Abstract
Objective:
We explored determinants of attrition a longitudinal cohort study in Nigeria.
Study Design and Setting:
We enroled 1,020 women into a prospective study. Of these, 973 were eligible to return for follow-up. We investigated the determinants of attrition among eligible women using a sequential mixed methods design. We used logistic regression models to compare the baseline characteristics of responders and non-responders. At the end of the parent study, we conducted 4 focus group discussions and 8 key informant interviews with non-responders
Results.
Of the 973 women included in the quantitative analysis, 26% were non-responders. From quantitative analysis, older women were less likely to drop out than younger women (reference: women ≤30 years; OR 0.46; 95%CI 0.30 – 0.70, p<0.001 women 31–44 years; and OR 0.31; 95%CI 0.17 – 0.56, p<0.001 women ≥45 years). HIV-positive women were also less likely to drop out of the study (OR 0.45; 95%CI 0.33 – 0.63, p<0.001). From qualitative analysis, contextual factors that influenced attrition were high cost of participation, therapeutic misconceptions, inaccurate expectations, spousal disapproval, unpleasant side effects, challenges in maintaining contact with participants and difficulties in locating the study clinic.
Conclusion:
Several participant, research and environment related factors influence attrition. Retention strategies which address these barriers are important to minimize attrition.
Keywords: Retention, Attrition, Drop-out, Loss to follow-up, Withdrawal, Longitudinal studies
1. Introduction
Longitudinal studies are important for understanding relationships between risk factors and health outcomes, and can be used to determine causal relationships [1]. However, selective attrition in longitudinal studies, where individuals who continue to participate are systematically different from those who are lost to follow up, may pose significant threats to the internal and external validity of results [2–4]. High levels of attrition can reduce the statistical power of a study to detect a difference among groups or treatments and may lead to biased effect estimates, especially when the loss to follow up is non-random with respect to exposure and outcome [5, 6]. High levels of attrition may also lead to other practical concerns such as prolongation of research studies to recruit more participants and increased costs. Therefore, focused efforts at optimizing participants’ retention are important in the design and conduct of studies to ensure that findings are valid and the study remains adequately powered.
Minimizing attrition in longitudinal studies can be very challenging and requires considerable effort and time during the design and implementation stages [7]. It is even more challenging for studies that require in-person visits to the study site. In a systematic review of studies that evaluated different retention methods to reduce loss to follow up, Booker et al reported that retention increased by an average of 18% when in person visit to study sites for follow-up was replaced with postal questionnaires[8]. In low and middle-income countries (LMIC), there are additional challenges to participants’ retention in prospective studies. These include limited public health and research infrastructure, poor follow-up culture, poverty, low levels of education and high mobility. In these settings, attrition may vary from 5 – 30% in studies with tracking strategies, to 40–52% in studies without tracking strategies [9]. Although there is no absolute standard for acceptable attrition levels, bias becomes a major concern if attrition exceeds 20% [10].
Recently, several articles have investigated the predictors of participant attrition in longitudinal studies [11–18]. All of the studies that were conducted in LMIC focused on the attrition of patients in HIV care programs [11–13]. The experiences in such situations may differ from prospective research cohorts, particularly when participants are free of disease at baseline. As HIV care programs are relatively better funded than research studies in most LMIC, many HIV programs have investigated and implemented various interventions, such as home visits, peer support, task shifting, decentralization of services, and motivational counselling, to minimize attrition [19]. Furthermore, a strong motivation for adherence in HIV care programs that may not be present in several research settings, is the desire of HIV patients to reduce their high risk of morbidity and mortality associated with untreated HIV [20]. In contrast, most of the studies on participants attrition in high income countries (HIC) have focused on hard to reach populations such as ethnic minorities, children, and the elderly[14–16].
In this study, we use sequential mixed methods design to identify determinants of attrition in a longitudinal study in Nigeria, which required in-person study site visits for follow-up.
2. Methods
2.1. Study Design
This study on the determinants of attrition was conducted within a parent prospective study that evaluated host and viral factors associated with persistent high risk human papillomavirus (hrHPV) infection in Nigerian women. Details of the parent study have been previously described [21]. Briefly we recruited 1,020 women who were at least 18 years old and had a prior history of penetrative vaginal intercourse, from cervical cancer screening clinics in Abuja, Nigeria. We excluded women who could not commit to in-person follow-up visits, or had a history of cervical cancer or hysterectomy or were pregnant. We used structured questionnaires to collect information on demographic and lifestyle risk factors; performed a pelvic examination and collected biological specimens for HPV detection; and screened for cervical cancer using visual inspection with acetic acid/Lugol’s Iodine. All participants were scheduled to return for follow-up visits 6 months after enrolment.
Within this parent study, we used sequential explanatory mixed methods design to evaluate the determinants of loss to follow-up. In this design, we collected and analysed quantitative data and followed up with analysis of qualitative data collected in focus group interviews and key informant interviews. Participant selection for the quantitative and qualitative data collection is described below.
2.2. Study Setting and Selection of Participants
Attrition was defined as attendance at the enrolment visit but failure to return for the scheduled follow up visit 6–9 months later, among women who were eligible to return for follow-up visits. Women who attended both the enrolment visit and the follow-up visits were defined as “responders” while women who attended only the enrolment visit were defined as “non-responders”.
Of the 1,020 women enrolled in the parent study, 47 (5%) became ineligible to continue in the study (27 became pregnant, 4 had hysterectomies, and 16 relocated) leaving 973 women eligible to return for follow-up visits.
2.2.1. Quantitative Study
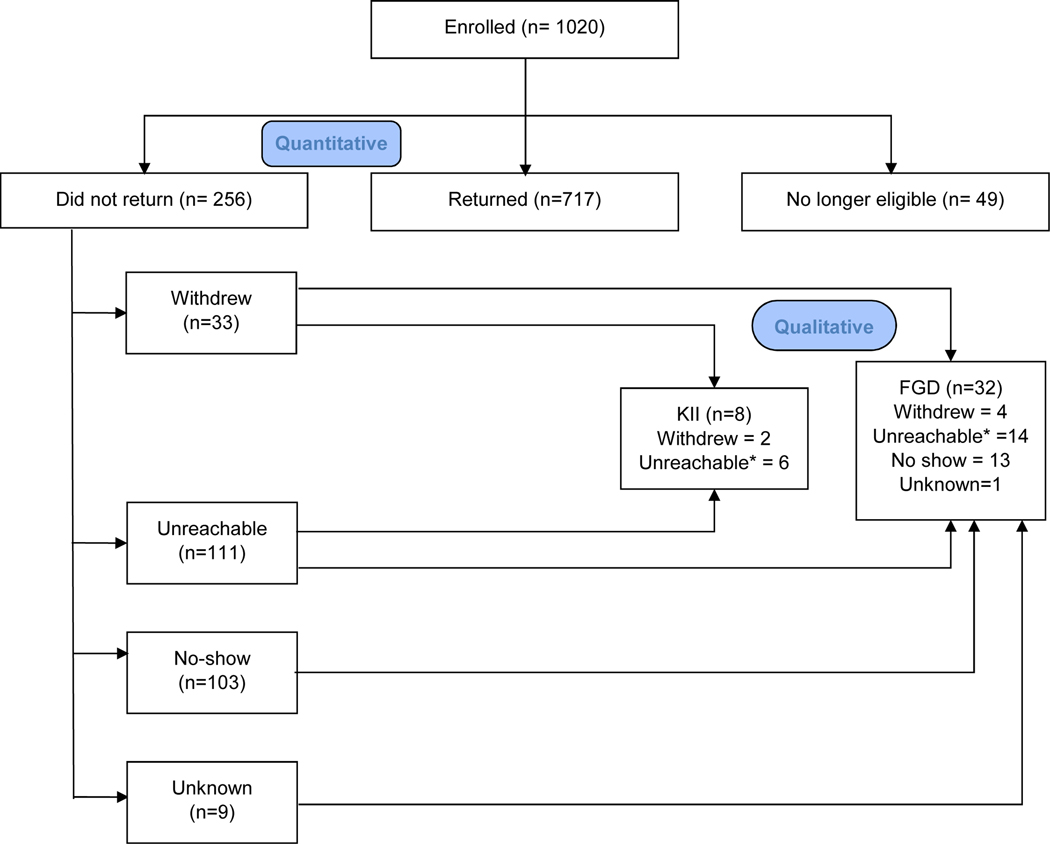
Of the remaining 973 women, 717 (74%) were responders while 256 (26%) were non-responders. These participants were included in the quantitative aspect of this study. Study flow diagram is provided in Figure 1. As part of the retention strategies in the study, we collected reasons for not returning for scheduled follow-up visits through phone calls. For non-responders that could not be reached by their primary phone numbers, we contacted members of their social network (spouses, relatives and friends). At the enrolment visit, all participants provided consent for study personnel to contact members of their social network for tracking purposes.
Figure 1: Participant flow chart.
*These participants could not be contacted during the parent study but contact was reestabished at the time of the qualitative studies.
2.2.2. Qualitative Study
To provide contextual information on the determinants of loss to follow-up, we conducted four focus group discussions (FGD) and eight key informant interviews (KII) 6 months after the end of the parent study in March 2016. We were able to re-establish contact with some women who had been lost to follow-up during the parent study’s duration.
We used simple random selection to identify 45 non-responders to participate in the FGDs. Of the 45 women approached, 40 agreed to participate in the FGDs but only 32 women turned up on the scheduled day and time. For the remaining 8 women, we conducted KIIs. Details of participant selection are provided in Figure 1. To prevent inadvertent HIV status disclosure, we stratified our population by HIV status and conducted two FGDs and four KIIs in each stratum.
Each FGD/KII was facilitated by a health research scientist, EMO and a physician, MKO who have both had training in qualitative research methods and together have over seven years’ experience in conducting and analysing qualitative research. Each FGD session lasted between 30 and 40 minutes while each KII lasted for about 20 minutes. Each FGD/KII was conducted in English, audio-recorded and transcribed verbatim. The facilitators took supplementary reflective field notes which were reviewed by other members of the team within 48 hours. None of the participants had met any of the facilitators prior to the FGDs/KIIs. The FGD/KII interview guide was piloted among five women who were not part of the cohort. In brief, the interview guide was comprised of four sections: motivations to participate; barriers to study completion; perception of effects of non-completion and strategies to improve retention in future studies.
2.3. Retention Strategies
We applied the social cognitive theory [22] as a conceptual framework to design our retention strategies (Table 1). This theory postulates that behaviour is a function of aspects of the environment and of the person, all of which are in constant interaction. Using this framework, we identified strategies that would foster a conducive research environment and enhance the self-efficacy of participants and their motivation to comply with the study protocol. These strategies were evaluated and revised in an iterative process at monthly review meetings. We implemented a combination of different strategies (Table 1) as the implementation of more than one retention strategy has been reported to be associated with better participant retention [23]. These strategies listed in Table 1 were implemented as preventive measures to minimize attrition levels in the study.
Table 1:
Retention Strategies
| Staff/Visit Characteristics | Incentives | Participant contact | Participant Bonding | Community Engagement | Tracking system |
|---|---|---|---|---|---|
| Flexibilty in scheduling to include early morning and late evening appointments | Reimbursement of transportation costs | Phone calls and text messages to schedule appointments and reminder calls two days before appointment and morning of appointment | Use of study logos on questionnaires and all communication materials | Attendance at town hall meetings | Use of robust electronic scheduling and contact software |
| Engagement of culturally competent and sensitive staff with strong interpersonal skills | Benefits of participation – free see and treat cervical cancer screening | Phone calls for missed appointments up to ten attempts | Continuity of contact via text messages and emails in betweem study visits | Designing health promotion activities for the community e.g health alks encouraging physical activity | |
| Detailed study description to include the study requirements, follow-up demands and potential benefits/harms of the study | Benefits of particaption – free physical and breast examination | Use of scripts for phone calls | Availability of a platform for communication between participants and study personnel in between study visits for rapid resolution of queries | Presentation of research updates at regligous gatherings and other social events | |
| Training and retraining of study personnel to maintain appropriate atttitudes in researcher- participant interactions | Breast Self Awareness education | Record of outcome of all phone calls in call logs | If outcome of previous calls documented welfare concerns, schedule follow-up call to enquire about welfare | Engagement with local community leadership | |
| Mainitaing appropriate respect for study participants | Provision of informational brochures promoting healthy lifestyles | Multiple calls at different times/days up to ten attempts for unanswered calls. | |||
| Research staff recognition for sites that maintain high retention rates | Altruisitc purposes: Discussion of the need for complete data to achieve study aims | Multiple contact phone numbers for each participant |
2.4. Statistical Analysis
2.4.1. Quantitative Analysis
The outcome variable for this analysis was response status: whether participants were responders or non-responders. We included 16 potential predictors grouped into 4 general categories: sociodemographic (6 predictors), lifestyle (2 predictors), reproductive and sexual health (6 predictors), and general health (2 predictors). The sociodemographic predictors were age, marital status, education, socioeconomic status, length of time in current residence and nature of dwelling (rural, semi urban or urban). To determine socioeconomic status (SES), we calculated the wealth index using principal component analysis (PCA) of data on household assets as described by Filmer and Pritchett [24]. Participants were categorized into low (lower 40%), middle (middle 40%) and high (top 20%) SES. Lifestyle predictors included smoking and alcohol use. Reproductive and sexual health predictors included lifetime number of sexual partners, sexual debut age, number of children, HIV status, HPV status at baseline and vaginal douching practice. General health predictors included presence of chronic ailments and self-rated health.
We used logistic regression models to evaluate association between potential predictors and non-response. All variables that were associated with non-response in age adjusted analysis at a p-value of <0.20 were included in a multivariable model. All analyses were performed in STATA 15 (Stata Corporation, College Station, Texas, USA).
2.4.2. Qualitative Analysis
We analysed the qualitative data using a content analysis approach and a combination of deductive and inductive methods to code transcripts and reflective field notes in an iterative process. We evaluated the coding frame for unidimensionality, mutual exclusiveness and exhaustiveness at a data review meeting. Recurrent themes were identified and classified into categories based on the ecological model[25]. This model consists of a series of nested layers that include participant factors at its core, researcher related factors and environmental factors, in its periphery, with interactions between the different layers. All analysis was conducted using ATLAS.ti version 7.5.10.
2.5. Ethics
Ethical approval to conduct this study was obtained from National Health Research Ethics Committee of Nigeria and the University of Maryland Institutional Research Board. We obtained written informed consent from all participants.
3. Results
3.1. Quantitative Study Results
Of the 973 women included in this study, 256 (26%) were non-responders (Figure 1). 89 (9%) of the 973 women included in this study required the use of other contacts to respond. Of the 256 non-responders, 33 (13%) withdrew, 111 (43%) were unreachable, 103 (40%) missed their scheduled appointments despite multiple attempts at rescheduling and 9 (4%) did not provide any reasons.
Participant characteristics of responders and non-responders are presented in Table 2. Mean age (SD) of the 973 participants included in the quantitative study was 38 (8) years. Most participants were married (67%) and had completed more than six years of formal education (92%). Prevalence of smoking was very low (1%) and slightly more than half (53%) of the study population were HIV negative. Median lifetime number of sexual partners in this population was 3 (IQR: 1– 4). Most participants (78%) rated their general health as good.
Table 2:
Baseline characteristics of study population by participation
| Characteristics | Total = 973 N (%) | Responder = 717 N (%) | Non-responder = 256 N (%) |
|---|---|---|---|
| Sociodemographic factors | |||
| Age, years (mean, SD) | 38 (8) | 38(8) | 36 (8) |
| Marital status | |||
| Married | 645 (67) | 472 (66) | 173 (68) |
| Unmarried | 320 (33) | 239 (34) | 81 (32) |
| Education | |||
| ≤6 years | 109 (11) | 84 (12) | 25 (10) |
| 7 – 12 years | 256 (27) | 178 (25) | 78 (30) |
| >12 years | 600 (62) | 449 (63) | 151 (60) |
| Socioeconomic status | |||
| Low | 382 (40) | 276 (39) | 106 (42) |
| Middle | 385 (40) | 288 (41) | 97 (39) |
| High | 189 (20) | 141 (20) | 48 (19) |
| Length of time in current residence, months (mean, SD) | 33.2 (32) | 32.8 (33) | 34.0(27) |
| Nature of dwelling | |||
| Urban | 427 (44) | 305 (43) | 122 (48) |
| Semi urban | 367 (38) | 280 (39) | 87 (35) |
| Rural | 168 (18) | 125 (18) | 43 (17) |
| Lifestyle factors | |||
| Smoking | |||
| Never smoked | 952 (99) | 701 (99) | 251 (99) |
| Ever smoked | 13 (1) | 10 (1) | 3 (1) |
| Alcohol Consumption* | |||
| No | 835 (87) | 612 (86) | 223 (88) |
| Yes | 125 (13) | 96 (14) | 29 (12) |
| Reproductive and sexual health factors | |||
| Lifetime number of sexual partners (median, IQR) | 3 (1 – 4) | 3 (1 – 4) | 3 (1–4) |
| Sexual debut age (median, IQR) | 20 (18 – 22) | 20 (18 – 22) | >20 (18 −23) |
| Number of children (median, IQR) | 1 (0 – 3) | 1 (0 – 3) | 1 (0 – 2) |
| HIV status | |||
| HIV negative | 511 (53) | 348 (49) | 195 (64) |
| HIV positive | 421 (44) | 345 (48) | 76 (30) |
| Any HPV status | |||
| HPV negative | 588 (62) | 427 (61) | 161 (63) |
| HPV positive | 367 (38) | 274 (39) | 93 (37) |
| Vaginal douching | |||
| No | 347 (36) | 251 (35) | 96 (38) |
| Yes | 619 (64) | 461 (65) | 158 (62) |
| General health | |||
| Presence of chronic ailments | 230 (24) | 179 (25) | 51 (20) |
| Self-rated health | |||
| Good health | 752 (78) | 559 (78) | 193 (76) |
| Poor health | 214 (22) | 153 (22) | 61 (24) |
Alcohol consumption within the three months preceding enrolment into the study
Compared to women less than 30 years old, the OR for the likelihood of dropping out of our study for women aged 31 to 44 years was 0.46 (95% CI: 0.33 – 0.63, p <0.001) while that for women over 45 years of age was 0.31 (95% CI 0.17 – 0.56, p <0.001) (Table 3). HIV positive women were less likely to drop out of the study than HIV negative women (OR 0.45;95% CI 0.33 – 0.70, p <0.001). Other sociodemographic (education, socioeconomic status, nature of dwelling, length of time in current residence), lifestyle (marital status, smoking), reproductive (number of children), sexual (lifetime number of sexual partners, any human papillomavirus infection) and general health (presence of chronic ailments, self rated health) factors were not associated with dropping out of the study (Table 3).
Table 3:
Association between participant characteristics and loss to follow-up
| Univariate model | Multivariate Model | |||
|---|---|---|---|---|
| Characteristic | Odds Ratio (95% CI) | p value | Odds Ratio (95% CI) | p value |
| Age (years) | pt = <0.001 | |||
| ≤ 30 | 1.00 | 1.00 | ||
| 31 – 44 | 0.53 (0.37 – 0.78) | <0.001 | 0.46 (0.30 – 0.70) | <0.001 |
| >44 | 0.38 (0.23 – 0.63) | <0.001 | 0.31 (0.17 – 0.56) | <0.001 |
| HIV status | ||||
| HIV negative | 1.00 | 1.00 | ||
| HIV positive | 0.47 (0.34 – 0.64) | <0.001 | 0.45 (0.33 – 0.63) | <0.001 |
| Nature of dwelling | pt = 0.27 | |||
| Urban | 1.00 | 1.00 | ||
| Semi urban | 0.78 (0.56 – 1.07) | 0.12 | 0.86 (0.61 – 1.21) | 0.44 |
| Rural | 0.86 (0.57 – 1.29) | 0.47 | 0.92 (0.58 – 1.44) | 0.26 |
| Length of time in current residence (months) | pt = 0.13 | |||
| ≤ 25 | 1.00 | |||
| 26– 60 | 1.07 (0.77 – 1.50) | 0.69 | 1.05 (0.74 – 1.50) | 0.79 |
| >60 | 1.38 (0.92 – 2.07) | 0.12 | 1.32 (0.86 – 2.04) | 0.20 |
| Number of children | pt = 0.10 | |||
| None | 1.00 | 1.00 | ||
| 1 – 4 | 0.94 (0.70 – 1.26) | 0.69 | 1.14 (0.81 – 1.61) | 0.44 |
| ≥5 | 0.55(0.27 – 1.12) | 0.10 | 0.60 (0.25 – 1.45) | 0.26 |
| Lifetime number of partners | pt = 0.23 | |||
| 1 | 1.00 | |||
| 2 – 4 | 1.15 (0.81 – 1.62) | 0.44 | ||
| ≥ 5 | 0.83 (0.54 – 1.28) | 0.41 | ||
| Marital status | ||||
| Married | 1.00 | |||
| Unmarried | 0.92 (0.68 – 1.26) | 0.62 | ||
| Education | pt = 0.73 | |||
| ≤6 years | 1.00 | |||
| 7 – 12 years | 1.47 (0.88 – 2.48) | 0.15 | ||
| >12 years | 1.13 (0.70 – 1.83) | 0.62 | ||
| Socioeconomic status | pt = 0.47 | |||
| Low | 1.00 | |||
| Middle | 0.87 (0.64 – 1.21) | 0.42 | ||
| High | 0.89 (0.60 – 1.32) | 0.55 | ||
| Smoking | ||||
| Never smoked | 1.00 | |||
| Ever smoked | 0.84 (0.23 – 3.07) | 0.79 | ||
| Any HPV status | ||||
| Negative | 1.00 | |||
| Positive | 0.84 (0.63 – 1.11) | 0.22 | ||
| Chronic ailments | ||||
| Absent | 1.00 | |||
| Present | 0.90 (0.67 – 1.21) | 0.49 | ||
| Self-rated health | ||||
| Good health | 1.00 | |||
| Poor health | 1.15 (0.82 – 1.62) | 0.41 | ||
3.2. Qualitative Study Results
The mean age (SD) was 38 (7) years for the FGD (32 participants) and 41 (6) years for the KII (8 participants). Most participants in the FGD (78%) and KII (87%) had more than 6 years of formal education. Slightly less than half of participants in the FGD (47%) were HIV positive while half of participants in the KII (50%) were HIV positive. Details of participant characteristics for the FGDs and KIIs are provided in Table 4.
Table 4:
Characteristics of Participants in the Focus Group Discussions and Key Informant Interviews
| FGD = 32 N (%) | KII = 8 N (%) | |
|---|---|---|
| Age, years (mean, SD) | 38 (7) | 41 (6) |
| Education | ||
| ≤6 years | 7 (22) | 1 (12) |
| 7 – 12 years | 7 (22) | 4 (50) |
| >12 years | 18 (56) | 3 (38) |
| Marital Status | ||
| Married | 22 (69) | 5 (62) |
| Unmarried | 10 (31) | 3 (38) |
| Socioeconomic Status | ||
| Low | 13 (41) | 3 (38) |
| Middle | 16 (50) | 2 (25) |
| High | 3 (9) | 3 (37) |
| HIV Status | ||
| Negative | 17 (53) | 4 (50) |
| Positive | 15 (47) | 4 (50) |
| Number of children (median, IQR) | 2 (0–3) | 2 (1–4) |
| Lifetime number of sexual partners (median, IQR) | 2 (1–4) | 3 (1–4) |
3.2.1. Focus Group Discussions
High Cost of Participation
One of the commonly cited explanations for failure to return for follow-up visit was the prohibitive cost of transportation to the study clinic despite monetary incentives.
“… one of the things is transport issue, like I myself in particular, I may not [cannot] afford it. Even the money wey you give me the first time [enrolment visit], e no reach [not enough] my transport”
Other demands for time, such as commitments at the workplace, marketplace and school were cited for dropping out of the study.
“It’s just because I am too busy at my workplace”
Therapeutic misconception
Some participants equated participation in the study to their need for cervical cancer screening, which was provided as an added benefit for participation in the research study. Therefore, when they tested negative for cervical cancer and its precursor lesions at the enrolment visit, their attitudes towards continued participation was less favourable.
“But like me, they did it [cervical cancer screening] the first time. They asked me to come after 6 months. I say to myself, there is no need”
Inaccurate expectations
A few participants had inaccurate expectations about the research study that did not reflect the actual processes and outcomes described during the informed consent procedure. The commonest of these was related to the dissemination of research findings obtained from blood tests. While all participants received results for cervical cancer screening and were treated if necessary, they were informed during the informed consent process that other research results would not be immediately available.
“… And eh… there was another time they [study personnel] took our blood samples they said they were taking it to the lab for test. So, I didn’t hear anything in respect of that test since then ehn … that’s why I didn’t come back”
Lack of understanding of risk of cervical cancer
Some participants had wrong notions about their personal risk of cervical cancer. They believed that they were not at risk of cervical cancer because they had been previously screened and have few sexual partners.
“I was told that I was supposed to come back again. After the first visit, I say there is no need. I believe I am not a single woman that ah…We [participant and sexual partner] are just faithful to each other so I didn’t see any reason for coming back again”
Study Clinic Characteristics
Some participants believed that the urban location of the study clinic was a deterrent to study completion for participants who need to travel from rural environments.
‘So, the people coming here from their lungu [villages] it might not be that easy [for them to return for follow-up visits]”
Some other participants opined that the location of the study clinic was not very visible and they had problems locating the study clinic.
“… I would love a more stable accommodation for you people [ the research study]. Because there is need for follow-up. You understand. That time, I came [for follow-up visit], I looked for those people [study clinic staff] that were here then [enrolment visit] … but I don’t really know the location of the place that I want. Even when you tell them in National Hospital [ask for directions at the hospital in which the study clinic is located], they find it difficult to locate this particular building, this particular place. So, I think you need to improve on your publicity [visibility].
3.2.2. Key Informant Interviews
Unpleasant side effects
Some non-responders opined that the pain and discomfort experienced during the speculum examination was an important determinant of attrition.
“I was afraid, I don’t want the pain [associated with the speculum examination of the cervix] again”
“Looking at the pain, it wasn’t funny for me that day. So, I say… I don’t want to believe that there is any problem…so let me just stay”
Spousal disapproval
An important normative influence on the decision to drop out of the study was spousal disapproval.
“...na my husband wey no agree say make I come back again [return for follow-up visit]”
Difficulties in maintaining contact between study personnel and participants
Loss of contact between study personnel and research participants was an important barrier to returning for scheduled follow-up visits even when participants were aware they were required to return to the study clinic.
“Because that time when I do the first one [enrolment visit], them [study personnel] say make I go. Them [study personnel] give me date when I go come back do the second one [follow-up visit]. Na that time [scheduled appointment period] the phone come lost, I no know the time wey I go come back. Toh…[after I found the phone] you come call me [for the KII].
4. Discussion
In this study, we used a multifaceted approach to investigate the determinants of attrition in a longitudinal study that required in-person follow-up visits to study clinics. From quantitative analysis, factors that increased the likelihood of attrition were younger age and HIV negative status. From qualitative analysis, we identified some participant related characteristics (high cost of participation, therapeutic misconceptions, inaccurate expectations, spousal dissaproval); research related characteristics (unpleasant side effects, and challenges in maintaining contact between study personnel and participants); and environmental factors (study clinic characteristics) that affected attrition.
Our findings of lower attrition among older women is similar to results from previous studies. In a prospective cohort study investigating sexual health among Australian women who have sex with women, attrition was associated with being less than 30 years of age at enrolment [26]. Similarly, in a nationally representative sample of women in a longitudinal study on women’s health in Australia, older women were less likely to drop out of the study [27]. Younger persons are more likely to migrate from study areas for a plethora of reasons, including family formation, education and job opportunities. Among participants in a longitudinal cohort study on aging in the United States of America, attrition among younger participants was more likely to be attributable to mobility compared to attrition among older participants who were more likely to drop out for biological reasons such as mortality [28].
Some previous studies have shown that initiation of antiretroviral therapy (ART) among HIV positive women is associated with lower attrition rates in HIV care and treatment programs [29, 30]. In our study, most of the HIV positive participants were on ART and this may explain our finding of higher attrition levels among HIV negative women compared to HIV positive women. It is possible that HIV positive women who are on ART and otherwise healthy are less likely to drop out of research studies, compared to HIV negative women, because the former may be better motivated to attend scheduled clinic visits and exhibit positive health seeking behaviours. The effect of the presence of comorbidities in research participants is likely to vary depending on the nature of the comorbidity and the research. Health disorders which limit the ability of patients to move or have a high risk of mortality within a short interval are likely to increase the chances of attrition [31]. While, chronic ailments which require routine clinic visits, such as HIV infection, are likely to foster positive health seeking behaviours with reduced chances of sudy drop out.
We did not find any strong associations between other sociodemographic (education, socioeconomic status, nature of dwelling, length of time in current residence), lifestyle (marital status, smoking) reproductive (number of children), general (presence of chronic ailments, self rated health) and sexual health (lifetime number of partners, any human papillomavirus (HPV) infection) factors with attrition. Previous studies have produced mixed results. Some studies show that attrition is higher among smokers [27], unmarried women [32], poorly educated individuals [33], and people with poor self-rated health [27] while other studies do not observe these relationships [26, 31, 33, 34]. As participation in longitudinal studies may be influenced by study attributes, local social and cultural factors and an interaction between various personal and contextual factors, the mixed results may reflect the varied study population and research protocols. For example, it has been suggested that more years of formal education, as a surrogate for English literacy, enables participants to have a better understanding of research protocols. Consequently participants with more years of formal education are less likely to drop out [27]. However, as our study personnel were culturally sensitive and able to communicate in local languages with participants who had low levels of literacy, level of education would be a poor surrogate for comprehension of research protocols masking any effects of education on attrition.
Our findings of high cost of participation as an important factor in attrition is similar to findings from previous research [35]. In a prospective randomised vaccine trial among healthy persons in Canada, it was observed that drawbacks to participation included time requirements and financial cost of participation. Even though we provided monetary incentive as a retention strategy, some participants reported that it wasn’t enough to cover their transportation cost. Our findings highlight the need to incorporate retention strategies that not only address the direct economic costs of participation but also the indirect costs of the time and effort taken to participate. Retention strategies that place an emphasis on the ethical principle of respect for persons may be better suited to addressing these indirect costs. These strategies may take the form of non-monetary incentives such as sufficient explanation during the informed consent process to ensure full disclosure and the right to self-determination; and providing participants with a sense of identification with the scientific community by providing project logo branded souvenirs to participants.
Higher levels of cognitive abilities in both fluid intelligence (reasoning, problem solving) and crystallized intelligence (comprehension, memory) have been linked to reduced levels of attrition [28, 31]. This is consistent with our findings in which therapeutic misconceptions and inaccurate expectations were important determinants of attrition. Despite provision of information on follow-up requirements and dissemination of research findings during the informed consent procedure, some participants did not fully understand the need for them to return for follow-up. Most research on informed consent has been focused on participants understanding of autonomy, voluntariness, risks, benefits, and assessment of the readability of the informed consent document [36]. However, our study shows that comprehension of follow-up requirements and accurate expectations is particularly important for minimizing attrition levels in longitudinal studies. These may be addressed by more complex informed consent procedures that incorporate decision support, assessment of participants’ understanding of follow-up requirements and expectations from the research.
Attrition due to negative experiences with research implementation has been described in previous studies [37, 38]. In a qualitative study on improving retention in clinical trials of cancer screening, prevention and treatment among minority women in the United States of America, it was observed that side effects from trial procedures and high participation burden were important barriers to continued participation in longitudinal studies [37]. The degree to which these factors affect studies would vary and be largely dependent of the nature of the research study. Research studies that collect highly sensitive and invasive personal information such as sexual behaviour, partner violence, substance abuse; or elicit painful memories may have higher attrition levels compared to other studies due to unpleasant experiences during the data collection. It is therefore important to consider retention strategies that are tailored to address the peculiar contextual factors that are relevant to any given study to minimize attrition.
We found that inability to contact participants was the commonest reason for attrition in our study. This finding is not unique to our study. In a longitudinal study of mothers of children with asthma, Zook et. al. [32], found that outdated contact information was the commonest reason for attrition accounting for 39% of all attrition. Although cell phones are commonly used, participants may lose their phones or change their phone numbers, may not answer if the incoming phone number is not recognized, or may be reluctant to use their phones for research study purposes if they have limited minutes. Errors in data entry may also result in inaccurate contact information for study participants. In a study evaluating the intensity and timing of contacts as part of a retention strategy, Senturia et.al., reported that retention was positively correlated with number of phone contacts provided [39]. This finding emphasizes the need to collect as much locator information as possible and the need to test phone numbers while participants are at the clinic to minimize data collection errors.
Accessibility to research study sites as a product of site location and characteristics of the built environment has been identified as an important factor in participant retention in a previous research study conducted among researchers and research participants in the United States of America [40]. In that study, it was observed that sites that were easily accessible, physically attractive, clean, welcoming, and with comfortable waiting rooms encouraged continued research participation. It is therefore important to incorporate strategies that improve the overall experience of participating in research studies by improving the physical characteristics of research sites.
4.1. Strengths and Limitations
Our study has some strengths and limitations. We included a qualitative approach which enabled us to investigate research related and environment related factors in addition to participant level factors that influence attrition. There is limited information about these contextual factors especially in developing countries and our findings would be helpful in the implementation of future longitudinal studies. Table 5 provides some suggestions based on our findings, to minimize attrition in future studies with similar populations.
Table 5:
Strategies to minimize attrition based on study findings
| Modifiable characteristics | Retention strategy |
|---|---|
|
Participant related | |
| High cost of participation | • Monetary incentives to cover transportation costs. Need for flexibility as transportation costs may vary by participants • Non-monetary incentives that provide a sense of identification with the project. For example, project logo branded souvenirs • Provision of child care options, particularly for studies that recruit women of reproductive age |
| Misconceptions about follow-up requirements and benefits of participation | • Include pictorial illustrations of follow-up requirements to improve comprehension • Dedicate more time to explaining the follow-up requirements during the informed consent procedure • Ensure that informed consent procedures incorporate decision support, assessment of participants’ understanding of follow up requirements and expectations from the research |
|
Research related | |
| Side effects from study | • Principal investigators need to think through all risk and side effect possibilities • Minimize risks to the fullest extent possible. • Provide an accurate and fair description of the risks/discomforts and the anticipated benefits to the participants |
| Maintaining contact with participants | • Collect as much locator information as possible – multiple phone numbers of participants • Test phone numbers as they are collected to prevent data entry errors • Use of mhealth technology to maintain contact with participants |
|
Environmental factors | |
| Study clinic characteristics | • Select study sites that are easily accessible • Ensure study clinics are physically attractive, clean, welcoming and with comfortable waiting rooms |
|
Non-modifiable characteristics | |
| Young age | • Oversample from this demographic to account for potential attrition • Include strategies that allow participants to continue participation even when they relocate. These include the use of online questionnaires, self-collection and mailing of samples where feasible |
| HIV status | • As this may be an indicator of health seeking behaviour, researchers should consider strategies to improve the overall health seeking behaviour of participants • Recruit participants who are easy to follow and highly motivated to participate. For example, nurses in hospitals; |
Our study was conducted among women in a prospective study on the host and viral factors associated with persistent high-risk HPV. Therefore, our findings may have limited generalizability to other populations. Despite this, we provide several retention strategies developed based on the social cognitive theory that can be adapted to suit other study populations.
We did not evaluate motivation for completion of study among participants who completed the study. To design effective retention strategies, it would be important to understand the reasons why participants complete longitudinal studies so that these motivational factors can be promoted in other studies to maximise retention.
5. Conclusion
This paper contributes to the understanding of barriers to retention, especially for longitudinal visits that require in-person follow-up visits in a developing country. We identify both participant related barriers and other contextual barriers that need to be addressed to optimise retention. We also provide several practical strategies that can be implemented to mitigate attrition. Our findings will be helpful to other researchers in the design and conduct of longitudinal studies.
Supplementary Material
What is New?
Existing information on determinants of attrition in prospective cohort studies in low and middle-income countries is limited.
We found that the likelihood of attrition was lower in older women compared to younger women less than 30 years old. HIV positive women were also less likely to be lost to follow-up than HIV positive women.
We identified high cost of participation, therapeutic misconceptions, inaccurate expectations, spousal disapproval, unpleasant side effects, challenges in maintaining contact with participants and participant difficulties in locating the study clinic as important contextual barriers to study retention.
Future studies in similar settings need to incorporate retention strategies that address the direct and indirect cost of research participation; provide more thorough and complex informed consent procedures to prevent participant misconceptions about requirements for follow-up; collect multiple contact information for participants and test participants’ phone numbers while they are at the clinic to minimize attrition.
Acknowledgement
We acknowledge the contribution of Chika Onyenakie, Ebimoboere Apreala, Joke Idowu, Dayo Satoye, Dorcas Jesse Bako, Jane Anichebe, Surraya Said Abdullahi, Clare Anyanwu, Thelma Ugorji, Gladys Omenuko, Temitope Filade, Patience Bamisaye, Miriam Anyogu and Nkiru Onwuka for their role in participant recruitment; Tolulope Gbolahan and Jesse James for their role in data management.
Funding
Research reported in this publication was supported by the UM-Capacity Development for Research in AIDS Associated Malignancy Grant (NIH/NCI 1D43CA153792-01) and African Collaborative Center for Microbiome and Genomics Research (NIH/NHGRI U54HG006947). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of interests.
All authors declare that there are no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention, Principles of epidemiology in public health practice: an introduction to applied epidemiology and biostatistics. 2006, Atlanta, GA: US Dept. of Health and Human Services, Centers for Disease Control and Prevention (CDC), Office of Workforce and Career Development. [Google Scholar]
- 2.Greenland S, Response and follow-up bias in cohort studies. Am J Epidemiol, 1977. 106(3): p. 184–7. [DOI] [PubMed] [Google Scholar]
- 3.Pizzi C, et al. , Sample selection and validity of exposure-disease association estimates in cohort studies. J Epidemiol Community Health, 2011. 65(5): p. 407–11. [DOI] [PubMed] [Google Scholar]
- 4.Kristman VL, Manno M, and Cote P, Methods to account for attrition in longitudinal data: do they work? A simulation study. Eur J Epidemiol, 2005. 20(8): p. 657–62. [DOI] [PubMed] [Google Scholar]
- 5.Kennison RF and Zelinski EM, Estimating age change in list recall in asset and health dynamics of the oldest-old: the effects of attrition bias and missing data treatment. Psychol Aging, 2005. 20(3): p. 460–75. [DOI] [PubMed] [Google Scholar]
- 6.Osler M, et al. , Rapid report on methodology: does loss to follow-up in a cohort study bias associations between early life factors and lifestyle-related health outcomes? Ann Epidemiol, 2008. 18(5): p. 422–4. [DOI] [PubMed] [Google Scholar]
- 7.Coday M, et al. , Strategies for retaining study participants in behavioral intervention trials: retention experiences of the NIH Behavior Change Consortium. Ann Behav Med, 2005. 29 Suppl: p. 55–65. [DOI] [PubMed] [Google Scholar]
- 8.Booker CL, Harding S, and Benzeval M, A systematic review of the effect of retention methods in population-based cohort studies. BMC Public Health, 2011. 11: p. 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill Z, Reducing attrition in panel studies in developing countries. Int J Epidemiol, 2004. 33(3): p. 493–8. [DOI] [PubMed] [Google Scholar]
- 10.Polit D. and Hungler B, Nursing research: Principles and methods. 5th Edition ed. 1995, Philadelphia: Lippincott. [Google Scholar]
- 11.Elul B, et al. , Attrition From Human Immunodeficiency Virus Treatment Programs in Africa: A Longitudinal Ecological Analysis Using Data From 307 144 Patients Initiating Antiretroviral Therapy Between 2005 and 2010. Clin Infect Dis, 2017. 64(10): p. 1309–1316. [DOI] [PubMed] [Google Scholar]
- 12.Plazy M, et al. , Retention in care prior to antiretroviral treatment eligibility in sub-Saharan Africa: a systematic review of the literature. BMJ Open, 2015. 5(6): p. e006927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox MP and Rosen S, Retention of Adult Patients on Antiretroviral Therapy in Low- and Middle-Income Countries: Systematic Review and Meta-analysis 2008–2013. J Acquir Immune Defic Syndr, 2015. 69(1): p. 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flores G, et al. , A successful approach to minimizing attrition in racial/ethnic minority, low-income populations. Contemp Clin Trials Commun, 2017. 5: p. 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seibold-Simpson S. and Morrison-Beedy D, Avoiding early study attrition in adolescent girls: impact of recruitment contextual factors. West J Nurs Res, 2010. 32(6): p. 761–78. [DOI] [PubMed] [Google Scholar]
- 16.Chatfield MD, Brayne CE, and Matthews FE, A systematic literature review of attrition between waves in longitudinal studies in the elderly shows a consistent pattern of dropout between differing studies. J Clin Epidemiol, 2005. 58(1): p. 13–9. [DOI] [PubMed] [Google Scholar]
- 17.Deeg DJ, et al. , Attrition in the Longitudinal Aging Study Amsterdam. The effect of differential inclusion in side studies. J Clin Epidemiol, 2002. 55(4): p. 319–28. [DOI] [PubMed] [Google Scholar]
- 18.Vega S, et al. , Several factors influenced attrition in a population-based elderly cohort: neurological disorders in Central Spain Study. J Clin Epidemiol, 2010. 63(2): p. 215–22. [DOI] [PubMed] [Google Scholar]
- 19.Mukumbang FC, et al. , Exploring ‘generative mechanisms’ of the antiretroviral adherence club intervention using the realist approach: a scoping review of research-based antiretroviral treatment adherence theories. BMC Public Health, 2017. 17(1): p. 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merten S, et al. , Patient-reported barriers and drivers of adherence to antiretrovirals in sub-Saharan Africa: a meta-ethnography. Trop Med Int Health, 2010. 15 Suppl 1: p. 16–33. [DOI] [PubMed] [Google Scholar]
- 21.Dareng EO, et al. , Test-Retest Reliability of Self-Reported Sexual Behavior History in Urbanized Nigerian Women. Front Public Health, 2017. 5: p. 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bandura A, Social cognitive theory: An agentic perspective. Annual review of psychology, 2001. 52(1): p. 1–26. [DOI] [PubMed] [Google Scholar]
- 23.Robinson KA, et al. , Updated systematic review identifies substantial number of retention strategies: using more strategies retains more study participants. J Clin Epidemiol, 2015. 68(12): p. 1481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filmer D. and Pritchett LH, Estimating wealth effects without expenditure data--or tears: an application to educational enrollments in states of India. Demography, 2001. 38(1): p. 115–32. [DOI] [PubMed] [Google Scholar]
- 25.Marcellus L, Are we missing anything? Pursuing research on attrition. CJNR (Canadian Journal of Nursing Research), 2004. 36(3): p. 82–98. [PubMed] [Google Scholar]
- 26.Forcey DS, et al. , Factors Associated with Participation and Attrition in a Longitudinal Study of Bacterial Vaginosis in Australian Women Who Have Sex with Women. PLoS ONE, 2014. 9(11): p. e113452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young AF, Powers JR, and Bell SL, Attrition in longitudinal studies: who do you lose? Aust N Z J Public Health, 2006. 30(4): p. 353–61. [DOI] [PubMed] [Google Scholar]
- 28.Salthouse TA, Selectivity of attrition in longitudinal studies of cognitive functioning. J Gerontol B Psychol Sci Soc Sci, 2014. 69(4): p. 567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clouse K, et al. , Patient retention from HIV diagnosis through one year on antiretroviral therapy at a primary healthcare clinic in Johannesburg, South Africa. J Acquie Immune Defic Syndr (1999), 2013. 62(2): p. e39–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox MP, et al. , Attrition through Multiple Stages of Pre-Treatment and ART HIV Care in South Africa. PLoS ONE, 2014. 9(10): p. e110252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Facal D, et al. , Characterizing Magnitude and Selectivity of Attrition in a Study of Mild Cognitive Impairment. J Nutr Health Aging, 2016. 20(7): p. 722–8. [DOI] [PubMed] [Google Scholar]
- 32.Zook PM, et al. , Retention strategies and predictors of attrition in an urban pediatric asthma study. Clin Trials, 2010. 7(4): p. 400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gustavson K, et al. , Attrition and generalizability in longitudinal studies: findings from a 15-year population-based study and a Monte Carlo simulation study. BMC Public Health, 2012. 12: p. 918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zunzunegui MV, Beland F, and Gutierrez-Cuadra P, Loss to follow-up in a longitudinal study on aging in Spain. J Clin Epidemiol, 2001. 54(5): p. 501–10. [DOI] [PubMed] [Google Scholar]
- 35.Russell ML, Moralejo DG, and Burgess ED, Paying research subjects: participants’ perspectives. J Med Ethics, 2000. 26(2): p. 126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Neill O, Informed consent and public health. Philos Trans R Soc Lond B Biol Sci, 2004. 359(1447): p. 1133–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown DR, et al. , Recruitment and retention of minority women in cancer screening, prevention, and treatment trials. Ann Epidemiol, 2000. 10(8 Suppl): p. S13–21. [DOI] [PubMed] [Google Scholar]
- 38.Naranjo LE and Dirksen SR, The recruitment and participation of Hispanic women in nursing research: a learning process. Public Health Nurs, 1998. 15(1): p. 25–9. [DOI] [PubMed] [Google Scholar]
- 39.Senturia YD, et al. , Successful techniques for retention of study participants in an inner-city population. Control Clin Trials, 1998. 19(6): p. 544–54. [DOI] [PubMed] [Google Scholar]
- 40.Odierna DH and Bero LA, Retaining Participants in Outpatient and Community-Based Health Studies: Researchers and Participants in Their Own Words. SAGE open, 2014. 4(4): p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.