Abstract
Chemotherapy using natural compounds, such as resveratrol, curcumin, paclitaxel, docetaxel, etoposide, doxorubicin, and camptothecin, is of importance in cancer therapy because of the outstanding therapeutic activity and multitargeting capability of these compounds. However, poor solubility and bioavailability of natural compounds have limited their efficacy in cancer therapy. To circumvent this hurdle, nanocarriers have been designed to improve the antitumor activity of the aforementioned compounds. Nevertheless, cancer treatment is still a challenge, demanding novel strategies. It is well-known that a combination of natural products and gene therapy is advantageous over monotherapy. Delivery of multiple therapeutic agents/small interfering RNA (siRNA) as a potent gene-editing tool in cancer therapy can maximize the synergistic effects against tumor cells. In the present review, co-delivery of natural compounds/siRNA using nanovehicles are highlighted to provide a backdrop for future research.
Keywords: anticancer therapy, chemotherapy, co-delivery platforms, nanocarriers, natural products, small interfering RNA
Introduction
According to the World Health Organization (WHO), 9.6 million deaths are attributed to cancer. This life-threatening disorder was the second leading cause of death worldwide in 2018.1 Despite considerable progress in anticancer therapy, many challenges still exist.2,3 One of the challenges is the off-targeting feature of conventional cancer therapeutics that significantly diminishes their therapeutic efficacy.4,5
In light of this, research scientists have focused on using targeted delivery in overcoming cancer cells. Notably, targeted delivery systems are able to inhibit tumor growth and reduce tumor burden.6 It is held that designing novel nanoscale delivery systems for delivery of siRNA can improve its efficacy in gene silencing. It appears that resistance of cancer cells to chemotherapy has limited the potential of targeted delivery systems. SiRNA is a powerful tool in reversing chemoresistance of cancer cells by down-regulation of oncogene factors, such as Survivin, Bcl-xl, and Mcl-1.7,8 Thus, understanding the mechanisms involved in drug resistance can help render anticancer therapy more efficacious.9 Another issue in anticancer therapy is the low efficacy of monotherapy in the eradication of cancer cells.10 These difficulties have spurred scientists toward developing co-delivery strategies for anticancer therapy. Combination cancer treatment indeed has significant appeal owing to its many advantages over monodelivery therapeutics, including improved efficacy by synergistic effects and overcoming drug resistance.11−13 In this regard, various siRNA and natural compounds co-delivery vehicles have been developed to achieve more effective therapy than conventional monodelivery.14 Natural compounds, because of their biobased origin, have attracted more attention than synthetic drugs.15 The present Review aims to provide a summary of the potential of natural compounds-siRNA co-delivery platforms in the elimination of cancer cells and suppression of their resistance to chemotherapy.
Natural Compounds in Anticancer Therapy: An Overview
Natural compounds have opened new vistas in anticancer therapy because of their structural and chemical diversity.15−18 These compounds are of importance in the field of drug discovery that can lead to the discovery of novel cancer therapeutics.19−21 More than 100 natural products and their analogs are currently applied clinically or in clinical trials.22,23 Between 1981 and 2010, up to 50% of antitumor drugs approved by the US Food and Drug Administration (FDA) are natural compounds or their analogs.24 Accordingly, natural products are important in anticancer therapy. Numerous experiments have evaluated the efficacy of natural products in anticancer therapy. Because of their multitargeting capability, natural compounds can negatively affect the different aspects of cancer cells, such as proliferation, viability, and metastasis.25−32 In this way, natural compounds target various molecular pathways. The most common manner in which natural products participate in anticancer therapy is stimulation of apoptotic cell death.33 Administration of natural products induces mitochondrial-mediated and endoplasmic reticulum (ER)-mediated apoptosis.34,35 Natural products enhance the production of reactive oxygen species (ROS) that stimulate mitochondrial dysfunction, as well as ER stress.36,37 By increasing ROS generation, the integrity of the mitochondrial membrane is disrupted. During this process, expression of the antiapoptotic factor Bcl-2 is down-regulated,38 while the pro-apoptotic factor Bax is up-regulated. This causes the release of cytochrome C (Cyt C) from the mitochondria and activation of the caspase cascade that results in apoptosis.39 Another pathway is the induction of ER stress-mediated apoptosis.40 Natural product supplements trigger ER stress by enhancing ROS generation. This, in turn, causes apoptotic cell death by upregulation of C/EBP homologous protein (CHOP).41 In addition to apoptotic cell death, natural products are capable of targeting molecular pathways involved in the proliferation of cancer cells. The PI3K/Akt signaling pathway is a vital axis for the proliferation and growth of cancer cells.42 This pathway can be inhibited by an onco-suppressor factor known as PTEN.43 Studies have demonstrated that natural products are capable of activating PTEN in suppressing the PI3K/Akt signaling pathway, thereby decreasing the proliferation and viability of cancerous cells.44 Manu natural products that can target molecular pathways involved in metastasis and invasion of cancer cells.
Epithelial-to-mesenchymal transition (EMT) is a process that causes metastasis of cancer cells via malignant transformation of epithelial cells into mesenchymal cells.45,46 Natural products have shown potential in suppressing EMT to minimize their migration and improve cancer prognosis.47 The upstream modulators of EMT can also be targeted by natural products. It is held that Wnt and STAT3 are upstream modulators of EMT in cancer.48,49 The administration of natural products inhibits both Wnt and STAT3 to suprress EMT.50,51 In addition, ZEB proteins that induce EMT during cancer metastasis are also down-regulated by natural products.52
Natural products are promising candidates in anticancer therapy due to their capacity in affecting diverse targets such as growth and migration of cancer cells as well as targeting different molecular pathways.53−55 However, the poor bioavailability of these valuable compounds has negative impact on their anticancer therapeutic activity.56 The application of nanocarriers can remarkably enhance the antitumor potential of natural products, protect them against degradation before reaching the tumor sites, and augment their accumulation in cancer cells via penetrating into the blood-tumor barrier (BTB).56−59 These benefits support the use of nanoparticles for natural product delivery in anticancer therapy.
SiRNA: Basics, Role in Anticancer Therapy, Challenges, and Possible Strategies
Conventional therapeutics have drawbacks, of which the limitation in targeting just one special molecular pathway or protein is the most important.60,61 Consequently, attention has been directed toward using genetic tools in anticancer therapy.62 RNA interference (RNAi) is one of the most powerful genetic tools used in anticancer therapy.63 Cancer occurs as a result of mutations in onco-suppressor and oncogene factors, leading to uncontrolled cell growth and inhibition of apoptosis.64,65 Different driver genes accounting for enhancing growth and malignancy of cancer have been identified.66 RNA interference is beneficial in the modulation of the aforementioned genes in anticancer therapy.67,68 The discovery of RNAi and its application have a long history; RNAi was first discovered in plants. Subsequently, scientists attempted to exploit the potential of RNAi in gene editing. In 2006, Fire and Mello received the Nobel prize in medicine because of their significant contribution in the field of RNAi.69 The extensive application of RNAi in anticancer therapy is not accidental. The high specificity, effectiveness, minimal adverse effects, and ease of preparation of RNAi has led to its use in anticancer therapy.70
Small interfering RNA (siRNA) is a subcategory of small RNA molecules with a length of 21–23 nucleotides.71 To adequately performing its function, siRNA requires a complete match with its target mRNA (mRNA).72 Furthermore, siRNA suppresses the expression of its target gene at the post-transcriptional level by mRNA degradation.73 Biogenesis of siRNA commences via the degradation of long double-stranded RNA in the cytoplasm via Dicer enzyme. For activation, siRNA is embedded into an RNA-induced silencing complex (RISC) to produce single-stranded RNA (ssRNA). This ssRNA functions as an antisense guide for the RISC complex. By binding to a complementary mRNA target, the ssRNA causes degradation via Argonaute proteins.74,75
Application of first synthetic siRNA dated back to 2001 when Elbashir and colleagues used siRNA for gene editing in mammalian cells.76 Other scientists followed by using siRNA for gene silencing in anticancer therapy.77,78 Because of the capability of siRNA in selective targeting, much attention has been directed toward using siRNA in treatment of different cancers, Examples include breast cancer,79 lung cancer,80 brain tumors,81 thyroid cancer,82 and bladder cancer.83 Recent publications have shed some light on using siRNA in anticancer therapy. Oncogene factors participating in cancer malignancy may be targeted via SiRNA. The remodeling and spacing factor-1 (RSF-1) is an oncogene factor that is high expressed in cancer cells. Up-regulation of RSF-1 enhances the proliferation of cancer cells and causes resistance of cancer cells to chemotherapy.84 The siRNA-mediated RSF-1 silencing in cervical cancer cells is associated with their enhanced sensitivity to radiotherapy. Down-regulation of RSF-1 by siRNA increases the efficacy of radiotherapy via stimulation of apoptosis, DNA damage, and cell cycle arrest in cervical cancer cells.85 Apart from RSF-1, glucose transporter-1 (GLUT-1) is also responsible for resistance of cancer cells to radiotherapy;86,87 siRNA-induced GLUT1 inhibition render cancer cells more responsive to radiotherapy by induce their DNA damage and apoptosis.88 These two studies illustrate that siRNA is a potential strategy in enhancing the efficacy of radiotherapy. Invasion and metastasis of cancer cells may be regulated with the use of siRNA. Matrix metalloproteinase-2 (MMP-2) is a proteinase that enhances the migration of cancer cells and promotes lymph node metastasis via the degradation of type IV basement membrane collagen.89 The siRNA-mediated Annexin A7 inhibition reduces proliferation and invasion of cancer cells via down-regulation of MMP-2 and proliferating cell nuclear antigen (PCNA).90 Ribonucleotide reductase (RR) is a potential target in anticancer therapy because of its role in DNA repair and replication via catalytic reduction.91 Ribonucleotide reductase regulatory subunit M2 (RRM2), a protein-coding gene, is expressed during the late G1/early S phase and participates in DNA repair.92 RRM2 induces chemoresistance of cancer cells because of its capabililty in DNA repair.93 In ovarian cancer cells, silencing of RRM2 via siRNA induces DNA damage and inhibits their repair. This, in turn, increases the sensitivity of cancer cells to cisplatin chemotherapy.94
The signaling networks responsible for proliferation, metastasis, radioresistance and chemoresistance of cancer cells have been reported in previous studies. Targeting molecular pathways is important in suppressing the aggressive behavior of cancer cells and in promoting their responses to chemotherapy and radiotherapy. However, siRNA suffers from off-targeting and are easily degraded by enzymes. These drawbacks may be circumvented by using nanosized vehicles. Similar to the encapsulation of natural product cargoes, encapsulation of siRNA by nanocarriers protect them against degradation during blood circulation. Nanomaterials can also provide targeted delivery of siRNA to the tumor site. Potential nanocarriers for delivery of siRNA in anticancer therapy will be reviewed in the next section.
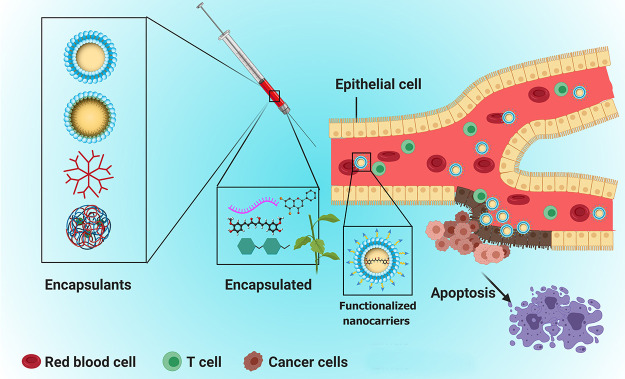
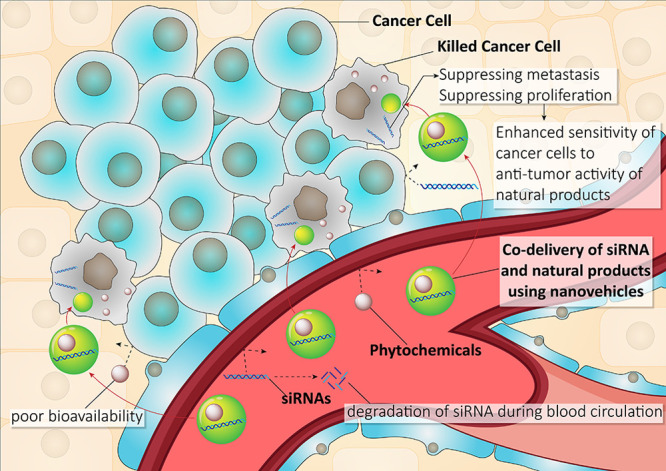
Because different therapeutics employed for combination cancer treatment have specific sites and mechanisms of action, nanovehicle-mediated co-delivery strategies are essential for maximizing the synergistic effects against tumor cells.14 In light of this, functionalized vehicles with site specific delivery have attracted substantial attention in precisely delivering multiple therapeutic agents/RNA for improved synergistic effects (Figure 1).
Figure 1.
Anticancer therapy using a site-specific co-delivery strategy. SiRNA and phytochemicals can be coloaded on nanoparticles for promoting their efficacy in cancer therapy. Encapsulation of siRNA in nanoparticles protects against degradation. Nanoparticles enhance bioavailability of natural products. Blood circulation time of siRNA and phytochemicals increases by nanoparticles. Various nanoparticles, such as micelles, liposomes, dendrimers, and polymeric nanoparticles can provide targeted delivery of siRNA and phytochemicals at tumor site, leading to an increase in their efficacy in apoptosis induction.
Although siRNAs are important in anticancer therapy, there are a number of extracellular and intracellular barriers that challenge their efficacy.71 Among these siRNA limitations, off-targeting, their instability in blood circulation, inadvertent stimulation of the host’s immune responses, as well as their incapability to enter cells (cell uptake) are the most important.95 With respect to off-targeting, it has been reported that one-tenth of siRNAs affect unintended genes.78 In addition, siRNAs triggers immunotoxicity by inducing inflammation and enhancing the levels of cytokines.96 Synthetic siRNAs may impair RNAi machinery by interfering with the function of microRNAs (miRs) and stimulating the overexpression of specific proteins.97 The most critical challenge of siRNAs is their hydrophilic and anionic features that inhibit their penetration through hydrophobic cellular membranes.98
To circumvent this issue, various delivery platforms have been developed for siRNAs. To date, polymeric nanoparticles, gold nanoparticles, iron oxide nanoparticles, silicon dioxide nanoparticles, carbon nanotubes, lipid nanoparticles, liposomal nanoparticles, hydrogel nanoparticles, and aptamers have been developed for delivery of siRNAs.99 Recent literatures have reported the usefulness of siRNA-delivery systems in anticancer therapy. Dendrimers are a subcategory of polymeric nanoparticles with three components, including a central core, an internaldendritic structure and an external surface with the functional surface group. Dendrimers are promising candidates for the delivery of anticancer drugs.100 SiRNA can be loaded into dendrimers for anticancer therapy. Dendrimers remarkably enhance the cellular uptake of siRNAs and their release from endosomes. This causes more effective up-regulation or down-regulation of their targets, resulting in decrease in cancer malignancy.101 Selenium nanoparticles are beneficial in drug and gene delivery. These nanoparticles overcome multidrug resistance (MDR) because of their great biocompatibility and high cellular uptake.102,103 Selenium nanoparticles not only reduce adverse effects, they also enable maximum gene silencing.104 Because of their low size (<100 nm), nanoparticles can infiltrate cellular impediments, such as the blood–tumor barrier (BTB), the blood–brain barrier (BBB), and the cell membrane.105,106 It has been reported that siRNA-loaded nanocarriers can penetrate BBB via endocytosis and transcytosis,107 resulting in more effective treatment of brain tumors. Reduction in off-targeting and adverse effects, enhancement of therapeutic capability and elevation of cellular uptake are the benefits of using nanoparticles for siRNA delivery.108−111Table 1 summarizes the different nanocarriers used for siRNA delivery in anticancer therapy.
Table 1. siRNA-Loaded Nanoparticles in Anticancer Therapy.
| nanovehicle | cancer type | cell line | target gene | size (nm) | zeta potential (mV) | encapsulation efficiency (EE) (%) | drug | results | ref |
|---|---|---|---|---|---|---|---|---|---|
| polymeric nanoparticles | pancreatic cancer | HEK293T cell line | GRP78 | 92 | +15.14 | 27–31 | high efficiency in silencing GRP78 gene (83.9% decrease in expression) and cytotoxicity against cancer cells | (112) | |
| lipid/polymer hybrid nanoassembles | prostate cancer | PC3 cells | EGFR | 120.2 | –8.8 | 98 | reducing growth and volume of cancer without making toxicity against normal cells | (113) | |
| lipid nanoparticle | ovarian cancer | human ovarian cancer SK-OV-3 cells | RPN2 | 66.5 | –9.1 | more than 80 | effective gene silencing, and excellent cellular uptake | (114) | |
| multifunctional nanoplatform | lung cancer | human lung adenocarcinoma A549 cells | PLK1 | 80–102 | 5–12 | 78–80 | providing endo/lysosomal escape, having a pH-responsive feature to release a drug in the tumor microenvironment, high cellular uptake, and cytotoxicity | (113) | |
| redox-responsive nanoparticles | liver cancer | human hepatic (L02) and hepatoma cells (HepG2) | Bcl-2 | 85 | 80 | camptothecin | accumulation and selective targeting of cancer cells, and induction of apoptosis via Bcl-2 down-regulation | (115) | |
| silica nanoparticles | breast cancer | human breast carcinoma cell line MDA-MB-231 | PLK1 | 100–200 | –19 | effective elimination of cancer cells via down-regulation of PLK1 | (116) | ||
| magnetic nanoparticles | prostate cancer | PC3 cell line | ADAM10 | 15.82–79.20 | 5–31 | high cellular uptake and reducing expression of ADAM10, leading to a decrease in cell viability | (115) | ||
| polymeric nanoparticles | liver cancer | Huh7 cells | survivin | 210 | –6.7 | 53 | stimulation of apoptosis in cancer cells via down-regulation of surviving and subsequent induction of Bax and caspase-3 | (117) | |
| selenium nanoparticles | cervical cancer | HeLa human cervical cancer cell | derlin-1 | less than 150 | 14.7 | enhancing generation of ROS, stimulation of mitochondrial dysfunction and induction of apoptotic cell death | (118) | ||
| magnetic nanoparticles | oral cancer | human oral cancer cell Ca9–22 and CAL 27 | Bcl-2 | 26.12 | 46.5 | decreasing viability and survival of cancer cells via down-regulation of Bcl-2 | (119) | ||
| pH-responsive micelles | liver cancer | human liver cancer cells SK-Hep1 | IL-8 | 83 | high biocompatibility, excellent cellular uptake and effective decrease in gene expression | (120) |
Natural Compounds–siRNA Co-delivery
Doxorubicin–siRNA Co-delivery
Doxorubicin (DOX) belongs to the family of anthracyclines and is extensively employed for the treatment of breast cancer, lung cancer, ovarian cancer, cervical cancer, and thyroid cancer.121 Doxorubicin is derived from bacteria belonging to the genus Streptomyces. It suppresses malignancy and proliferation of cancer cells via inhibition of DNA topoisomerases, DNA intercalation, and free radical generation.122 Despite its excellent antitumor activity, DOX adversely affects normal cells because of its off-targeting feature.123,124 This has resulted in using nanoplatforms for the targeted delivery of DOX.125 In addition, cancer cells are capable of developing resistance against DOX chemotherapy.126 These two issues have resulted in the use of combination therapy and nanoparticles. It has been shown that siRNAs are helpful in reversing DOX chemoresistance by targeting the genes involved in DOX resistance.
A combination of DOX and siRNA has been used for enhancing the antitumor activity of DOX against cancer cells. Chemotherapeutic agents can reduce the malignancy of cancer cells via EMT induction.127 Different molecular pathways function as an upstream regulators of EMT in cancer. The Ras-related C3 botulinum toxin substrate 1 (RAC1) is considered as a key player in the regulation of invasion and metastasis of cancer cells.128,129 The RAC1 attaches to nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) and increases the production of ROS.130 Formation of actin stress fibers subsequently occurs by cytoskeleton reorganization.131 Down-regulation of RAC1 suppresses metastasis of cancer cells via inhibition of EMT. The use of DOX and siRNA-RAC1 enhances the antitumor activity of DOX against breast cancer cells via inhibition of EMT.132 The antitumor effect of DOX is augmented by elevating its accumulation in cancer cells by inhibition of P-gp activity via siRNA.133 The use of siRNA enables negative targeting of oncogene factors, such as STAT3, β-catenin, and Notch-1, which increases the antitumor activity of DOX.134 Molecular pathways involved in proliferation and growth of cancer cells, such as PI3K/Akt, may be targeted using siRNA, resulting in an increase in cytotoxicity of DOX against cancer cells.135 These studies are in support of the value of collaborative antitumor therapy via DOX and siRNA.136−138 Previous studies have examined the potential of co-delivery of siRNA and DOX using nanoparticles in anticancer therapy.139
The advent of nanotechnology facilitates simultaneous chemotherapy and immunotherapy. Programmed death-ligand 1 (PD-L1) is the key element of the PD-1/PD-L1 axis that induces apoptosis of T cells, inhibits their proliferation and provides immune escape of cancer cells.140,141 Down-regulation of PD-L1 is a potential strategy in the elimination of cancer cells by enhancing the cytotoxicity of T cells against tumor cells.142 The combination of DOX and siRNA-PD-L1 is beneficial in anticancer therapy. Cancer cell membrane-coated nanoparticles (CCMNPs) are capable of codelivering DOX and siRNA–PD-L1. Improved cellular uptake of CCMNPs enhances the internalization of PD-L1 and DOX, resulting in concomitant chemotherapy and immunotherapy.143 Internalization of DOX in cancer cells may be improved by targeting transporters. The role of P-gp in exporting chemotherapeutic agents out of the cell has previously been reported.144 Loading siRNA–MDR1 on nanoparticles for co-delivery with DOX is important for enhancing the antitumor activity of DOX. Expression and activity of P-gp are reduced by down-regulation of MDR1. This results in increased accumulation of DOX in cancer cells to improve its antitumor activity.145
Surface modification of nanoparticles with receptors and ligands can be made to enhance their targeted delivery. The EphA10 demonstrates high expression in cancers and is correlated with the progression and malignancy of cancer cells.146 Surface modification of nanoparticles with EphA10–antibody enhances their cellular uptake, leading to effective inhibition of P-gp and cytotoxicity of DOX.147 Following the design of nanoparticles that are capable of increasing intracellular DOX uptake, the next step should be devoted to developing strategies in reducing the viability and proliferation of cancer cells to maximize the antitumor activity of DOX. In this way, siRNA–Bcl-2- and DOX-loaded liposomes have been designed. By down-regulation of the antiapototic factor Bcl-2, the cancer cells undergo apoptosis and increase their sensitivity to DOX-mediated cell death.148 Nanoparticles are valuable for targeted delivery and enhanced cellular uptake of siRNA–Bcl-2 and DOX in anticancer therapy.149
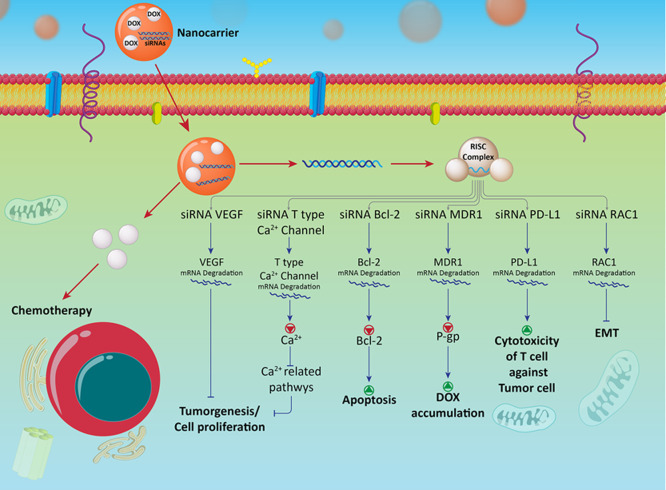
Cytosolic Ca2+ is a vital signal transduction regulator that has a variety of biological functions, such as modulation of cell proliferation, tumorigenesis, and migration.150−152 The Ca2+ channels and pumps accounting for Ca2+ transportation are up-regulated in different cancers.153−155 These pumps increase the concentration of Ca2+ in the cells to activate Ca2+-related pathways.156 Activation of Ca2+-related pathways induces drug resistance.157 As a consequence, attention has been directed toward inhibition of Ca2+ pumps, such as low-voltage activated T-type Ca2+ channels in anticancer therapy.158−160 Encapsulation of siRNA against T-type Ca2+ channels and DOX by mesoporous silica nanoparticles reduces the activity of these channels, resulting in inhibition of DOX resistance in breast cancer cells.161 In addition to siRNA, other plant derived-natural compounds may be loaded into nanoparticles. The co-delivery of siRNA, quercetin and DOX suppresses proliferation and malignancy of cancer cells by providing collaborative antitumor therapy (Figure 2).162,163
Figure 2.
Co-delivery of DOX–siRNA in anticancer therapy and affected molecular pathways. Nanovehicles facilitate the penetration of siRNA and DOX through the cell membrane. SiRNA down-regulates molecular pathways that are responsible for cancer progression to promote antitumor activity of DOX.
The JNK-interacting protein 1 (JIP1) is an oncogene factor involved in the development of resistance against DOX by cancer cells. Down-regulation of JIP1 enhances the sensitivity of DOX chemotherapy.164 Co-delivery of JIP1 and DOX by cationic nanoliposomes inhibits the resistance of osteosarcoma cells to chemotherapy via induction of apoptosis and cytotoxicity.165 The erythropoietin-producing human hepatocellular receptor A2 (EphA2) undergoes up-regulation in osteosarcoma cells. Loading of the histidine-tagged EphA2 receptor-specific peptide (YSA peptide) as a ligand of EphA2 into cationic nanoliposomes enhances the efficacy of delivery of siRNA and DOX into cancer cells.165 In addition to liposomes, graphene oxide may be used for DOX delivery. Graphene oxide is an oxidative product of graphite. The excellent biocompatibility and biodegradability of graphene oxide have made it valuable for drug delivery.166−171 Co-delivery of siRNA–VEGF and DOX using graphene oxide enhances their cellular uptake and targeted delivery, resulting in suppressing growth and metastasis of cancer cells.172
Apart from side effects, chemoresistance is a major problem associated with DOX-related chemotherapy. Enhanced metastasis is correlated with DOX resistance. EMT inhibition via siRNA leads to DOX sensitivity. Furthermore, P-gp that contributes to pumping out DOX from cancer cells and triggering chemoresistance is inhibited by siRNA.
Encapsulants offer a platform for co-delivery of DOX and siRNA to promote siRNA efficiency in gene silencing, and to increase DOX accumulation in cancer cells. The advantage of using siRNA is simultaneous chemotherapy and immunotherapy. For instance, siRNA-PD-L1 can be applied for preventing immune evasion of cancer cells to support the use of DOX in chemotherapy. siRNA-Bcl-2 may be used to promote the efficacy of DOX in apoptosis induction. To increase the selective targeting capability of nanocarriers, surface modification of nanoparticles with receptors, such as EphA10 has been adapted to promote their cellular uptake. Apart from DOX and siRNA, other antitumor agents, such as quercetin, can be loaded into nanoparticles to increase their efficacy against cancer cells. However, one of the drawbacks is the large particle size of nanoparticles. As shown in Table 2, most of synthesized NPs have particle size that are more than 100 nm. Future studies have to be focused on reducing the particle size of nanocarriers to enhance cellular uptake. Table 2 summarizes the DOX–siRNA co-delivery platforms used experimentally in anticancer therapy.
Table 2. DOX-siRNA Co-delivery Platforms in Anticancer Therapya.
| nanovehicle | cancer type | cell line | target gene | size (nm) | zeta potential (mV) | encapsulation efficiency (EE) (%) | results | ref |
|---|---|---|---|---|---|---|---|---|
| ROS-sensitive NPs | breast cancer | 4T1 cells | PD-L1 | 139.9 | 28.1 | down-regulation of PD-L1, and providing simultaneous chemotherapy and immunotherapy | (173) | |
| polymeric NPs | breast cancer | human breast cancer MCF-7 and MCF-7/ADR cell lines | P-gp | 74.7 | 13.6 | enhancing intracellular accumulation in cancer cells via down-regulation of P-gp | (174) | |
| polymeric NPs | liver cancer | HepG2 cells | Bcl-2 | 60–90 | less than 25 | 79.4 | induction of apoptosis via down-regulation of Bcl-2 | (175) |
| mesoporous silica NPs | oral cancer | human oral squamous carcinoma DOX-resistant cell line (KBV) | MDR1 | 170.5 | +34.7 | 70% decrease in expression of MDR1, enhanced accumulation of DOX in cancer cells and stimulation of apoptosis | (176) | |
| selenium NPs | liver cancer | HCC (HepG2) and human normal liver cell (Lo2) | Nanog | 12 | cellular uptake via clathrin-mediated endocytosis, down-regulation of Nanog, and inhibition of proliferation and migration | (177) | ||
| micelle | lung cancer | A549 cells | TLR4 | 125.9 | +24.66 | 85.81 (DOX) | releasing drug and siRNA in a pH/redox-sensitive manner, and suppressing tumor growth | (178) |
| polymeric NPs | breast cancer | MCF-7 cells | MDR1 | 65.7 | +13.9 | 67.4 (DOX) | inhibition of drug resistance via down-regulation of P-gp, and enhancing the antitumor activity of DOX | (179) |
| self-assembled polyjuglanin NPs | lung cancer | human lung cancer cell lines, A549 and H69 | Kras | 81.8 | –18.62 | down-regulation of oncogene factor Kras, inhibition of c-Myc and P-gp, and enhanced cytotoxicity of DOX | (180) | |
| gold NPs | ovarian cancer | SK-OV-3 cells | erbB2 | 105 | –48 | targeted delivery, high biodistribution, and great antitumor activity | (181) | |
| mesoporous silica NPs | breast cancer | human breast adenocarcinoma cell line MCF-7 | Bcl-2 | 125 | –47.4 | targeted delivery and inhibition of cancer proliferation | (182) | |
| gold NPs | cervical cancer | HeLa cells | EGFP | 150 | –35.4 | 82.5 | inhibition of EGFP expression, high intracellular accumulation and suppressing cancer malignancy | (183) |
| polymeric NPs | breast cancer | MCF-7 cells | Bcl-2 | 187 | +22.5 | induction of apoptotic cell death via down-regulation of Bcl-2 | (184) | |
| chitosan NPs | lung cancer | A549 cells | IGF-1R | 176 | +11 | 86 (siRNA)75 (DOX) | suppressing invasion and migration of cancer cells via down-regulation of MMP-9, VEGF, and STAT3 | (185) |
| micelles | breast cancer | 4T1 and WRL-68 cells | MDR | 92–101 | +7 to +10 | 72 (DOX) | inhibition of resistance via down-regulation of MDR | (186) |
| micelles | breast cancer | MCF-7 cells | PLK-1 | 98.74 | +21.62 to +44.5 | suppressing proliferation of cancer cells | (187) | |
| chitosan NPs | breast cancer | MDA-MB361 metastatic breast cancer cell line | IL17RB | 114 | +10.1 | enhancing cytotoxicity of DOX via down-regulation of IL17RB, and inhibition of NF-κB and Bcl-2 | (188) |
NP: Nanoparticles.
Curcumin–siRNA Co-delivery
Curcumin is a naturally occurring nutraceutical compound derived from Curcuma longa.189 This compound is responsible for the yellow color of turmeric and is responsible for the purported therapeutic activities of Curcuma longa.190,191 Curcumin has a number of pharmacological effects such as neuroprotective,192 cardioprotective,193 hepatoprotective,194 antitumor,195,196 antioxidant,197 and anti-inflammatory effects.198 In terms of antitumor activity, many studies have reported the efficacy of curcumin in suppressing the proliferation, viability, and migration of cancer cells via targeting molecular pathways and mechanisms, such as apoptosis, autophagy, STAT3, Bcl-2, Bax, caspase, Wnt, and Nrf2.199−203 Similar to other plant-derived natural compounds, curcumin suffers from poor bioavailability.204 Loading curcumin into nanoparticles has been reported to remarkably enhance its antitumor activity.205 Curcumin has been used with gene therapy to augment its antitumor activity.206,207 Because of curcumin’s poor bioavailabililty, studies have focused on developing nanosized encapsulants for co-delivery of curcumin and siRNAs. To date, four studies have evaluated curcumin–siRNA co-delivery in anticancer therapy, which are summarized below.
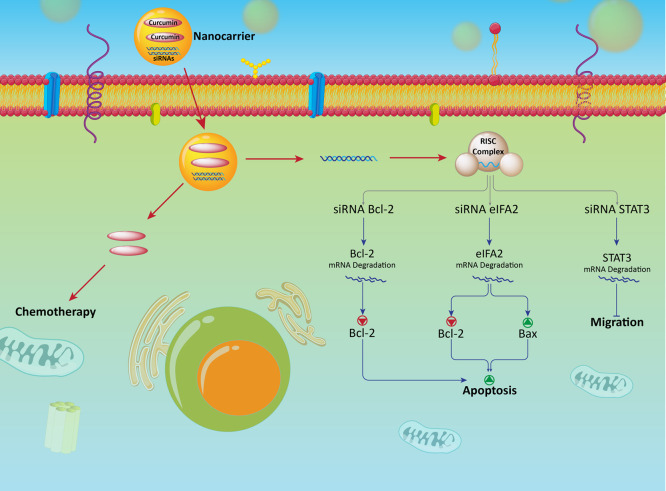
Polyamidoamine (PAMAM) dendrimers are promising candidates in drug and gene delivery because of the high density of surface groups, capability of sustained cargo release, spherical shape, low polydispersity, and water solubility.208,209 The hydrophobic interior of PAMAM dendrimers is ideal for the encapsulation of hydrophobic compounds, while their hydrophilic surface provides sites for attachment of siRNA.210 Both siRNA and curcumin can be codelivered by PAMAM dendrimers into cancer cells. Anticancerous effect was achieved by synergistic inhibition of Bcl-2 expression by the siRNA and antitumor acivity of curcumin (Figure 3).211
Figure 3.
Co-delivery of curcumin and siRNA in cancer therapy with focus on molecular signaling pathways. Down-regulation of Bcl-2, elF5A2, and STAT3 by siRNA increases the antitumor activity of curcumin against cancer cells. Nanoparticles promote cellular accumulation of siRNA and curcumin to enhance their antitumor potential.
The STAT3 signaling pathway is an oncogene factor that enhances the proliferation and invasion of cancer cells.212,213 Down-regulation of STAT3 causes apoptosis of skin cancer cells and inhibits their migration and growth.214,215 Because curcumin targets the STAT3 signaling pathway in anticancer therapy, co-delivery of curcumin and STAT3-targeting siRNA can provide synergistic effects. In vitro and in vivo experiments demonstrate that curcumin- and siRNA–STAT3-loaded cationic liposomes are capable of suppress skin cancer progression and malignancy via down-regulation of STAT3 and disruption of cancer growth.216 The efficacy of cationic liposomes in the co-delivery of curcumin and siRNA-STAT3 in therapy against skin cancer was also investigated in another study. This combination remarkably suppressed skin cancer proliferation, growth, and survival.217 Because STAT3 in an oncogene for skin cancer (melanoma), silencing of STAT3 using siRNA interferes with cancer growth and invasion.
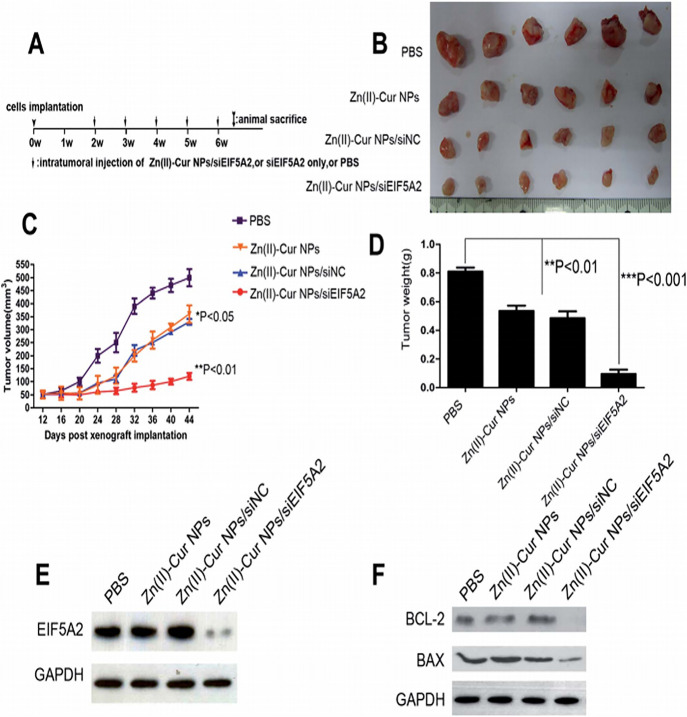
Delivery of curcumin also enhances the inhibitory impact of STAT3 on melanoma cells. Nonviral vehicles, such as Au nanoparticles, carbon nanotubes, and silica nanoparticles are not biodegradable.218−220 Degradation of biodegradable polymers, such as poly(lactic-co-glycolic acid) nanoparticles, results in the production of acidic oligomers and creation of a low pH environment that are toxic for cells.221 Zinc–curcumin nanoparticles are free of the aforementioned drawbacks. Zinc ions enhance the solubility of curcumin and increases it cellular uptake. Zinc nanoparticles release drug in tumor sites in response to pH. Because of its high cellular uptake, siRNA–elF5A2 enters readily into cancer cells. Co-delivery of curcumin and siRNA–elF5A2 inhibits proliferation and malignancy of bladder cancer cells both in vitro and in vivo. The combination induces apoptosis of the bladder cancer cells via upregulation of Bax and down-regulation of Bcl-2 (Figures 2 and 4).222
Figure 4.
(A) Evaluation of antitumor potential of Zn(II)–Cur NP/siELF5A2 complex in a xenograft model. (B) Size of tumor treated with different therapeutics is shown. (C) Tumor volume based on days post xenograft implantation with Zn(II)–Cur NPs, Zn(II)–Cur NPs/siNC, and Zn(II)–Cur NPs/siEIF5A2 (20 mg of siEIF5A2 per injection, 50:1 mass ratio). (D) Mean tumor weights implanted with Zn(II)–Cur NPs, Zn(II)–Cur NPs/siNC, and Zn(II)–Cur NPs/siEIF5A2. (E) Western blots of specimens using anti-EIF5A2 and anti-GAPDH antibodies. (F) Western blots of the tissue specimens using anti-BCL-2, anti-BAX, and anti-GAPDH antibodies. Reproduced from ref (222) with permission from Royal Society of Chemistry.
One of the most well-known phytochemicals in anticancer therapy is curcumin. Many cell culture and animal experiments have been performed to evaluate its antitumor activity against different types of cancer. The poor bioavailability of curcumin may be resolved by coadministration with piperine derived from black pepper or using nanoparticles that significantly promote curcumin accumulation in cancer cells.223
Antitumor activity of curcumin may be improved by its coapplication with siRNA. For instance, siRNAs can down-regulate expression of Bcl-2, STAT3, and elF5A2 to interfere with cancer cell proliferation. This paves the way for enhanced antitumor activity of curcumin against cancer cells. A combination of curcumin and siRNA, and their co-delivery by nanoparticles can provide effective anticancer therapy. To date, only a few studies have evaluated the efficiency of this combination. Further studies should focus on the ability of curcumin and siRNA in down-regulation of other signaling networks, such as Nrf2, Wnt, c-Myc, and SOX in anticancer therapy. Other nanocarriers, such as micelles, liposomes, and carbon nanotubes can be designed for co-delivery of curcumin and siRNA. Table 3 represents curcumin–siRNA co-delivery in anticancer therapy.
Table 3. Curcumin–siRNA Co-delivery in Anticancer Therapy.
| nanovehicle | cancer type | cell line | target gene | size (nm) | zeta potential (mV) | encapsulation efficiency (EE) (%) | remarks | refs |
|---|---|---|---|---|---|---|---|---|
| PAMAM dendrimer | liver cancer | HeLa cells | Bcl-2 | 180 | –48 | 82 | high cellular uptake, synergistic impact, down-regulation of Bcl-2 and stimulation of apoptosis | (211) |
| cationic liposome | skin cancer | mouse melanoma cells (B16F10) | STAT3 | 276.9 | 42.8 | 86.8 | down-regulation of STAT3 and effective inhibition of tumor growth and viability | (216) |
| cationic liposome | skin cancer | human epidermoid carcinoma cells (A431) | STAT3 | 195 | 58.8 | 87.5 | significant reduction in STAT3 expression, resulting in inhibition of cancer growth and invasion | (217) |
| Zn nanoparticle | bladder cancer | human bladder cancer cell line | elF5A2 | 80–500 | +22.3 | effective knock-down of elF5A2, induction and apoptosis and reducing proliferation and growth of cancer cells | (222) |
Taxane–siRNA Co-delivery
Docetaxel–siRNA Co-delivery
Docetaxel (DTX) is a semisynthetic taxane derived from the needles of the European yew tree.224 This chemotherapeutic agent functions by inhibiting cell replication via interfering with microtubule network and stimulation of cell cycle arrest.225 The US FDA has approved the application of docetaxel for the treatment of lung cancer,226 prostate cancer,227 ovarian cancer,228 and breast cancer.229 Several clinical trials have evaluated the efficacy of docetaxel in anticancer therapy, and it is considered as an ideal candidate in chemotherapy of cancer patients.230−232 Different pathways and mechanisms contribute to the resistance of cancer cells in docetaxel chemotherapy. Regulation of these molecular pathways and mechanisms is important in the reversal of docetaxel resistance. Modulation of miR expression, Nrf2, and Klotho demonstrated promising results in inhibition of docetaxel resistance.230,233,234 More importantly, genes may be modulated by siRNA to improve the antitumor activity of docetaxel. Knockout of the oncogenes Notch1 and CIP2A by siRNA enhances the efficacy of etoposide in eradication of cancer cells.235,236 The antitumor activity of etoposide and potential of siRNA in gene silencing may be promoted using nanoplatforms. To date, different studies have evaluated the efficacy of co-delivery of docetaxel and siRNA using nanoparticles.
The ERK-1 (p42-MAPK) and ERK-2 (p44-MAPK) kinases can be induced by growth factors through Ras-Raf-dependent pathways and are upregulated in prostate cancer cells.237 Suppressing the expression of these MAPL kinases for elimination of prostate cancer cells.238,239 To optimize therapy against prostate cancer, a combination of etoposide and siRNA-MAPK has been experimental codelivered by polymeric nanoparticles into cancer cells. The codelivered siRNA-MAPK diminished the expression of ERK-1 and ERK-2 and suppressed the proliferation and invasion of prostate cancer cells, while the codelivered etoposide induced apoptosis and cell cycle arrest via down-regulation of α-tubulin.240
Matrix metalloproteinase-9 (MMP-9) is involved in the metastasis of cancer cells. This protease degrades the cell membrane of cancer cells and enhances their mobility and progression, resulting in poor prognosis.241,242 Because MMP-9 increases the resistance of cancer cells to chemotherapy,243,244 it is a suitable target in anticancer therapy. A potential strategy combining docetaxel and siRNA–MMP-9 has been used experimentally for the treatment of breast cancer. The docetaxel- and siRNA–MMP-9-loaded polymeric nanoparticles inhibit migration and viability of breast cancer cells by down-regulation of MMP-9 (inhibition of metastasis) and cellular uptake of docetaxel (apoptosis induction).245 Because MMP-9 induces epithelial-to-mesenchymal transition via extracellular matrix degradation,246 it is rational to down-regulate MMP-9 to control malignancy and sensitize the cancer cells to chemotherapy.247 Breast cancer cells have been found in the lung due to metastasis. Down-regulation of MMP-9 enhances the overall survival of patients with breast cancer. Loading docetaxel and siRNA–p65 into nanoparticles significantly suppresses lung metastasis of breast cancer cells via inhibition of MMP-2 and Bcl-2, and stimulation of apoptosis (Figure 5).248 These studies indicate that nanoplatforms are beneficial in co-delivery of etoposide and siRNAs to enhance internalization of etoposide by promoting its antitumor activity and suppressing the migration and proliferation of cancer cells.249
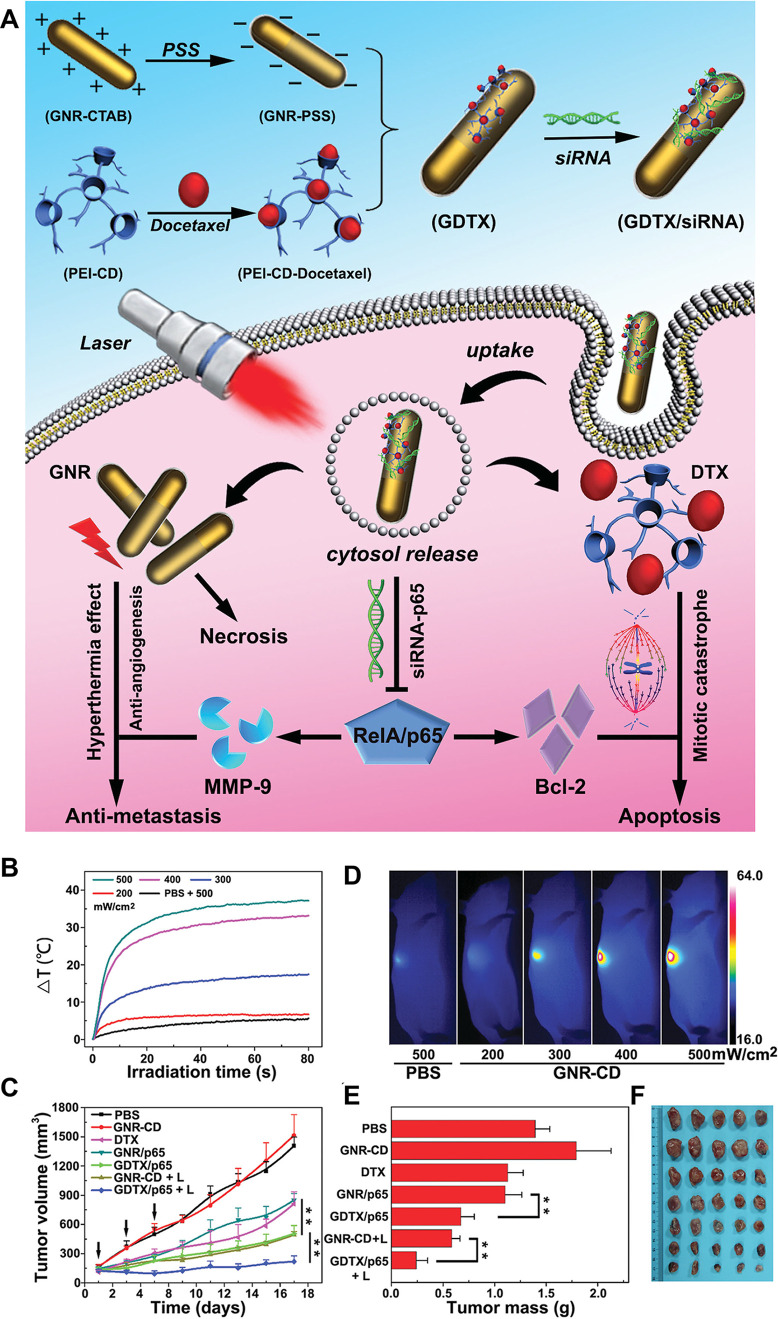
Figure 5.
(A) Schematic illustration of the fabrication and therapeutic mechanism of siRNA and DTX coloaded host–guest gold nanorods (GNRs). (B) Temperature elevation, (C) infrared thermal images of 4T1 tumors upon laser irradiation at various power densities (200, 300, 400, and 500 mW cm –2), (D) tumor growth curves, and (E) change of tumor weight after treated with GDTX/siRNA nanoparticles and 655 nm laser; the black arrows indicated the time points for DTX/siRNA injection and laser irradiation. (F) Tumor photographs with different treatment. Reproduced from ref (248) with permission from Wiley.
Autophagy is a type II programmed cell death and plays a pivotal role in the degradation of proteins and organelles, such as the Golgi apparatus, mitochondria, and endoplasmic reticulum.250 Autophagy is correlated with metabolic stress, genomic damage and tumorigenesis.251 Autophagy is not only involved in survival and progression of cancer cells, but can increase the resistance of cancer cells to chemotherapy.252,253 For example, autophagy increases the resistance of cancer cells to docetaxel chemotherapy.254,255 Consequently, regulation of autophagy is important in cancer therapy. A combination of docetaxel and siRNA–ATG7 has been used experimentally for the treatment of breast cancer. ATG7 is an upstream inducer of autophagy.256 Administration of docetaxel stimulates autophagy and suppresses the proliferation and migration of breast cancer cells. Co-delivery of siRNA-ATG7 and docetaxel using micelles suppresses prosurvival autophagy in breast cancer cells and improves the efficacy of docetaxel in the stimulation of apoptosis.257
The surface of nanoparticles may be modified with receptors to enhance the cellular uptake of siRNA- and docetaxel-loaded nanoparticles. The low-density lipoprotein receptor-related protein (LRP) receptor undergoes up-regulation in BBB and glioblastoma cells.258−260 Angiopep-2 and tLyp-1 are ligands that on bind to receptors on cancer cells and penetrate these cells.261−264 Surface modification of liposomes with Angiopep-2 and tLyp-1 has been performed to enhance their penetration into glioblastoma cells, resulting to increase in the internalization of docetaxel and siRNA–VEGF.265 Liposomes provide an effective platform for coloading of siRNA and docetaxel. This co-delivery remarkably reduced the proliferation and viability of cancer cells via induction of apoptosis.266 Micelles are another potential candidate for drug delivery. They are capable of encapsulating chemotherapeutic agents to improve their antitumor activity.267,268 The antitumor activity of siRNA–Bcl-2- and docetaxel-loaded micelles against breast cancer cells has been investigated in a recent study. The micelles codelivered siRNA and docetaxel to the tumor site. This targeted delivery significantly reduced the growth of cancer cells via induction of apoptosis and down-regulation of the antiapoptotic factor Bcl-2 (Figure 6).269
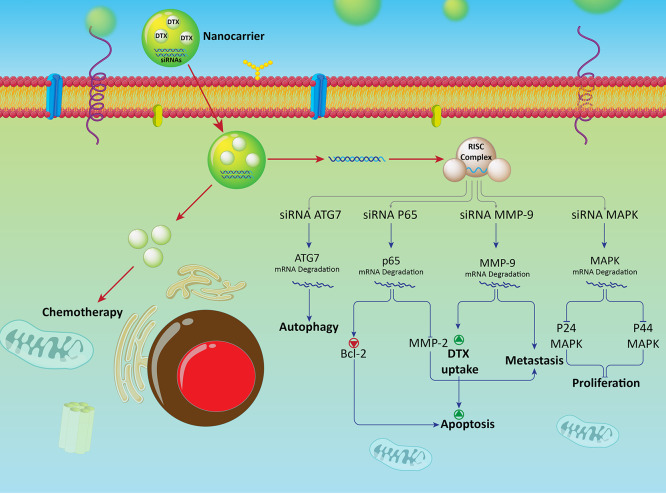
Figure 6.
Co-delivery of docetaxel–siRNA in treatment of cancer. Suppression of the proliferation and metastasis of cancer cells is provided using siRNA-ATG7, p65, MMP-9, and MAPK. This results in increase in cytotoxicity of DTX docetaxel against cancer cells. Nanoparticles provide a platform for co-delivery of docetaxel and siRNA in triggering chemosensitivity.
Similar to other antitumor agents, siRNA and nanoparticles have been successful in promoting the inhibitory effect of docetaxel against cancer cells. Proliferation (MAPK) and metastasis (MMP-9) have been down-regulated by siRNA in promoting antitumor activity of docetaxel. Nanocarriers such as polymeric nanoparticles and micelles have been used for siRNA and docetaxel co-delivery. Autophagy induction following docetaxel chemotherapy functions as a pro-survival factor. SiRNA–ATG7 inhibits autophagy in promoting the antitumor activity of docetaxel against cancer cells. Nanoparticles are potentially useful in anticancer therapy because they are capable of inducing autophagy270−273 and that autophagy has both oncogene and onco-suppressor functions.274−277Table 4 summarizes currently published docetaxel–siRNA co-delivery platforms in anticancer therapy.
Table 4. Docetaxel–siRNA Co-delivery Platforms in Anticancer Therapy.
| nanovehicle | cancer type | cell line | target gene | size (nm) | zeta potential (mV) | encapsulation efficiency (EE) (%) | remarks | ref |
|---|---|---|---|---|---|---|---|---|
| micelle | prostate cancer | PCa cells | SREBP1 | 100 | +20.3 to +26.9 | high cellular uptake via lysosome escape, and suppressing invasion, metastasis and proliferation of cancer cells | (278) | |
| polymeric NPs | prostate cancer | PC-3 cell line | GRP78 | 39.7 | –24.2 | 83.8 (DTX) | targeted delivery using RGD segment, high biocompatibility, excellent EE, prolonged-release and high antitumor activity | (279) |
| 82.4 (siRNA) | ||||||||
| chitosan NPs | breast cancer | Mucin1+ SKBR3 and mucin1– CHO cells | cMET | 110.5 | +11.6 | 90.7 (siRNA) | high cellular uptake, effective down-regulation of cMET, suppressing the expression of STAT3, IL-8, MMP-2, MMP-9, and VEGF, leading to a decrease in invasion and proliferation of cancer cells | (280) |
| 88.3 (DTX) | ||||||||
| chitosan NPs | breast cancer | SKBR3 breast cancer cells | IGF-1R | 110–118 | +12 to +14 | 91.2 (siRNA) | high cellular uptake, reducing cancer viability, and down-regulation of IGF-1R, STAT3, MMP-9 and VEGF | (281) |
| 87.6 (DTX) | ||||||||
| liposome | laryngeal cancer | Hep-2 cells | ABCG2 | 180 | inhibiting tumor growth for in vitro and in vivo | (282) | ||
| polymeric NPs | nasopharyngeal carcinoma | HEN-1 cells | MMP-9 | down-regulation of MMP-9, stimulation of apoptosis and suppressing metastasis | (283) |
Paclitaxel–siRNA Co-delivery
Paclitaxel (PTX) is the first member of the taxane family that was approved by the FDA for use in clinical trials.284 This chemotherapeutic agent is exclusively applied in the treatment of malignancies such as breast cancer,285 lung cancer,286 brain tumors,287 ovarian cancer,288 and cervical cancer.289 Nevertheless, the resistance of cancer cells to paclitaxel has resulted in unfavorable outcomes in its clinical applications.290 Different factors are responsible for the resistance of cancer cells to paclitaxel chemotherapy, including drug transporters and miRs.291 Identification of these pathways and mechanisms, as well as further targeting, are beneficial for the reversal of paclitaxel resistance.292 For the treatment of lung cancer, siRNA–Beclin inhibits prosurvival autophagy in lung cancer cells and sensitizes the cells to paclitaxel chemotherapy. By down-regulating Beclin/autophagy, the expression and activities of P-gp and multidrug resistance protein 7 (ABCC10) are reduced. This generates the conditions for enhanced intracellular accumulation of paclitaxel to promote its potent antitumor activity.293 Using a combination of siRNA–VEGF and paclitaxel is also beneficial in anticancer therapy. The siRNA–VEGF suppresses metastasis of cancer cells, as well as angiogenesis and neovascularization of cancerous tissues, while paclitaxel exerts its inhibitory effect on the growth and viability of cancer cells.294
Stathmin 1 (STMN1) is an oncogene that promotes growth and differentiation of cancer cells.295 Targeting STMN1 is important in anticancer therapy. siRNA-mediated STMN1 down-regulation is correlated with enhanced sensitivity of cancer cells to paclitaxel chemotherapy.296 These studies support the use of paclitaxel and siRNA to promote the antitumor activity of paclitaxel and to inhibit the resistance of cancer cells to paclitaxel chemotherapy.297 Future research in improving the antitumor activity of paclitaxel and siRNA should be directed at the use of nanotechnology. Nanoplatforms can effectively encapsulate siRNA and paclitaxel, protecting them against degradation and providing targeted delivery to the tumorous sites.298 Studies that evaluated the efficacy of nanoparticles in co-delivery of siRNA and paclitaxel will be reviewed below.
Solid lipid nanoparticles are potential nanocarriers containing physiological and biocompatible lipids. These nanocarriers have a size of 10–1000 nm and are capable of encapsulating both hydrophilic and hydrophobic drugs.299,300 Biocompatibility, sustained release, and biodegradability are additional beneficial characteristics of solid lipid nanoparticles.301 Co-delivery of siRNA–Bcl-2 and paclitaxel has been used in experimental therapy against cervical cancer. These nanocarriers induced apoptosis in cancer cells and reduced their viability and proliferation via down-regulation of Bcl-2 and stimulation of paclitaxel-mediated apoptosis.302 Gold nanoparticles may also be used for the delivery of siRNA because of their adjustable physicochemical features.303 Bifunctional polyethylene glycol moieties on the gold nanoparticles enhance targeted delivery and cellular internalization.304 These nanocarriers are used for co-delivery of siRNA–NF-κB and paclitaxel. Surface modification of gold nanoparticles with anisamide enhances their cellular uptake by prostate cancer cells. Anisamide acts as a ligand for up-regulation of sigma receptors in prostate cancer cells.305,306 Co-delivery of siRNA–NF-κB and paclitaxel via anisamide-modified gold nanoparticles effectively down-regulate NF-κB and enhanced intracellular accumulation of paclitaxel in prostate cancer cells. This resulted in halting the proliferation and invasion of cancer cells.307 Similar to docetaxel, there have been intense interest in targeting genes involved in the viability and survival of cancer cells, to render the cells more conducive to paclitaxel chemotherapy. For example, siRNA–survivin and paclitaxel have been loaded into cationic liposomes for antiglioma therapy. Surface modification of these liposomes by CD133 enhances their cellular uptake by cancer cells. Down-regulation of survivin, induction of apoptosis, and inhibition of proliferation result from the use of these cationic liposomes.308 Apart from inhibiting the proliferation and growth of cancer cells, regulating the migration of cancer cells is also of interest in anticancer therapy. This is because cancer cells with high motility result in poor prognosis.309,310 Inhibition of cancer cell metastasis controlling factors involved in angiogenesis. The co-delivery of siRNA–VEGF and paclitaxel by micelles suppressed the proliferation and invasion (siRNA–VEGF) of cancer cells, improving the overall prognosis (Figure 7).311
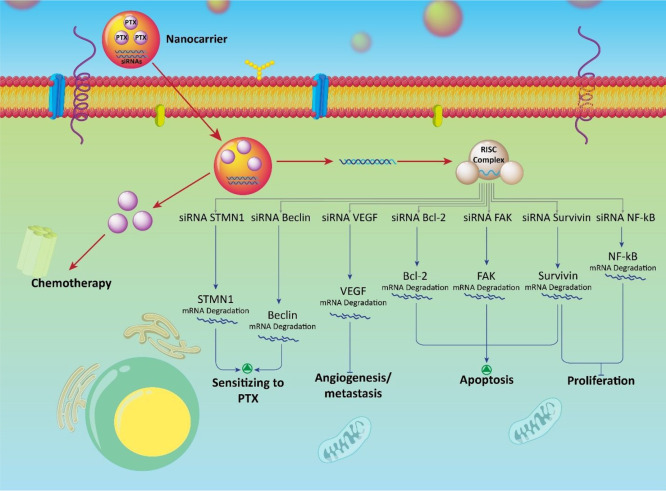
Figure 7.
Targeting molecular pathways in anticancer therapy using paclitaxel–siRNA-loaded nanoparticles. SiRNA–Beclin inhibits autophagy and enhances the antitumor activity of paclitaxel. SiRNA–STMN1, VEGF, Bcl-2, FAK, survivin, and NF-κB sensitize cancer cells to paclitaxel chemotherapy. The potential of siRNA and paclitaxel in anticancer therapy is boosted when they are loaded into nanoparticles.
Focal adhesion kinase (FAK) is a novel target in anticancer therapy because its expression is up-regulated in different cancers.312 Overexpression of FAK increases the resistance of cancer cells to chemotherapy. Accordingly, modulation of FAK expression can provide new therapeutic venues in inhibiting chemoresistance.313 Surface modification of nanoparticles with hyaluronic acid (HA) enhances their penetration into cancer cells because HA binds to CD44, which is highly expressed on cancer cells.314,315 The HA-modified poly(lactic-co-glycolic acid) nanoparticles are able to target ovarian cancer cells, and have high cellular uptake because they target CD44 receptors. The siRNA–FAK reduces the resistance of ovarian cancer cells to chemotherapy and paclitaxel induces apoptosis in cancer cells.316 Efflux transporters and Bcl-2 are the most common targets used to render cancerous cells more susceptible to paclitaxel chemotherapy. Efflux transports such as P-gp inhibit intracellular accumulation of chemotherapeutic agents while Bcl-2 suppresses apoptosis, thereby increasing the viability and survival of cancer cells.317,318 Co-delivery of siRNA–Bcl-2, siRNA–MDR1, and paclitaxel via poly(lactic-co-glycolic acid) nanoparticles is associated with improvement in the antitumor activity of paclitaxel, inhibition of growth and proliferation of cancer cells, and increased accumulation of paclitaxel within cancer cells.319 Paclitaxel resistance is gradually becoming an increasing challenge in anticancer therapeutics. Overcoming paclitaxel resistance requires designing a collaborative antitumor therapy in which siRNA inhibits expression of genes involved in paclitaxel resistance. Other hurdles include eliminating the poor bioavailability of paclitaxel and enhancing its targeted delivery. Nanoplatforms are able to release paclitaxel at the tumor site and enhance its internalization.320−330 Co-delivery of paclitaxel and siRNA has been extensively investigated in anticancer therapy. Overall, proliferation and metastasis are negatively affected by paclitaxel and siRNA. Paclitaxel and siRNA impede angiogenesis via VEGF down-regulation to disrupt cancer metastasis. Nanoparticles are used to promote siRNA in gene silencing and paclitaxel internalization into cancer cells. Table 5 is a summary of currently reported paclitaxel–siRNA co-delivery platforms in anticancer therapy.
Table 5. PTX–siRNA Co-delivery Platforms in Cancer Therapya.
| nanovehicle | cancer type | cell line | target gene | size (nm) | zeta potential (mV) | encapsulation efficiency (EE) (%) | remarks | ref |
|---|---|---|---|---|---|---|---|---|
| solid lipid NPs | cervical cancer | HeLa cells | Bcl-2 | 180 | +22.2 to +48.16 | 97–98 | down-regulation of Bcl-2, and induction of apoptosis | (331) |
| liposome | melanoma | B16F10 cells | Bcl-2 | 136 | 34.5 | 94 (siRNA) | down-regulation of Bcl-2, and inhibition of growth and proliferation | (332) |
| 91.2 (PTX) | ||||||||
| lipid NPs | breast cancer | human triple-negative breast cancer MDA-MB-231 cells | elF4E | 10–60 | reversal of PTX resistance and induction of apoptosis | (333) | ||
| polymeric NPs | cervical cancer | HeLa cells | E7 | 100–1000 | –14.4 to −30 | 88.4 (siRNA) | effective delivery into cancer cells, enhanced accumulation of siRNA and PTX in cancer cells, down-regulation of E7 and suppressing cancer proliferation and malignancy | (334) |
| 90.2 (PTX) | ||||||||
| liposome | ovarian cancer | HeyA8-MDR cells | KSP | 150.7 | 12.1 | high cellular uptake, down-regulation of KSP, and more inhibitory effect on cancer cells compared to PTX alone | (335) | |
| micelle | breast cancer | MCF-7 | MDR1 | 171.6 | –22.52 | 93.92 | protection of siRNA against degradation by macrophages, down-regulation of MDR1 and suppressing tumor volume | (336) |
| micelle | breast cancer | MDA-MB-231 cells | AURKA | 135 | +14 | 86 | delivering cargo in an HA-receptor mediated endocytosis, and high antitumor activity | (337) |
| polymeric NPs | breast cancer | mouse breast cancer cell lines 4T1 | twist | 80–140 | +16 to +36 | 92.79 | suppressing metastasis of cancer cells via down-regulation of twist | (337) |
| polymeric NPs | ovarian cancer | MDR ovarian cancer cell lines SKOV3TR | MDR1 | 173.3 | –22.5 | inhibiting expressions and activities of P-gp and MDR1, and suppressing PTX resistance | (338) | |
| micelle | ovarian cancer | human ovarian adenocarcinoma resistant cell line, SKOV3-tr PXL resistant cells | survivin | 25 | 50 (siRNA) | down-regulation of survivin, and exerting antitumor activity | (339) | |
| 90 (PTX) | ||||||||
| micelle | liver cancer | human hepatocellular carcinoma (HCC) HepG2 cell | Bcl-2 | 394.3–427 | +22 | high cellular uptake, exerting antitumor activity and inhibition of Bcl-2 expression | (340) | |
| polymeric NPs | breast cancer | human breast cancer MCF-7 cells | VEGF | 120.48 | +47.60 | suppressing tumor growth for in vitro and in vivo | (341) |
NP: Nanoparticles.
Etoposide–siRNA Co-delivery
Etoposide is a member of epipodophyllotoxins that are capable of suppressing the activity of DNA topoisomerase II.342 This chemotherapeutic agent exerts its antitumor activity by inhibition of DNA topoisomerase and subsequent induction of DNA damage and apoptotic cell death.343,344 To date, etoposide has been applied in the treatment of different cancers with excellent results achieved in clinical trials.345−347 There is still a long way in improving the antitumor activity of etoposide. Similar to other chemotherapeutic agents, cancer cells are capable of acquiring resistance to etoposide chemotherapy.348,349 Studies have looked at the use of combined etoposide and gene therapy in the treatment of cancer. This regime demonstrated satisfactory results in cancer therapy. The ABCB1 is a drug transporter involved in imparting cancer cells with resistance to chemotherapy. This is achieved by controlling efflux of chemotherapeutic agents and reducing their accumulation in cancer cells that results in chemoresistance.350 The siRNA–ABCB1 effectively suppresses this transporter and enhances etoposide accumulation in cancer cells, thereby decreasing the viability and proliferation of cancer cells.351 In addition to transporters, genes participating in the survival of cancer cells may also be targeted. Silencing survivin gene using siRNA remarkably decreases the viability of leukemia cancer cells and induces their apoptosis.352 Another apoptotic factor is p53. The oncoprotein inhibitory member of the ASPP family (iASPP) functions as an upstream modulator of p53; iASPP reduces the expression of p53 and renders cancer cells resistant to apoptosis.353,354 Knock-down of iASPP by siRNA stimulates the expression of p53 and make cancer cells susceptible to etoposide-mediated apoptosis.355 Although the combination of etoposide and siRNA is beneficial in cancer elimination,356 further progress has to be made to enhance the efficacy of these agents. This may be achieved by using nanotechnology as platforms for targeted delivery of etoposide and siRNA.
Small interfering RNA may be used to knockout the genes involved in malignancy. Vascular endothelial growth factor (VEGF) is an oncogene involved in enhancing tumor neovascularization and is up-regulated in different types of cancer.357,358 Because of the role of VEGF in promoting cancer growth and viability, studies have been performed on the inhibition of VEGF expression in anticancer therapy.359,360 The combination of siRNA–VEGF and etoposide appears to be beneficial in the treatment of lung cancer. Multifunctional nanoparticles have been used as platforms for coloading of siRNA–VEGF and etoposide. The multifunctional nanoparticles are capable of codelivering siRNA–VEGF and etoposide to tumor cells because of their excellent internalization potential. The mild acidic pH of the tumor microenvironment induces the release of siRNA–VEGF and etoposide, providing targeted delivery. Effective co-delivery of siRNA–VEGF and etoposide resulted in suppression of angiogenesis and metastasis of lung cancer cells.361 Another oncogene in lung cancer cells is the enhancer of zeste homologue 2 (EZH2) belonging to the family of the Polycomb Group (PcG) gene. This protein is overexpressed in lung cancer,362 breast cancer,363 thyroid cancer,364 as well as in brain tumors.365 Co-delivery of siRNA–EZH2 and etoposide using multifunctional nanoparticles has been experimental used for fighting lung cancer. In vitro and in vivo experiments demonstrated that the multifunctional nanoparticles provide targeted co-delivery of siRNA–EZH2 and etoposide, decreasing the proliferation, and metastasis of lung cancer cells.366
Because etoposide is frequently used for anticancer therapy, cancer cells may develop resistance to this chemotherapeutic agent. There is a need to identify the molecular pathways involved in the development of etoposide resistance. This will facilitate the design of relevant siRNA and selection of appropriate nanoparticles for targeted co-delivery of etoposide and siRNA (Figure 8).367Table 6 represents etoposide–siRNA co-delivery platforms in cancer therapy.
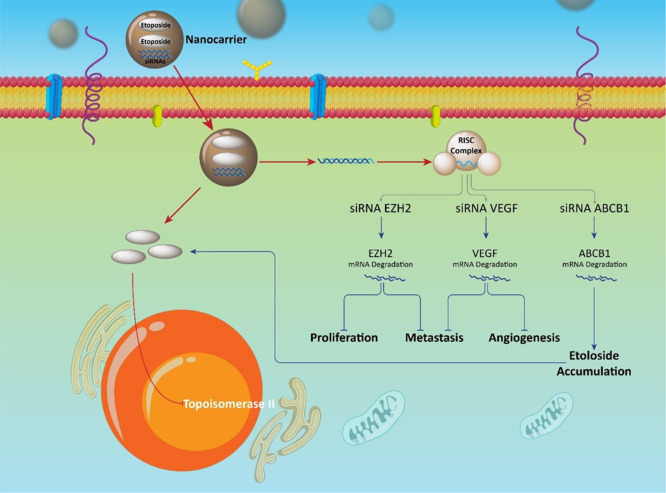
Figure 8.
Down-stream targets of etoposide–siRNA nanoparticles in anticancer therapy. Promotion of etoposide accumulation by nanoparticles and down-regulation of ABCB1 by siRNA. This demonstrates how a combination of nanoparticles and siRNA promotes internalization of etoposide into cancerous cells. Metastasis, angiogenesis, and proliferation are suppressed following co-delivery of siRNA and etoposide by nanoparticles.
Table 6. Etoposide–siRNA Co-delivery Platforms in Cancer Therapy.
| nanovehicle | cancer type | cell line | target gene | size (nm) | zeta potential (mV) | encapsulation efficiency (EE) (%) | remarks | ref |
|---|---|---|---|---|---|---|---|---|
| multifunctional nanoparticles | lung cancer | A549 cells | VEGF | 161.3 | +15.5 to +25.5 | down-regulation of VEGF, inhibition of metastasis and angiogenesis, and stimulation of apoptotic cell death | (368) | |
| multifunctional nanoparticles | lung cancer | A549 cells | EZH2 | 111.7 | +7.3 | inhibition of EZH2, and reduction in proliferation and invasion of cancer cells | (369) |
Resveratrol–siRNA Co-delivery
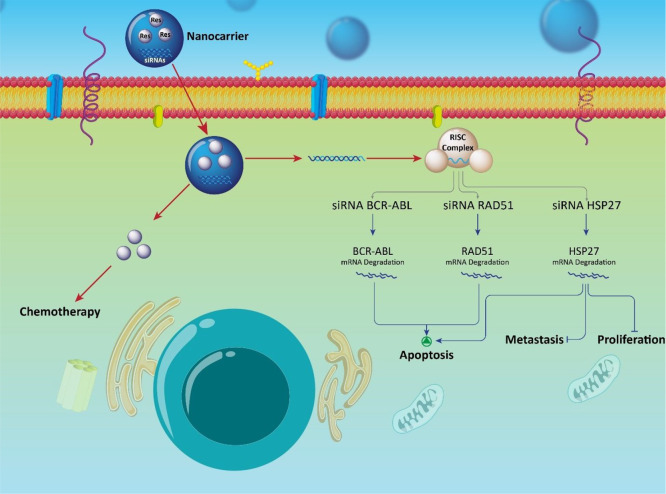
Resveratrol is a plant derived-chemical compound belonging to the flavonoid family.372 It has two distinct isoforms, trans-resveratrol and cis-resveratrol.373 These isoforms can be transformed into one another under certain circumstances. For example, exposure to ultraviolet irradiation changes the cis isoform into the trans form.374 Resveratrol is secreted by plants in response to biotic and abiotic stresses.375 This naturally occurring polyphenol provides defense against pathogens and is produced by edible plants such as hops.376 Resveratrol possesses excellent antioxidant, anti-inflammatory, antidiabetic, and neuroprotective activities.377−380 The antitumor activity of resveratrol has provided a valuable option in anticancer therapy.381,382 Similar to curcumin, the therapeutic effects of resveratrol are limited by its poor bioavailability.383 The antitumor activity of resveratrol may be accelerated by combining its use with siRNA-based gene therapy.384 An example if the combination of Res and siRNA–RAD51 in anticancer therapy. RAD51 is an oncogene that is involved in cancer progression and chemoresistance.385 Silencing of RAD51 together with the administration of resveratrol effectively induce apoptosis in cancer cells.386 Heat shock proteins (HSPs) are involved in malignancy and HSP27 is one of these proteins. Overexpression of HSP27 causes metastasis of cancer cells via induction of epithelial–mesenchymal transition.387,388 A combination of resveratrol and siRNA–HSP27 significantly inhibited the proliferation and migration of glioblastoma cells via down-regulation of HSP27 and activation of caspase-3, which, in turn, causes apoptosis of the cancer cells.389
The use of nanoplatforms for co-delivery of Res and siRNA enhances their antitumor activity. Over the past decades, electrospun fibers have been considered ideal candidates for drug delivery because of their potential in acting as platforms for sustained drug release.390 Multilayered core–shell fibers can be formed using multiaxial electrospinning. Drugs with different release kinetics may be incorporated in different compartments of the core–shell fibers.391 These electrospun fibers for delivery of resveratrol to cancer cells. Resveratrol- and siRNA-loaded electrospun fibers have been reported to reduce the viability and proliferation of leukemia cells. This is due to prolonged-release of resveratrol in 5 days and effective delivery of resveratrol and siRNA to the tumor cells.370 Apart from incorporating into a single nanoplatform, resveratrol and siRNA may be loaded into two distinct nanocarriers. siRNA–BCR-ABL liposomes and resveratrol-loaded electrospun fibers have been prepared to reduce the viability and growth of leukemia cancer cells via sustained drug release.371 To date, only two studies have investigated the co-delivery of resveratrol and siRNA in anticancer therapy. Further studies should focus on the development of other nanocarriers, such as polymeric nanoparticles, solid lipid nanoparticles, niosomes, or carbon dots for co-delivery of resveratrol and siRNA currently reported (Figure 9). Table 7 represents resveratrol–siRNA co-delivery platforms that have been used experimental in anticancer therapy.
Figure 9.
Targeting molecular signaling pathways in cancer therapy using resveratrol–siRNA-loaded nanoparticles. Apoptosis induction via siRNA–RAD51 and HSP27 results in increase in the antitumor activity of resveratrol. Co-delivery of resveratrol and siRNA by nanoparticles enhances their cellular uptake and antitumor potential.
Table 7. Resveratrol–siRNA Co-delivery Platforms in Cancer Therapy.
| nanovehicle | cancer type | cell line | target gene | size (nm) | zeta potential (mV) | encapsulation efficiency (EE) (%) | remarks | ref |
|---|---|---|---|---|---|---|---|---|
| electrospun fiber | leukemia | K562 cells | BCR-ABL | 76.9–88.3 | effective delivery of Res and siRNA, and reducing proliferation and viability of cancer cells | (370) | ||
| electrospun fiberLiposome | leukemia | K562 cells | BCR-ABL | 117.2 | –11 | 85.9 | releasing Res in a prolonged-release behavior, knock-down of BCR-ABL gene and decreasing viability and proliferation of cancer cells | (371) |
Camptothecin–siRNA Co-delivery
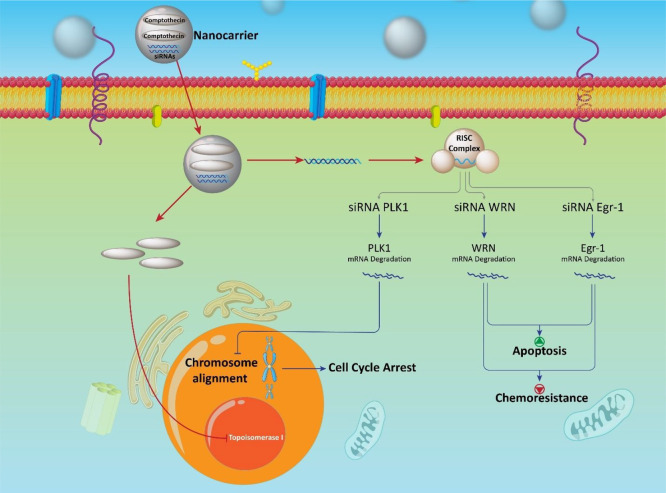
Adverse effects and off-targeting of chemotherapeutic agents are two critical drawbacks associated with their use.392 Camptothecin is a potential chemotherapeutic agent capable of targeting DNA topoisomerase I by suppressing its activities in DNA transcription, replication, and chromosome condensation.393−395 Because the antitumor activity of camptothecin is not affected by P-gp/MDR1 resistance, it is a valuable option for anticancer therapy.396 Nevertheless, modifications in the administration of camptothecin should be performed to enhance its antitumor activity. Camptothecin and siRNAs (siRNA–WRN and siRNA–Egr-1) can be coadministered in anticancer therapy to induce apoptosis of cancer cells, impair their proliferation, and suppress chemoresistance.397,398 Nanocarriers may be used to overcome the drawbacks associated with camptothecin (side effects and off-targeting). Different nanoparticles have been used for delivery of camptothecin in anticancer therapy. Examples include polymeric nanoparticles, dendrimers, micelles, nanofibers, carbon nanotube, and multifunctional nanocarriers (Figure 10).173,399−405
Figure 10.
Camptothecin and its co-delivery with siRNA in the treatment of cancer.
Although camptothecin-loaded nanocarriers demonstrate potential in reducing the survival and proliferation of cancer cells, the antitumor activity of camptothecin may be further optimized via co-delivery of siRNA. Polo-like kinase 1 (PLK1) is a key member of the PLK family and has important biological functions, such as bipolar arrangement of centrosomes, spindle assembly checkpoint, and cytokinesis.406−408 Targeting of PLK-1 offers novel opportunities for anticancer therapy because of its roles in chromosome alignment and the cell cycle.409−411 The liposomes are capable of release siRNA–PLK1 and camptothecin at tumor in a sustained-release behavior. Release occurs in response to the low pH of the tumor microenvironment. The siRNA–PLK-1 and camptothecin accumulate at the tumor site, the toxicity of which causes apoptosis of the cancel cells.412 Apoptosis results from the inhibitory effect of camptothecin on DNA topoisomerases and silencing of PLK1. Liposomes enhance the accumulation of siRNA and camptothecin at the tumor sites. Camptothecin is the least investigated antitumor agents that we have discussed so far. Liposomes are the only nanocarriers that have been applied for co-delivery of camptothecin and siRNA against cancer. Other nanoparticles such as micelles, polymeric nanoparticles, carbon nanotubes and metal nanoparticles may also be applied for co-delivery of siRNA and camptothecin. Further studies will help in identifying the efficacy of different types of nanocarriers in promoting the antitumor activity of codelivered camptothecin and siRNA.
Conclusion and Remarks
The efficacy of nanocarriers for the co-delivery of siRNA and natural products in the treatment of cancer was examined in the present Review. Cancer cells develop resistance against chemotherapeutic agents. Thus, ushering scientists to provide new regimes and strategies in field of anticancer therapeutics. Natural products are used in chemotherapy because of their excellent antitumor activity and their capability to target different molecular pathways. Two strategies may be considered in the investigation of the antitumor activity of natural products. The first strategy should focus on targeted delivery of chemotherapeutic agents and enhancement in their intracellular accumulation via the use of nanoparticles. The poor bioavailability of many phytochemicals may be overcome using nanoparticles. There are other barriers that limit the antitumor activity of natural products. Nanosized encapsulants derived from polymer and lipid organic nanomaterials, as well as inorganic-based nanometals, have been designed to carry siRNA and natural compounds. Encapsulants can inhibit proliferation and of cancer cells via co-delivery of natural compounds and siRNA. On one hand, this enhances the effectiveness of siRNA in gene silencing. On the other hand, the nanocarriers ameliorate the accumulation of natural products in tumor cells. As an example, in treatment of brain tumors, the BBB restricts the infiltration of antitumor agents into the brain. Nanocarriers promote penetration of the anticancer therapeutic agents through the BBB. Different receptors, such as transferrin, can be incorporated on nanoparticles for promoting their infiltration through the BBB. The BTB is another impediment that limits the penetration of antitumor agents into tumors. Nanoparticles can facilitate the penetration through BTB and promote internalization of antitumor agents. Hence, nanotechnology is an inevitable part of anticancer therapy.
Uncontrolled metastasis and proliferation of cancer cells are responsible chemoresistance. SiRNAs suppress cancer cell metastasis (MMP-9) and proliferation (Bcl-2). They improve the sensitivity of cancer cells to natural products with anticancer properties. The off-targeting limitation of siRNA may be improved via the use of nanotechnology. Nanovehicles also protect siRNA and natural compounds from degradation during blood circulation. Thus, nanocarriers, siRNA, and natural products may be combined for effective treatment against cancer. Nevertheless, these treatment regimes are still at their infancy of development. Additional animal studies are required to improve their efficacy prior to the implementation of human clinical trials.
Some studies have examined the overexpression of specific receptors on cancer cells, and have designed novel nanoencapsulant for targeting those receptors via surface modification. In addition to targeted delivery, the second strategy may be directed toward targeting molecular pathways and mechanisms involved in chemoresistance. These pathways may be utilized for increasing the sensitivity of cancer cells to chemotherapy. The siRNAs may be used for realizing the second strategy.
Glossary
Abbreviations
- WHO
World Health Organization
- P-gp
P-glycorprotein
- FDA
Food and Drug Administration
- ER
endoplasmic reticulum
- ROS
reactive oxygen species
- cyt C
cytochrome C
- EMT
epithelial-to-mesenchymal transition
- BTB
blood–tumor barrier
- RNAi
RNA interference
- siRNA
small interfering RNA
- ssRNA
single stranded RNA
- RISC
RNA-induced silencing complex
- RSF-1
remodeling and spacing factor-1
- GLUT-1
glucose transporter-1
- MMP-2
matrix metalloproteinase-2
- RR
ribonucleotide reductase
- miRs
microRNAs
- MDR
multidrug resistance
- BBB
blood-brain barrier
- DOX
doxorubicin
- RAC1
Ras-related C3 botulinum toxin substrate 1
- NADPH
nicotinamide adenine dinucleotide phosphate
- NOX
NADPH oxidase
- PD-L1
programmed death-ligand 1
- CCMNPs
cancer cell membrane-coated nanoparticles
- JIP1
JNK-interacting protein 1
- GO
graphene oxide
- PAMAM
polyamidoamine
- DTX
docetaxel
- MMP-9
matrix metalloproteinase-9
- ECM
extracellular matrix
- PCD
programmed cell death
- LRP
low-density lipoprotein receptor-related protein
- PTX
paclitaxel
- STMN1
stathmin1
- SLNs
sold lipid nanoparticles
- FAK
focal adhesion kinase
- HA
hyaluronic acid
- iASPP
inhibitory member of ASPP family
- VEGF
vascular endothelial growth factor
- EZH2
enhancer of zeste homologue 2
- PcG
polycomb group
- Res
resveratrol
- PLK
polo-like kinase
- CHOP
C/EBP homologous protein
Authors received no funding for this article.
The authors declare no competing financial interest.
References
- Zhang T.; Li Y.; Hong W.; Chen Z.; Peng P.; Yuan S.; Qu J.; Xiao M.; Xu L. Glucose Oxidase and Polydopamine Functionalized Iron Oxide Nanoparticles: Combination of the Photothermal Effect and Reactive Oxygen Species Generation for Dual-Modality Selective Cancer Therapy. J. Mater. Chem. B 2019, 7, 2190–2200. 10.1039/C8TB03320J. [DOI] [PubMed] [Google Scholar]
- Rabiee S.; Tavakol S.; Barati M.; Joghataei M. T. Autophagic, Apoptotic, and Necrotic Cancer Cell Fates Triggered by Acidic Ph Microenvironment. J. Cell. Physiol. 2019, 234, 12061–12069. 10.1002/jcp.27876. [DOI] [PubMed] [Google Scholar]
- Tavakol S. Acidic Ph Derived from Cancer Cells May Induce Failed Reprogramming of Normal Differentiated Cells Adjacent Tumor Cells and Turn Them into Cancer Cells. Med. Hypotheses 2014, 83, 668–672. 10.1016/j.mehy.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Yang X.; An J.; Luo Z.; Yang R.; Yan S.; Liu D.-E.; Fu H.; Gao H. A Cyanine-Based Polymeric Nanoplatform with Microenvironment-Driven Cascaded Responsiveness for Imaging-Guided Chemo-Photothermal Combination Anticancer Therapy. J. Mater. Chem. B 2020, 8, 2115–2122. 10.1039/C9TB02890K. [DOI] [PubMed] [Google Scholar]
- Park J. J.; Hsu G.; Siden E. G.; Thorlund K.; Mills E. J. An Overview of Precision Oncology Basket and Umbrella Trials for Clinicians. Ca-Cancer J. Clin. 2020, 70 (2), 125–37. 10.3322/caac.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y.; Sun X.; Wang Y.; Yan C.; Liu Y.; Li J.; Zhang D.; Zheng M.; Chung R. S.; Shi B. Single Sirna Nanocapsules for Effective Sirna Brain Delivery and Glioblastoma Treatment. Adv. Mater. (Weinheim, Ger.) 2020, e2000416 10.1002/adma.202000416. [DOI] [PubMed] [Google Scholar]
- Meryet-Figuiere M.; Lecerf C.; Varin E.; Coll J. L.; Louis M. H.; Dutoit S.; Giffard F.; Blanc-Fournier C.; Hedir S.; Vigneron N.; Brotin E.; Pelletier L.; Josserand V.; Denoyelle C.; Poulain L. Atelocollagen-Mediated in Vivo Sirna Transfection in Ovarian Carcinoma Is Influenced by Tumor Site, Sirna Target and Administration Route. Oncol. Rep. 2017, 38, 1949–1958. 10.3892/or.2017.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattheolabakis G.; Ling D.; Ahmad G.; Amiji M. Enhanced Anti-Tumor Efficacy of Lipid-Modified Platinum Derivatives in Combination with Survivin Silencing Sirna in Resistant Non-Small Cell Lung Cancer. Pharm. Res. 2016, 33, 2943–2953. 10.1007/s11095-016-2016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Liu J.; Movahedi F.; Gu W.; Xu T.; Xu Z. P. Enhanced Prevention of Breast Tumor Metastasis by Nanoparticle-Delivered Vitamin E in Combination with Interferon-Gamma. Adv. Healthcare Mater. 2020, 9 (6), 1901706. 10.1002/adhm.201901706. [DOI] [PubMed] [Google Scholar]
- Lucky S. S.; Soo K. C.; Zhang Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- Shen X.; Wang Y.; Xi L.; Su F.; Li S. Biocompatibility and Paclitaxel/Cisplatin Dual-Loading of Nanotubes Prepared from Poly (Ethylene Glycol)-Polylactide-Poly (Ethylene Glycol) Triblock Copolymers for Combination Cancer Therapy. Saudi Pharm. J. 2019, 27, 1025–1035. 10.1016/j.jsps.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Combination Treatment of Cervical Cancer Using Folate-Decorated, Ph-Sensitive, Carboplatin and Paclitaxel Co-Loaded Lipid-Polymer Hybrid Nanoparticles. Drug Des., Dev. Ther. 2020, 14, 823. 10.2147/DDDT.S235098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamaledin R.; Di Natale C.; Onesto V.; Taraghdari Z. B.; Zare E. N.; Makvandi P.; Vecchione R.; Netti P. A. Progress in Microneedle-Mediated Protein Delivery. J. Clin. Med. 2020, 9, 542. 10.3390/jcm9020542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S.; Liu M.; Li T.; Lin S.; Mo R. Recent Progress in Nanomedicine-Based Combination Cancer Therapy Using a Site-Specific Co-Delivery Strategy. Biomater. Sci. 2017, 5, 1367–1381. 10.1039/C7BM00297A. [DOI] [PubMed] [Google Scholar]
- Wang L.; Li L.; Han Q.; Wang X.; Zhao D.; Liu J. Identification and Biological Evaluation of Natural Product Biochanin A. Bioorg. Chem. 2020, 97, 103674. 10.1016/j.bioorg.2020.103674. [DOI] [PubMed] [Google Scholar]
- Liskova A.; Koklesova L.; Samec M.; Smejkal K.; Samuel S. M.; Varghese E.; Abotaleb M.; Biringer K.; Kudela E.; Danko J.; Shakibaei M.; Kwon T. K.; Büsselberg D.; Kubatka P. Flavonoids in Cancer Metastasis. Cancers 2020, 12, 1498. 10.3390/cancers12061498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abotaleb M.; Liskova A.; Kubatka P.; Büsselberg D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. 10.3390/biom10020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese E.; Liskova A.; Kubatka P.; Samuel S. M.; Büsselberg D. Anti-Angiogenic Effects of Phytochemicals on Mirna Regulating Breast Cancer Progression. Biomolecules 2020, 10, 191. 10.3390/biom10020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Wu S.; Dong G.; Chen S.; Ma Z.; Liu D.; Sheng C. Natural Product Evodiamine with Borate Trigger Unit: Discovery of Potent Antitumor Agents against Colon Cancer. ACS Med. Chem. Lett. 2020, 11, 439–444. 10.1021/acsmedchemlett.9b00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solárová Z.; Liskova A.; Samec M.; Kubatka P.; Büsselberg D.; Solár P. Anticancer Potential of Lichens’ Secondary Metabolites. Biomolecules 2020, 10, 87. 10.3390/biom10010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskova T.; Kubatka P.; Büsselberg D.; Kassayova M. The Plant-Derived Compound Resveratrol in Brain Cancer: A Review. Biomolecules 2020, 10, 161. 10.3390/biom10010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M. S.; Robertson A. A.; Cooper M. A. Natural Product and Natural Product Derived Drugs in Clinical Trials. Nat. Prod. Rep. 2014, 31, 1612–1661. 10.1039/C4NP00064A. [DOI] [PubMed] [Google Scholar]
- Butler M. S. Natural Products to Drugs: Natural Product-Derived Compounds in Clinical Trials. Nat. Prod. Rep. 2008, 25, 475–516. 10.1039/b514294f. [DOI] [PubMed] [Google Scholar]
- Newman D. J.; Cragg G. M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Fang K.; Cheng J.; Li Y.; Huang Y.; Chen S.; Dong G.; Wu S.; Sheng C. Scaffold Hopping of Natural Product Evodiamine: Discovery of a Novel Antitumor Scaffold with Excellent Potency against Colon Cancer. J. Med. Chem. 2020, 63 (2), 696–713. 10.1021/acs.jmedchem.9b01626. [DOI] [PubMed] [Google Scholar]
- Li X.; Zhang W.; Liang L.; Duan X.; Deng J.; Zhou Y. Natural Product-Derived Icaritin Exerts Anti-Glioblastoma Effects by Positively Modulating Estrogen Receptor B. Exp. Ther. Med. 2020, 19, 2841–2850. 10.3892/etm.2020.8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefayat A.; Ghahremani F.; Safavi A.; Hajiaghababa A.; Moshtaghian J. C-Phycocyanin: A Natural Product with Radiosensitizing Property for Enhancement of Colon Cancer Radiation Therapy Efficacy through Inhibition of Cox-2 Expression. Sci. Rep. 2019, 9, 19161. 10.1038/s41598-019-55605-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. F.; Yanez M.; Moghadam S. E.; Moridi Farimani M.; Soroury S.; Ebrahimi S. N.; Tabefam M.; Jabbarzadeh E. 7-Epi-Clusianone, a Multi-Targeting Natural Product with Potential Chemotherapeutic, Immune-Modulating, and Anti-Angiogenic Properties. Molecules 2019, 24, 4415. 10.3390/molecules24234415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D.; Wang Z.; Lin M.; Shang Y.; Wang F.; Zhou J.; Wang F.; Zhang X.; Luo X.; Huang W. In Vitro and in Vivo Antitumor Activity of Cucurbitacin C, a Novel Natural Product from Cucumber. Front. Pharmacol. 2019, 10, 1287. 10.3389/fphar.2019.01287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch V. C.; Orgler C.; Braig S.; Jeremias I.; Auerbach D.; Müller R.; Vollmar A. M.; Sieber S. A. The Cytotoxic Natural Product Vioprolide a Targets Nucleolar Protein 14, Which Is Essential for Ribosome Biogenesis. Angew. Chem., Int. Ed. 2020, 59, 1595–1600. 10.1002/anie.201911158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangachari B.; Jeong Hwa K.; Mi-Na J.; Chenglian X.; Jin Kyu P.; Jae Kwon L. Bee Wax Coated Water-Soluble Fraction of Bee Venom Improved Altered Glucose Homeostasis in Streptozotocin-Induced Diabetic Rats. Journal of Traditional Chinese Medicine 2019, 39, 842–852. [PubMed] [Google Scholar]
- Liu L. Y.; Zhu H. R.; Wu W.; Shen Y. Y.; Wu Y.; Zhou Y. J.; Liu L.; Tang J.; Sun F.; Lin H. W.; et al. Neoantimycin F, a Streptomyces-Derived Natural Product Induces Mitochondria-Related Apoptotic Death in Human Non-Small Cell Lung Cancer Cells. Front. Pharmacol. 2019, 10, 1042. 10.3389/fphar.2019.01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi M.; Movahedpour A.; Tayarani A.; Shabaninejad Z.; Pourhanifeh M. H.; Mortezapour E.; Nickdasti A.; Mottaghi R.; Davoodabadi A.; Khan H.; Savardashtaki A.; Mirzaei H. Therapeutic Potentials of Curcumin in the Treatment of Non-Small-Cell Lung Carcinoma. Phytother. Res. 2020, 34, 2557. 10.1002/ptr.6704. [DOI] [PubMed] [Google Scholar]
- Lai K. C.; Chueh F. S.; Hsiao Y. T.; Cheng Z. Y.; Lien J. C.; Liu K. C.; Peng S. F.; Chung J. G. Gefitinib and Curcumin-Loaded Nanoparticles Enhance Cell Apoptosis in Human Oral Cancer Sas Cells in Vitro and Inhibit Sas Cell Xenografted Tumor in Vivo. Toxicol. Appl. Pharmacol. 2019, 382, 114734. 10.1016/j.taap.2019.114734. [DOI] [PubMed] [Google Scholar]
- Wang B.; Gao X.; Liu B.; Li Y.; Bai M.; Zhang Z.; Xu E.; Xiong Z.; Hu Y. Protective Effects of Curcumin against Chronic Alcohol-Induced Liver Injury in Mice through Modulating Mitochondrial Dysfunction and Inhibiting Endoplasmic Reticulum Stress. Food Nutr. Res. 2019, 10.29219/fnr.v63.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansourizadeh F.; Alberti D.; Bitonto V.; Tripepi M.; Sepehri H.; Khoee S.; Geninatti Crich S. Efficient Synergistic Combination Effect of Quercetin with Curcumin on Breast Cancer Cell Apoptosis through Their Loading into Apo Ferritin Cavity. Colloids Surf., B 2020, 191, 110982. 10.1016/j.colsurfb.2020.110982. [DOI] [PubMed] [Google Scholar]
- Rajamanickam V.; Yan T.; Wu L.; Zhao Y.; Xu X.; Zhu H.; Chen X.; Wang M.; Liu Z.; Liu Z.; Liang G.; Wang Y. Allylated Curcumin Analog Ca6 Inhibits Trxr1 and Leads to Ros-Dependent Apoptotic Cell Death in Gastric Cancer through Akt-Foxo3a. Cancer Manage. Res. 2020, 12, 247–263. 10.2147/CMAR.S227415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong S. J. F.; Marchi S.; Petroni G.; Kroemer G.; Galluzzi L.; Pervaiz S. Noncanonical Cell Fate Regulation by Bcl-2 Proteins. Trends Cell Biol. 2020, 30, 537. 10.1016/j.tcb.2020.03.004. [DOI] [PubMed] [Google Scholar]
- Yao H.; Fan M.; He X. Autophagy Suppresses Resveratrol-Induced Apoptosis in Renal Cell Carcinoma 786-O Cells. Oncol. Lett. 2020, 19, 3269–3277. 10.3892/ol.2020.11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. A.; Crowther C. M.; Andrus M. B.; Kenealey J. D. Resveratrol Derivatives Increase Cytosolic Calcium by Inhibiting Plasma Membrane Atpase and Inducing Calcium Release from the Endoplasmic Reticulum in Prostate Cancer Cells. Biochemistry and biophysics reports 2019, 19, 100667. 10.1016/j.bbrep.2019.100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J. R.; Kim S. M.; Hwang K. A.; Kang J. H.; Choi K. C. Resveratrol Induced Reactive Oxygen Species and Endoplasmic Reticulum Stressmediated Apoptosis, and Cell Cycle Arrest in the A375sm Malignant Melanoma Cell Line. Int. J. Mol. Med. 2018, 42, 1427–1435. 10.3892/ijmm.2018.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q.; Wang N.; Ren L.; Tian J.; Yang S.; Cheng H. Mir-125a-5p Post-Transcriptionally Suppresses Galnt7 to Inhibit Proliferation and Invasion in Cervical Cancer Cells Via the Egfr/Pi3k/Akt Pathway. Cancer Cell Int. 2020, 20, 117. 10.1186/s12935-020-01209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M.; Li G.; Zhu L.; Zhou H.; Lu L. Arctiin Attenuates High Glucose-Induced Human Retinal Capillary Endothelial Cell Proliferation by Regulating Rock1/Pten/Pi3k/Akt/Vegf Pathway in Vitro. J. Cell. Mol. Med. 2020, 24, 5695. 10.1111/jcmm.15232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang Z.; Meng L.; Yi C.; Yu L.; Chen W.; Sha W. Curcumin Regulates the Mir-21/Pten/Akt Pathway and Acts in Synergy with Pd98059 to Induce Apoptosis of Human Gastric Cancer Mgc-803 Cells. J. Int. Med. Res. 2019, 47, 1288–1297. 10.1177/0300060518822213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Wu Y.; Yang S.; Liu X.; Lu Y.; Liu F.; Li G.; Tian G. Mir-874 Directly Targets Aqp3 to Inhibit Cell Proliferation, Mobility and Emt in Non-Small Cell Lung Cancer. Thorac. Cancer 2020, 11, 1550. 10.1111/1759-7714.13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H.; Mohan C. D.; Deivasigamani A.; Jung Y. Y.; Rangappa S.; Basappa S.; Chinnathambi A.; Alahmadi T. A.; Alharbi S. A.; Garg M.; Lin Z.-X.; Rangappa K. S.; Sethi G.; Hui K. M.; Ahn K. S. Brusatol Suppresses Stat3-Driven Metastasis by Downregulating Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma. Journal of Advanced Research 2020, 10.1016/j.jare.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q. H.; Xiao Y.; Li X. Q.; Fan L.; Zhou C. C.; Cheng L.; Jiang Z. D.; Wang G. H. Resveratrol Counteracts Hypoxia-Induced Gastric Cancer Invasion and Emt through Hedgehog Pathway Suppression. Anti-Cancer Agents Med. Chem. 2020, 20, 1105. 10.2174/1871520620666200402080034. [DOI] [PubMed] [Google Scholar]
- Sun L.; Shi C.; Liu S.; Zhang E.; Yan L.; Ji C.; Zhao Y. Overexpression of Nusap1 Is Predictive of an Unfavourable Prognosis and Promotes Proliferation and Invasion of Triple-Negative Breast Cancer Cells Via the Wnt/Beta-Catenin/Emt Signalling Axis. Gene 2020, 747, 144657. 10.1016/j.gene.2020.144657. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Shao Z.; Jiang E.; Zhou X.; Wang L.; Wang H.; Luo X.; Chen Q.; Liu K.; Shang Z. Ccl21/Ccr7 Interaction Promotes Emt and Enhances the Stemness of Oscc Via a Jak2/Stat3 Signaling Pathway. J. Cell. Physiol. 2020, 235, 5995. 10.1002/jcp.29525. [DOI] [PubMed] [Google Scholar]
- Yen H. Y.; Tsao C. W.; Lin Y. W.; Kuo C. C.; Tsao C. H.; Liu C. Y. Regulation of Carcinogenesis and Modulation through Wnt/Beta-Catenin Signaling by Curcumin in an Ovarian Cancer Cell Line. Sci. Rep. 2019, 9, 17267. 10.1038/s41598-019-53509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.; Liu L.; Wang Y.; He A.; Hu H.; Zhang J.; Han M.; Huang Y. Curcumin Inhibits the Proliferation and Invasion of Mg-63 Cells through Inactivation of the P-Jak2/P-Stat3 Pathway. OncoTargets Ther. 2019, 12, 2011–2021. 10.2147/OTT.S172909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi Dermani F.; Saidijam M.; Amini R.; Mahdavinezhad A.; Heydari K.; Najafi R. Resveratrol Inhibits Proliferation, Invasion, and Epithelial-Mesenchymal Transition by Increasing Mir-200c Expression in Hct-116 Colorectal Cancer Cells. J. Cell. Biochem. 2017, 118, 1547–1555. 10.1002/jcb.25816. [DOI] [PubMed] [Google Scholar]
- Wen Q.; Zhang Y.; Luo J.; Xiong K.; Lu Y.; Wu Z.; Wang B. Q.; Wu J.; Chen Y.; Fu S. Therapeutic Efficacy of Thermosensitive Pluronic Hydrogel for Co-delivery of Resveratrol Microspheres and Cisplatin in the Treatment of Liver Cancer Ascites. Int. J. Pharm. 2020, 582, 119334. 10.1016/j.ijpharm.2020.119334. [DOI] [PubMed] [Google Scholar]
- Sudha T.; El-Far A. H.; Mousa D. S.; Mousa S. A. Resveratrol and Its Nanoformulation Attenuate Growth and the Angiogenesis of Xenograft and Orthotopic Colon Cancer Models. Molecules 2020, 25, 1412. 10.3390/molecules25061412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T. C.; Wu J. M. Resveratrol Suppresses Prostate Cancer Epithelial Cell Scatter/Invasion by Targeting Inhibition of Hepatocyte Growth Factor (Hgf) Secretion by Prostate Stromal Cells and Upregulation of E-Cadherin by Prostate Cancer Epithelial Cells. Int. J. Mol. Sci. 2020, 21, 1760. 10.3390/ijms21051760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bano S.; Ahmed F.; Khan F.; Chaudhary S. C.; Samim M. Enhancement of the Cancer Inhibitory Effect of the Bioactive Food Component Resveratrol by Nanoparticle Based Delivery. Food Funct. 2020, 11, 3213. 10.1039/C9FO02445J. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Cai C.; Liu M.; Zhao Y.; Wu Y.; Fan Z.; Ding Z.; Zhang H.; Wang Z.; Han J. Drug-Binding Albumins Forming Stabilized Nanoparticles for Co-Delivery of Paclitaxel and Resveratrol: In Vitro/in Vivo Evaluation and Binding Properties Investigation. Int. J. Biol. Macromol. 2020, 153, 873–882. 10.1016/j.ijbiomac.2020.03.060. [DOI] [PubMed] [Google Scholar]
- Senthil Kumar C.; Thangam R.; Mary S. A.; Kannan P. R.; Arun G.; Madhan B. Targeted Delivery and Apoptosis Induction of Trans-Resveratrol-Ferulic Acid Loaded Chitosan Coated Folic Acid Conjugate Solid Lipid Nanoparticles in Colon Cancer Cells. Carbohydr. Polym. 2020, 231, 115682. 10.1016/j.carbpol.2019.115682. [DOI] [PubMed] [Google Scholar]
- Zhao Y. N.; Cao Y. N.; Sun J.; Liang Z.; Wu Q.; Cui S. H.; Zhi D. F.; Guo S. T.; Zhen Y. H.; Zhang S. B. Anti-Breast Cancer Activity of Resveratrol Encapsulated in Liposomes. J. Mater. Chem. B 2020, 8, 27–37. 10.1039/C9TB02051A. [DOI] [PubMed] [Google Scholar]
- Ngamcherdtrakul W.; Yantasee W. Sirna Therapeutics for Breast Cancer: Recent Efforts in Targeting Metastasis, Drug Resistance, and Immune Evasion. Translational Research 2019, 214, 105–20. 10.1016/j.trsl.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slastnikova T. A.; Ulasov A. V.; Rosenkranz A. A.; Sobolev A. S. Targeted Intracellular Delivery of Antibodies: The State of the Art. Front. Pharmacol. 2018, 9, 1208. 10.3389/fphar.2018.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Dou Y.; Tang Y.; Zhang X. Folate Receptor-Targeted Rnai Nanoparticles for Silencing Stat3 in Tumor-Associated Macrophages and Tumor Cells. Nanomedicine 2020, 25, 102173. 10.1016/j.nano.2020.102173. [DOI] [PubMed] [Google Scholar]
- Smekalova E. M.; Gerashchenko M. V.; O’Connor P. B. F.; Whittaker C. A.; Kauffman K. J.; Fefilova A. S.; Zatsepin T. S.; Bogorad R. L.; Baranov P. V.; Langer R.; Gladyshev V. N.; Anderson D. G.; Koteliansky V. In Vivo Rnai-Mediated Eif3m Knockdown Affects Ribosome Biogenesis and Transcription but Has Limited Impact on Mrna-Specific Translation. Mol. Ther.--Nucleic Acids 2020, 19, 252–266. 10.1016/j.omtn.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirkol Canli S.; Dedeoglu E.; Akbar M. W.; Kucukkaraduman B.; Isbilen M.; Erdogan O. S.; Erciyas S. K.; Yazici H.; Vural B.; Gure A. O. A Novel 20-Gene Prognostic Score in Pancreatic Adenocarcinoma. PLoS One 2020, 15, e0231835 10.1371/journal.pone.0231835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S.; Li M.; Liu J.; Yang Y.; Li G. Identification of Immunologic Subtype and Prognosis of Gbm Based on Tnfsf14 and Immune Checkpoint Gene Expression Profiling. Aging 2020, 12, 7112. 10.18632/aging.103065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehexige E.; Bao M.; Bazarjav P.; Yu X.; Xiao H.; Han S.; Baigude H. Silencing of Stat3 Via Peptidomimetic Lnp-Mediated Systemic Delivery of Rnai Downregulates Pd-L1 and Inhibits Melanoma Growth. Biomolecules 2020, 10, 285. 10.3390/biom10020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz T. K.; Kleczko E. K.; Singleton K. R.; Calhoun J.; Marek L. A.; Kim J.; Tan A. C.; Heasley L. E. Functional Rnai Screens Define Distinct Protein Kinase Vulnerabilities in Egfr-Dependent Hnscc Cell Lines. Mol. Pharmacol. 2019, 96, 862–870. 10.1124/mol.119.117804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. C.; Zhang H.; Jakymiw A. Peptide Carriers to the Rescue: Overcoming the Barriers to Sirna Delivery for Cancer Treatment. Translational Research 2019, 214, 92–104. 10.1016/j.trsl.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C.-f.; Wang J. Delivery Systems for Sirna Drug Development in Cancer Therapy. Asian J. Pharm. Sci. 2015, 10, 1–12. 10.1016/j.ajps.2014.08.011. [DOI] [Google Scholar]
- Subhan M. A.; Torchilin V. Efficient Nanocarriers of Sirna Therapeutics for Cancer Treatment. Translational Research 2019, 214, 62–91. 10.1016/j.trsl.2019.07.006. [DOI] [PubMed] [Google Scholar]
- Halbur C.; Choudhury N.; Chen M.; Kim J. H.; Chung E. J. Sirna-Conjugated Nanoparticles to Treat Ovarian Cancer. SLAS TECHNOLOGY: Translating Life Sciences Innovation 2019, 24, 137–150. 10.1177/2472630318816668. [DOI] [PubMed] [Google Scholar]
- Zhang P.; An K.; Duan X.; Xu H.; Li F.; Xu F. Recent Advances in Sirna Delivery for Cancer Therapy Using Smart Nanocarriers. Drug Discovery Today 2018, 23, 900–911. 10.1016/j.drudis.2018.01.042. [DOI] [PubMed] [Google Scholar]
- Wang M.; Wang J.; Li B.; Meng L.; Tian Z. Recent Advances in Mechanism-Based Chemotherapy Drug-Sirna Pairs in Co-Delivery Systems for Cancer: A Review. Colloids Surf., B 2017, 157, 297–308. 10.1016/j.colsurfb.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Hammond S. M.; Bernstein E.; Beach D.; Hannon G. J. An Rna-Directed Nuclease Mediates Post-Transcriptional Gene Silencing in Drosophila Cells. Nature 2000, 404, 293–296. 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- Cuccato G.; Polynikis A.; Siciliano V.; Graziano M.; di Bernardo M.; di Bernardo D. Modeling Rna Interference in Mammalian Cells. BMC Syst. Biol. 2011, 5, 19. 10.1186/1752-0509-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir S. M.; Harborth J.; Lendeckel W.; Yalcin A.; Weber K.; Tuschl T. Duplexes of 21-Nucleotide Rnas Mediate Rna Interference in Cultured Mammalian Cells. Nature 2001, 411, 494–498. 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- McCaffrey A. P.; Meuse L.; Pham T.-T. T.; Conklin D. S.; Hannon G. J.; Kay M. A. Rna Interference in Adult Mice. Nature 2002, 418, 38–39. 10.1038/418038a. [DOI] [PubMed] [Google Scholar]
- Qiu S.; Adema C. M.; Lane T. A Computational Study of Off-Target Effects of Rna Interference. Nucleic acids research 2005, 33, 1834–1847. 10.1093/nar/gki324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu M.; Gao F.; Yu C.; Zeng M.; He G.; Wu Y.; Su Y.; Hu N.; Zhou Z.; Lin X.; Yang Z. Dual Targeted Therapy in Her2-Positive Breast Cancer Cells with the Combination of Carbon Dots/Her3 Sirna and Trastuzumab. Nanotechnology 2020, 31, 335102. 10.1088/1361-6528/ab8a8a. [DOI] [PubMed] [Google Scholar]
- Song Y.; Zhou B.; Du X.; Wang Y.; Zhang J.; Ai Y.; Xia Z.; Zhao G. Folic Acid (Fa)-Conjugated Mesoporous Silica Nanoparticles Combined with Mrp-1 Sirna Improves the Suppressive Effects of Myricetin on Non-Small Cell Lung Cancer (Nsclc). Biomed. Pharmacother. 2020, 125, 109561. 10.1016/j.biopha.2019.109561. [DOI] [PubMed] [Google Scholar]
- Ravi V.; Madhankumar A. B.; Abraham T.; Slagle-Webb B.; Connor J. R. Liposomal Delivery of Ferritin Heavy Chain 1 (Fth1) Sirna in Patient Xenograft Derived Glioblastoma Initiating Cells Suggests Different Sensitivities to Radiation and Distinct Survival Mechanisms. PLoS One 2019, 14, e0221952 10.1371/journal.pone.0221952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo G. E.; Maggisano V.; Celano M.; Cosco D.; Mignogna C.; Baldan F.; Lepore S. M.; Allegri L.; Moretti S.; Durante C.; Damante G.; Fresta M.; Russo D.; Bulotta S.; Puxeddu E. Anti-Htert Sirna-Loaded Nanoparticles Block the Growth of Anaplastic Thyroid Cancer Xenograft. Mol. Cancer Ther. 2018, 17, 1187–1195. 10.1158/1535-7163.MCT-17-0559. [DOI] [PubMed] [Google Scholar]
- Pandey P.; Siddiqui M. H.; Behari A.; Kapoor V. K.; Mishra K.; Sayyed U.; Tiwari R. K.; Shekh R.; Bajpai P. Jab1-Sirna Induces Cell Growth Inhibition and Cell Cycle Arrest in Gall Bladder Cancer Cells Via Targeting Jab1 Signalosome. Anti-Cancer Agents Med. Chem. 2020, 19, 2019–2033. 10.2174/1871520619666190725122400. [DOI] [PubMed] [Google Scholar]
- Wang X.; Sheu J. J.; Lai M. T.; Yin-Yi Chang C.; Sheng X.; Wei L.; Gao Y.; Wang X.; Liu N.; Xie W.; Chen C. M.; Ding W. Y.; Sun L. Rsf-1 Overexpression Determines Cancer Progression and Drug Resistance in Cervical Cancer. BioMedicine 2018, 8, 4. 10.1051/bmdcn/2018080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J.; Kong E.; Wang X.; Xie Z.; Chang C. Y.; Sheu J. J.; Hao Q.; Sun L. Rsf-1 Sirna Enhances Tumor Radiosensitivity in Cervical Cancer Via Enhanced DNA Damage, Cell Cycle Redistribution, and Promotion of Apoptosis. OncoTargets Ther. 2020, 13, 3061–3071. 10.2147/OTT.S246632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X. M.; Xu B.; Zhou M. L.; Bao Y. Y.; Zhou S. H.; Fan J.; Lu Z. J. Co-Inhibition of Glut-1 Expression and the Pi3k/Akt Signaling Pathway to Enhance the Radiosensitivity of Laryngeal Carcinoma Xenografts in Vivo. PLoS One 2015, 10, e0143306 10.1371/journal.pone.0143306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J.; Zhou S. H.; Fan J.; Yan S. X. Roles of Glucose Transporter-1 and the Phosphatidylinositol 3kinase/Protein Kinase B Pathway in Cancer Radioresistance (Review). Mol. Med. Rep. 2015, 11, 1573–81. 10.3892/mmr.2014.2888. [DOI] [PubMed] [Google Scholar]
- Zhong J. T.; Yu Q.; Zhou S. H.; Yu E.; Bao Y. Y.; Lu Z. J.; Fan J. Glut-1 Sirna Enhances Radiosensitization of Laryngeal Cancer Stem Cells Via Enhanced DNA Damage, Cell Cycle Redistribution, and Promotion of Apoptosis in Vitro and in Vivo. OncoTargets Ther. 2019, 12, 9129–9142. 10.2147/OTT.S221423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L.; Duan Y.; Ma F.; Lou L. Punicalagin Inhibits the Viability, Migration, Invasion, and Emt by Regulating Golph3 in Breast Cancer Cells. J. Recept. Signal Transduction Res. 2020, 40, 173–180. 10.1080/10799893.2020.1719152. [DOI] [PubMed] [Google Scholar]
- Yuan H. F.; Li Y.; Tan B. B.; Zhao Q.; Fan L. Q.; An Z. J. Inhibitory Effect of Sirna-Annexin A7 on Growth, Migration, and Invasion in Bgc823 Cells and Gastric Cancer Xenograftsin Nude Mice. International Journal of Clinical and Experimental Pathology 2020, 13, 122–131. [PMC free article] [PubMed] [Google Scholar]
- Wu X.; An X.; Zhang C.; Huang M. Clb6-Cdc28 Promotes Ribonucleotide Reductase Subcellular Redistribution During S Phase. Mol. Cell. Biol. 2018, 10.1128/MCB.00497-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman J. E.; Hsueh T.; Koya R. C.; Davis M. E.; Ribas A. Sirna Knockdown of Ribonucleotide Reductase Inhibits Melanoma Cell Line Proliferation Alone or Synergistically with Temozolomide. J. Invest. Dermatol. 2011, 131, 453–60. 10.1038/jid.2010.310. [DOI] [PubMed] [Google Scholar]
- Huang N.; Guo W.; Ren K.; Li W.; Jiang Y.; Sun J.; Dai W.; Zhao W. Lncrna Afap1-As1 Supresses Mir-139–5p and Promotes Cell Proliferation and Chemotherapy Resistance of Non-Small Cell Lung Cancer by Competitively Upregulating Rrm2. Front. Oncol. 2019, 9, 1103. 10.3389/fonc.2019.01103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue T.; Wang L.; Li Y.; Song H.; Chu H.; Yang H.; Guo A.; Jiao J. Sirna-Mediated Rrm2 Gene Silencing Combined with Cisplatin in the Treatment of Epithelial Ovarian Cancer in Vivo: An Experimental Study of Nude Mice. Int. J. Med. Sci. 2019, 16, 1510–1516. 10.7150/ijms.33979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt S. Sirna-Based Nanoparticles for Cancer Therapy: Hurdles and Hopes. MOJ. Proteomics Bioinform 2016, 4, 00142. 10.15406/mojpb.2016.04.00142. [DOI] [Google Scholar]
- de Fougerolles A.; Vornlocher H.-P.; Maraganore J.; Lieberman J. Interfering with Disease: A Progress Report on Sirna-Based Therapeutics. Nat. Rev. Drug Discovery 2007, 6, 443–453. 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. H.; Rossi J. J. Strategies for Silencing Human Disease Using Rna Interference. Nat. Rev. Genet. 2007, 8, 173–184. 10.1038/nrg2006. [DOI] [PubMed] [Google Scholar]
- Oh Y.-K.; Park T. G. Sirna Delivery Systems for Cancer Treatment. Adv. Drug Delivery Rev. 2009, 61, 850–862. 10.1016/j.addr.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Acharya R.; Saha S.; Ray S.; Hazra S.; Mitra M. K.; Chakraborty J. Sirna-Nanoparticle Conjugate in Gene Silencing: A Future Cure to Deadly Diseases?. Mater. Sci. Eng., C 2017, 76, 1378–1400. 10.1016/j.msec.2017.03.009. [DOI] [PubMed] [Google Scholar]
- Almuqbil R. M.; Heyder R. S.; Bielski E. R.; Durymanov M.; Reineke J. J.; da Rocha S. R. P. Dendrimer Conjugation Enhances Tumor Penetration and Efficacy of Doxorubicin in Extracellular Matrix-Expressing 3d Lung Cancer Models. Mol. Pharmaceutics 2020, 17, 1648. 10.1021/acs.molpharmaceut.0c00083. [DOI] [PubMed] [Google Scholar]
- Dong Y.; Chen Y.; Zhu D.; Shi K.; Ma C.; Zhang W.; Rocchi P.; Jiang L.; Liu X. Self-Assembly of Amphiphilic Phospholipid Peptide Dendrimer-Based Nanovectors for Effective Delivery of Sirna Therapeutics in Prostate Cancer Therapy. J. Controlled Release 2020, 322, 416–425. 10.1016/j.jconrel.2020.04.003. [DOI] [PubMed] [Google Scholar]
- Chen Q.; Yu Q.; Liu Y.; Bhavsar D.; Yang L.; Ren X.; Sun D.; Zheng W.; Liu J.; Chen L. M. Multifunctional Selenium Nanoparticles: Chiral Selectivity of Delivering Mdr-Sirna for Reversal of Multidrug Resistance and Real-Time Biofluorescence Imaging. Nanomedicine 2015, 11, 1773–84. 10.1016/j.nano.2015.04.011. [DOI] [PubMed] [Google Scholar]
- Zheng W.; Cao C.; Liu Y.; Yu Q.; Zheng C.; Sun D.; Ren X.; Liu J. Multifunctional Polyamidoamine-Modified Selenium Nanoparticles Dual-Delivering Sirna and Cisplatin to A549/Ddp Cells for Reversal Multidrug Resistance. Acta Biomater. 2015, 11, 368–80. 10.1016/j.actbio.2014.08.035. [DOI] [PubMed] [Google Scholar]
- Maiyo F.; Singh M. Polymerized Selenium Nanoparticles for Folate-Receptor-Targeted Delivery of Anti-Luc-Sirna: Potential for Gene Silencing. Biomedicines 2020, 8, 76. 10.3390/biomedicines8040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofolini T.; Dalmina M.; Sierra J. A.; Silva A. H.; Pasa A. A.; Pittella F.; Creczynski-Pasa T. B. Multifunctional Hybrid Nanoparticles as Magnetic Delivery Systems for Sirna Targeting the Her2 Gene in Breast Cancer Cells. Mater. Sci. Eng., C 2020, 109, 110555. 10.1016/j.msec.2019.110555. [DOI] [PubMed] [Google Scholar]
- Xu C.; Wang Y.; Tu Q.; Zhang Z.; Chen M.; Mwangi J.; Li Y.; Jin Y.; Zhao X.; Lai R. Targeting Surface Nucleolin Induces Autophagy-Dependent Cell Death in Pancreatic Cancer Via Ampk Activation. Oncogene 2019, 38, 1832–1844. 10.1038/s41388-018-0556-x. [DOI] [PubMed] [Google Scholar]
- Kim T.; Viard M.; Afonin K. A.; Gupta K.; Popov M.; Salotti J.; Johnson P. F.; Linder C.; Heldman E.; Shapiro B. A. Characterization of Cationic Bolaamphiphile Vesicles for Sirna Delivery into Tumors and Brain. Mol. Ther.--Nucleic Acids 2020, 20, 359–372. 10.1016/j.omtn.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A. K.; Gudipati S.; Pettenuzzo A.; Ronconi L.; Rouge J. L. Chimeric Sirna-DNA Surfactants for the Enhanced Delivery and Sustained Cytotoxicity of a Gold(Iii) Metallodrug. Bioconjugate Chem. 2020, 31, 1063–1069. 10.1021/acs.bioconjchem.0c00047. [DOI] [PubMed] [Google Scholar]
- Claveau S.; Nehlig E.; Garcia-Argote S.; Feuillastre S.; Pieters G.; Girard H. A.; Arnault J. C.; Treussart F.; Bertrand J. R. Delivery of Sirna to Ewing Sarcoma Tumor Xenografted on Mice, Using Hydrogenated Detonation Nanodiamonds: Treatment Efficacy and Tissue Distribution. Nanomaterials 2020, 10, 553. 10.3390/nano10030553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez-Rivera A.; Fournier P. G. J.; Arellano D. L.; Rodriguez-Hernandez A. G.; Vazquez-Duhalt R.; Cadena-Nava R. D. Brome Mosaic Virus-Like Particles as Sirna Nanocarriers for Biomedical Purposes. Beilstein J. Nanotechnol. 2020, 11, 372–382. 10.3762/bjnano.11.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W.; Sun J.; Guo X.-Y.; Chen X.; Wang R.; Qiu C.; Zhang H.-T.; Pang W.; Wang J.-C.; Zhang Q. Microfluidic-Based Holonomic Constraints of Sirna in Kernel of Lipid/Polymer Hybrid Nano-Assembles for Improving in Vivo Stable and Safe Delivery. ACS Appl. Mater. Interfaces 2020, 12 (13), 14839–54. 10.1021/acsami.9b22781. [DOI] [PubMed] [Google Scholar]
- Ceylan S.; Bahadori F.; Akbas F. Engineering of Sirna Loaded Plga Nano-Particles for Highly Efficient Silencing of Gpr87 Gene as a Target for Pancreatic Cancer Treatment. Pharm. Dev. Technol. 2020, 25, 855–864. 10.1080/10837450.2020.1745232. [DOI] [PubMed] [Google Scholar]
- Shi M.; Zhang J.; Huang Z.; Chen Y.; Pan S.; Hu H.; Qiao M.; Chen D.; Zhao X. Stimuli-Responsive Release and Efficient Sirna Delivery in Non-Small Cell Lung Cancer by a Poly (L-Histidine)-Based Multifunctional Nanoplatform. J. Mater. Chem. B 2020, 8, 1616–1628. 10.1039/C9TB02764E. [DOI] [PubMed] [Google Scholar]
- Sakurai Y.; Mizumura W.; Ito K.; Iwasaki K.; Katoh T.; Goto Y.; Suga H.; Harashima H. Improved Stability of Sirna-Loaded Lipid Nanoparticles Prepared with a Peg-Monoacyl Fatty Acid Facilitates Ligand-Mediated Sirna Delivery. Mol. Pharmaceutics 2020, 17, 1397–1404. 10.1021/acs.molpharmaceut.0c00087. [DOI] [PubMed] [Google Scholar]
- Panday R.; Abdalla A. M. E.; Yu M.; Li X.; Ouyang C.; Yang G. Functionally Modified Magnetic Nanoparticles for Effective Sirna Delivery to Prostate Cancer Cells in Vitro. J. Biomater. Appl. 2020, 34, 952–964. 10.1177/0885328219886953. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Xie Y.; Kilchrist K. V.; Li J.; Duvall C. L.; Oupický D. Endosomolytic and Tumor-Penetrating Mesoporous Silica Nanoparticles for Sirna/Mirna Combination Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12 (4), 4308–22. 10.1021/acsami.9b21214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. A.; Alanazi A. M.; Jabeen M.; Chauhan A.; Ansari M. A. Therapeutic Potential of Functionalized Sirna Nanoparticles on Regression of Liver Cancer in Experimental Mice. Sci. Rep. 2019, 9, 15825. 10.1038/s41598-019-52142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y.; Tang G.; Wang C.; Zhong J.; Chen Y.; Hua L.; Li Y.; Liu H.; Zhu B. Functionalized Selenium Nanoparticles for Targeted Sirna Delivery Silence Derlin1 and Promote Antitumor Efficacy against Cervical Cancer. Drug Delivery 2020, 27, 15–25. 10.1080/10717544.2019.1667452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L.; Wang Q.; Chen J.; Wang Z.; Xin H.; Zhang D. Efficient Delivery of Therapeutic Sirna by Fe3o4Magnetic Nanoparticles into Oral Cancer Cells. Pharmaceutics 2019, 11, 615. 10.3390/pharmaceutics11110615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z.; Xiao H.; Li L.; Liu M.; Lin G.; Zhai P.; Yong K.-T.; Wang X.; Xu G. The Co-Delivery of Sirna and Qds by Ph Responsive Micelle for Hepatoma Cancer Cells. Front. Pharmacol. 2019, 10, 1194. 10.3389/fphar.2019.01194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanna P. L.; Renu K.; Valsala Gopalakrishnan A. New Molecular and Biochemical Insights of Doxorubicin-Induced Hepatotoxicity. Life Sci. 2020, 250, 117599. 10.1016/j.lfs.2020.117599. [DOI] [PubMed] [Google Scholar]
- Prathumsap N.; Shinlapawittayatorn K.; Chattipakorn S. C.; Chattipakorn N. Effects of Doxorubicin on the Heart: From Molecular Mechanisms to Intervention Strategies. Eur. J. Pharmacol. 2020, 866, 172818. 10.1016/j.ejphar.2019.172818. [DOI] [PubMed] [Google Scholar]
- Yee C.; McCoy D.; Yu J.; Losey A.; Jordan C.; Moore T.; Stillson C.; Oh H. J.; Kilbride B.; Roy S.; Patel A.; Wilson M. W.; Hetts S. W. Endovascular Ion Exchange Chemofiltration Device Reduces Off-Target Doxorubicin Exposure in a Hepatic Intra-Arterial Chemotherapy Model. Radiology. Imaging cancer 2019, 1, e190009 10.1148/rycan.2019190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z.; Guo J.; Hu M.; Gao Y.; Huang L. Icaritin Exacerbates Mitophagy and Synergizes with Doxorubicin to Induce Immunogenic Cell Death in Hepatocellular Carcinoma. ACS Nano 2020, 14, 4816. 10.1021/acsnano.0c00708. [DOI] [PubMed] [Google Scholar]
- Ibiyeye K. M.; Zuki A. B. Z. Cockle Shell-Derived Aragonite Caco3 Nanoparticles for Co-Delivery of Doxorubicin and Thymoquinone Eliminates Cancer Stem Cells. Int. J. Mol. Sci. 2020, 21, 1900. 10.3390/ijms21051900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. W.; Ma K. L.; Liu H.; Zhou J. Y. Reversal of Multidrug Resistance in Leukemia Cells Using a Transferrin-Modified Nanomicelle Encapsulating Both Doxorubicin and Psoralen. Aging 2020, 12, 6018–6029. 10.18632/aging.102992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X.; Zhang H. Doxorubicin-Induced Cancer Cell Senescence Shows a Time Delay Effect and Is Inhibited by Epithelial-Mesenchymal Transition (Emt). Med. Sci. Monit. 2019, 25, 3617–3623. 10.12659/MSM.914295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol A.; Taiyab A.; West-Mays J. A. Rhoa/Rock Signaling Regulates Tgfbeta-Induced Epithelial-Mesenchymal Transition of Lens Epithelial Cells through Mrtf-A. Mol. Med. (Manhasset, NY, U. S.) 2016, 22, 713–723. 10.2119/molmed.2016.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. D. Both Sides of the Same Coin: Rac1 Splicing Regulating by Egf Signaling. Cell Res. 2017, 27, 455–456. 10.1038/cr.2017.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas S. Ros and Redox Signaling in Myocardial Ischemia-Reperfusion Injury and Cardioprotection. Free Radical Biol. Med. 2018, 117, 76–89. 10.1016/j.freeradbiomed.2018.01.024. [DOI] [PubMed] [Google Scholar]
- Grobe H.; Wustenhagen A.; Baarlink C.; Grosse R.; Grikscheit K. A Rac1-Fmnl2 Signaling Module Affects Cell-Cell Contact Formation Independent of Cdc42 and Membrane Protrusions. PLoS One 2018, 13, e0194716 10.1371/journal.pone.0194716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Zhou X. Q.; Shang X. W.; Wang L.; Li Y.; Yuan H.; Hu F. Q. Inhibition of Chemotherapy-Related Breast Tumor Emt by Application of Redox-Sensitive Sirna Delivery System Cso-Ss-Sa/Sirna Along with Doxorubicin Treatment. J. Zhejiang Univ., Sci., B 2020, 21, 218–233. 10.1631/jzus.B1900468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.; Luo Y.; Lv H.; Wang J.; Zhang Y.; Pei R. Aptamer-Based Erythrocyte-Derived Mimic Vesicles Loaded with Sirna and Doxorubicin for the Targeted Treatment of Multidrug-Resistant Tumors. ACS Appl. Mater. Interfaces 2019, 11, 45455–45466. 10.1021/acsami.9b16637. [DOI] [PubMed] [Google Scholar]
- Alshaer W.; Alqudah D. A.; Wehaibi S.; Abuarqoub D.; Zihlif M.; Hatmal M. M.; Awidi A. Downregulation of Stat3, Beta-Catenin, and Notch-1 by Single and Combinations of Sirna Treatment Enhance Chemosensitivity of Wild Type and Doxorubicin Resistant Mcf7 Breast Cancer Cells to Doxorubicin. Int. J. Mol. Sci. 2019, 20, 3696. 10.3390/ijms20153696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L.; Han H.; Wang H.; Cao L.; Feng W. H. Il-10 Knockdown with Sirna Enhances the Efficacy of Doxorubicin Chemotherapy in Ebv-Positive Tumors by Inducing Lytic Cycle Via Pi3k/P38 Mapk/Nf-Kb Pathway. Cancer Lett. 2019, 462, 12–22. 10.1016/j.canlet.2019.07.016. [DOI] [PubMed] [Google Scholar]
- Mohammed A. F. A.; Higashi T.; Motoyama K.; Ohyama A.; Onodera R.; Khaled K. A.; Sarhan H. A.; Hussein A. K.; Arima H. In Vitro and in Vivo Co-Delivery of Sirna and Doxorubicin by Folate-Peg-Appended Dendrimer/Glucuronylglucosyl-Beta-Cyclodextrin Conjugate. AAPS J. 2019, 21, 54. 10.1208/s12248-019-0327-9. [DOI] [PubMed] [Google Scholar]
- Zheng S.; Wang X.; Weng Y. H.; Jin X.; Ji J. L.; Guo L.; Hu B.; Liu N.; Cheng Q.; Zhang J.; Bai H.; Yang T.; Xia X. H.; Zhang H. Y.; Gao S.; Huang Y. Sirna Knockdown of Rrm2 Effectively Suppressed Pancreatic Tumor Growth Alone or Synergistically with Doxorubicin. Mol. Ther.--Nucleic Acids 2018, 12, 805–816. 10.1016/j.omtn.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W.; Tan W.; Huang X.; Yu H. G. Doxorubicin Combined with Notch1-Targeting Sirna for the Treatment of Gastric Cancer. Oncol. Lett. 2018, 16, 2805–2812. 10.3892/ol.2018.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.; Wang L.; Wang F.; Li F.; Xia W.; Gu H.; Chen Y. Quick Synthesis of a Novel Combinatorial Delivery System of Sirna and Doxorubicin for a Synergistic Anticancer Effect. Int. J. Nanomed. 2019, 14, 3557. 10.2147/IJN.S198511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chocarro de Erauso L.; Zuazo M.; Arasanz H.; Bocanegra A.; Hernandez C.; Fernandez G.; Garcia-Granda M. J.; Blanco E.; Vera R.; Kochan G.; Escors D. Resistance to Pd-L1/Pd-1 Blockade Immunotherapy. A Tumor-Intrinsic or Tumor-Extrinsic Phenomenon?. Front. Pharmacol. 2020, 11, 441. 10.3389/fphar.2020.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.; Zhang P.; Wang Y.; Wang J.; Su M.; Wang Y.; Zhou L.; Zhou J.; Xiong W.; Zeng Z.; Zhou Y.; Nie S.; Liao Q. The Biogenesis, Biology, and Clinical Significance of Exosomal Pd-L1 in Cancer. Front. Immunol. 2020, 11, 604. 10.3389/fimmu.2020.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Yu Y.; Lu S. Effectiveness of Pd-1/Pd-L1 Inhibitors in the Treatment of Lung Cancer: Brightness and Challenge. Sci. China: Life Sci. 2020, 63, 1499. 10.1007/s11427-019-1622-5. [DOI] [PubMed] [Google Scholar]
- Chen M.; Chen M.; He J. Cancer Cell Membrane Cloaking Nanoparticles for Targeted Co-Delivery of Doxorubicin and Pd-L1 Sirna. Artif. Cells, Nanomed., Biotechnol. 2019, 47, 1635–1641. 10.1080/21691401.2019.1608219. [DOI] [PubMed] [Google Scholar]
- Teodori E.; Braconi L.; Bua S.; Lapucci A.; Bartolucci G.; Manetti D.; Romanelli M. N.; Dei S.; Supuran C. T.; Coronnello M. Dual P-Glycoprotein and Ca Xii Inhibitors: A New Strategy to Reverse the P-Gp Mediated Multidrug Resistance (Mdr) in Cancer Cells. Molecules 2020, 25, 1748. 10.3390/molecules25071748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y.; Jia L.; Wang Q.; Hu H.; Zhao X.; Chen D.; Qiao M. Ph/Redox Dual-Responsive Polyplex with Effective Endosomal Escape for Co-delivery of Sirna and Doxorubicin against Drug-Resistant Cancer Cells. ACS Appl. Mater. Interfaces 2019, 11, 16296–16310. 10.1021/acsami.9b02016. [DOI] [PubMed] [Google Scholar]
- Nagano K.; Maeda Y.; Kanasaki S.-i.; Watanabe T.; Yamashita T.; Inoue M.; Higashisaka K.; Yoshioka Y.; Abe Y.; Mukai Y.; et al. Ephrin Receptor A10 Is a Promising Drug Target Potentially Useful for Breast Cancers Including Triple Negative Breast Cancers. J. Controlled Release 2014, 189, 72–79. 10.1016/j.jconrel.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Du Z.; Pan S.; Shi M.; Li J.; Yang C.; Hu H.; Qiao M.; Chen D.; Zhao X. Overcoming Multidrug Resistance by Co-delivery of Mdr1-Targeting Sirna and Doxorubicin Using Epha10-Mediated Ph-Sensitive Lipoplexes: In Vitro and in Vivo Evaluation. ACS Appl. Mater. Interfaces 2018, 10, 21590–21600. 10.1021/acsami.8b01806. [DOI] [PubMed] [Google Scholar]
- Tan X.; Fang Y.; Ren Y.; Li Y.; Wu P.; Yang X.; Liu W. D-A-Tocopherol Polyethylene Glycol 1000 Succinate-Modified Liposomes with an Sirna Corona Confer Enhanced Cellular Uptake and Targeted Delivery of Doxorubicin Via Tumor Priming. Int. J. Nanomed. 2019, 14, 1255. 10.2147/IJN.S191858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G.; Pan R.; Zhang B.; Qu M.; Lian B.; Jiang H.; Gao Z.; Wu J. Liver-Targeted Combination Therapy Basing on Glycyrrhizic Acid-Modified Dspe-Peg-Pei Nanoparticles for Co-Delivery of Doxorubicin and Bcl-2 Sirna. Front. Pharmacol. 2019, 10, 4. 10.3389/fphar.2019.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteith G. R.; McAndrew D.; Faddy H. M.; Roberts-Thomson S. J. Calcium and Cancer: Targeting Ca 2+ Transport. Nat. Rev. Cancer 2007, 7, 519–530. 10.1038/nrc2171. [DOI] [PubMed] [Google Scholar]
- Ma X.; Cai Y.; He D.; Zou C.; Zhang P.; Lo C. Y.; Xu Z.; Chan F. L.; Yu S.; Chen Y.; et al. Transient Receptor Potential Channel Trpc5 Is Essential for P-Glycoprotein Induction in Drug-Resistant Cancer Cells. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 16282–16287. 10.1073/pnas.1202989109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L.; Liang C.; Chen E.; Chen W.; Liang F.; Zhi X.; Wei T.; Xue F.; Li G.; Yang Q.; et al. Regulation of Multi-Drug Resistance in Hepatocellular Carcinoma Cells Is Trpc6/Calcium Dependent. Sci. Rep. 2016, 6, 23269. 10.1038/srep23269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderick H. L.; Cook S. J. Ca 2+ Signalling Checkpoints in Cancer: Remodelling Ca 2+ for Cancer Cell Proliferation and Survival. Nat. Rev. Cancer 2008, 8, 361–375. 10.1038/nrc2374. [DOI] [PubMed] [Google Scholar]
- Schreiber R. Ca 2+ Signaling, Intracellular Ph and Cell Volume in Cell Proliferation. J. Membr. Biol. 2005, 205, 129. 10.1007/s00232-005-0778-z. [DOI] [PubMed] [Google Scholar]
- Monteith G. R.; Prevarskaya N.; Roberts-Thomson S. J. The Calcium-Cancer Signalling Nexus. Nat. Rev. Cancer 2017, 17, 373. 10.1038/nrc.2017.18. [DOI] [PubMed] [Google Scholar]
- Berridge M. J.; Bootman M. D.; Roderick H. L. Calcium Signalling: Dynamics, Homeostasis and Remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Schwab B. L.; Guerini D.; Didszun C.; Bano D.; Ferrando-May E.; Fava E.; Tam J.; Xu D.; Xanthoudakis S.; Nicholson D. W.; et al. Cleavage of Plasma Membrane Calcium Pumps by Caspases: A Link between Apoptosis and Necrosis. Cell Death Differ. 2002, 9, 818–831. 10.1038/sj.cdd.4401042. [DOI] [PubMed] [Google Scholar]
- Taylor J. T.; Huang L.; Pottle J. E.; Liu K.; Yang Y.; Zeng X.; Keyser B. M.; Agrawal K. C.; Hansen J. B.; Li M. Selective Blockade of T-Type Ca2+ Channels Suppresses Human Breast Cancer Cell Proliferation. Cancer Lett. 2008, 267, 116–124. 10.1016/j.canlet.2008.03.032. [DOI] [PubMed] [Google Scholar]
- Lu F.; Chen H.; Zhou C.; Liu S.; Guo M.; Chen P.; Zhuang H.; Xie D.; Wu S. T-Type Ca2+ Channel Expression in Human Esophageal Carcinomas: A Functional Role in Proliferation. Cell Calcium 2008, 43, 49–58. 10.1016/j.ceca.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziegielewska B.; Casarez E. V.; Yang W. Z.; Gray L. S.; Dziegielewski J.; Slack-Davis J. K. T-Type Ca2+ Channel Inhibition Sensitizes Ovarian Cancer to Carboplatin. Mol. Cancer Ther. 2016, 15, 460–470. 10.1158/1535-7163.MCT-15-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Liu X.; Chen S.; Liu Z.; Zhang X.; Liang X.-J.; Li L. Regulation of Ca2+ Signaling for Drug-Resistant Breast Cancer Therapy with Mesoporous Silica Nanocapsule Encapsulated Doxorubicin/Sirna Cocktail. ACS Nano 2019, 13, 274–283. 10.1021/acsnano.8b05639. [DOI] [PubMed] [Google Scholar]
- Pan Q.-S.; Chen T.-T.; Nie C.-P.; Yi J.-T.; Liu C.; Hu Y.-L.; Chu X. In Situ Synthesis of Ultrathin Zif-8 Film-Coated Msns for Codelivering Bcl 2 Sirna and Doxorubicin to Enhance Chemotherapeutic Efficacy in Drug-Resistant Cancer Cells. ACS Appl. Mater. Interfaces 2018, 10, 33070–33077. 10.1021/acsami.8b13393. [DOI] [PubMed] [Google Scholar]
- Hemati M.; Haghiralsadat F.; Yazdian F.; Jafari F.; Moradi A.; Malekpour-Dehkordi Z. Development and Characterization of a Novel Cationic Pegylated Niosome-Encapsulated Forms of Doxorubicin, Quercetin and Sirna for the Treatment of Cancer by Using Combination Therapy. Artif. Cells, Nanomed., Biotechnol. 2019, 47, 1295–1311. 10.1080/21691401.2018.1489271. [DOI] [PubMed] [Google Scholar]
- Posthuma De Boer J.; van Egmond P. W.; Helder M. N.; de Menezes R. X.; Cleton-Jansen A.-M.; Belien J. A.M.; Verheul H. M. W.; van Royen B. J.; Kaspers G.-J. J.L.; van Beusechem V. W. Targeting Jnk-Interacting Protein 1 (Jip1) Sensitises Osteosarcoma to Doxorubicin. Oncotarget 2012, 3, 1169. 10.18632/oncotarget.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghiralsadat F.; Amoabediny G.; Naderinezhad S.; Zandieh-Doulabi B.; Forouzanfar T.; Helder M. N. Co-delivery of Doxorubicin and Jip1 Sirna with Novel Epha2-Targeted Pegylated Cationic Nanoliposomes to Overcome Osteosarcoma Multidrug Resistance. Int. J. Nanomed. 2018, 13, 3853. 10.2147/IJN.S150017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G.; Shen H.; Mao J.; Zhang L.; Jiang Z.; Sun T.; Lan Q.; Zhang Z. Transferrin Modified Graphene Oxide for Glioma-Targeted Drug Delivery: In Vitro and in Vivo Evaluations. ACS Appl. Mater. Interfaces 2013, 5, 6909–6914. 10.1021/am402128s. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Robinson J. T.; Sun X.; Dai H. Pegylated Nanographene Oxide for Delivery of Water-Insoluble Cancer Drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. 10.1021/ja803688x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Gu W.; Xiao N.; Ye L.; Xu Q. Chlorotoxin-Conjugated Graphene Oxide for Targeted Delivery of an Anticancer Drug. Int. J. Nanomed. 2014, 9, 1433. 10.2147/IJN.S58783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavitha T.; Haider Abdi S. I.; Park S.-Y. Ph-Sensitive Nanocargo Based on Smart Polymer Functionalized Graphene Oxide for Site-Specific Drug Delivery. Phys. Chem. Chem. Phys. 2013, 15, 5176–5185. 10.1039/c3cp00008g. [DOI] [PubMed] [Google Scholar]
- Wen H.; Dong C.; Dong H.; Shen A.; Xia W.; Cai X.; Song Y.; Li X.; Li Y.; Shi D. Engineered Redox-Responsive Peg Detachment Mechanism in Pegylated Nano-Graphene Oxide for Intracellular Drug Delivery. Small 2012, 8, 760–769. 10.1002/smll.201101613. [DOI] [PubMed] [Google Scholar]
- Xu H.; Fan M.; Elhissi A. M.; Zhang Z.; Wan K.-W.; Ahmed W.; Phoenix D. A.; Sun X. Pegylated Graphene Oxide for Tumor-Targeted Delivery of Paclitaxel. Nanomedicine 2015, 10, 1247–1262. 10.2217/nnm.14.233. [DOI] [PubMed] [Google Scholar]
- Sun Q.; Wang X.; Cui C.; Li J.; Wang Y. Doxorubicin and Anti-Vegf Sirna Co-Delivery Via Nano-Graphene Oxide for Enhanced Cancer Therapy in Vitro and in Vivo. Int. J. Nanomed. 2018, 13, 3713. 10.2147/IJN.S162939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.; Piao Y.; Hao L.; Wang G.; Zhou Z.; Shen Y. Acidity-Responsive Shell-Sheddable Camptothecin-Based Nanofibers for Carrier-Free Cancer Drug Delivery. Nanoscale 2019, 11, 15907–15916. 10.1039/C9NR03872H. [DOI] [PubMed] [Google Scholar]
- Wu M.; Li J.; Lin X.; Wei Z.; Zhang D.; Zhao B.; Liu X.; Liu J. Reduction/Photo Dual-Responsive Polymeric Prodrug Nanoparticles for Programmed Sirna and Doxorubicin Delivery. Biomater. Sci. 2018, 6, 1457–1468. 10.1039/C8BM00226F. [DOI] [PubMed] [Google Scholar]
- Sun W.; Chen X.; Xie C.; Wang Y.; Lin L.; Zhu K.; Shuai X. Co-Delivery of Doxorubicin and Anti-Bcl-2 Sirna by Ph-Responsive Polymeric Vector to Overcome Drug Resistance in in Vitro and in Vivo Hepg2 Hepatoma Model. Biomacromolecules 2018, 19, 2248–2256. 10.1021/acs.biomac.8b00272. [DOI] [PubMed] [Google Scholar]
- Wang D.; Xu X.; Zhang K.; Sun B.; Wang L.; Meng L.; Liu Q.; Zheng C.; Yang B.; Sun H. Co-delivery of Doxorubicin and Mdr1-Sirna by Mesoporous Silica Nanoparticles-Polymerpolyethylenimine to Improve Oral Squamous Carcinoma Treatment. Int. J. Nanomed. 2018, 13, 187–198. 10.2147/IJN.S150610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y.; Xu T.; Wang C.; Li Y.; Lin Z.; Zhao M.; Zhu B. Novel Functionalized Nanoparticles for Tumor-Targeting Co-Delivery of Doxorubicin and Sirna to Enhance Cancer Therapy. Int. J. Nanomed. 2018, 13, 143. 10.2147/IJN.S148960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.; Ren Z.; Chen M.; Wang Y.; You B.; Chen W.; Qu C.; Liu Y.; Zhang X. Nucleolin-Targeting As1411-Aptamer-Modified Graft Polymeric Micelle with Dual Ph/Redox Sensitivity Designed to Enhance Tumor Therapy through the Co-delivery of Doxorubicin/Tlr4 Sirna and Suppression of Invasion. Mol. Pharmaceutics 2018, 15, 314–325. 10.1021/acs.molpharmaceut.7b01093. [DOI] [PubMed] [Google Scholar]
- Meng T.; Lu B.; Shao S.; Yuan M.; Liu X.; Yuan H.; Huang X.; Hu F. Sequential Therapy with Redox-Responsive Glucolipid Nanocarrier Separately Delivering Sirna and Doxorubicin to Overcome Multidrug Resistance. Int. J. Pharm. 2017, 534, 368–377. 10.1016/j.ijpharm.2017.10.036. [DOI] [PubMed] [Google Scholar]
- Wen Z.-M.; Jie J.; Zhang Y.; Liu H.; Peng L.-P. A Self-Assembled Polyjuglanin Nanoparticle Loaded with Doxorubicin and Anti-Kras Sirna for Attenuating Multidrug Resistance in Human Lung Cancer. Biochem. Biophys. Res. Commun. 2017, 493, 1430–1437. 10.1016/j.bbrc.2017.09.132. [DOI] [PubMed] [Google Scholar]
- Kotcherlakota R.; Srinivasan D. J.; Mukherjee S.; Haroon M. M.; Dar G. H.; Venkatraman U.; Patra C. R.; Gopal V. Engineered Fusion Protein-Loaded Gold Nanocarriers for Targeted Co-Delivery of Doxorubicin and Erbb2-Sirna in Human Epidermal Growth Factor Receptor-2+ Ovarian Cancer. J. Mater. Chem. B 2017, 5, 7082–7098. 10.1039/C7TB01587A. [DOI] [PubMed] [Google Scholar]
- Zhao S.; Xu M.; Cao C.; Yu Q.; Zhou Y.; Liu J. A Redox-Responsive Strategy Using Mesoporous Silica Nanoparticles for Co-Delivery of Sirna and Doxorubicin. J. Mater. Chem. B 2017, 5, 6908–6919. 10.1039/C7TB00613F. [DOI] [PubMed] [Google Scholar]
- Kumar K.; Vulugundam G.; Jaiswal P. K.; Shyamlal B. R. K.; Chaudhary S. Efficacious Cellular Co-delivery of Doxorubicin and Egfp Sirna Mediated by the Composition of Plga and Pei Protected Gold Nanoparticles. Bioorg. Med. Chem. Lett. 2017, 27, 4288–4293. 10.1016/j.bmcl.2017.08.037. [DOI] [PubMed] [Google Scholar]
- Suo A.; Qian J.; Xu M.; Xu W.; Zhang Y.; Yao Y. Folate-Decorated Pegylated Triblock Copolymer as a Ph/Reduction Dual-Responsive Nanovehicle for Targeted Intracellular Co-Delivery of Doxorubicin and Bcl-2 Sirna. Mater. Sci. Eng., C 2017, 76, 659–672. 10.1016/j.msec.2017.03.124. [DOI] [PubMed] [Google Scholar]
- Shali H.; Shabani M.; Pourgholi F.; Hajivalili M.; Aghebati-Maleki L.; Jadidi-Niaragh F.; Baradaran B.; Movassaghpour Akbari A. A.; Younesi V.; Yousefi M. Co-Delivery of Insulin-Like Growth Factor 1 Receptor Specific Sirna and Doxorubicin Using Chitosan-Based Nanoparticles Enhanced Anticancer Efficacy in A549 Lung Cancer Cell Line. Artif. Cells, Nanomed., Biotechnol. 2018, 46, 293–302. 10.1080/21691401.2017.1307212. [DOI] [PubMed] [Google Scholar]
- Butt A. M.; Amin M. C. I. M.; Katas H.; Abdul Murad N. A.; Jamal R.; Kesharwani P. Doxorubicin and Sirna Co-delivery Via Chitosan-Coated Ph-Responsive Mixed Micellar Polyplexes for Enhanced Cancer Therapy in Multidrug-Resistant Tumors. Mol. Pharmaceutics 2016, 13, 4179–4190. 10.1021/acs.molpharmaceut.6b00776. [DOI] [PubMed] [Google Scholar]
- Aji Alex M. R.; Veeranarayanan S.; Poulose A. C.; Nehate C.; Kumar D. S.; Koul V. Click Modified Amphiphilic Graft Copolymeric Micelles of Poly (Styrene-Alt-Maleic Anhydride) for Combinatorial Delivery of Doxorubicin and Plk-1 Sirna in Cancer Therapy. J. Mater. Chem. B 2016, 4, 7303–7313. 10.1039/C6TB02094A. [DOI] [PubMed] [Google Scholar]
- Alinejad V.; Hossein Somi M.; Baradaran B.; Akbarzadeh P.; Atyabi F.; Kazerooni H.; Samadi Kafil H.; Aghebati Maleki L.; Siah Mansouri H.; Yousefi M. Co-Delivery of Il17rb Sirna and Doxorubicin by Chitosan-Based Nanoparticles for Enhanced Anticancer Efficacy in Breast Cancer Cells. Biomed. Pharmacother. 2016, 83, 229–240. 10.1016/j.biopha.2016.06.037. [DOI] [PubMed] [Google Scholar]
- Weng W.; Goel A. Curcumin and Colorectal Cancer: An Update and Current Perspective on This Natural Medicine. Semin. Cancer Biol. 2020, 10.1016/j.semcancer.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd. Wahab N. A.; H. Lajis N.; Abas F.; Othman I.; Naidu R. Mechanism of Anti-Cancer Activity of Curcumin on Androgen-Dependent and Androgen-Independent Prostate Cancer. Nutrients 2020, 12, 679. 10.3390/nu12030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. S.; Acharya A.; Ray R. S.; Agrawal R.; Raghuwanshi R.; Jain P. Cellular and Molecular Mechanisms of Curcumin in Prevention and Treatment of Disease. Crit. Rev. Food Sci. Nutr. 2020, 60, 887–939. 10.1080/10408398.2018.1552244. [DOI] [PubMed] [Google Scholar]
- Kamali Dolatabadi L.; Emamghoreishi M.; Namavar M. R.; Badeli Sarkala H. Curcumin Effects on Memory Impairment and Restoration of Irregular Neuronal Distribution in the Hippocampal Ca1 Region after Global Cerebral Ischemia in Male Rats. Basic and clinical neuroscience 2019, 10, 527–539. 10.32598/bcn.9.10.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarinezhad Z.; Rafati A.; Ketabchi F.; Noorafshan A.; Karbalay-Doust S. Cardioprotective Effects of Curcumin and Carvacrol in Doxorubicin-Treated Rats: Stereological Study. Food Sci. Nutr. 2019, 7, 3581–3588. 10.1002/fsn3.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapelle I. B. D.; Manalu W.; Souhoka F. A. Effect of Curcumin Analogue Synthetic Product from Cullilawan Oil for the Liver Damage Treatment in Male Mice (Mus Musculus L.). Journal of Basic and Clinical Physiology and Pharmacology 2020, 10.1515/jbcpp-2019-0241. [DOI] [PubMed] [Google Scholar]
- DiMarco-Crook C.; Rakariyatham K.; Li Z.; Du Z.; Zheng J.; Wu X.; Xiao H. Synergistic Anticancer Effects of Curcumin and 3′,4′-Didemethylnobiletin in Combination on Colon Cancer Cells. J. Food Sci. 2020, 85, 1292–1301. 10.1111/1750-3841.15073. [DOI] [PubMed] [Google Scholar]
- Piwowarczyk L.; Stawny M.; Mlynarczyk D. T.; Muszalska-Kolos I.; Goslinski T.; Jelińska A. Role of Curcumin and (−)-Epigallocatechin-3-O-Gallate in Bladder Cancer Treatment: A Review. Cancers 2020, 12, 1801. 10.3390/cancers12071801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.; Dutta P. K. Green Synthesis, Characterization and Biological Evaluation of Chitin Glucan Based Zinc Oxide Nanoparticles and Its Curcumin Conjugation. Int. J. Biol. Macromol. 2020, 156, 514. 10.1016/j.ijbiomac.2020.04.081. [DOI] [PubMed] [Google Scholar]
- Osali A. Aerobic Exercise and Nano-Curcumin Supplementation Improve Inflammation in Elderly Females with Metabolic Syndrome. Diabetol. Metab. Syndr. 2020, 12, 26. 10.1186/s13098-020-00532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaddoni A.; Mohammadi E.; Sedaghat F.; Qujeq D.; As’Habi A. The Anticancer Effects of Curcumin Via Targeting the Mammalian Target of Rapamycin Complex 1 (Mtorc1) Signaling Pathway. Pharmacol. Res. 2020, 156, 104798. 10.1016/j.phrs.2020.104798. [DOI] [PubMed] [Google Scholar]
- Li N.; Wen S.; Chen G.; Wang S. Antiproliferative Potential of Piperine and Curcumin in Drug-Resistant Human Leukemia Cancer Cells Are Mediated Via Autophagy and Apoptosis Induction, S-Phase Cell Cycle Arrest and Inhibition of Cell Invasion and Migration. Journal of B.U.ON.: Official Journal of the Balkan Union of Oncology 2020, 25, 401–406. [PubMed] [Google Scholar]
- Jiang X.; Li S.; Qiu X.; Cong J.; Zhou J.; Miu W. Curcumin Inhibits Cell Viability and Increases Apoptosis of Sw620 Human Colon Adenocarcinoma Cells Via the Caudal Type Homeobox-2 (Cdx2)/Wnt/Beta-Catenin Pathway. Med. Sci. Monit. 2019, 25, 7451–7458. 10.12659/MSM.918364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi Kian M.; Salemi M.; Bahadoran M.; Haghi A.; Dashti N.; Mohammadi S.; Rostami S.; Chahardouli B.; Babakhani D.; Nikbakht M. Curcumin Combined with Thalidomide Reduces Expression of Stat3 and Bcl-Xl, Leading to Apoptosis in Acute Myeloid Leukemia Cell Lines. Drug Des., Dev. Ther. 2020, 14, 185–194. 10.2147/DDDT.S228610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke S.; Zhang Y.; Lan Z.; Li S.; Zhu W.; Liu L. Curcumin Protects Murine Lung Mesenchymal Stem Cells from H2o2 by Modulating the Akt/Nrf2/Ho-1 Pathway. J. Int. Med. Res. 2020, 48, 030006052091066. 10.1177/0300060520910665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T.; Xiao P.; Yu N.; Zhou Y.; Mao J.; Peng H.; Deng S. A Novel Pectin from Akebia Trifoliata Var. Australis Fruit Peel and Its Use as a Wall-Material to Coat Curcumin-Loaded Zein Nanoparticle. Int. J. Biol. Macromol. 2020, 152, 40–49. 10.1016/j.ijbiomac.2020.02.234. [DOI] [PubMed] [Google Scholar]
- Cheng Y.; Zhang Y.; Deng W.; Hu J. Antibacterial and Anticancer Activities of Asymmetric Lollipop-Like Mesoporous Silica Nanoparticles Loaded with Curcumin and Gentamicin Sulfate. Colloids Surf., B 2020, 186, 110744. 10.1016/j.colsurfb.2019.110744. [DOI] [PubMed] [Google Scholar]
- Kantara C.; O’Connell M.; Sarkar S.; Moya S.; Ullrich R.; Singh P. Curcumin Promotes Autophagic Survival of a Subset of Colon Cancer Stem Cells, Which Are Ablated by Dclk1-Sirna. Cancer Res. 2014, 74, 2487–2498. 10.1158/0008-5472.CAN-13-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F.; Zhang C.; Tian W.; Jiang Y.; Zhang X. Comparison of the Effect of P65 Sirna and Curcumin in Promoting Apoptosis in Esophageal Squamous Cell Carcinoma Cells and in Nude Mice. Oncol. Rep. 2012, 28, 232–240. 10.3892/or.2012.1777. [DOI] [PubMed] [Google Scholar]
- Abedi-Gaballu F.; Dehghan G.; Ghaffari M.; Yekta R.; Abbaspour-Ravasjani S.; Baradaran B.; Ezzati Nazhad Dolatabadi J.; Hamblin M. R. Pamam Dendrimers as Efficient Drug and Gene Delivery Nanosystems for Cancer Therapy. Applied materials today 2018, 12, 177–190. 10.1016/j.apmt.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D.; Yang J.; Xing Z.; Han H.; Wang T.; Zhang A.; Yang Y.; Li Q. Phenylboronic Acid-Functionalized Polyamidoamine-Mediated Bcl-2 Sirna Delivery for Inhibiting the Cell Proliferation. Colloids Surf., B 2016, 146, 318–325. 10.1016/j.colsurfb.2016.06.034. [DOI] [PubMed] [Google Scholar]
- Li J.; Liang H.; Liu J.; Wang Z. Poly (Amidoamine)(Pamam) Dendrimer Mediated Delivery of Drug and Pdna/Sirna for Cancer Therapy. Int. J. Pharm. 2018, 546, 215–225. 10.1016/j.ijpharm.2018.05.045. [DOI] [PubMed] [Google Scholar]
- Ghaffari M.; Dehghan G.; Baradaran B.; Zarebkohan A.; Mansoori B.; Soleymani J.; Ezzati Nazhad Dolatabadi J.; Hamblin M. R. Co-Delivery of Curcumin and Bcl-2 Sirna by Pamam Dendrimers for Enhancement of the Therapeutic Efficacy in Hela Cancer Cells. Colloids Surf., B 2020, 188, 110762. 10.1016/j.colsurfb.2019.110762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R.; Wang S.; Wang N.; Zheng Y.; Zhou J.; Yang B.; Wang X.; Zhang J.; Guo L.; Wang S.; Chen Z.; Wang Z.; Xiang S. Ccl5 Derived from Tumor-Associated Macrophages Promotes Prostate Cancer Stem Cells and Metastasis Via Activating Beta-Catenin/Stat3 Signaling. Cell Death Dis. 2020, 11, 234. 10.1038/s41419-020-2435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M. H.; Jung S. H.; Chinnathambi A.; Alahmadi T. A.; Alharbi S. A.; Sethi G.; Ahn K. S. Attenuation of Stat3 Signaling Cascade by Daidzin Can Enhance the Apoptotic Potential of Bortezomib against Multiple Myeloma. Biomolecules 2020, 10, 23. 10.3390/biom10010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. X.; Xu B. W.; Chen Y. J.; Fu X. Q.; Zhu P. L.; Bai J. X.; Chou J. Y.; Yin C. L.; Li J. K.; Wang Y. P.; Wu J. Y.; Wu Y.; Chan K. K.; Liang C.; Yu Z. L. Inhibiting the Src/Stat3 Signaling Pathway Contributes to the Anti-Melanoma Mechanisms of Dioscin. Oncol. Lett. 2020, 19, 2508–2514. 10.3892/ol.2020.11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari S.; Lavasanifar A.; Hejazi M. S.; Maleki-Dizaji N.; Mesgari M.; Molavi O. Stat3 Inhibitory Stattic Enhances Immunogenic Cell Death Induced by Chemotherapy in Cancer Cells. Daru: Journal of Faculty of Pharmacy 2020, 10.1007/s40199-020-00326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose A.; Labala S.; Ninave K. M.; Gade S. K.; Venuganti V. V. K. Effective Skin Cancer Treatment by Topical Co-Delivery of Curcumin and Stat3 Sirna Using Cationic Liposomes. AAPS PharmSciTech 2018, 19, 166–175. 10.1208/s12249-017-0833-y. [DOI] [PubMed] [Google Scholar]
- Jose A.; Labala S.; Venuganti V. V. K. Co-Delivery of Curcumin and Stat3 Sirna Using Deformable Cationic Liposomes to Treat Skin Cancer. J. Drug Targeting 2017, 25, 330–341. 10.1080/1061186X.2016.1258567. [DOI] [PubMed] [Google Scholar]
- Thomas M.; Klibanov A. M. Conjugation to Gold Nanoparticles Enhances Polyethylenimine’s Transfer of Plasmid DNA into Mammalian Cells. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 9138–9143. 10.1073/pnas.1233634100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Sun X.; Nakayama-Ratchford N.; Dai H. Supramolecular Chemistry on Water-Soluble Carbon Nanotubes for Drug Loading and Delivery. ACS Nano 2007, 1, 50–56. 10.1021/nn700040t. [DOI] [PubMed] [Google Scholar]
- Radu D. R.; Lai C.-Y.; Jeftinija K.; Rowe E. W.; Jeftinija S.; Lin V. S.-Y. A Polyamidoamine Dendrimer-Capped Mesoporous Silica Nanosphere-Based Gene Transfection Reagent. J. Am. Chem. Soc. 2004, 126, 13216–13217. 10.1021/ja046275m. [DOI] [PubMed] [Google Scholar]
- Woodrow K. A.; Cu Y.; Booth C. J.; Saucier-Sawyer J. K.; Wood M. J.; Saltzman W. M. Intravaginal Gene Silencing Using Biodegradable Polymer Nanoparticles Densely Loaded with Small-Interfering Rna. Nat. Mater. 2009, 8, 526–533. 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z.-H.; Wei J.-H.; Cheang T.-Y.; Wang Z.-R.; Zhou X.; Wang S.-S.; Chen W.; Wang S.-M.; Luo J.-H.; Xu A.-W. Bifunctional Ph-Sensitive Zn (Ii)-Curcumin Nanoparticles/Sirna Effectively Inhibit Growth of Human Bladder Cancer Cells in Vitro and in Vivo. J. Mater. Chem. B 2014, 2, 2714–2724. 10.1039/c3tb21625j. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Rauf Khan A.; Fu M.; Zhai Y.; Ji J.; Bobrovskaya L.; Zhai G. Advances in Curcumin-Loaded Nanopreparations: Improving Bioavailability and Overcoming Inherent Drawbacks. J. Drug Targeting 2019, 27, 917–931. 10.1080/1061186X.2019.1572158. [DOI] [PubMed] [Google Scholar]
- Sohail M. F.; Rehman M.; Sarwar H. S.; Naveed S.; Qureshi O. S.; Bukhari N. I.; Hussain I.; Webster T. J; Shahnaz G. Advancements in the Oral Delivery of Docetaxel: Challenges, Current State-of-the-Art and Future Trends. Int. J. Nanomed. 2018, 13, 3145–3161. 10.2147/IJN.S164518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino B.; Boggiani D.; Tommasi C.; Palli D.; Musolino A. Nab-Paclitaxel after Docetaxel Hypersensitivity Reaction: Case Report and Literature Review. Acta bio-medica: Atenei Parmensis 2017, 88, 329–333. 10.23750/abm.v88i3.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T.; Furuya N.; Ito K.; Hida N.; Morikawa K.; Komase Y.; Inoue T.; Hataji O.; Mineshita M. The Efficacy and Safety of Ramucirumab Plus Docetaxel in Older Patients with Advanced Non-Small Cell Lung Cancer. Thorac. Cancer 2020, 11, 1559. 10.1111/1759-7714.13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y.; Narita S.; Nara T.; Mingguo H.; Sato H.; Koizumi A.; Kanda S.; Numakura K.; Saito M.; Inoue T.; Hiroshima Y.; Nanjo H.; Satoh S.; Tsuchiya N.; Habuchi T. Impact of Nuclear Yap1 Expression in Residual Cancer after Neoadjuvant Chemohormonal Therapy with Docetaxel for High-Risk Localized Prostate Cancer. BMC Cancer 2020, 20, 302. 10.1186/s12885-020-06844-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G.; Zhu C.; Li B.; Wang T.; Wan J.; Zhang Y.; Huang J.; Yang D.; Shen Y. Improving the Anti-Ovarian Cancer Activity of Docetaxel by Self-Assemble Micelles and Thermosensitive Hydrogel Drug Delivery System. J. Biomed. Nanotechnol. 2020, 16, 40–53. 10.1166/jbn.2020.2867. [DOI] [PubMed] [Google Scholar]
- Li J.; Yu K.; Pang D.; Wang C.; Jiang J.; Yang S.; Liu Y.; Fu P.; Sheng Y.; Zhang G.; Cao Y.; He Q.; Cui S.; Wang X.; Ren G.; Li X.; Yu S.; Liu P.; Qu X.; Tang J.; Wang O.; Fan Z.; Jiang G.; Zhang J.; Wang J.; Zhang H.; Wang S.; Zhang J.; Jin F.; Rao N.; Ma B.; He P.; Xu B.; Zhuang Z.; Wang J.; Sun Q.; Guo X.; Mo M.; Shao Z. Adjuvant Capecitabine with Docetaxel and Cyclophosphamide Plus Epirubicin for Triple-Negative Breast Cancer (Cbcsg010): An Open-Label, Randomized, Multicenter, Phase Iii Trial. J. Clin. Oncol. 2020, 38, 1774. 10.1200/JCO.19.02474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J.; Wang H.; Zhang X.; Sun X.; Zhang J.; Ma J. Exosomal Mir-200c Suppresses Chemoresistance of Docetaxel in Tongue Squamous Cell Carcinoma by Suppressing Tubb3 and Ppp2r1b. Aging 2020, 12, 6756. 10.18632/aging.103036. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gonzalez-Ochoa E.; Verduzco-Aguirre H.; Crawford E. D.; Bourlon M. T. 69-Year-Old Man with Castration-Resistant Prostate Cancer Progressing after Docetaxel and Androgen Receptor-Targeting Agent. Oncology (Williston Park, N.Y.) 2020, 34, 125. 10.46883/ONC.2020.3404.0125. [DOI] [PubMed] [Google Scholar]
- Cortot A. B.; Audigier-Valette C.; Molinier O.; Le Moulec S.; Barlesi F.; Zalcman G.; Dumont P.; Pouessel D.; Poulet C.; Fontaine-Delaruelle C.; Hiret S.; Dixmier A.; Renault P. A.; Becht C.; Raffy O.; Dayen C.; Mazieres J.; Pichon E.; Langlais A.; Morin F.; Moro-Sibilot D.; Besse B. Weekly Paclitaxel Plus Bevacizumab Versus Docetaxel as Second- or Third-Line Treatment in Advanced Non-Squamous Non-Small-Cell Lung Cancer: Results of the Ifct-1103 Ultimate Study. Eur. J. Cancer 2020, 131, 27–36. 10.1016/j.ejca.2020.02.022. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Meng H.; Zhang M.; Zhang C.; Huang M.; Yan C.; Wang Z.; Hou L.; Yang L.; Ling R. Regulation of Docetaxel Chemosensitivity by Nr2f6 in Breast Cancer. Endocr.-Relat. Cancer 2020, 27, 309. 10.1530/ERC-19-0229. [DOI] [PubMed] [Google Scholar]
- Onishi K.; Miyake M.; Hori S.; Onishi S.; Iida K.; Morizawa Y.; Tatsumi Y.; Nakai Y.; Tanaka N.; Fujimoto K. Gamma-Klotho Is Correlated with Resistance to Docetaxel in Castration-Resistant Prostate Cancer. Oncol. Lett. 2020, 19, 2306–2316. 10.3892/ol.2020.11308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q. F.; Zhang Y. C.; Peng X. Q.; Long Z.; Ming Y. Z.; He L. Y. Sirna-Mediated Silencing of Notch-1 Enhances Docetaxel Induced Mitotic Arrest and Apoptosis in Prostate Cancer Cells. Asian Pacific journal of cancer prevention: APJCP 2012, 13, 2485–9. 10.7314/APJCP.2012.13.6.2485. [DOI] [PubMed] [Google Scholar]
- Razi Soofiyani S.; Mohammad Hoseini A.; Mohammadi A.; Khaze Shahgoli V.; Baradaran B.; Hejazi M. S. Sirna-Mediated Silencing of Cip2a Enhances Docetaxel Activity against Pc-3 Prostate Cancer Cells. Adv. Pharm. Bull. 2017, 7, 637–643. 10.15171/apb.2017.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.-H.; Sharrocks A. D.; Whitmarsh A. J. Transcriptional Regulation by the Map Kinase Signaling Cascades. Gene 2003, 320, 3–21. 10.1016/S0378-1119(03)00816-3. [DOI] [PubMed] [Google Scholar]
- Shen X.; Shen P.; Yang Q.; Yin Q.; Wang F.; Cong H.; Wang X.; Ju S. Knockdown of Long Non-Coding Rna Pcat-1 Inhibits Myeloma Cell Growth and Drug Resistance Via P38 and Jnk Mapk Pathways. J. Cancer 2019, 10, 6502–6510. 10.7150/jca.35098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z.; Hong Y.; Zhang F.; An L.; Yang Q.; Huang X.; Xu Q. Knockdown of Cops3 Inhibits the Progress of Prostate Cancer through Reducing Phosphorylated P38 Mapk Expression and Impairs the Epithelial-Mesenchymal Transition Process. Prostate 2019, 79, 1823–1831. 10.1002/pros.23907. [DOI] [PubMed] [Google Scholar]
- Pang S. T.; Lin F. W.; Chuang C. K.; Yang H. W. Co-Delivery of Docetaxel and P44/42 Mapk Sirna Using Psma Antibody-Conjugated Bsa-Pei Layer-by-Layer Nanoparticles for Prostate Cancer Target Therapy. Macromol. Biosci. 2017, 17, 1600421. 10.1002/mabi.201600421. [DOI] [PubMed] [Google Scholar]
- Mondal S.; Adhikari N.; Banerjee S.; Amin S. A.; Jha T. Matrix Metalloproteinase-9 (Mmp-9) and Its Inhibitors in Cancer: A Minireview. Eur. J. Med. Chem. 2020, 194, 112260. 10.1016/j.ejmech.2020.112260. [DOI] [PubMed] [Google Scholar]
- Liu T.; Xue W.; Ke B.; Xie M.-Q.; Ma D. Star-Shaped Cyclodextrin-Poly (L-Lysine) Derivative Co-Delivering Docetaxel and Mmp-9 Sirna Plasmid in Cancer Therapy. Biomaterials 2014, 35, 3865–3872. 10.1016/j.biomaterials.2014.01.040. [DOI] [PubMed] [Google Scholar]
- Zhao F.; Evans K.; Xiao C.; DeVito N.; Theivanthiran B.; Holtzhausen A.; Siska P. J.; Blobe G. C.; Hanks B. A. Stromal Fibroblasts Mediate Anti-Pd-1 Resistance Via Mmp-9 and Dictate Tgfbeta Inhibitor Sequencing in Melanoma. Cancer Immunol. Res. 2018, 6, 1459–1471. 10.1158/2326-6066.CIR-18-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.; Wu X.; Wang Y.; Hou X.; Jiang G.; Wu T.; Xie H.; Xie M. Cd-Plld Co-Delivering Docetaxel and Mmp-9 Sirna Plasmid for Nasopharyngeal Carcinoma Therapy in Vivo. Mol. Med. Rep. 2017, 16, 1383–1388. 10.3892/mmr.2017.6715. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Zheng Q.; Wang C.; Xu J.; Wu J.-P.; Kirk T. B.; Ma D.; Xue W. Star-Shaped Amphiphilic Hyperbranched Polyglycerol Conjugated with Dendritic Poly (L-Lysine) for the Co-delivery of Docetaxel and Mmp-9 Sirna in Cancer Therapy. ACS Appl. Mater. Interfaces 2016, 8, 12609–12619. 10.1021/acsami.6b01611. [DOI] [PubMed] [Google Scholar]
- Rajesh Y.; Banerjee A.; Pal I.; Biswas A.; Das S.; Dey K. K.; Kapoor N.; Ghosh A. K.; Mitra P.; Mandal M. Delineation of Crosstalk between Hsp27 and Mmp-2/Mmp-9: A Synergistic Therapeutic Avenue for Glioblastoma Management. Biochim. Biophys. Acta, Gen. Subj. 2019, 1863, 1196–1209. 10.1016/j.bbagen.2019.04.015. [DOI] [PubMed] [Google Scholar]
- Luo D. J.; Li L. J.; Huo H. F.; Liu X. Q.; Cui H. W.; Jiang D. M. Microrna-29b Sensitizes Osteosarcoma Cells to Doxorubicin by Targeting Matrix Metalloproteinase 9 (Mmp-9) in Osteosarcoma. European Review for Medical and Pharmacological Sciences 2019, 23, 1434–1442. 10.26355/eurrev_201902_17100. [DOI] [PubMed] [Google Scholar]
- Wang D.; Wang T.; Xu Z.; Yu H.; Feng B.; Zhang J.; Guo C.; Yin Q.; Zhang Z.; Li Y. Cooperative Treatment of Metastatic Breast Cancer Using Host-Guest Nanoplatform Coloaded with Docetaxel and Sirna. Small 2016, 12, 488–498. 10.1002/smll.201502913. [DOI] [PubMed] [Google Scholar]
- Pérez-Martínez F. C.; Carrión B.; Lucío M. I.; Rubio N.; Herrero M. A.; Vázquez E.; Ceña V. Enhanced Docetaxel-Mediated Cytotoxicity in Human Prostate Cancer Cells through Knockdown of Cofilin-1 by Carbon Nanohorn Delivered Sirna. Biomaterials 2012, 33, 8152–8159. 10.1016/j.biomaterials.2012.07.038. [DOI] [PubMed] [Google Scholar]
- Hazari Y.; Bravo-San Pedro J. M.; Hetz C.; Galluzzi L.; Kroemer G. Autophagy in Hepatic Adaptation to Stress. J. Hepatol. 2020, 72, 183–196. 10.1016/j.jhep.2019.08.026. [DOI] [PubMed] [Google Scholar]
- Degenhardt K.; Mathew R.; Beaudoin B.; Bray K.; Anderson D.; Chen G.; Mukherjee C.; Shi Y.; Gélinas C.; Fan Y.; et al. Autophagy Promotes Tumor Cell Survival and Restricts Necrosis, Inflammation, and Tumorigenesis. Cancer Cell 2006, 10, 51–64. 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K.; Yuan Y.; Wen J.; Chen D.; Zhu W.; Ouyang Z.; Wang W. Lncrna Sox2ot-V7 Promotes Doxorubicin-Induced Autophagy and Chemoresistance in Osteosarcoma Via Tumor-Suppressive Mir-142/Mir-22. Aging 2020, 12, 6644. 10.18632/aging.103004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K. T.; Lu Z. B.; Lv J. Q.; Zhang J. G. The Role of Long Non-Coding Rnas in Mediating Chemoresistance by Modulating Autophagy in Cancer. RNA Biol. 2020, 1–14. 10.1080/15476286.2020.1737787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S.; Shao L.; Chen Z.; Hu D.; Jiang L.; Tang W. Nprl2 Promotes Docetaxel Chemoresistance in Castration Resistant Prostate Cancer Cells by Regulating Autophagy through the Mtor Pathway. Exp. Cell Res. 2020, 390, 111981. 10.1016/j.yexcr.2020.111981. [DOI] [PubMed] [Google Scholar]
- Lin J. Z.; Wang W. W.; Hu T. T.; Zhu G. Y.; Li L. N.; Zhang C. Y.; Xu Z.; Yu H. B.; Wu H. F.; Zhu J. G. Foxm1 Contributes to Docetaxel Resistance in Castration-Resistant Prostate Cancer by Inducing Ampk/Mtor-Mediated Autophagy. Cancer Lett. 2020, 469, 481–489. 10.1016/j.canlet.2019.11.014. [DOI] [PubMed] [Google Scholar]
- Li J.; Lin W.; Zhuang L. Cd5l-Induced Activation of Autophagy Is Associated with Hepatoprotection in Ischemic Reperfusion Injury Via the Cd36/Atg7 Axis. Exp. Ther. Med. 2020, 19, 2588–2596. 10.3892/etm.2020.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong C.; Hu C.; Gu F.; Xia Q.; Yao C.; Zhang L.; Qiang L.; Gao S.; Gao Y. Co-Delivery of Autophagy Inhibitor Atg7 Sirna and Docetaxel for Breast Cancer Treatment. J. Controlled Release 2017, 266, 272–286. 10.1016/j.jconrel.2017.09.042. [DOI] [PubMed] [Google Scholar]
- Bell R. D.; Sagare A. P.; Friedman A. E.; Bedi G. S.; Holtzman D. M.; Deane R.; Zlokovic B. V. Transport Pathways for Clearance of Human Alzheimer’s Amyloid B-Peptide and Apolipoproteins E and J in the Mouse Central Nervous System. J. Cereb. Blood Flow Metab. 2007, 27, 909–918. 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M.; Ikeda K.; Ohshima K.; Tsugu H.; Kimura H.; Tomonaga M. Increased Expression of Low Density Lipoprotein Receptor-Related Protein/A2-Macroglobulin Receptor in Human Malignant Astrocytomas. Cancer Research 1997, 57, 2799–2805. [PubMed] [Google Scholar]
- Maletínská L.; Blakely E. A.; Bjornstad K. A.; Deen D. F.; Knoff L. J.; Forte T. M. Human Glioblastoma Cell Lines: Levels of Low-Density Lipoprotein Receptor and Low-Density Lipoprotein Receptor-Related Protein. Cancer Research 2000, 60, 2300–2303. [PubMed] [Google Scholar]
- Demeule M.; Currie J. C.; Bertrand Y.; Ché C.; Nguyen T.; Régina A.; Gabathuler R.; Castaigne J. P.; Béliveau R. Involvement of the Low-Density Lipoprotein Receptor-Related Protein in the Transcytosis of the Brain Delivery Vector Angiopep-2. J. Neurochem. 2008, 106, 1534–1544. 10.1111/j.1471-4159.2008.05492.x. [DOI] [PubMed] [Google Scholar]
- Demeule M.; Regina A.; Che C.; Poirier J.; Nguyen T.; Gabathuler R.; Castaigne J.-P.; Beliveau R. Identification and Design of Peptides as a New Drug Delivery System for the Brain. J. Pharmacol. Exp. Ther. 2008, 324, 1064–1072. 10.1124/jpet.107.131318. [DOI] [PubMed] [Google Scholar]
- Roth L.; Agemy L.; Kotamraju V.; Braun G.; Teesalu T.; Sugahara K.; Hamzah J.; Ruoslahti E. Transtumoral Targeting Enabled by a Novel Neuropilin-Binding Peptide. Oncogene 2012, 31, 3754–3763. 10.1038/onc.2011.537. [DOI] [PubMed] [Google Scholar]
- Teesalu T.; Sugahara K. N.; Kotamraju V. R.; Ruoslahti E. C-End Rule Peptides Mediate Neuropilin-1-Dependent Cell, Vascular, and Tissue Penetration. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 16157–16162. 10.1073/pnas.0908201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.-Z.; Li J.-Q.; Wang Z.-Z.; Dong D.-W.; Qi X.-R. Tumor-Targeting Dual Peptides-Modified Cationic Liposomes for Delivery of Sirna and Docetaxel to Gliomas. Biomaterials 2014, 35, 5226–5239. 10.1016/j.biomaterials.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Qu M.-H.; Zeng R.-F.; Fang S.; Dai Q.-S.; Li H.-P.; Long J.-T. Liposome-Based Co-Delivery of Sirna and Docetaxel for the Synergistic Treatment of Lung Cancer. Int. J. Pharm. 2014, 474, 112–122. 10.1016/j.ijpharm.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Cheng D.; Cao N.; Chen J.; Yu X.; Shuai X. Multifunctional Nanocarrier Mediated Co-Delivery of Doxorubicin and Sirna for Synergistic Enhancement of Glioma Apoptosis in Rat. Biomaterials 2012, 33, 1170–1179. 10.1016/j.biomaterials.2011.10.057. [DOI] [PubMed] [Google Scholar]
- Yap T. A.; Carden C. P.; Kaye S. B. Beyond Chemotherapy: Targeted Therapies in Ovarian Cancer. Nat. Rev. Cancer 2009, 9, 167–181. 10.1038/nrc2583. [DOI] [PubMed] [Google Scholar]
- Zheng C.; Zheng M.; Gong P.; Deng J.; Yi H.; Zhang P.; Zhang Y.; Liu P.; Ma Y.; Cai L. Polypeptide Cationic Micelles Mediated Co-Delivery of Docetaxel and Sirna for Synergistic Tumor Therapy. Biomaterials 2013, 34, 3431–3438. 10.1016/j.biomaterials.2013.01.053. [DOI] [PubMed] [Google Scholar]
- Ning F.; Yang Z.; Xu L.; Sun Y. Targeted Tumor Therapy by Autophagy of Nanoparticles. Future Oncol. 2020, 16, 793–803. 10.2217/fon-2019-0712. [DOI] [PubMed] [Google Scholar]
- Pérez-Arizti J. A.; Ventura-Gallegos J. L.; Galván Juárez R. E.; Ramos-Godinez M. D. P.; Colín-Val Z.; López-Marure R. Titanium Dioxide Nanoparticles Promote Oxidative Stress, Autophagy and Reduce Nlrp3 in Primary Rat Astrocytes. Chem.-Biol. Interact. 2020, 317, 108966. 10.1016/j.cbi.2020.108966. [DOI] [PubMed] [Google Scholar]
- Wang P.; Qiao P.; Xing H.; Zhang R.; Lingling E.; Liu H. Cytotoxicity, Oxidative Stress, and Autophagy Effects of Tantalum Nanoparticles on Mc3t3-E1Mouse Osteoblasts. J. Nanosci. Nanotechnol. 2020, 20, 1417–1424. 10.1166/jnn.2020.17158. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Yang T.; Chen S.; Qi S.; Zhang Z.; Xu Y. Silver Nanoparticles Regulate Autophagy through Lysosome Injury and Cell Hypoxia in Prostate Cancer Cells. J. Biochem. Mol. Toxicol. 2020, 34, e22474 10.1002/jbt.22474. [DOI] [PubMed] [Google Scholar]
- Xu H.; Zhang L.; Qian X.; Zhou X.; Yan Y.; Zhou J.; Ge W.; Albahde M.; Wang W. Gsk343 Induces Autophagy and Downregulates the Akt/Mtor Signaling Pathway in Pancreatic Cancer Cells. Exp. Ther. Med. 2019, 18, 2608–2616. 10.3892/etm.2019.7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Yang L.; Zhong C.; Zhou L. Ezh2 Regulates H2b Phosphorylation and Elevates Colon Cancer Cell Autophagy. J. Cell. Physiol. 2020, 235, 1494–1503. 10.1002/jcp.29069. [DOI] [PubMed] [Google Scholar]
- Yin C.; Ke X.; Zhang R.; Hou J.; Dong Z.; Wang F.; Zhang K.; Zhong X.; Yang L.; Cui H. G9a Promotes Cell Proliferation and Suppresses Autophagy in Gastric Cancer by Directly Activating Mtor. FASEB J. 2019, 33, 14036–14050. 10.1096/fj.201900233RR. [DOI] [PubMed] [Google Scholar]
- Hu Z.; Cai M.; Zhang Y.; Tao L.; Guo R. Mir-29c-3p Inhibits Autophagy and Cisplatin Resistance in Ovarian Cancer by Regulating Foxp1/Atg14 Pathway. Cell Cycle 2020, 19, 193–206. 10.1080/15384101.2019.1704537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Wu Z.; Ding W.; Xiao C.; Zhang Y.; Gao S.; Gao Y.; Cai W. Srebp1 Sirna Enhance the Docetaxel Effect Based on a Bone-Cancer Dual-Targeting Biomimetic Nanosystem against Bone Metastatic Castration-Resistant Prostate Cancer. Theranostics 2020, 10, 1619–1632. 10.7150/thno.40489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; He Z.; Xiang L.; Li L.; Zhang H.; Lin F.; Cao H. Co-delivery of Grp78 Sirna and Docetaxel Via Rgd-Peg-Dspe/Dopa/Cap Nanoparticles for the Treatment of Castration-Resistant Prostate Cancer. Drug Des., Dev. Ther. 2019, 13, 1357–1372. 10.2147/DDDT.S198400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majidi Zolbanin N.; Jafari R.; Majidi J.; Atyabi F.; Yousefi M.; Jadidi-Niaragh F.; Aghebati-Maleki L.; Shanehbandi D.; Soltani Zangbar M. S.; Nayebi A. M. Targeted Co-Delivery of Docetaxel and Cmet Sirna for Treatment of Mucin1 Overexpressing Breast Cancer Cells. Adv. Pharm. Bull. 2018, 8, 383–393. 10.15171/apb.2018.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari R.; Majidi Zolbanin N.; Majidi J.; Atyabi F.; Yousefi M.; Jadidi-Niaragh F.; Aghebati-Maleki L.; Shanehbandi D.; Soltani Zangbar M. S.; Rafatpanah H. Anti-Mucin1 Aptamer-Conjugated Chitosan Nanoparticles for Targeted Co-Delivery of Docetaxel and Igf-1r Sirna to Skbr3Metastatic Breast Cancer Cells. Iran. Biomed. J. 2019, 23, 21–33. 10.29252/ibj.23.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W. W.; Liu D. Y.; Cao Y. C.; Wang X. Y. Ge11 Peptide-Conjugated Nanoliposomes to Enhance the Combinational Therapeutic Efficacy of Docetaxel and Sirna in Laryngeal Cancers. Int. J. Nanomed. 2017, 12, 6461–6470. 10.2147/IJN.S129946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.; Wu X.; Wang Y.; Zhang T.; Wu T.; Liu F.; Wang W.; Jiang G.; Xie M. Folate-Targeted Star-Shaped Cationic Copolymer Co-Delivering Docetaxel and Mmp-9 Sirna for Nasopharyngeal Carcinoma Therapy. Oncotarget 2106, 7, 42017–42030. 10.18632/oncotarget.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M. A.; Thiruvengadam M.; Farooqui Z.; Rajakumar G.; Jamal Q. M. S.; Alzohairy M. A.; Almatroudi A.; Alomary M. N.; Chung I.-M.; Al-Suhaimi E. A.. In Nanotechnology, in Silico and Endocrine-Based Strategy for Delivering Paclitaxel and Mirna: Prospects for the Therapeutic Management of Breast Cancer, Seminars in Cancer Biology; Elsevier, 2019. [DOI] [PubMed] [Google Scholar]
- Potthoff K.; Stotzer O.; Soling U.; Hansen R.; Harde J.; Dille S.; Nusch A.; Marschner N. Effectiveness and Tolerability of Nab-Paclitaxel in Younger Versus Elderly Patients with Metastatic Hr-Positive/Her2-Negative Breast Cancer: Results from the Noninterventional, Prospective Study Nabucco. Clin. Breast Cancer 2020, xxx. 10.1016/j.clbc.2019.11.003. [DOI] [PubMed] [Google Scholar]
- Jotte R.; Cappuzzo F.; Vynnychenko I.; Stroyakovskiy D.; Rodriguez-Abreu D.; Hussein M.; Soo R.; Conter H. J.; Kozuki T.; Huang K. C.; Graupner V.; Sun S. W.; Hoang T.; Jessop H.; McCleland M.; Ballinger M.; Sandler A.; Socinski M. A. Atezolizumab in Combination with Carboplatin and Nab-Paclitaxel in Advanced Squamous Non-Small-Cell Lung Cancer (Impower131): Results from a Randomized Phase Iii Trial. J. Thorac. Oncol. 2020, 15, 1351. 10.1016/j.jtho.2020.03.028. [DOI] [PubMed] [Google Scholar]
- Graham-Gurysh E. G.; Moore K. M.; Schorzman A. N.; Lee T.; Zamboni W. C.; Hingtgen S. D.; Bachelder E. M.; Ainslie K. M. Tumor Responsive and Tunable Polymeric Platform for Optimized Delivery of Paclitaxel to Treat Glioblastoma. ACS Appl. Mater. Interfaces 2020, 12, 19345. 10.1021/acsami.0c04102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S. Q.; Jiang F. F.; Hong T.; Zhuang Y.; Chen L.; Huang X. L. Comparison of Pegylated Liposomal Doxorubicin and Paclitaxel Plus Carboplatin-Based Chemotherapy as First Line Treatment for Patients with Ovarian Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. European Review for Medical and Pharmacological Sciences 2020, 24, 2911–2927. 10.26355/eurrev_202003_20655. [DOI] [PubMed] [Google Scholar]
- Della Corte L.; Barra F.; Foreste V.; Giampaolino P.; Evangelisti G.; Ferrero S.; Bifulco G. Advances in Paclitaxel Combinations for Treating Cervical Cancer. Expert Opin. Pharmacother. 2020, 21, 663. 10.1080/14656566.2020.1724284. [DOI] [PubMed] [Google Scholar]
- Ying S.-Y.; Chang C. P.; Lin S.-L.. Intron-Mediated Rna Interference, Intronic Micrornas, and Applications. In RNA Therapeutics; Springer, 2010; pp 203–235. [DOI] [PubMed] [Google Scholar]
- Khalifa A.; Elsheikh M. A.; Khalifa A.; Elnaggar Y. S. Current Strategies for Different Paclitaxel-Loaded Nano-Delivery Systems Towards Therapeutic Applications for Ovarian Carcinoma: A Review Article. J. Controlled Release 2019, 311, 125–37. 10.1016/j.jconrel.2019.08.034. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Tang M.; Wu Y.; Weng X.; Yang L.; Xu W.; Yi W.; Gao J.; Bode A. M.; Dong Z.; et al. A Combination of Paclitaxel and Sirna-Mediated Silencing of Stathmin Inhibits Growth and Promotes Apoptosis of Nasopharyngeal Carcinoma Cells. Cell. Oncol. 2014, 37, 53–67. 10.1007/s13402-013-0163-3. [DOI] [PubMed] [Google Scholar]
- Liu W.; Lo Y. L.; Hsu C.; Wu Y. T.; Liao Z. X.; Wu W. J.; Chen Y. J.; Kao C.; Chiu C. C.; Wang L. F. Cs-Pei/Beclin-Sirna Downregulate Multidrug Resistance Proteins and Increase Paclitaxel Therapeutic Efficacy against Nsclc. Mol. Ther.--Nucleic Acids 2019, 17, 477–490. 10.1016/j.omtn.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.; Zhao Z.; Han Y.; Hu S.; Opoku-Damoah Y.; Zhou J.; Yin L.; Ding Y. Natural Particulates Inspired Specific-Targeted Co-delivery of Sirna and Paclitaxel for Collaborative Antitumor Therapy. Mol. Pharmaceutics 2017, 14, 2999–3012. 10.1021/acs.molpharmaceut.7b00192. [DOI] [PubMed] [Google Scholar]
- Chakravarthi B.; Chandrashekar D. S.; Agarwal S.; Balasubramanya S. A. H.; Pathi S. S.; Goswami M. T.; Jing X.; Wang R.; Mehra R.; Asangani I. A.; Chinnaiyan A. M.; Manne U.; Sonpavde G.; Netto G. J.; Gordetsky J.; Varambally S. Mir-34a Regulates Expression of the Stathmin-1 Oncoprotein and Prostate Cancer Progression. Mol. Cancer Res. 2018, 16, 1125–1137. 10.1158/1541-7786.MCR-17-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H. W.; Jiang D.; Xie Z. Y.; Zhou M. H.; Sun D. Y.; Zhao Y. G. Effects of Stathmin 1 Silencing by Sirna on Sensitivity of Esophageal Cancer Cells Eca-109 to Paclitaxel. GMR, Genet. Mol. Res. 2015, 14, 18695–702. 10.4238/2015.December.28.18. [DOI] [PubMed] [Google Scholar]
- Jones S. K.; Lizzio V.; Merkel O. M. Folate Receptor Targeted Delivery of Sirna and Paclitaxel to Ovarian Cancer Cells Via Folate Conjugated Triblock Copolymer to Overcome Tlr4 Driven Chemotherapy Resistance. Biomacromolecules 2016, 17, 76–87. 10.1021/acs.biomac.5b01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R.; Yao X.; Chen Y.; Sun X.; Lin Y. [Cytological Study in Vitro on Co-Delivery of Sirna and Paclitaxel within Solid Lipid Nanoparticles to Overcome Multidrug Resistance in Tumors]. Sheng wu yi xue gong cheng xue za zhi = Journal of biomedical engineering = Shengwu yixue gongchengxue zazhi 2016, 33, 108–14. [PubMed] [Google Scholar]
- Chimmiri P.; Rajalakshmi R.; Mahitha B.; Ramesh G.; Noor Ahmed V. Solid Lipid Nanoparticles: A Novel Carrier for Cancer Therapy. International Journal of Biological and Pharmaceutical Research 2012, 3, 405–413. [Google Scholar]
- Serpe L.; Catalano M. G.; Cavalli R.; Ugazio E.; Bosco O.; Canaparo R.; Muntoni E.; Frairia R.; Gasco M. R.; Eandi M.; et al. Cytotoxicity of Anticancer Drugs Incorporated in Solid Lipid Nanoparticles on Ht-29 Colorectal Cancer Cell Line. Eur. J. Pharm. Biopharm. 2004, 58, 673–680. 10.1016/j.ejpb.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Shah R.; Eldridge D.; Palombo E.; Harding I. Optimisation and Stability Assessment of Solid Lipid Nanoparticles Using Particle Size and Zeta Potential. Journal of Physical Science 2014, 25 (1), 59–75. [Google Scholar]
- Büyükköroğlu G.; Şenel B.; Yenilmez E.. Vaginal Suppositories with Sirna and Paclitaxel-Incorporated Solid Lipid Nanoparticles for Cervical Cancer: Preparation and in Vitro Evaluation. In RNA Interference and Cancer Therapy; Springer, 2019; pp 303–328. [DOI] [PubMed] [Google Scholar]
- Guo J.; Rahme K.; Fitzgerald K. A.; Holmes J. D.; O’Driscoll C. M. Biomimetic Gold Nanocomplexes for Gene Knockdown: Will Gold Deliver Dividends for Small Interfering Rna Nanomedicines?. Nano Res. 2015, 8, 3111–3140. 10.1007/s12274-015-0829-4. [DOI] [Google Scholar]
- Guo J.; O’Driscoll C. M.; Holmes J. D.; Rahme K. Bioconjugated Gold Nanoparticles Enhance Cellular Uptake: A Proof of Concept Study for Sirna Delivery in Prostate Cancer Cells. Int. J. Pharm. 2016, 509, 16–27. 10.1016/j.ijpharm.2016.05.027. [DOI] [PubMed] [Google Scholar]
- Banerjee R.; Tyagi P.; Li S.; Huang L. Anisamide-Targeted Stealth Liposomes: A Potent Carrier for Targeting Doxorubicin to Human Prostate Cancer Cells. Int. J. Cancer 2004, 112, 693–700. 10.1002/ijc.20452. [DOI] [PubMed] [Google Scholar]
- Guo J.; Ogier J. R.; Desgranges S.; Darcy R.; O'Driscoll C. Anisamide-Targeted Cyclodextrin Nanoparticles for Sirna Delivery to Prostate Tumours in Mice. Biomaterials 2012, 33, 7775–7784. 10.1016/j.biomaterials.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Luan X.; Rahme K.; Cong Z.; Wang L.; Zou Y.; He Y.; Yang H.; Holmes J. D.; O’Driscoll C. M.; Guo J. Anisamide-Targeted Pegylated Gold Nanoparticles Designed to Target Prostate Cancer Mediate: Enhanced Systemic Exposure of Sirna, Tumour Growth Suppression and a Synergistic Therapeutic Response in Combination with Paclitaxel in Mice. Eur. J. Pharm. Biopharm. 2019, 137, 56–67. 10.1016/j.ejpb.2019.02.013. [DOI] [PubMed] [Google Scholar]
- Sun X.; Chen Y.; Zhao H.; Qiao G.; Liu M.; Zhang C.; Cui D.; Ma L. Dual-Modified Cationic Liposomes Loaded with Paclitaxel and Survivin Sirna for Targeted Imaging and Therapy of Cancer Stem Cells in Brain Glioma. Drug Delivery 2018, 25, 1718–1727. 10.1080/10717544.2018.1494225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K.; Zheng J.; Yu J.; Wu Y.; Guo J.; Xu Z.; Sun X. Knockdown of Mmp1 Inhibits the Progression of Colorectal Cancer by Suppressing the Pi3k/Akt/Cmyc Signaling Pathway and Emt. Oncology Reports 2020, 10.3892/or.2020.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W.; Sun L.; Liu T.; Zhu J.; Zeng Y.; Zhang Y.; Wang X.; Liu Z.; Huang J. A. The Mir6253p/Axl Axis Induces Nont790m Acquired Resistance to Egfrtki Via Activation of the Tgfbeta/Smad Pathway and Emt in Egfrmutant Nonsmall Cell Lung Cancer. Oncology Reports 2020, 10.3892/or.2020.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Meng Y.; Ye J.; Xia X.; Wang H.; Li L.; Dong W.; Jin D.; Liu Y. Sequential Delivery of Vegf Sirna and Paclitaxel for Pvn Destruction, Anti-Angiogenesis, and Tumor Cell Apoptosis Procedurally Via a Multi-Functional Polymer Micelle. J. Controlled Release 2018, 287, 103–120. 10.1016/j.jconrel.2018.08.028. [DOI] [PubMed] [Google Scholar]
- Franke F. C.; Slusarenko B. O.; Engleitner T.; Johannes W.; Laschinger M.; Rad R.; Nitsche U.; Janssen K. P. Novel Role for Crk Adaptor Proteins as Essential Components of Src/Fak Signaling for Epithelial-Mesenchymal Transition and Colorectal Cancer Aggressiveness. Int. J. Cancer 2020, 147, 1715. 10.1002/ijc.32955. [DOI] [PubMed] [Google Scholar]
- Worthmuller J.; Salicio V.; Oberson A.; Blum W.; Schwaller B. Modulation of Calretinin Expression in Human Mesothelioma Cells Reveals the Implication of the Fak and Wnt Signaling Pathways in Conferring Chemoresistance Towards Cisplatin. Int. J. Mol. Sci. 2019, 20, 5391. 10.3390/ijms20215391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J.; Ghosh S. C.; Han H. D.; Stone R. L.; Bottsford-Miller J.; Auzenne E. J.; Lopez-Araujo A.; Lu C.; Nishimura M.; Pecot C. V.; et al. Metronomic Activity of Cd44-Targeted Hyaluronic Acid-Paclitaxel in Ovarian Carcinoma. Clin. Cancer Res. 2012, 18, 4114–4121. 10.1158/1078-0432.CCR-11-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios de la Rosa J. M.; Tirella A.; Gennari A.; Stratford I. J.; Tirelli N. The Cd44-Mediated Uptake of Hyaluronic Acid-Based Carriers in Macrophages. Adv. Healthcare Mater. 2017, 6, 1601012. 10.1002/adhm.201601012. [DOI] [PubMed] [Google Scholar]
- Byeon Y.; Lee J.-W.; Choi W. S.; Won J. E.; Kim G. H.; Kim M. G.; Wi T. I.; Lee J. M.; Kang T. H.; Jung I. D.; et al. Cd44-Targeting Plga Nanoparticles Incorporating Paclitaxel and Fak Sirna Overcome Chemoresistance in Epithelial Ovarian Cancer. Cancer Res. 2018, 78, 6247–6256. 10.1158/0008-5472.CAN-17-3871. [DOI] [PubMed] [Google Scholar]
- Dean M.; Hamon Y.; Chimini G. The Human Atp-Binding Cassette (Abc) Transporter Superfamily. Journal of Lipid Research 2001, 42, 1007–1017. [PubMed] [Google Scholar]
- Shi Z.; Liang Y.-J.; Chen Z.-S.; Wang X.-H.; Ding Y.; Chen L.-M.; Fu L.-W. Overexpression of Survivin and Xiap in Mdr Cancer Cells Unrelated to P-Glycoprotein. Oncol. Rep. 2007, 17, 969–976. 10.3892/or.17.4.969. [DOI] [PubMed] [Google Scholar]
- Risnayanti C.; Jang Y.-S.; Lee J.; Ahn H. J. Plga Nanoparticles Co-Delivering Mdr1 and Bcl2 Sirna for Overcoming Resistance of Paclitaxel and Cisplatin in Recurrent or Advanced Ovarian Cancer. Sci. Rep. 2018, 8, 7498. 10.1038/s41598-018-25930-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou W.; Byeon J. H.; Soe Z. C.; Kim B. K.; Thapa R. K.; Gupta B.; Poudel B. K.; Ku S. K.; Yong C. S.; Kim J. O. Tailored Black Phosphorus for Erythrocyte Membrane Nanocloaking with Interleukin-1α Sirna and Paclitaxel for Targeted, Durable, and Mild Combination Cancer Therapy. Theranostics 2019, 9, 6780. 10.7150/thno.37123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M.; Jin G.; Kang L.; Chen L.; Gao Z.; Huang W. Smart Polymeric Nanoparticles with Ph-Responsive and Peg-Detachable Properties for Co-Delivering Paclitaxel and Survivin Sirna to Enhance Antitumor Outcomes. Int. J. Nanomed. 2018, 13, 2405. 10.2147/IJN.S161426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S.; Bi X.; Yang L.; Wu S.; Yu Y.; Jiang B.; Zhang A.; Lan K.; Duan S. Co-Delivery of Paclitaxel and Plk1-Targeted Sirna Using Aptamer-Functionalized Cationic Liposome for Synergistic Anti-Breast Cancer Effects in Vivo. J. Biomed. Nanotechnol. 2019, 15, 1135–1148. 10.1166/jbn.2019.2751. [DOI] [PubMed] [Google Scholar]
- Chen X.; Zhang Y.; Tang C.; Tian C.; Sun Q.; Su Z.; Xue L.; Yin Y.; Ju C.; Zhang C. Co-Delivery of Paclitaxel and Anti-Survivin Sirna Via Redox-Sensitive Oligopeptide Liposomes for the Synergistic Treatment of Breast Cancer and Metastasis. Int. J. Pharm. 2017, 529, 102–115. 10.1016/j.ijpharm.2017.06.071. [DOI] [PubMed] [Google Scholar]
- Zhu W.-j.; Yang S.-d.; Qu C.-x.; Zhu Q.-l.; Chen W.-l.; Li F.; Yuan Z.-q.; Liu Y.; You B.-g.; Zhang X.-n. Low-Density Lipoprotein-Coupled Micelles with Reduction and Ph Dual Sensitivity for Intelligent Co-Delivery of Paclitaxel and Sirna to Breast Tumor. Int. J. Nanomed. 2017, 12, 3375. 10.2147/IJN.S126310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.; Xu Z.; Chen X.; Xu L.; Yin Q.; Zhang Z.; Li Y. Reversal of Lung Cancer Multidrug Resistance by P H-R Esponsive Micelleplexes Mediating Co-D Elivery of Si Rna and Paclitaxel. Macromol. Biosci. 2014, 14, 100–109. 10.1002/mabi.201300282. [DOI] [PubMed] [Google Scholar]
- Wei W.; Lv P.-P.; Chen X.-M.; Yue Z.-G.; Fu Q.; Liu S.-Y.; Yue H.; Ma G.-H. Co-delivery of Mtert Sirna and Paclitaxel by Chitosan-Based Nanoparticles Promoted Synergistic Tumor Suppression. Biomaterials 2013, 34, 3912–3923. 10.1016/j.biomaterials.2013.02.030. [DOI] [PubMed] [Google Scholar]
- Bae K. H.; Lee J. Y.; Lee S. H.; Park T. G.; Nam Y. S. Optically Traceable Solid Lipid Nanoparticles Loaded with Sirna and Paclitaxel for Synergistic Chemotherapy with in Situ Imaging. Adv. Healthcare Mater. 2013, 2, 576–584. 10.1002/adhm.201200338. [DOI] [PubMed] [Google Scholar]
- Jang Y. L.; Yun U. J.; Lee M. S.; Kim M. G.; Son S.; Lee K.; Chae S. Y.; Lim D. W.; Kim H. T.; Kim S. H.; et al. Cell-Penetrating Peptide Mimicking Polymer-Based Combined Delivery of Paclitaxel and Sirna for Enhanced Tumor Growth Suppression. Int. J. Pharm. 2012, 434, 488–493. 10.1016/j.ijpharm.2012.04.083. [DOI] [PubMed] [Google Scholar]
- Yu Y. H.; Kim E.; Park D. E.; Shim G.; Lee S.; Kim Y. B.; Kim C.-W.; Oh Y.-K. Cationic Solid Lipid Nanoparticles for Co-Delivery of Paclitaxel and Sirna. Eur. J. Pharm. Biopharm. 2012, 80, 268–273. 10.1016/j.ejpb.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Su W.-P.; Cheng F.-Y.; Shieh D.-B.; Yeh C.-S.; Su W.-C. Plga Nanoparticles Codeliver Paclitaxel and Stat3 Sirna to Overcome Cellular Resistance in Lung Cancer Cells. Int. J. Nanomed. 2012, 7, 4269. 10.2147/IJN.S33666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büyükköroğlu G.; Şenel B.; Başaran E.; Yenilmez E.; Yazan Y. Preparation and in Vitro Evaluation of Vaginal Formulations Including Sirna and Paclitaxel-Loaded Slns for Cervical Cancer. Eur. J. Pharm. Biopharm. 2016, 109, 174–183. 10.1016/j.ejpb.2016.10.017. [DOI] [PubMed] [Google Scholar]
- Reddy T. L.; Garikapati K. R.; Reddy S. G.; Reddy B. S.; Yadav J.; Bhadra U.; Bhadra M. P. Simultaneous Delivery of Paclitaxel and Bcl-2 Sirna Via Ph-Sensitive Liposomal Nanocarrier for the Synergistic Treatment of Melanoma. Sci. Rep. 2016, 6, 35223. 10.1038/srep35223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujrati M.; Vaidya A. M.; Mack M.; Snyder D.; Malamas A.; Lu Z. R. Targeted Dual Ph-Sensitive Lipid Eco/Sirna Self-Assembly Nanoparticles Facilitate in Vivo Cytosolic Sieif4e Delivery and Overcome Paclitaxel Resistance in Breast Cancer Therapy. Adv. Healthcare Mater. 2016, 5, 2882–2895. 10.1002/adhm.201600677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C.; Liu W.; Hu Y.; Li W.; Di W. Bioinspired Tumor-Homing Nanoplatform for Co-Delivery of Paclitaxel and Sirna-E7 to Hpv-Related Cervical Malignancies for Synergistic Therapy. Theranostics 2020, 10, 3325. 10.7150/thno.41228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Cho Y. J.; Lee J.-W.; Ahn H. J. Ksp Sirna/Paclitaxel-Loaded Pegylated Cationic Liposomes for Overcoming Resistance to Ksp Inhibitors: Synergistic Antitumor Effects in Drug-Resistant Ovarian Cancer. J. Controlled Release 2020, 321, 184–197. 10.1016/j.jconrel.2020.02.013. [DOI] [PubMed] [Google Scholar]
- Yang S. D.; Zhu W. J.; Zhu Q. L.; Chen W. L.; Ren Z. X.; Li F.; Yuan Z. Q.; Li J. Z.; Liu Y.; Zhou X. F.; et al. Binary-Copolymer System Base on Low-Density Lipoprotein-Coupled N-Succinyl Chitosan Lipoic Acid Micelles for Co-Delivery Mdr1 Sirna and Paclitaxel, Enhances Antitumor Effects Via Reducing Drug. J. Biomed. Mater. Res., Part B 2017, 105, 1114–1125. 10.1002/jbm.b.33636. [DOI] [PubMed] [Google Scholar]
- Tang S.; Yin Q.; Su J.; Sun H.; Meng Q.; Chen Y.; Chen L.; Huang Y.; Gu W.; Xu M.; et al. Inhibition of Metastasis and Growth of Breast Cancer by Ph-Sensitive Poly (B-Amino Ester) Nanoparticles Co-Delivering Two Sirna and Paclitaxel. Biomaterials 2015, 48, 1–15. 10.1016/j.biomaterials.2015.01.049. [DOI] [PubMed] [Google Scholar]
- Yang X.; lyer A. K.; Singh A.; Choy E.; Hornicek F. J.; Amiji M. M.; Duan Z. Mdr1 Sirna Loaded Hyaluronic Acid-Based Cd44 Targeted Nanoparticle Systems Circumvent Paclitaxel Resistance in Ovarian Cancer. Sci. Rep. 2015, 5, 8509. 10.1038/srep08509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzano G.; Navarro G.; Trivedi M. S.; De Rosa G.; Torchilin V. P. Multifunctional Polymeric Micelles Co-Loaded with Anti-Survivin Sirna and Paclitaxel Overcome Drug Resistance in an Animal Model of Ovarian Cancer. Mol. Cancer Ther. 2015, 14, 1075–84. 10.1158/1535-7163.MCT-14-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T.; Wang P.; Li J.; Wang Y.; Zheng B.; Zheng R.; Cheng D.; Shuai X. Tumor-Penetrating Co-delivery of Sirna and Paclitaxel with Ultrasound-Responsive Nanobubbles Hetero-Assembled from Polymeric Micelles and Liposomes. Biomaterials 2014, 35, 5932–5943. 10.1016/j.biomaterials.2014.03.072. [DOI] [PubMed] [Google Scholar]
- Feng Q.; Yu M.-Z.; Wang J.-C.; Hou W.-J.; Gao L.-Y.; Ma X.-F.; Pei X.-W.; Niu Y.-J.; Liu X.-Y.; Qiu C.; et al. Synergistic Inhibition of Breast Cancer by Co-Delivery of Vegf Sirna and Paclitaxel Via Vapreotide-Modified Core-Shell Nanoparticles. Biomaterials 2014, 35, 5028–5038. 10.1016/j.biomaterials.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Ni W.; Luo L.; Zuo P.; Li R.; Xu X.; Wen F.; Hu D. Mir-374a Inhibitor Enhances Etoposide-Induced Cytotoxicity against Glioma Cells through Upregulation of Foxo1. Oncol. Res. 2019, 27, 703–712. 10.3727/096504018X15426775024905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M.; Nakashima S.; Banno Y.; Yamakawa H.; Hayashi K.; Takenaka K.; Nishimura Y.; Sakai N.; Nozawa Y. Ordering of Ceramide Formation, Caspase Activation, and Bax/Bcl-2 Expression During Etoposide-Induced Apoptosis in C6 Glioma Cells. Cell Death Differ. 2000, 7, 761–772. 10.1038/sj.cdd.4400711. [DOI] [PubMed] [Google Scholar]
- Wang F.; Bhat K.; Doucette M.; Zhou S.; Gu Y.; Law B.; Liu X.; Wong E. T.; Kang J. X.; Hsieh T.-C.; et al. Docosahexaenoic Acid (Dha) Sensitizes Brain Tumor Cells to Etoposide-Induced Apoptosis. Curr. Mol. Med. 2011, 11, 503–511. 10.2174/156652411796268740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y.; Ma H.; Hui Z.; Zhao L.; Li D.; Liang J.; Wang X.; Xu L.; Chen B.; Tang Y.; Wu R.; Xu Y.; Pang Q.; Chen M.; Wang L. Helper Study: A Phase Ii Trial of Continuous Infusion of Endostar Combined with Concurrent Etoposide Plus Cisplatin and Radiotherapy for Treatment of Unresectable Stage Iii Non-Small-Cell Lung Cancer. Radiother. Oncol. 2019, 131, 27–34. 10.1016/j.radonc.2018.10.032. [DOI] [PubMed] [Google Scholar]
- Reck M.; Horn L.; Novello S.; Barlesi F.; Albert I.; Juhasz E.; Kowalski D.; Robinet G.; Cadranel J.; Bidoli P.; Chung J.; Fritsch A.; Drews U.; Wagner A.; Govindan R. Phase Ii Study of Roniciclib in Combination with Cisplatin/Etoposide or Carboplatin/Etoposide as First-Line Therapy in Patients with Extensive-Disease Small Cell Lung Cancer. J. Thorac. Oncol. 2019, 14, 701–711. 10.1016/j.jtho.2019.01.010. [DOI] [PubMed] [Google Scholar]
- Owonikoko T. K.; Dahlberg S. E.; Sica G. L.; Wagner L. I.; Wade J. L. 3rd; Srkalovic G.; Lash B. W.; Leach J. W.; Leal T. B.; Aggarwal C.; Ramalingam S. S. Randomized Phase Ii Trial of Cisplatin and Etoposide in Combination with Veliparib or Placebo for Extensive-Stage Small-Cell Lung Cancer: Ecog-Acrin 2511 Study. J. Clin. Oncol. 2019, 37, 222–229. 10.1200/JCO.18.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania E. E.; Carvajal-Moreno J.; Hernandez V. A.; English A.; Papa J. L.; Shkolnikov N.; Ozer H. G.; Yilmaz A. S.; Yalowich J. C.; Elton T. S. Hsa-Mir-9–3p and Hsa-Mir-9–5p as Post-Transcriptional Modulators of DNA Topoisomerase Iialpha in Human Leukemia K562 Cells with Acquired Resistance to Etoposide. Mol. Pharmacol. 2020, 97, 159–170. 10.1124/mol.119.118315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z.; Lin A.; Li K.; Lin W.; Wang Q.; Wei T.; Zhu W.; Luo P.; Zhang J. A Novel Mutation Panel for Predicting Etoposide Resistance in Small-Cell Lung Cancer. Drug Des., Dev. Ther. 2019, 13, 2021–2041. 10.2147/DDDT.S205633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultang N.; Illendula A.; Lin J.; Pandey M. K.; Klase Z.; Peethambaran B. Ror1 Regulates Chemoresistance in Breast Cancer Via Modulation of Drug Efflux Pump Abcb1. Sci. Rep. 2020, 10, 1821. 10.1038/s41598-020-58864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachalaki S.; Baradaran B.; Majidi J.; Yousefi M.; Shanehbandi D.; Mohammadinejad S.; Mansoori B. Reversal of Chemoresistance with Small Interference Rna (Sirna) in Etoposide Resistant Acute Myeloid Leukemia Cells (Hl-60). Biomed. Pharmacother. 2015, 75, 100–4. 10.1016/j.biopha.2015.08.032. [DOI] [PubMed] [Google Scholar]
- Jafarlou M.; Baradaran B.; Shanehbandi D.; Saedi T. A.; Jafarlou V.; Ismail P.; Othman F. Sirna-Mediated Inhibition of Survivin Gene Enhances the Anti-Cancer Effect of Etoposide in U-937 Acute Myeloid Leukemia Cells. Cellular and Molecular Biology (Noisy-le-Grand, France) 2016, 62, 44–9. [PubMed] [Google Scholar]
- Chan K. K.; Wong O. G.; Wong E. S.; Chan K. K.; Ip P. P.; Tse K. Y.; Cheung A. N. Impact of Iaspp on Chemoresistance through Plk1 and Autophagy in Ovarian Clear Cell Carcinoma. Int. J. Cancer 2018, 143, 1456–1469. 10.1002/ijc.31535. [DOI] [PubMed] [Google Scholar]
- Wu J.; Liang Y.; Tan Y.; Tang Y.; Song H.; Wang Z.; Li Y.; Lu M. Cdk9 Inhibitors Reactivate P53 by Downregulating Iaspp. Cell. Signalling 2020, 67, 109508. 10.1016/j.cellsig.2019.109508. [DOI] [PubMed] [Google Scholar]
- Liu H.; Wang M.; Diao S.; Rao Q.; Zhang X.; Xing H.; Wang J. Sirna-Mediated Down-Regulation of Iaspp Promotes Apoptosis Induced by Etoposide and Daunorubicin in Leukemia Cells Expressing Wild-Type P53. Leuk. Res. 2009, 33, 1243–8. 10.1016/j.leukres.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Karami H.; Baradaran B.; Esfahani A.; Estiar M. A.; Naghavi-Behzad M.; Sakhinia M.; Sakhinia E. Sirna-Mediated Silencing of Survivin Inhibits Proliferation and Enhances Etoposide Chemosensitivity in Acute Myeloid Leukemia Cells. Asian Pacific journal of cancer prevention: APJCP 2013, 14, 7719–24. 10.7314/APJCP.2013.14.12.7719. [DOI] [PubMed] [Google Scholar]
- Zhang P. L.; Hou X. X.; Liu M. R.; Huang F. P.; Qin X. Y. Two Novel Chiral Tetranucleate Copper-Based Complexes: Crystal Structures, Nanoparticles, and Inhibiting Angiogenesis and the Growth of Human Breast Cancer by Regulating the Vegf/Vegfr2 Signal Pathway in Vitro. Dalton Transactions (Cambridge, England: 2003) 2020, 49, 6043. 10.1039/D0DT00380H. [DOI] [PubMed] [Google Scholar]
- de Almeida P. E.; Mak J.; Hernandez G.; Jesudason R.; Herault A.; Javinal V.; Borneo J.; Kim J. M.; Walsh K. B. Anti-Vegf Treatment Enhances Cd8+ T-Cell Antitumor Activity by Amplifying Hypoxia. Cancer Immunol. Res. 2020, 8, 806. 10.1158/2326-6066.CIR-19-0360. [DOI] [PubMed] [Google Scholar]
- Xie H.; Lafky J. M.; Morlan B. W.; Stella P. J.; Dakhil S. R.; Gross G. G.; Loui W. S.; Hubbard J. M.; Alberts S. R.; Grothey A. Dual Vegf Inhibition with Sorafenib and Bevacizumab as Salvage Therapy in Metastatic Colorectal Cancer: Results of the Phase Ii North Central Cancer Treatment Group Study N054c (Alliance). Therapeutic advances in medical oncology 2020, 12, 175883592091091. 10.1177/1758835920910913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Ramirez J.; Morera Diaz Y.; Bequet-Romero M.; Hernandez-Bernal F.; Martin Bauta Y.; Selman-Housein Bernal K. H.; de la Torre Santos A. V.; Perez de la Iglesia M.; Trimino Lorenzo L.; Ayala Avila M. Specific Humoral Response in Cancer Patients Treated with a Vegf-Specific Active Immunotherapy Procedure within a Compassionate Use Program. BMC Immunol. 2020, 21, 12. 10.1186/s12865-020-0338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Lee J. I.; Ahn T. G. Effect of Quercetin on the Anti-Tumor Activity of Cisplatin in Emt6 Breast Tumor-Bearing Mice. Obstetrics & gynecology science 2019, 62, 242–248. 10.5468/ogs.2019.62.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang X.; Gu J.; Zhang J.; Shi H.; Hou S.; Xu X.; Chen Y.; Zhang Y.; Mao F.; Qian H.; Zhu T.; Xu W.; Zhang X. Exosome-Transmitted Lncrna Ufc1 Promotes Non-Small-Cell Lung Cancer Progression by Ezh2-Mediated Epigenetic Silencing of Pten Expression. Cell Death Dis. 2020, 11, 215. 10.1038/s41419-020-2409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu W.; Zhang X.; Qi L.; Fu Y.; Wang P.; Zhao W.; Du J.; Zhang J.; Zhan J.; Wang Y.; Zhu W. G.; Yu Y.; Zhang H. The Ezh2-Phactr2-As1-Ribosome Axis Induces Genomic Instability and Promotes Growth and Metastasis in Breast Cancer. Cancer Res. 2020, 80, 2737. 10.1158/0008-5472.CAN-19-3326. [DOI] [PubMed] [Google Scholar]
- Pellecchia S.; Sepe R.; Decaussin-Petrucci M.; Ivan C.; Shimizu M.; Coppola C.; Testa D.; Calin G. A.; Fusco A.; Pallante P. The Long Non-Coding Rna Prader Willi/Angelman Region Rna5 (Par5) Is Downregulated in Anaplastic Thyroid Carcinomas Where It Acts as a Tumor Suppressor by Reducing Ezh2 Activity. Cancers 2020, 12, 235. 10.3390/cancers12010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stazi G.; Taglieri L.; Nicolai A.; Romanelli A.; Fioravanti R.; Morrone S.; Sabatino M.; Ragno R.; Taurone S.; Nebbioso M.; Carletti R.; Artico M.; Valente S.; Scarpa S.; Mai A. Dissecting the Role of Novel Ezh2 Inhibitors in Primary Glioblastoma Cell Cultures: Effects on Proliferation, Epithelial-Mesenchymal Transition, Migration, and on the Pro-Inflammatory Phenotype. Clin. Epigenet. 2019, 11, 173. 10.1186/s13148-019-0763-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yuan Z. Q.; Chen W. L.; You B. G.; Liu Y.; Yang S. D.; Li J. Z.; Zhu W. J.; Zhou X. F.; Liu C.; Zhang X. N. Multifunctional Nanoparticles Co-Delivering Ezh2 Sirna and Etoposide for Synergistic Therapy of Orthotopic Non-Small-Cell Lung Tumor. J. Controlled Release 2017, 268, 198–211. 10.1016/j.jconrel.2017.10.025. [DOI] [PubMed] [Google Scholar]
- Popova P.; Notabi M. K.; Code C.; Arnspang E. C.; Andersen M. O. Co-Delivery of Sirna and Etoposide to Cancer Cells Using an Mdea Esterquat Based Drug Delivery System. Eur. J. Pharm. Sci. 2019, 127, 142–150. 10.1016/j.ejps.2018.10.023. [DOI] [PubMed] [Google Scholar]
- Mortezaee K.; Potes Y.; Mirtavoos-Mahyari H.; Motevaseli E.; Shabeeb D.; Musa A. E.; Najafi M.; Farhood B. Boosting Immune System against Cancer by Melatonin: A Mechanistic Viewpoint. Life Sci. 2019, 238, 116960. 10.1016/j.lfs.2019.116960. [DOI] [PubMed] [Google Scholar]
- Yuan Z.-q.; Chen W.-l.; You B.-g.; Liu Y.; Li J.-z.; Zhu W.-j.; Zhou X.-f.; Liu C.; Zhang X.-n.; et al. Multifunctional Nanoparticles Co-Delivering Ezh2 Sirna and Etoposide for Synergistic Therapy of Orthotopic Non-Small-Cell Lung Tumor. J. Controlled Release 2017, 268, 198–211. 10.1016/j.jconrel.2017.10.025. [DOI] [PubMed] [Google Scholar]
- Al-Attar T.; Madihally S. V. Influence of Controlled Release of Resveratrol from Electrospun Fibers in Combination with Sirna on Leukemia Cells. Eur. J. Pharm. Sci. 2018, 123, 173–183. 10.1016/j.ejps.2018.07.043. [DOI] [PubMed] [Google Scholar]
- Al-Attar T.; Madihally S. V. Targeted Cancer Treatment Using a Combination of Sirna-Liposomes and Resveratrol-Electrospun Fibers in Co-Cultures. Int. J. Pharm. 2019, 569, 118599. 10.1016/j.ijpharm.2019.118599. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Wang W.; Wu X.; Li C.; Huang Y.; Zhou H.; Cui Y. Resveratrol Sensitizes Colorectal Cancer Cells to Cetuximab by Connexin 43 Upregulation-Induced Akt Inhibition. Front. Oncol. 2020, 10, 383. 10.3389/fonc.2020.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B.; Liu J. Resveratrol: A Review of Plant Sources, Synthesis, Stability, Modification and Food Application. J. Sci. Food Agric. 2020, 100 (4), 1392–404. 10.1002/jsfa.10152. [DOI] [PubMed] [Google Scholar]
- Vervandier-Fasseur D.; Latruffe N. The Potential Use of Resveratrol for Cancer Prevention. Molecules 2019, 24, 4506. 10.3390/molecules24244506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langcake P.; Pryce R. The Production of Resveratrol by Vitis Vinifera and Other Members of the Vitaceae as a Response to Infection or Injury. Physiol. Plant Pathol. 1976, 9, 77–86. 10.1016/0048-4059(76)90077-1. [DOI] [Google Scholar]
- Callemien D.; Jerkovic V.; Rozenberg R.; Collin S. Hop as an Interesting Source of Resveratrol for Brewers: Optimization of the Extraction and Quantitative Study by Liquid Chromatography/Atmospheric Pressure Chemical Ionization Tandem Mass Spectrometry. J. Agric. Food Chem. 2005, 53, 424–429. 10.1021/jf040179n. [DOI] [PubMed] [Google Scholar]
- Wang X.; Fang H.; Xu G.; Yang Y.; Xu R.; Liu Q.; Xue X.; Liu J.; Wang H. Resveratrol Prevents Cognitive Impairment in Type 2 Diabetic Mice by Upregulating Nrf2 Expression and Transcriptional Level. Diabetes, Metab. Syndr. Obes.: Targets Ther. 2020, 13, 1061–1075. 10.2147/DMSO.S243560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.; Zhen L.; Li Z.; Xu W.; Leng H.; Xu W.; Zheng V.; Luria V.; Pan J.; Tao Y.; Zhang H.; Cao S.; Xu Y. Trans-Resveratrol Ameliorates Anxiety-Like Behaviors and Neuropathic Pain in Mouse Model of Post-Traumatic Stress Disorder. J. Psychopharmacol. (London, U. K.) 2020, 34, 726. 10.1177/0269881120914221. [DOI] [PubMed] [Google Scholar]
- Akyuva Y.; Naziroglu M. Resveratrol Attenuates Hypoxia-Induced Neuronal Cell Death, Inflammation and Mitochondrial Oxidative Stress by Modulation of Trpm2 Channel. Sci. Rep. 2020, 10, 6449. 10.1038/s41598-020-63577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D. J.; Kang J. B.; Shah F. A.; Koh P. O. Resveratrol Modulates the Akt/Gsk-3beta Signaling Pathway in a Middle Cerebral Artery Occlusion Animal Model. Laboratory animal research 2019, 35, 18. 10.1186/s42826-019-0019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.; Chen L.; Zhu F.; Han X.; Sun L.; Chen K. The Cytotoxicity Effect of Resveratrol: Cell Cycle Arrest and Induced Apoptosis of Breast Cancer 4t1 Cells. Toxins 2019, 11, 731. 10.3390/toxins11120731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.; Li C.; Ma L.; Jin F. Resveratrol Inhibits Viability and Induces Apoptosis in the Small-Cell Lung Cancer H446 Cell Line Via the Pi3k/Akt/C-Myc Pathway. Oncology Reports 2020, 10.3892/or.2020.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H. J.; Han J. M.; Choi Y. S.; Jung H. J. Pterostilbene Suppresses Both Cancer Cells and Cancer Stem-Like Cells in Cervical Cancer with Superior Bioavailability to Resveratrol. Molecules 2020, 25, 228. 10.3390/molecules25010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S. X. M.; Caballero R.; Ali H.; Roy D. L. F.; Cassol E.; Kumar A. Transfection of Hard-to-Transfect Primary Human Macrophages with Bax Sirna to Reverse Resveratrol-Induced Apoptosis. RNA Biol. 2020, 17, 755–764. 10.1080/15476286.2020.1730081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y.; Guo W.; Xu N.; Li F.; Li J. Ctbp1 Transactivates Rad51 and Confers Cisplatin Resistance to Breast Cancer Cells. Mol. Carcinog. 2020, 59, 512–519. 10.1002/mc.23175. [DOI] [PubMed] [Google Scholar]
- Ruiz G.; Valencia-Gonzalez H. A.; Leon-Galicia I.; Garcia-Villa E.; Garcia-Carranca A.; Gariglio P. Inhibition of Rad51 by Sirna and Resveratrol Sensitizes Cancer Stem Cells Derived from Hela Cell Cultures to Apoptosis. Stem Cells Int. 2018, 2018, 2493869. 10.1155/2018/2493869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadad L. J.; Harisa G. I.; Alanazi F. K. Design and Encapsulation of Anticancer Dual Hsp27 and Her2 Inhibitor into Low Density Lipoprotein to Target Ovarian Cancer Cells. Saudi Pharm. J. 2020, 28, 387–396. 10.1016/j.jsps.2020.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K.; He L.; Gan Y.; Liu J.; Tang J.; Long Z.; Tan J. Hmgn5 Promotes Il-6-Induced Epithelial-Mesenchymal Transition of Bladder Cancer by Interacting with Hsp27. Aging 2020, 12, 7282. 10.18632/aging.103076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Önay Uçar E. Ö.; Şengelen A. Resveratrol and Sirna in Combination Reduces Hsp27 Expression and Induces Caspase-3 Activity in Human Glioblastoma Cells. Cell Stress Chaperones 2019, 24, 763–775. 10.1007/s12192-019-01004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A.; Yadav S. K.; Yadav S. C. Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems. Colloids Surf., B 2010, 75, 1–18. 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Khalf A.; Madihally S. V. Recent Advances in Multiaxial Electrospinning for Drug Delivery. Eur. J. Pharm. Biopharm. 2017, 112, 1–17. 10.1016/j.ejpb.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Landgraf M.; Lahr C. A.; Kaur I.; Shafiee A.; Sanchez-Herrero A.; Janowicz P. W.; Ravichandran A.; Howard C. B.; Cifuentes-Rius A.; McGovern J. A.; et al. Targeted Camptothecin Delivery Via Silicon Nanoparticles Reduces Breast Cancer Metastasis. Biomaterials 2020, 240, 119791. 10.1016/j.biomaterials.2020.119791. [DOI] [PubMed] [Google Scholar]
- Tsuchihashi Y.; Abe S.; Miyamoto L.; Tsunematsu H.; Izumi T.; Hatano A.; Okuno H.; Yamane M.; Yasuoka T.; Ikeda Y.; et al. Novel Hydrophilic Camptothecin Derivatives Conjugated to Branched Glycerol Trimer Suppress Tumor Growth without Causing Diarrhea in Murine Xenograft Models of Human Lung Cancer. Mol. Pharmaceutics 2020, 17 (4), 1049–58. 10.1021/acs.molpharmaceut.9b00249. [DOI] [PubMed] [Google Scholar]
- Follmann H. D.; Oliveira O. N. Jr.; Martins A. C.; Lazarin-Bidóia D.; Nakamura C. V.; Rubira A. F.; Silva R.; Asefa T. Nanofibrous Silica Microparticles/Polymer Hybrid Aerogels for Sustained Delivery of Poorly Water-Soluble Camptothecin. J. Colloid Interface Sci. 2020, 567, 92–102. 10.1016/j.jcis.2020.01.110. [DOI] [PubMed] [Google Scholar]
- Ren L.; Jiang Q.; Chen Z.; Chen K.; Xu S.; Gao J.; Jiang L. Flexible Microneedle Array Electrode Using Magnetorheological Drawing Lithography for Bio-Signal Monitoring. Sens. Actuators, A 2017, 268, 38–45. 10.1016/j.sna.2017.10.042. [DOI] [Google Scholar]
- Dancey J.; Eisenhauer E. Current Perspectives on Camptothecins in Cancer Treatment. Br. J. Cancer 1996, 74, 327–338. 10.1038/bjc.1996.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra E.; Ferreira J. The Effect of Sirna-Egr-1 and Camptothecin on Growth and Chemosensitivity of Breast Cancer Cell Lines. Oncol. Rep. 2010, 23, 1159–65. 10.3892/or_00000746. [DOI] [PubMed] [Google Scholar]
- Futami K.; Takagi M.; Shimamoto A.; Sugimoto M.; Furuichi Y. Increased Chemotherapeutic Activity of Camptothecin in Cancer Cells by Sirna-Induced Silencing of Wrn Helicase. Biol. Pharm. Bull. 2007, 30, 1958–61. 10.1248/bpb.30.1958. [DOI] [PubMed] [Google Scholar]
- Shabeeb D.; Keshavarz M.; Shirazi A.; Hassanzadeh G.; Hadian M. R.; Nowrouzi A.; Najafi M.; Musa A. E. Evaluation of the Radioprotective Effects of Melatonin against Ionizing Radiation-Induced Muscle Tissue Injury. Curr. Radiopharm. 2019, 12, 247–255. 10.2174/1874471012666190219120329. [DOI] [PubMed] [Google Scholar]
- Alinejad A.; Raissi H.; Hashemzadeh H. Understanding Co-Loading of Doxorubicin and Camptothecin on Graphene and Folic Acid-Conjugated Graphene for Targeting Drug Delivery: Classical Md Simulation and Dft Calculation. J. Biomol. Struct. Dyn. 2020, 38, 2737–2745. 10.1080/07391102.2019.1645044. [DOI] [PubMed] [Google Scholar]
- Koh B.; Park S. B.; Yoon E.; Yoo H. M.; Lee D.; Heo J. N.; Ahn S. Alphavbeta3-Targeted Delivery of Camptothecin-Encapsulated Carbon Nanotube-Cyclic Rgd in 2d and 3d Cancer Cell Culture. J. Pharm. Sci. 2019, 108, 3704–3712. 10.1016/j.xphs.2019.07.011. [DOI] [PubMed] [Google Scholar]
- Gao Y. E.; Bai S.; Ma X.; Zhang X.; Hou M.; Shi X.; Huang X.; Chen J.; Wen F.; Xue P.; Kang Y.; Xu Z. Co-delivery of Doxorubicin and Camptothecin by Dual-Responsive Unimolecular Micelle-Based Beta-Cyclodextrin for Enhanced Chemotherapy. Colloids Surf., B 2019, 183, 110428. 10.1016/j.colsurfb.2019.110428. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Huang Y.; Lu W.; Liu S.; Xiao Y.; Yu J. 4-Carboxyphenylboronic Acid-Decorated, Redox-Sensitive Rod-Shaped Nano-Micelles Fabricated through Co-Assembling Strategy for Active Targeting and Synergistic Co-Delivery of Camptothecin and Gemcitabine. Eur. J. Pharm. Biopharm. 2019, 144, 193–206. 10.1016/j.ejpb.2019.09.019. [DOI] [PubMed] [Google Scholar]
- Laskar P.; Somani S.; Campbell S. J.; Mullin M.; Keating P.; Tate R. J.; Irving C.; Leung H. Y.; Dufes C. Camptothecin-Based Dendrimersomes for Gene Delivery and Redox-Responsive Drug Delivery to Cancer Cells. Nanoscale 2019, 11, 20058–20071. 10.1039/C9NR07254C. [DOI] [PubMed] [Google Scholar]
- Zhan H.; Zhao H.; Muhammad N.; Li T.; Liu Y.; Wang J. Lytic Peptide-Grafted Beta-Cyclodextrin Polymer Based Nano-Scaled Drug Delivery System with Enhanced Camptothecin Anti-Cancer Efficacy. Nanotechnology 2020, 31, 075101. 10.1088/1361-6528/ab529b. [DOI] [PubMed] [Google Scholar]
- Petronczki M.; Lénárt P.; Peters J.-M. Polo on the Rise—from Mitotic Entry to Cytokinesis with Plk1. Dev. Cell 2008, 14, 646–659. 10.1016/j.devcel.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Wang G.; Jiang Q.; Zhang C. The Role of Mitotic Kinases in Coupling the Centrosome Cycle with the Assembly of the Mitotic Spindle. J. Cell Sci. 2014, 127, 4111–4122. 10.1242/jcs.151753. [DOI] [PubMed] [Google Scholar]
- Zitouni S.; Nabais C.; Jana S. C.; Guerrero A.; Bettencourt-Dias M. Polo-Like Kinases: Structural Variations Lead to Multiple Functions. Nat. Rev. Mol. Cell Biol. 2014, 15, 433–452. 10.1038/nrm3819. [DOI] [PubMed] [Google Scholar]
- Jemaa M.; Kifagi C.; Serrano S. S.; Massoumi R. Preferential Killing of Tetraploid Colon Cancer Cells by Targeting the Mitotic Kinase Plk1. Cell. Physiol. Biochem. 2020, 54, 303–320. 10.33594/000000221. [DOI] [PubMed] [Google Scholar]
- Parrilla A.; Barber M.; Majem B.; Castellvi J.; Morote J.; Sanchez J. L.; Perez-Benavente A.; Segura M. F.; Gil-Moreno A.; Santamaria A. Aurora Borealis (Bora), Which Promotes Plk1 Activation by Aurora a, Has an Oncogenic Role in Ovarian Cancer. Cancers 2020, 12, 886. 10.3390/cancers12040886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlen A.; Martin C.; Miron S.; Julien M.; Theillet F. X.; Ropars V.; Sessa G.; Beaurepere R.; Boucherit V.; Duchambon P.; El Marjou A.; Zinn-Justin S.; Carreira A. Proper Chromosome Alignment Depends on Brca2 Phosphorylation by Plk1. Nat. Commun. 2020, 11, 1819. 10.1038/s41467-020-15689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Liu R.; Yang J.; Ma G.; Zhang Z.; Zhang X. Dual Sensitive and Temporally Controlled Camptothecin Prodrug Liposomes Co-delivery of Sirna for High Efficiency Tumor Therapy. Biomaterials 2014, 35, 9731–9745. 10.1016/j.biomaterials.2014.08.022. [DOI] [PubMed] [Google Scholar]