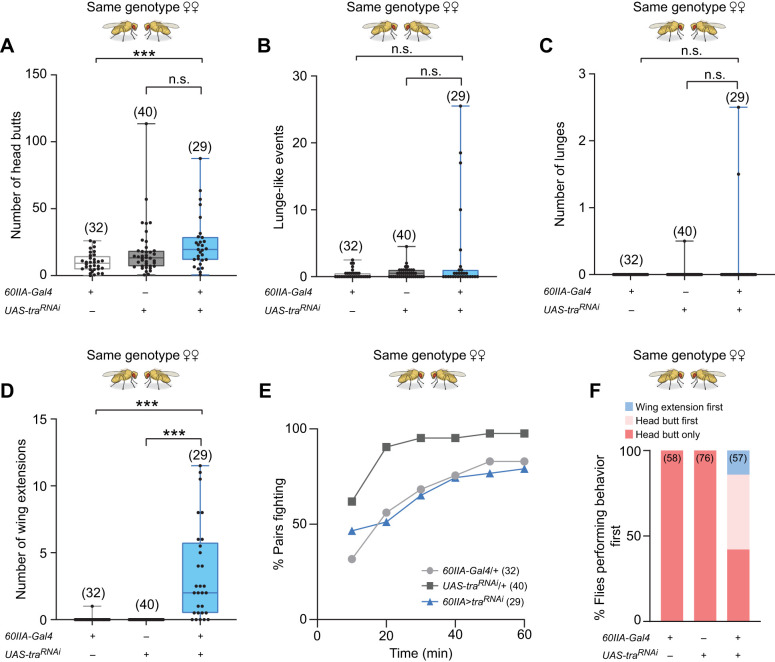
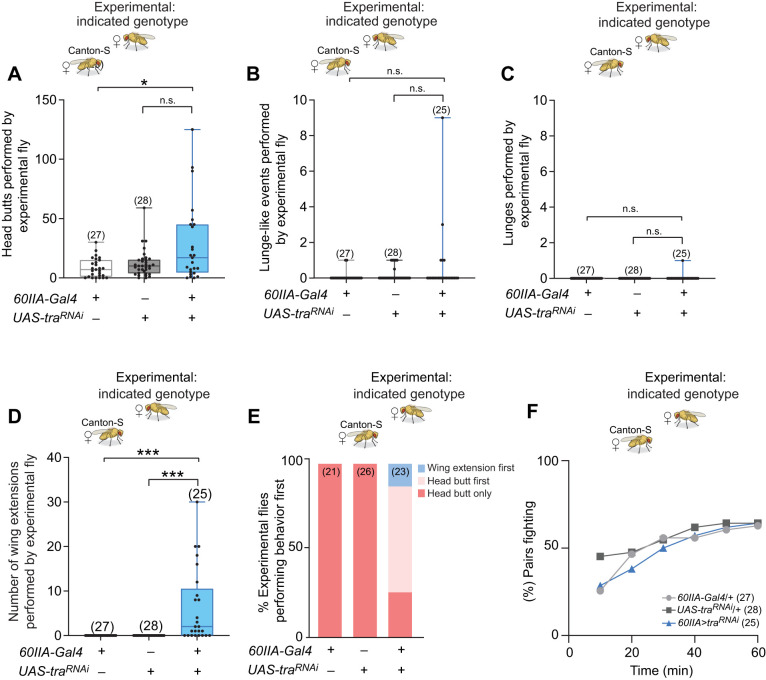
In these experiments, we paired 60IIA>traRNAi masculinized females with second females of the same genotype. As Chan and Kravitz (2007) reported previously, 60IIA>traRNAi masculinized females directed many head butts (Fig. 1A), a female aggressive behavior, towards their opponents. The numbers of head butts performed by the masculinized females were significantly higher than the Gal4 controls, but were not higher than their UAS controls. Although the 60IIA>traRNAi masculinized females clearly showed female aggressive behavioral patterns towards other females, as anticipated, they displayed no enhancement of male aggressive behavioral patterns (lunges or lunge-like behaviors) in these bouts. Instead, we observed very low levels of male aggression patterns similar to those observed in the UAS and Gal4 controls (Fig. 1B,C). We did note, however, that although the male-like aggressive behaviors of the 60IIA>traRNAi masculinized females were not significantly different from the behaviors of the controls, they appeared to show an increased variability in both lunging and lunge-like behaviors (Fig. 1B,C). Although the aggressive behavior of the 60IIA>traRNAi masculinized females was not significantly masculinized, their courtship behavior was. The 60IIA>traRNAi masculinized females displayed multiple wing extensions (Fig. 1D), a behavior seen frequently in males courting females, but rarely or never seen in wild-type or parental control females. The 60IIA>traRNA masculinized females thus appeared to view their opponents both as potential aggressors (from a female perspective) and as potential mates (from a masculine perspective).
Fig. 1.
Pairs of 60IIA>traRNAi masculinized female Drosophila melanogaster use primarily female aggressive behavioral patterns and exhibit male-like courtship during fights. Fights between pairs of 60IIA>traRNAi masculinized females, pairs of 60IIA control females or pairs of traRNAi control females. Behavioral patterns were scored for 10 min from the first encounter (meeting between the flies). (A) Average number of head butts performed by females of the indicated genotypes. Each dot represents the average for one fight (Kruskal–Wallis test with Dunn's multiple comparison test). (B) Average number of lunge-like behavioral patterns displayed (Kruskal–Wallis test with Dunn's multiple comparison test). (C) Average number of lunges displayed (Kruskal–Wallis test with Dunn's multiple comparison test). (D) Average number of wing extensions performed (Kruskal–Wallis test with Dunn's multiple comparison test). (E) Percent of pairs that began fighting (showing head butts) over a 1-h period. (F) Percent of flies that performed a wing extension before a head butt (wing extension first), a head butt before a wing extension (head butt first), or that showed only head butts and no wing extensions (head butt only). For all histograms, *P<0.05, **P<0.01, ***P<0.001, n.s., not significantly different.
Averaging the numbers of head butts or wing extensions as in Fig. 1A–D, provides little information on what happens during a fight (the dynamics of fights) between paired flies. Examining the dynamics is important in determining whether the experimental females initially view each other as potential mating partners or as potential agonistic opponents and/or whether they might switch or vary their behavioral patterns during fights. To ask whether switching between sex-selective patterns of courtship and aggression might take place during fights, we examined the fight dynamics. First, over a 1-h time period, we recorded the times at which the pairs of flies first began to fight. The results showed that 60IIA>traRNAi masculinized and control female pairs began fighting and continued to initiate fighting along almost identical timelines (Fig. 1E). This suggests that the 60IIA>traRNAi masculinized females do not have an increased arousal threshold. It also suggests that even though they appear to view other females as potential mates, that does not inhibit or enhance their aggression towards each other. To try to understand whether 60IIA>traRNAi masculinized females view other females primarily as mates or as opponents, we asked whether the 60IIA>traRNAi masculinized females courted or struck other females first. We found that the majority (∼44%) initiated their encounters with aggression rather than courtship (∼14%), while the other ∼42% performed no courtship behavior at all (Fig. 1F), indicating that the 60IIA>traRNAi masculinized females primarily view other females as opponents. Finally, we examined how aggressive and courtship behavioral patterns were used by these flies during the entire 1-h time period during which they were together. For this purpose, we selected five experiments in which the pairs of 60IIA>traRNAi masculinized females displayed high levels of both courtship and aggression and recorded the behaviors performed throughout the fights. We found that courtship and aggression frequently occurred in quick succession (Fig. S1A,B, red and blue bars), suggesting that 60IIA>traRNAi masculinized females likely receive mixed signals from each other that provoke them to both fight and attempt to court at the same time.
The male courtship behavior observed with the 60IIA>traRNAi masculinized females sets them apart from wild-type females, but interestingly, in several cases even their aggressive behavior was not entirely like that of normal females. In a few of the 60IIA>traRNAi masculinized female pairings, we observed a high intensity aggressive behavior that we have not seen before in females (Movie 1). Several pairs also exhibited a distinctive circling behavior that appeared more like aggression than like the normal circling displayed by males during courtship (Movie 1, Fig. S1A,B). In addition, although the amount of lunge-like behavior performed by the 60IIA>traRNAi masculinized females was not significantly different from that of control females, three pairs displayed an average of 15 or more lunge-like movements during fights (Fig. 1B). Numbers as high as that have never been seen before in even the most intense of wild-type female fights. These observations suggest that under certain circumstances and with different behavioral cues, the 60IIA>traRNAi masculinized females might have the potential to alter their usage of patterns of aggression.
60IIA>traRNAi masculinized females switch to male-like aggression when paired with wild-type males
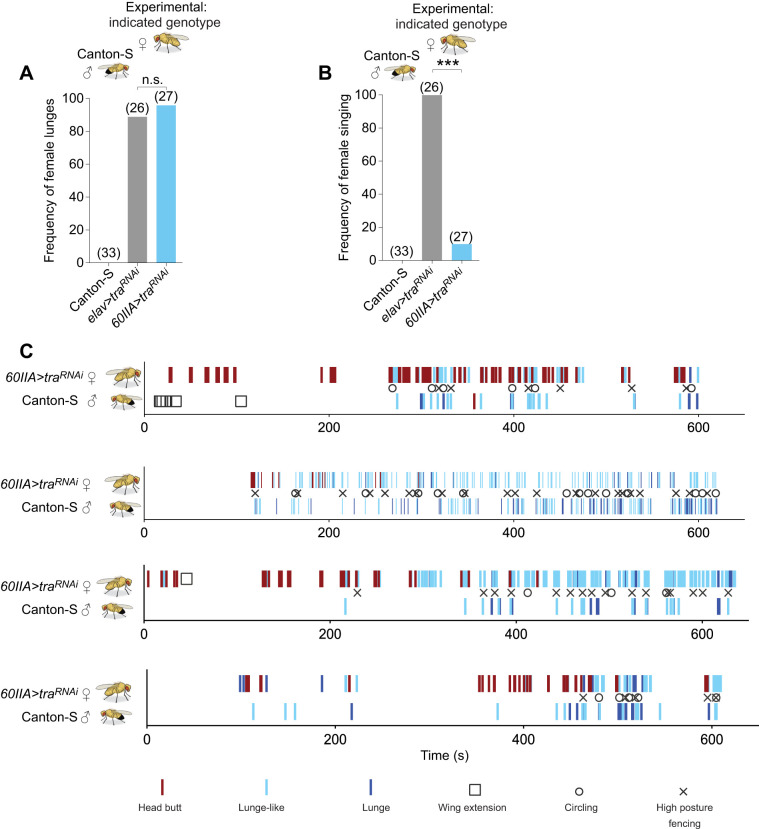
Next, we asked whether an opposite sex opponent might provoke different responses from the experimental females. In these experiments, we paired 60IIA>traRNAi masculinized females with wild-type (CS) males and measured their behavior. In these pairings, almost all females exhibited male-like aggression, and were as likely to lunge in fights as pan-neuronally masculinized elav>traRNAi females (Fig. 2A). The latter females have previously been shown to fight like males (Chan and Kravitz, 2007; Fernandez et al., 2010). Unlike the elav>traRNAi females, however, the 60IIA>traRNAi masculinized females only infrequently displayed wing extensions during fights with males (Fig. 2B). This suggests that the 60IIA>traRNAi masculinized females may directly target wing extensions to female partners as would be expected of a wild-type male fly. In the next experiments, we analyzed the dynamics of five fights in which 60IIA>traRNAi masculinized females showed high levels of high intensity male aggression. This was to determine whether the 60IIA>traRNAi masculinized females fought like males throughout their bouts with male opponents. To our surprise, in most of these fights, the 60IIA>traRNAi masculinized females started the fights predominantly using female patterns of aggression, and then switched to using mainly male patterns of aggression (Fig. 2C, Fig. S2). Hence, these results suggest that 60IIA>traRNAi masculinized females change their aggressive behavior based on cues they receive from their opponents, raising questions of how and why this change occurs.
Fig. 2.
60IIA>traRNAi females fight using male patterns of aggression when paired with Canton-S males. Fights between Canton-S males and females of the indicated genotypes. (A) Female lunge frequencies (Fisher's two-tailed test). (B) Frequency of female singing (Fisher's two-tailed test). (C) Behaviors performed by four pairs of 60IIA>traRNAi females versus Canton-S males. Scoring is shown for the first 10 min of the 1-h fight period. Circling was scored when flies rapidly chased each other in a tight circle; wing extension, unilateral wing extension; high posture fencing was scored when flies fought on their hind legs. Although high posture fencing and circling behaviors can last for many seconds, in these fights the duration of the behaviors was brief, so it was not recorded. For all histograms, ***P<0.001, n.s., not significantly different at >0.05.
As a first step to try to understand why 60IIA>traRNAi females changed their behavior based on information supplied by opponents, we attempted to examine the circuitry that was masculinized by using the Gal4 driver with the nuclear-localized UAS-nls-GFP (Fig. S3A). As noted in our previous report (Chan and Kravitz, 2007), the driver was broadly expressed, making it difficult to identify the neurons relevant to the aggression phenotype. One possible explanation for sexually dimorphic behavior is morphological differences in neurons between males and females, so to identify possibly relevant neurons, we used the membrane-bound UAS-mCD8::GFP to determine whether the 60IIA-Gal4 expression pattern differed in male and female brains and ventral nerve cords. Because the 60IIA-Gal4 driver is so widely expressed, it was difficult to discern the neural morphology of all labeled neurons; however, we did not see any overt morphological differences between females and males (Fig. S3B,C). Although we did not observe any sexually dimorphic structures, we noted that both males and females showed clear expression in the β-lobes of the mushroom bodies (Fig. S3D), a region of the fly brain most frequently associated with learning and memory (de Belle and Heisenberg, 1994; Dubnau et al., 2001; McGuire et al., 2001), but also suggested to play a role in aggression (Baier et al., 2002; Zwarts et al., 2015).
Wild-type males attack rather than court masculinized females
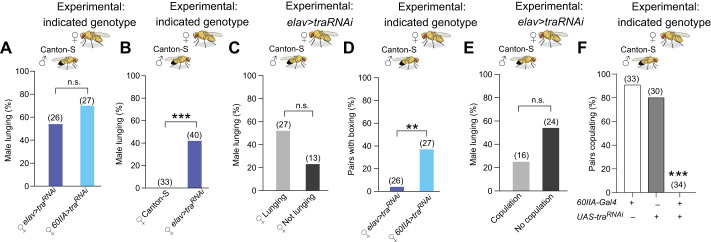
The importance of behavioral cues to aggression is seen not only in the switch the 60IIA>traRNAi masculinized females make from female to male aggression, but also in the switch wild-type males make from courtship to aggression. Wild-type males will lunge at females displaying male-like aggressive behavior, an observation first reported in Fernandez et al. (2010). This finding is replicated here with the 60IIA>traRNAi masculinized females, as CS males were as likely to lunge in fights with these females as they were with elav>traRNAi females (Fig. 3A). Thus, both genotypes of masculinized females have similar abilities to elicit male aggression. Wild-type males never lunged when paired with CS females, but lunged in approximately 50% of the fights when paired with elav>traRNA masculinized females (Fig. 3B). When elav>traRNAi females lunged, the CS-control males lunged more often, an effect that did not achieve statistical significance (Fig. 3C). We also saw significantly more boxing in the 60IIA>traRNAi masculinized females than in the original elav>traRNAi females (Fig. 3D), suggesting that the 60IIA>traRNAi masculinized females might be using additional unique behavioral cues that escalate the fights to higher intensity levels. Another behavioral cue that could be responsible for provoking aggression by wild-type males towards females is a reluctance on the part of the females to copulate. We examined this possibility, but did not see a statistically significant link between the percentage of males that lunged and their success at copulating with elav>traRNAi females (Fig. 3E). Strikingly, when we examined copulation rates in 60IIA>traRNAi masculinized females, we found that they failed to copulate during a 1-h observation period (Fig. 3F). It should be noted, however, that these females will copulate in some circumstances, albeit at greatly reduced rates compared with wild-type flies. Differences in gene expression in individual cells or neuron populations labeled by the two Gal4 drivers (60IIA and elav) used in these experiments could contribute to variances in the masculinized expression phenotype. Further experiments reducing the populations of neurons involved in retaining the phenotype will be important in clarifying the differences observed.
Fig. 3.
Canton-S males respond to masculinized females with male-like aggression. Fights between a Canton-S male and a female of the indicated genotype. (A,B) Percentage of fights in which the male lunged at the female (Fisher's two-tailed test). (C) Percentage of fights in which the male lunged at the elav>traRNAi female when the masculinized female was observed lunging or not lunging (Fisher's two-tailed test). (D) Percentage of fights in which the aggressive encounter escalated to boxing (Fisher's two-tailed test). (E) Percentage of fights in which the male lunged at the female when copulation was observed and when copulation was not observed (Fisher's two-tailed test). (F) Percentage of females copulating with a Canton-S male (chi-squared test). For all histograms, **P<0.01, ***P<0.001, n.s., not significantly different.
Is there specificity in the social behavior influenced by masculinization of 60IIA>traRNAi females?
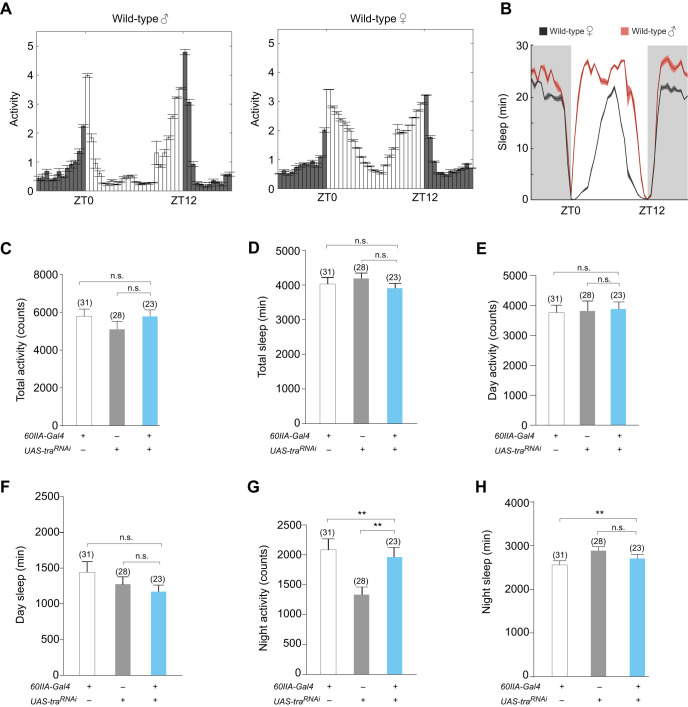
We have established that 60IIA>traRNAi masculinized females can display male-like aggression and courtship behavior depending on the identity of their opponent. We next wanted to determine whether the 60IIA>traRNAi females might be masculinized in other aspects of behavior. Adult D. melanogaster exhibit sexually dimorphic differences in amounts of sleep and levels of activity (Huber et al., 2004). Therefore, we measured these parameters in isolated 60IIA>traRNAi masculinized females. Wild-type female fruit flies exhibit more overall activity and less overall sleep than males (Fig. 4A,B), largely because the males take a siesta during the day (Huber et al., 2004). When we measured combined activity and sleep, 60IIA>traRNAi masculinized females displayed similar levels to female controls over a 24-h period (Fig. 4C,D). However, these observations do not preclude the possibility that there might be changes in finer details within their day- or night-time activity and sleep. To address this possibility, we examined day- and night-time activity and sleep separately. When we measured daytime behavior, we found that 60IIA>traRNAi masculinized females exhibited sleep and activity levels comparable to those of control females (Fig. 4E,F). Similarly, when we examined night-time behavior, we found that the sleep and activity levels of 60IIA>traRNAi masculinized females were no different than those of control females (Fig. 4G,H). These results suggest that the masculinization of 60IIA>traRNAi females has no influence on sleep or activity.
Fig. 4.
60IIA>traRNAi masculinized females exhibit sleep and activity patterns similar to those seen in wild-type females. Activity and sleep profiles of experimental and control flies. (A) Average activity profiles of wild-type males and females for days 3–5 of a 12 h:12 h light:dark cycle (LD). Zeitgeber time 0 (ZT0) indicates lights-on, ZT12 indicates lights-off. Dark gray bars indicate night. (B) Sleep patterns of Canton-S males and females during LD 3–5. (C) Total activity for days 3–5 of a 12 h:12 h LD cycle, measured by total number of beam crossings. (D) Average total daily sleep for days 3–5 during a LD cycle. (E) Total activity during the day phase (ZT0–ZT12), LD 3–5. (F) Total amount of sleep during the day phase (ZT0–ZT11). (G) Total activity during the night phase (ZT12–ZT23), LD 3–5. (H) Total amount of sleep during the night phase (ZT12–ZT23). For all histograms, *P<0.05, **P<0.01, ***P<0.001, n.s., not significantly different. For all activity plots, error bars represent means±s.e.m.
60IIA>traRNAi masculinized females use male fighting strategies towards more aggressive opponents
The above experiments established that 60IIA>traRNAi masculinized females show female aggression toward same-genotype female opponents, and a switch towards male aggression against male opponents. Next, we asked how they would behave towards control and wild-type female opponents, because such females normally show no patterns of male aggression. When the 60IIA>traRNAi masculinized females were paired with wild-type females, they displayed head butts (normal aggression for females) toward their wild-type opponents (Fig. 5A), and did not show significantly more lunge-like or lunging behaviors, although they appeared to show more variability in these behaviors than the controls (Fig. 5B,C). This confirmed that 60IIA>traRNAi masculinized females use female aggression with female opponents.
Fig. 5.
60IIA>traRNAi masculinized females paired with Canton-S females use primarily female aggressive behavior and show male courtship. Fights between a Canton-S female and females of indicated genotypes (experimental flies). Behaviors were scored for 10 min from the first encounter. (A) Number of head butts performed by experimental fly (Kruskal–Wallis test with Dunn's multiple comparison test). Note a much wider variation in the distribution of experimental flies. (B) Number of lunge-like behaviors performed by experimental fly (Kruskal–Wallis test with Dunn's multiple comparison test). Again, note the wider distribution of lunge-like behaviors in experimental flies. (C) Number of lunges performed by experimental fly (Kruskal–Wallis test with Dunn's multiple comparison test). (D) Number of wing extensions performed by experimental fly (Kruskal–Wallis test with Dunn's multiple comparison test). (E) Percent of flies that performed a wing extension before a head butt (wing extension first), a head butt before a wing extension (head butt first), or that showed only head butts and no wing extensions (head butt only). (F) Percent of pairs that began fighting (showing head butts) over a 1-h period. For all histograms, *P<0.05, ***P<0.001, n.s., not significantly different.
We then examined courtship behavior, to see whether the 60IIA>traRNAi masculinized females view wild-type females more like potential mates than they do same-genotype females. Not surprisingly, the 60IIA>traRNAi masculinized females performed wing extensions toward wild-type females (Fig. 5D), just as they had against other masculinized females. Close to 61% showed head butts before wing extensions, and a further 26% showed only aggressive behavior without any courtship behavior (Fig. 5E). Thus, even though the wild-type females showed no male aggressive behavior, the 60IIA>traRNAi masculinized females still viewed them primarily as opponents. Therefore, 60IIA>traRNAi masculinized females appear to behave similarly towards wild-type females as they do towards same-genotype females.
To examine the behavior of 60IIA>traRNAi masculinized females versus wild-type and parental control females in greater detail, we examined the dynamics of the fights. First, we measured the percentage of pairs fighting over a 1-h period, and found that 60IIA>traRNAi/CS female pairs started fighting at similar rates to UAS and Gal4 control/CS female pairs (Fig. 5F). We also examined whether the 60IIA>traRNAi masculinized females were more likely to strike before the CS females. In male fights, the fly that lunges first typically controls and ultimately wins the fight (Chen et al., 2002). Therefore, we wondered whether the 60IIA>traRNAi masculinized females would initiate fights by striking their opponents before the controls. The 60IIA>traRNAi masculinized females were more likely to strike before their CS opponents, but the UAS and Gal4 control females also invariably struck before the CS females (Fig. S4A). We have no explanation for the finding that both the parental controls and the 60IIA>traRNAi masculinized female flies appear to be more aggressive in initiating fights. When we examined the numbers of head butts performed by all genotypes, we found that CS females performed significantly fewer head butts than 60IIA>traRNAi masculinized females (Fig. S4B). These results present the opportunity for us to ask how behavior of the 60IIA>traRNAi masculinized females differs when paired with less aggressive opponents (CS females) and more aggressive same-genotype females.
We first asked whether the 60IIA>traRNAi masculinized females would court less if their opponent was more aggressive. We found, however, that there was no difference in the number of wing extensions performed by 60IIA>traRNAi masculinized females towards same-genotype or wild-type females (Fig. S4C). Next, we asked whether aggression levels would be altered by a less aggressive opponent. We found no difference in the number of head butts performed towards a same-genotype or wild-type opponent (Fig. S4D), but a small, yet significantly greater number of lunge-like behaviors were performed towards same-genotype females (Fig. S4E). To confirm the validity of this result, we asked whether a correlation existed between the number of head butts or lunge-like behaviors performed by one 60IIA>traRNAi masculinized female with the number of behaviors performed by the second fly in the pairing. When we measured the head butts performed by each fly, we saw no correlation in either the controls or the 60IIA>traRNAi masculinized female pairings (Fig. S4F). In contrast, when we scored the lunge-like behaviors, we saw a significant correlation in fights between the 60IIA>traRNAi masculinized females. This suggests that although these flies seldom use male aggressive patterns, when they do, it provokes male behavior from their opponents (Fig. S4G). This suggests that with the 60IIA>traRNAi masculinized females, male-like aggressive behavior may be more susceptible to change based on behavior of their opponents.
DISCUSSION
One way to better understand increased aggression in fruit flies is to look at the differences between male and female fighting behavior; one striking difference in normal flies is that male fights commonly go to much higher intensity levels than female fights. Here, we have identified a line of masculinized females that will use different aggressive strategies depending on their opponent. When these females are paired with a female opponent, they show female patterns of aggressive behavior exclusively (Figs 1, 5), but when paired with male opponents, they transition to using male patterns of aggression (Fig. 2). The 60IIA>traRNAi masculinized females appear to be fully capable of going to the highest levels of male aggressive behavior, as a high proportion of pairs exhibited boxing (Fig. 3D). Boxing is rare and is seen in only the most intense male–male fights. The results suggest that the 60IIA>traRNAi masculinized females possess a versatile repertoire of aggressive behaviors, which they can selectively employ based on the sexual identity and behavioral patterns displayed by their opponents.
What causes the behavioral switch from female to male aggression? We know from our previous experiments that both behavioral and pheromonal cues can stimulate male aggression (Fernandez et al., 2010). Male and female fruit flies display sexually dimorphic hydrocarbon profiles on their cuticular surfaces, and these have been proposed as pheromonal signals that allow other flies to identify the sex and species of conspecifics (Coyne and Oyama, 1995; Ferveur et al., 1997; Savarit et al., 1999). Our observation that 60IIA>traRNAi masculinized females use female patterns of aggression with females and male patterns with males suggests that pheromonal cues are an important part of the decision to alter strategy. Our laboratory showed further that females with masculinized cuticular hydrocarbon profiles will trigger lunging from males (Fernandez et al., 2010). It is likely, therefore, that male pheromonal profiles, when on the surfaces of 60IIA>traRNAi masculinized females, serve as important cues that trigger lunging.
However, when we examined closely the dynamics of the fights between 60IIA>traRNAi masculinized females and wild-type males, we saw that the 60IIA>traRNAi masculinized females typically began fights using female patterns of aggression, and progressed to using male patterns later in the fight. The switch in pattern usage typically began in conjunction with the males beginning to lunge (Fig. 2C, Fig. S2). These findings suggest that pheromonal cues alone may not be sufficient to trigger behavioral switching, and that behavioral cues from the opponent are also important for the switch in strategy. It is also notable that the wild-type males override the pheromonal cues promoting courtship and attack the 60IIA>traRNAi masculinized females, presumably based on behavioral cues presented by the masculinized females (Fernandez et al., 2010). Lunging and lunge-like behaviors are clear cues that could promote a transition, but if that is the case, what triggers the first lunge and who delivers it? Perhaps behavioral signals more subtle than lunging are involved in stimulating male aggressive behavior. We know that wing movements (such as wing threats or wing flicking) and leg movements (such as fencing) are components of D. melanogaster fights (Chen et al., 2002; Nilsen et al., 2004), but it is not known whether these less overtly aggressive movements could trigger male aggression in an opponent.
In trying to better understand how female aggression is changed to male aggression, we should also ask whether the switch to male aggression occurs because female and male aggression serve different functions. We were surprised to find that masculinized females paired with more aggressive opponents significantly increased the numbers of lunge-like behaviors performed, but did not significantly increase the number of head butts (Fig. S4). Because lunge-like behaviors are primarily performed by males, these results raise an interesting question: are female aggressive behaviors less dependent on the antagonism of the opponent, and if so, why? Male aggression appears to occur with the goal of driving an opponent away from a desirable resource, as male fights usually go to higher intensity levels and are characterized by the establishment of dominance relationships that allow them to control a won territory (Chen et al., 2002). Females, in contrast, commonly share territory after a few encounters during which one or the other fly appears to drive its opponent away from a resource. In these fights, dominance relationships are not usually established (Nilsen et al., 2004) (for an exception, see Palavicino-Maggio et al., 2019). One possible explanation for this difference may relate to the finding that females commonly display communal egg-laying (Dumenil et al., 2016), a behavior not shown by males and believed to be essential for the propagation of the species. This behavior might involve initial competition for egg-laying sites and then communal egg-laying by the strongest or healthiest females to help in the future survival of their progeny. If such is the case, female flies fight for a different reason than males. Female D. melanogaster consistently increase aggression levels for up to a week after mating, an action that may be protective of early egg development. This process has been reported to be due to the transfer of seminal fluid containing sex peptides and cis-vaccenyl acetate along with sperm cells during copulation (Bath et al., 2020, 2017; Nilsen et al., 2004). Therefore, female aggression may be more focused on protecting developing embryos and ensuring the best egg-laying sites and not on whether a female outperforms her opponent when fighting. It then becomes possible that the propensity of females to continue fighting regardless of the aggressiveness of their opponent may lead the 60IIA>traRNAi masculinized females to refrain from yielding in a fight when males would typically retreat. Perhaps that leads to the high levels of boxing we observed when the 60IIA>traRNAi masculinized females were paired with male opponents (Fig. 3D). In some cases, females that show high intensity behavior can drive wild-type opponents off the food cup (Palavicino-Maggio et al., 2019), but we did not observe this behavior with the 60IIA>traRNAi masculinized females. These results may indicate that the 60IIA>traRNAi females are masculinized with respect to the behavior they use in fights, but not with respect to their desire to control resources. In future studies, we will search for circuitry controlling both of these components of masculinization to try to understand what drives the behavior of the 60IIA>traRNAi females.
Many questions arise from the observations presented here. One is whether circuitry for both male and female aggression normally is present within adult brains of both sexes, with one or the other held in a latent state depending on the genetically determined sex of the fly. Related results have been described by Rezaval et al. (2016), who generated female flies that demonstrated male courtship behavior when a subpopulation of dsx+-expressing neurons were activated in these females. Neurons have been found by numerous investigators that are unique to either male or female brains along with other neurons that display differing morphological profiles in the brains of males and females (Cachero et al., 2010; Kimura et al., 2005, 2008; Palavicino-Maggio et al., 2019). Differences of all of these types have been suggested to lead to sex-specific behavioral differences between male and female flies. To determine whether any of these disparities might explain the aggression phenotype of the 60IIA>traRNAi masculinized females, we examined the expression pattern of 60IIA-Gal4 in male and female brains and ventral nerve cords, but did not see any variation in morphology (Fig. S3B,C). It should be noted, however, that sexual dimorphism may be present as changes in gene expression rather than structure, and thus might not be detectable by visual inspection of the expression pattern. Also importantly, we examined the Gal4 expression pattern in the adult nervous system, but our previous experiments suggest that the neurons most likely to be sexually dimorphic are labeled during development and not adulthood (Chan and Kravitz, 2007). Because the 60IIA-Gal4 is broadly expressed in the brain, it is difficult to definitively determine which cells are important for the masculinized aggression phenotype. To find the neurons that cause the 60IIA>traRNAi masculinized females to use male aggressive strategies, it will be necessary to narrow down the population of neurons labeled and to determine the developmental time period most relevant for the phenotype.
Although we have little information regarding what specific cells mediate the transition from female to male aggression in 60IIA>traRNAi masculinized females, our previous work does suggest that these cells may not be as important for changing male aggression into female aggression. Expressing the feminizing transcription factor traF in 60IIA-Gal4 neurons in male flies results in male–male courtship but does not result in female aggression (Chan and Kravitz, 2007), suggesting that these neurons may be more important for promoting male aggressive strategies than female aggressive strategies. Further work will be necessary to pinpoint the identity of these cells, and better understand their role in sexually dimorphic aggressive behavior.
In this paper, we identified a line of masculinized females that switch between female and male aggression depending on the identity of their opponents. Our data suggest that the switch in aggressive strategy requires both behavioral and pheromonal cues, but the exact cues required and the way in which they interact with brain circuits to change behavior are unknown. These flies have the potential to give us insight into the circuitry specifying male and female behaviors and the identification of cues important for differing aggression strategies. Our future work will focus on precisely determining the nature of these cues, and elucidating the circuitry of the female–male aggression switch.
Acknowledgements
We thank Barry Dickson for the UAS-traRNAi fly line. We also thank Orie Shafer for imaging advice and access to the confocal microscope in the Shafer Lab at ASRC/GC-CUNY. We also thank the Neurobiology Department and the Neurobiology Imaging Facility for consultation and instrument availability, as well as the Drosophila Genomic Resource Center. Finally, we thank other members of the Kravitz Laboratory for encouragement and for critical advice during the generation of the results presented in this paper.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: R.E.M., N.M.G., Y.C., M.P.F., E.A.K.; Methodology: R.E.M., Y.C., M.P.F.; Validation: R.E.M., N.M.G.; Formal analysis: R.E.M., N.M.G., A.P., G.T., M.P.F.; Investigation: R.E.M., N.M.G., Y.C., A.P., G.T.; Data curation: R.E.M., N.M.G., A.P., G.T., M.P.F.; Writing - original draft: R.E.M., E.A.K.; Writing - review & editing: R.E.M., Y.C., M.P.F., E.A.K.; Visualization: R.E.M., A.P., G.T., M.P.F.; Supervision: M.P.F., E.A.K.; Project administration: M.P.F., E.A.K.; Funding acquisition: E.A.K.
Funding
This research was primarily supported by the National Institute of General Medical Sciences grant no. R35 GM118137 (to E.A.K.). The Neurobiology Imaging Facility is supported in part by the Neural Imaging Center as part of a National Institute of Neurological Disorders and Stroke P30 Core Center grant no. NS072030. The Drosophila Genomics Resource Center is supported by National Institutes of Health grant 2P40OD010949. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at https://jeb.biologists.org/lookup/doi/10.1242/jeb.238006.supplemental
References
- Agrawal, S., Safarik, S. and Dickinson, M. (2014). The relative roles of vision and chemosensation in mate recognition of Drosophila melanogaster. J. Exp. Biol. 217, 2796-2805. 10.1242/jeb.105817 [DOI] [PubMed] [Google Scholar]
- Alekseyenko, O. V., Chan, Y.-B., Li, R. and Kravitz, E. A. (2013). Single dopaminergic neurons that modulate aggression in Drosophila. Proc. Natl. Acad. Sci. USA 110, 6151-6156. 10.1073/pnas.1303446110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseyenko, O. V., Chan, Y.-B., Fernandez, M. P., Bülow, T., Pankratz, M. J. and Kravitz, E. A. (2014). Single serotonergic neurons that modulate aggression in Drosophila. Curr. Biol. 24, 2700-2707. 10.1016/j.cub.2014.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseyenko, O. V., Chan, Y.-B., Okaty, B. W., Chang, Y., Dymecki, S. M. and Kravitz, E. A. (2019). Serotonergic modulation of aggression in Drosophila involves GABAergic and cholinergic opposing pathways. Curr. Biol. 29, 2145-2156.e5. 10.1016/j.cub.2019.05.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina, K., Watanabe, K., Duistermars, B. J., Hoopfer, E., González, C. R., Eyjólfsdóttir, E. A., Perona, P. and Anderson, D. J. (2014). Tachykinin-expressing neurons control male-specific aggressive arousal in Drosophila. Cell 156, 221-235. 10.1016/j.cell.2013.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier, A., Wittek, B. and Brembs, B. (2002). Drosophila as a new model organism for the neurobiology of aggression? J. Exp. Biol. 205, 1233-1240. [DOI] [PubMed] [Google Scholar]
- Bath, E., Bowden, S., Peters, C., Reddy, A., Tobias, J. A., Easton-Calabria, E., Seddon, N., Goodwin, S. F. and Wigby, S. (2017). Sperm and sex peptide stimulate aggression in female Drosophila. Nat. Ecol. Evol. 1, 0154. 10.1038/s41559-017-0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath, E., Biscocho, E. R., Easton-Calabria, A. and Wigby, S. (2020). Temporal and genetic variation in female aggression after mating. PLoS ONE 15, e0229633. 10.1371/journal.pone.0229633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter, J.-C., Villella, A., Allendorfer, J. B., Dornan, A. J., Richardson, M., Gailey, D. A. and Goodwin, S. F. (2006). Isoform-specific control of male neuronal differentiation and behavior in Drosophila by the fruitless gene. Curr. Biol. 16, 1063-1076. 10.1016/j.cub.2006.04.039 [DOI] [PubMed] [Google Scholar]
- Burtis, K. C. and Baker, B. S. (1989). Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell 56, 997-1010. 10.1016/0092-8674(89)90633-8 [DOI] [PubMed] [Google Scholar]
- Cachero, S., Ostrovsky, A. D., Yu, J. Y., Dickson, B. J. and Jefferis, G. S. X. E. (2010). Sexual dimorphism in the fly brain. Curr. Biol. 20, 1589-1601. 10.1016/j.cub.2010.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certel, S. J., Savella, M. G., Schlegel, D. C. F. and Kravitz, E. A. (2007). Modulation of Drosophila male behavioral choice. Proc. Natl. Acad. Sci. USA 104, 4706-4711. 10.1073/pnas.0700328104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, Y.-B. and Kravitz, E. A. (2007). Specific subgroups of FruM neurons control sexually dimorphic patterns of aggression in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 104, 19577-19582. 10.1073/pnas.0709803104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S., Lee, A. Y., Bowens, N. M., Huber, R. and Kravitz, E. A. (2002). Fighting fruit flies: a model system for the study of aggression. Proc. Natl. Acad. Sci. USA 99, 5664-5668. 10.1073/pnas.082102599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury, B., Chan, Y.-B. and Kravitz, E. A. (2017). Putative transmembrane transporter modulates higher-level aggression in Drosophila. Proc. Natl. Acad. Sci. USA 114, 2373-2378. 10.1073/pnas.1618354114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne, J. A. and Oyama, R. (1995). Localization of pheromonal sexual dimorphism in Drosophila melanogaster and its effect on sexual isolation. Proc. Natl. Acad. Sci. USA 92, 9505-9509. 10.1073/pnas.92.21.9505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Belle, J. S. and Heisenberg, M. (1994). Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science 263, 692-695. 10.1126/science.8303280 [DOI] [PubMed] [Google Scholar]
- Dierick, H. A. and Greenspan, R. J. (2006). Molecular analysis of flies selected for aggressive behavior. Nat. Genet. 38, 1023-1031. 10.1038/ng1864 [DOI] [PubMed] [Google Scholar]
- Dierick, H. A. and Greenspan, R. J. (2007). Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat. Genet. 39, 678-682. 10.1038/ng2029 [DOI] [PubMed] [Google Scholar]
- Dow, M. A. and von Schilcher, F. (1975). Aggression and mating success in Drosophila melanogaster. Nature 254, 511-512. 10.1038/254511a0 [DOI] [PubMed] [Google Scholar]
- Dubnau, J., Grady, L., Kitamoto, T. and Tully, T. (2001). Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature 411, 476-480. 10.1038/35078077 [DOI] [PubMed] [Google Scholar]
- Duménil, C., Woud, D., Pinto, F., Alkema, J. T., Jansen, I., Van Der Geest, A. M., Roessingh, S. and Billeter, J.-C. (2016). Pheromonal cues deposited by mated females convey social information about egg-laying sites in Drosophila melanogaster. J. Chem. Ecol. 42, 259-269. 10.1007/s10886-016-0681-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, A. C., Zwarts, L., Yamamoto, A., Callaerts, P. and Mackay, T. F. C. (2009). Mutations in many genes affect aggressive behavior in Drosophila melanogaster. BMC Biol. 7, 29. 10.1186/1741-7007-7-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández, M. P., Chan, Y.-B., Yew, J. Y., Billeter, J.-C., Dreisewerd, K., Levine, J. D. and Kravitz, E. A. (2010). Pheromonal and behavioral cues trigger male-to-female aggression in Drosophila. PLoS Biol. 8, e1000541. 10.1371/journal.pbio.1000541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez, M. P., Pettibone, H. L., Bogart, J. T., Roell, C. J., Davey, C. E., Pranevicius, A., Huynh, K. V., Lennox, S. M., Kostadinov, B. S. and Shafer, O. T. (2020). Sites of circadian clock neuron plasticity mediate sensory integration and entrainment. Curr. Biol. 30, 2225-2237.e5. 10.1016/j.cub.2020.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur, J.-F., Savarit, F., O'Kane, C. J., Sureau, G., Greenspan, R. J. and Jallon, J.-M. (1997). Genetic feminization of pheromones and its behavioral consequences in Drosophila males. Science 276, 1555-1558. 10.1126/science.276.5318.1555 [DOI] [PubMed] [Google Scholar]
- Hoyer, S. C., Eckart, A., Herrel, A., Zars, T., Fischer, S. A., Hardie, S. L. and Heisenberg, M. (2008). Octopamine in male aggression of Drosophila. Curr. Biol. 18, 159-167. 10.1016/j.cub.2007.12.052 [DOI] [PubMed] [Google Scholar]
- Huber, R., Hill, S. L., Holladay, C., Biesiadecki, M., Tononi, G. and Cirelli, C. (2004). Sleep homeostasis in Drosophila melanogaster. Sleep 27, 628-639. 10.1093/sleep/27.4.628 [DOI] [PubMed] [Google Scholar]
- Kimura, K.-I., Ote, M., Tazawa, T. and Yamamoto, D. (2005). Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature 438, 229-233. 10.1038/nature04229 [DOI] [PubMed] [Google Scholar]
- Kimura, K.-I., Hachiya, T., Koganezawa, M., Tazawa, T. and Yamamoto, D. (2008). Fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron 59, 759-769. 10.1016/j.neuron.2008.06.007 [DOI] [PubMed] [Google Scholar]
- McGuire, S. E., Le, P. T. and Davis, R. L. (2001). The role of Drosophila mushroom body signaling in olfactory memory. Science 293, 1330-1333. 10.1126/science.1062622 [DOI] [PubMed] [Google Scholar]
- Nilsen, S. P., Chan, Y.-B., Huber, R. and Kravitz, E. A. (2004). Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 101, 12342-12347. 10.1073/pnas.0404693101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palavicino-Maggio, C. B., Chan, Y.-B., McKellar, C. and Kravitz, E. A. (2019). A small number of cholinergic neurons mediate hyperaggression in female Drosophila. Proc. Natl. Acad. Sci. USA 116, 17029-17038. 10.1073/pnas.1907042116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn, J. K. M., Zito, M. F. and Kravitz, E. A. (2010). A single social defeat reduces aggression in a highly aggressive strain of Drosophila. Proc. Natl. Acad. Sci. USA 107, 12682-12686. 10.1073/pnas.1007016107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezával, C., Pattnaik, S., Pavlou, H. J., Nojima, T., Brüggemeier, B., D'Souza, L. A. D., Dweck, H. K. M. and Goodwin, S. F. (2016). Activation of latent courtship circuitry in the brain of Drosophila females induces male-like behaviors. Curr. Biol. 26, 2508-2515. 10.1016/j.cub.2016.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryner, L. C., Goodwin, S. F., Castrillon, D. H., Anand, A., Villella, A., Baker, B. S., Hall, J. C., Taylor, B. J. and Wasserman, S. A. (1996). Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell 87, 1079-1089. 10.1016/S0092-8674(00)81802-4 [DOI] [PubMed] [Google Scholar]
- Savarit, F., Sureau, G., Cobb, M. and Ferveur, J.-F. (1999). Genetic elimination of known pheromones reveals the fundamental chemical bases of mating and isolation in Drosophila. Proc. Natl. Acad. Sci. USA 96, 9015-9020. 10.1073/pnas.96.16.9015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter, J., Couch, C., Huang, W., Carbone, M. A., Peiffer, J., Anholt, R. R. H. and Mackay, T. F. C. (2015). Genetic architecture of natural variation in Drosophila melanogaster aggressive behavior. Proc. Natl. Acad. Sci. USA 112, E3555-E3563. 10.1073/pnas.1510104112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villella, A. and Hall, J. C. (1996). Courtship anomalies caused by doublesex mutations in Drosophila melanogaster. Genetics 143, 331-344. 10.1093/genetics/143.1.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkovic, A., Wang, O., Basu, A. C. and Kravitz, E. A. (2006). Learning and memory associated with aggression in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 103, 17519-17524. 10.1073/pnas.0608211103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwarts, L., Vanden Broeck, L., Cappuyns, E., Ayroles, J. F., Magwire, M. M., Vulsteke, V., Clements, J., Mackay, T. F. C. and Callaerts, P. (2015). The genetic basis of natural variation in mushroom body size in Drosophila melanogaster. Nat. Commun. 6, 10115. 10.1038/ncomms10115 [DOI] [PMC free article] [PubMed] [Google Scholar]