Abstract
BACKGROUND
Non-responsive celiac disease (NRCD) is defined as the persistence of symptoms in individuals with celiac disease (CeD) despite being on a gluten-free diet (GFD). There is scant literature about NRCD in the pediatric population.
AIM
To determine the incidence, clinical characteristics and underlying causes of NRCD in children.
METHODS
Retrospective cohort study performed at Boston Children’s Hospital (BCH). Children < 18 years diagnosed with CeD by positive serology and duodenal biopsies compatible with Marsh III histology between 2008 and 2012 were identified in the BCH’s Celiac Disease Program database. Medical records were longitudinally reviewed from the time of diagnosis through September 2015. NRCD was defined as persistent symptoms at 6 mo after the initiation of a GFD and causes of NRCD as well as symptom evolution were detailed. The children without symptoms at 6 mo (responders) were compared with the NRCD group. Additionally, presenting signs and symptoms at the time of diagnosis of CeD among the responders and NRCD patients were collected and compared to identify any potential predictors for NRCD at 6 mo of GFD therapy.
RESULTS
Six hundred and sixteen children were included. Ninety-one (15%) met criteria for NRCD. Most were female (77%). Abdominal pain [odds ratio (OR) 1.8 95% confidence interval (CI) 1.1-2.9], constipation (OR 3.1 95%CI 1.9-4.9) and absence of abdominal distension (OR for abdominal distension 0.4 95%CI 0.1-0.98) at diagnosis were associated with NRCD. NRCD was attributed to a wide variety of diagnoses with gluten exposure (30%) and constipation (20%) being the most common causes. Other causes for NRCD included lactose intolerance (9%), gastroesophageal reflux (8%), functional abdominal pain (7%), irritable bowel syndrome (3%), depression/anxiety (3%), eosinophilic esophagitis (2%), food allergy (1%), eating disorder (1%), gastric ulcer with Helicobacter pylori (1%), lymphocytic colitis (1%), aerophagia (1%) and undetermined (13%). 64% of children with NRCD improved on follow-up.
CONCLUSION
NRCD after ≥ 6 mo GFD is frequent among children, especially females, and is associated with initial presenting symptoms of constipation and/or abdominal pain. Gluten exposure is the most frequent cause.
Keywords: Celiac disease, Non-responsive celiac disease, Children, Gluten-free diet, Constipation, Abdominal pain
Core Tip: This is a retrospective chart review study of children < 18 years of age diagnosed with celiac disease (CeD) by positive serology and biopsies showing Marsh III histology to characterize non-responsive CeD (NRCD). NRCD was attributed to a wide variety of diagnoses with gluten exposure (30%) and constipation (20%) being the most common causes. Most (64%) patients improved on follow-up. Our study highlights the importance of performing a diligent search for etiologies of NRCD when there are persistent symptoms despite following a gluten-free diet and reinforces the need for close follow up in the first year of the diagnosis of CeD.
INTRODUCTION
Celiac disease (CeD) is a chronic immune-mediated enteropathy precipitated by exposure to gluten in genetically predisposed individuals[1]. CeD is diagnosed based on clinical symptoms and serological markers in conjunction with specific histological changes found in the duodenum, including villous atrophy, crypt hyperplasia and intraepithelial lymphocytosis[2].
Strict elimination of gluten is currently the only effective treatment for CeD[2,3]. A gluten-free diet (GFD) leads to significant improvement in most people with CeD within weeks[4]. Failure to respond after six to twelve months of a GFD is defined as non-responsive CeD (NRCD), and may occur in both adults and children[5]. NRCD incorporates a range of specific and distinct underlying diagnoses, such as other food intolerance, small intestinal bacterial overgrowth, microscopic colitis, and irritable bowel syndrome[6,7]; nevertheless, the most common etiology of NRCD is continued gluten exposure[6,8]. Studies suggest that 7% to 52% of adult CeD patients may have NRCD[2,5,6]. A recent retrospective study showed that 34% of children adhering to a GFD still report at least one symptoms more than 24 mo after their diagnosis[5]; however, little is known about the spectrum and etiologies of NRCD in children.
The objective of this study was to determine the incidence, clinical characteristics and underlying causes of NRCD in children.
MATERIALS AND METHODS
In this retrospective cohort study, we used the Boston Children’s Hospital (BCH) Celiac Disease Program database to identify children less than 18 years of age with an initial biopsy-confirmed CeD diagnosis at BCH between January 1, 2008 and December 31, 2012. Celiac antibodies included tissue transglutaminase (tTG) immunoglobulin (Ig) A, deamidated gliadin peptide (DGP) IgG and endomysial antibody (EMA) IgA. Included patients had Marsh III lesions, defined by increased intraepithelial lymphocytes, villous atrophy, and crypt hyperplasia[2]. Children diagnosed elsewhere and seen for a second opinion, and those who were asymptomatic at the time of diagnosis were excluded.
Data extracted from medical records included demographics, clinical presentation, serologic results, comorbidities, family history of CeD and number of dietitian visits at BCH within 120 d of CeD diagnosis. Medical records were reviewed longitudinally from the time of diagnosis through September 2015.
Adherence to the GFD was determined from review of medical records, primarily from dietician documentation/notes of their clinical assessment which included a dietary interview and is reported using a previously published assessment scale graded as follows: (1) Excellent = patient never ate gluten intentionally and/or had rare exposure, (2) Good = inadvertent exposure once per month, (3) Fair = exposure 2-3 times per month, (4) Poor = exposure 1-2 times per week, (5) Noncompliant = not on a GFD, or (6) Unable to assess GFD adherence from the medical record[9].
NRCD was defined as persistent clinical symptoms six months or more after the initiation of a GFD. Children with clinical improvement while on a GFD were considered as responders and compared with the NCRD group. This study was approved by the BCH Institutional Review Board.
Statistical analysis
Categorical data were summarized as frequency (percentage) and comparisons between responders and children with NRCD made with Fisher’s exact test. Continuous data were presented as median with interquartile range (IQR) and compared across groups by Wilcoxon rank-sum test. A stepwise logistic regression model was used to investigate symptoms at the time of diagnosis that are independently associated with NRCD signs and symptoms included abdominal pain, constipation (hard or infrequent stools), nausea/vomiting, diarrhea, weight loss or poor weight gain, fatigue, short stature, gassiness, abdominal distension, loss of appetite, arthritis/arthralgia, rash, and mouth ulcers. P < 0.20 was required for a symptom to enter the model, and P < 0.05 was required for the symptom to remain in the model. All comparisons were two-sided, with statistical significance established a priori as P < 0.05. Data analysis was performed with SAS v9.4 (Cary, NC, United States).
RESULTS
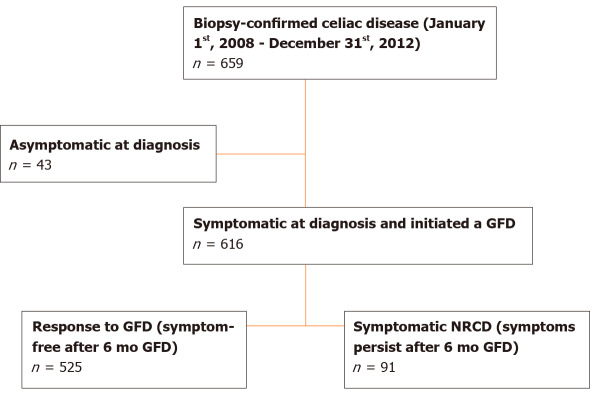
A total of 659 children under 18 years of age were diagnosed with biopsy-confirmed CeD at BCH between January 1, 2008 and December 31, 2012 (Figure 1). All had elevated serum tTG IgA except for nine subjects. These nine were diagnosed with CeD according to the presence of other celiac serology, HLA typing compatible with CeD or response to a GFD. Forty-three who did not have symptoms at the time of the diagnosis (evaluated for CeD because of type 1 diabetes, family history of CeD or short stature) were excluded from the primary analysis. Of the remaining 616 children included, 525 (85%) subjects showed complete response as determined by resolution of clinical symptoms in the 6 (± 2) mo following the initiation of the GFD (responders). Ninety-one children (15%) met the criteria for NRCD, with persistent symptoms 6 mo after the diagnosis.
Figure 1.
Patient selection flowchart. GFD: Gluten-free diet; NRCD: Non-responsive celiac disease.
Characteristics of responders (n = 525) and NRCD (n = 91) groups are shown in Table 1. Significantly more subjects with NRCD were female (77% vs 65%, respectively; P = 0.02). There was no difference in age at time of biopsy, and subjects of all ages were included: < 3 year (9%), 3-6 year (25%), 7-11 year (31%), and 12-17 year (35%). There was a trend towards more Hispanic patients among NRCD subjects compared to responders (5% vs 1%, respectively; P = 0.06). Family history of CeD (27%) was less common than family history of other autoimmune disorders (46%), but the incidence for each was similar between NRCD subjects and responders. Finally, at the time of CeD diagnosis, subjects with NRCD had lower body mass index (BMI) z-score compared to responders (median 0.00 (IQR -1.12, 0.58) vs 0.18 (IQR -0.48, 0.82); P = 0.02) (Table 1).
Table 1.
Characteristics of responders to the gluten-free diet and children with non-responsive celiac disease based on persistent symptoms after 6 mo on a gluten-free diet
|
Characteristic
|
Responders (n = 525)
|
NRCD (n = 91)
|
P
value
|
| Female sex, n (%) | 339 (65) | 70 (77) | 0.02 |
| Age at biopsy (yr), median (IQR) | 9.4 (5.9, 13.4) | 9.6 (5.7, 13.4) | 0.73 |
| Caucasian race1 (%) | 410/438 (92) | 75/85 (91) | 1.00 |
| Hispanic2 (%) | 6/438 (1) | 4/84 (5) | 0.06 |
| Medical history | |||
| Diabetes mellitus, n (%) | 39 (7) | 3 (3) | 0.18 |
| Thyroid condition, n (%) | 20 (4) | 1 (1) | 0.34 |
| Down syndrome, n (%) | 12 (2) | 0 (0) | 0.23 |
| Family history of celiac disease, n (%) | 144 (27) | 22 (24) | 0.53 |
| Family history of other autoimmune disease3 , n (%) | 239 (46) | 43 (47) | 0.73 |
| BMI at diagnosis4, median (IQR) | 17.0 (15.6, 20.0) | 16.4 (15.1, 19.3) | 0.03 |
| BMI z-score at diagnosis5, median (IQR) | 0.18 (-0.48, 0.82) | 0.00 (-1.12, 0.58) | 0.02 |
| ≥ 1 nutrition visit within first 120 d of GFD, n (%) | 342 (65) | 75 (82) | 0.001 |
| Time between diagnosis and first nutrition visit (wk), median (IQR) | 4.0 (2.1, 7.0) | 3.4 (2.2, 5.6) | 0.36 |
Data unknown:
n = 86.
n = 84.
n = 33.
n = 39.
Lupus, rheumatoid arthritis, type 1 diabetes, auto-immune thyroid diseases. P value from Fisher’s exact test or Wilcoxon rank-sum test. IQR: Interquartile range; GFD: Gluten-free diet; NRCD: Non-responsive celiac disease.
Abdominal pain was the most common presenting symptom at diagnosis and was more likely in children with NRCD compared to responders (70% vs 54%, P < 0.006). Constipation (hard or infrequent stools) was the second most common presenting symptom and was also more frequent with NRCD (54% vs 28%, P < 0.0001). Psychiatric manifestations (anxiety and restrictive eating disorder) were also more prevalent at diagnosis among children with NRCD compared to responders (Table 2).
Table 2.
Signs and symptoms at diagnosis of celiac disease among responders to the gluten-free diet and non-responsive celiac disease patients
|
|
Responders (n = 525)
|
NRCD (n = 91)
|
P
value
|
| Abdominal pain, n (%) | 286 (54) | 64 (70) | < 0.006 |
| Hard or infrequent stools, n (%) | 147 (28) | 49 (54) | < 0.0001 |
| Nausea/vomiting, n (%) | 127 (24) | 26 (29) | 0.43 |
| Diarrhea, n (%) | 133 (25) | 22 (24) | 0.90 |
| Weight loss, poor weight gain, n (%) | 126 (24) | 21 (23) | 0.89 |
| Fatigue, n (%) | 71 (14) | 15 (16) | 0.51 |
| Short stature, n (%) | 78 (15) | 8 (9) | 0.14 |
| Gassiness, n (%) | 58 (11) | 9 (10) | 0.86 |
| Abdominal distention, n (%) | 63 (12) | 5 (5) | 0.07 |
| Loss of appetite, n (%) | 53 (10) | 14 (15) | 0.15 |
| Neurologic1, n (%) | 4 (1) | 3 (3) | 0.07 |
| Psychiatric2, n (%) | 0 (0) | 2 (2) | 0.02 |
| Arthritis, arthralgia, n (%) | 28 (5) | 9 (10) | 0.10 |
| Rash, n (%) | 25 (5) | 4 (4) | 1.00 |
| Mouth ulcers, n (%) | 18 (3) | 5 (5) | 0.37 |
| Other3, n (%) | 5 (1) | 10 (11) | < 0.0001 |
Neurologic symptoms include: Headache (n = 6), blurry vision (n = 1).
Psychiatric symptoms include: anxiety (n = 1) and restrictive eating disorder (n = 1).
Other signs and symptoms include: Fecal soiling (n = 2) dysphagia (n = 2), symptomatic anemia (n = 2), rectal bleeding (n = 3), encopresis (n = 2), hair loss (n = 2) delayed puberty (n = 1), easy bruising (n = 1). P value from Fisher’s exact test. NRCD: Non-responsive celiac disease.
Symptoms at the time of diagnosis that were determined by stepwise logistic regression to be independently associated with NRCD included constipation, lack of abdominal distension, and abdominal pain. The odds of NRCD were 3.1 (95% confidence interval (CI) 1.9-4.9) times higher for subjects with constipation compared to those with normal bowel movements. Likewise, absence of abdominal distension (odds ratio (OR) for abdominal distension 0.4, 95%CI 0.1-0.98) and presence of abdominal pain (OR 1.8, 95%CI 1.1-2.9) were independently associated with NRCD.
When examining serum tTG IgA antibody levels, 14 (15%) of NRCD patients had a less than 20% decrease in tTG IgA at 6 mo, 11 of whom continued to have tTG IgA levels greater than 1.5 × the upper limit of normal (ULN) at 1 year after diagnosis.
Formal evaluation and counselling by a dietitian within the first 120 d following CeD diagnosis was performed for 75 (82%) of patients with NRCD, compared to 342 (67%) of responders (P = 0.001). Additional nutrition counselling after the first 120 d occurred for 41% (n = 255) children and was more common among NRCD patients than responders (55% vs 39%, P < 0.006). GFD adherence was classified as excellent in 54 (66%) of symptomatic NRCD patients and poor in 12 (15%). We were unable to assess adherence to the diet in 9 patients based on medical record review. Seventeen of the 91 patients (19%) had repeat endoscopy as part of their evaluation, a median of 18.9 (IQR 9.5-25.4) months after their initial endoscopy. The majority showed normal villous architecture (82%, n = 14). Two children with persistent villous atrophy had on-going gluten exposure; a third one had follow-up biopsies only 2.8 mo after CeD diagnosis (to follow-up on coexisting eosinophilic esophagitis).
An etiology of NRCD was identified for 88/91 children. Gluten exposure was the most common cause (30%, n = 27) (Figure 2). These children had similar rates of elevated tTG IgA at 6 mo (52% vs 46%) and tTG IgA > 1.5 × ULN at one year (15% vs 11%) to the rest of the cohort.
Figure 2.
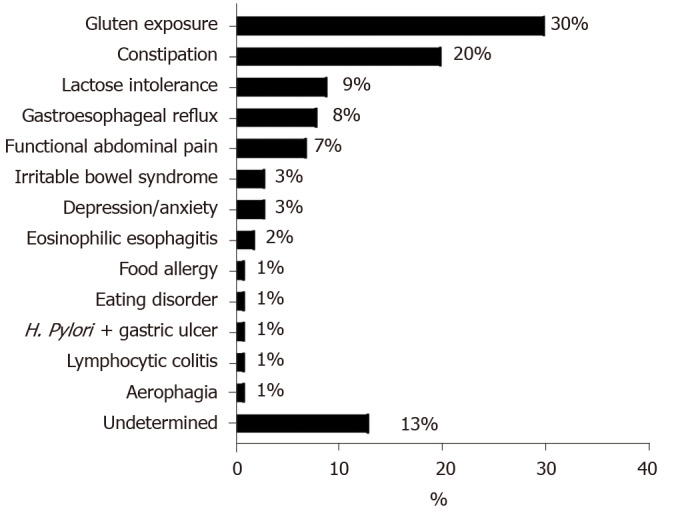
Reasons for non-responsive celiac disease in subjects with persistent symptoms after 6 mo on a gluten-free diet (n = 91). H. Pylori: Helicobacter pylori.
Chronic constipation was the underlying cause of NRCD in 18 (20%) patients, and was already present (n = 15) and treated with laxatives (n = 14) at the time of CeD diagnosis. Other causes for NRCD which were identified are detailed in Figure 2.
Forty-three (64%) of the 67 NRCD children with follow-up had eventual resolution of their clinical symptoms, whereas the remainder (n = 24, 26%) were lost to follow-up before symptoms resolved. Of the 11 patients for whom the etiology of NRCD was unclear, 4 were lost to follow-up, and 5 resolved with time. Only 2 children had persistent symptoms of undetermined etiology lasting 3 years or longer. Symptom resolution was frequent in patients with NRCD secondary to gluten exposure (n = 14, 67%) and constipation (n = 10, 71%). Those with functional abdominal pain (n = 6), irritable bowel syndrome (IBS) (n = 3), depression or anxiety (n = 3) fared worse. Only 1 patient in each group had resolution of symptoms at 3 years of follow-up despite treatment.
DISCUSSION
We report the incidence, etiology, and clinical characteristics of 91 children with NRCD who were diagnosed and treated at BCH between January 2008 and December 2012. The incidence of symptomatic NRCD 6 mo after initiation of a GFD was 15%. Pediatric NRCD was more prevalent in females and associated with a lower BMI z-score, greater abdominal pain, constipation and absence of abdominal distension at diagnosis. Etiologies of NRCD mirrored those reported in adults, with gluten exposure as the most common cause; however, there were no cases of refractory CeD.
Although definitions may vary, our results were consistent with previously published data on NRCD in adults of the same geographic region[6]. We found a lower incidence of NRCD than in a recent pediatric cohort study, in which 34% of children had at least one symptom of CeD more than 24 mo after initiating a GFD[5]. It is unclear whether the prevalence of NRCD increases with time, or if the apparently higher rate of NRCD at 24 mo reflects that the responders were lost to follow-up. Our experience with a pediatric population followed at an academic center that had persisting symptoms six months after the initiation of a GFD may also differ from children who had recurrence of symptoms 12 mo or 18 mo after the diagnosis. Although resolution of symptoms may be slow after starting the GFD, our clinical conclusion is that most children are generally symptom-free after six months on a GFD. Accordingly, 85% of pediatric patients diagnosed at BCH had a good clinical response to the GFD.
Some definitions of NRCD include isolated elevation of tTG[2,6]; however, we did not include such children if they were asymptomatic because tTG is not a reliable proxy for intestinal histology on a GFD[10]. Coexisting disorders, GFD adherence, the initial degree of tTG elevation and severity of villous atrophy all affect time to normalization of tTG which frequently takes longer than 1 year in children[11,12]. Moreover, the presence of villous atrophy is not a sine qua non for NRCD, since the formal definition is based on signs and symptoms and not histology results[2,6]. On the other hand, by only selecting children with positive serology at the time of the diagnosis of CeD, we may have missed some seronegative NRCD[13]. However, seronegative CD is rare, accounting for less than 1% of cases of pediatric biopsy-confirmed (Marsh III) CD in a recent study, so whether they have a different course than seropositive CD remains unclear[14].
As in previous adult and pediatric studies, females were more likely to be diagnosed with NRCD[5,6]. This trend has also been reported among children with functional abdominal pain, but not constipation[14,15]. Females may be more likely to experience, or report GI symptoms. We did not observe any difference in the age at the time of CeD diagnosis. Intriguingly, an association between longstanding symptoms before the diagnosis of CeD and the risk of NRCD was recently reported[5]. Due to the retrospective design of our study, we unfortunately do not have access to this information; however, we did observe lower BMI z-scores at CeD diagnosis among the NRCD group. Lower BMI may be a marker of disease severity, thus explaining a prolonged clinical recovery. This underscores the need to monitor CeD patients after diagnosis because malabsorption due to persistent disease and decreased food intake in relation to GI symptoms may further affect BMI and growth. Moreover, the NRCD group had significantly more symptoms at diagnosis which may reflect visceral hypersensitivity or portend a functional gastrointestinal disorder. Although these manifestations are frequently described among pediatric CeD patients[5], the rate and response to a GFD is variable[16]. Identification of a cause other than gluten exposure for NRCD in most of our patients highlights the importance of considering a coexisting disorder.
Gluten exposure was the most common cause of NRCD in our cohort, as previously reported in adults[6,8]. A strict GFD is the only current treatment for CeD. Consultation with a registered dietitian for specialized GFD education at the time of CeD diagnosis is recommended in clinical practice guidelines and standard practice at our institution[2,3]. Nonetheless, only 65% of the responder group and 82% of the NRCD group had a dietitian visit at our institution within the first 4 mo after diagnosis of CD. Due to the retrospective nature of this study, we were unable to ascertain whether some individuals received GFD education outside of our institution, or if families opted out of dietitian visits because a family member had received GFD education. Nevertheless, additional nutrition counselling after the first 120 days was also more common among NRCD patients than responders, perhaps because ongoing symptoms prompt families to seek out additional education/support. Recent American College of Gastroenterology guidelines recommend reevaluation of diet as a first step in the management of NRCD[2].
Eliminating gluten ingestion, both intentional and unintentional, is a significant challenge for many pediatric CeD patients. Even with specialized GFD education, a sizable number of patients had ongoing gluten exposure. Avoiding gluten cross-contact, which occurs during processing, preparation and serving of foods, is especially difficult. Non-adherence to a strict GFD in children and teenagers has been estimated at 8% when disclosed openly[5], but may be nearer 30% when stools are assessed for gluten immunogenic peptides[17] and may increase with GFD duration[18]. Interestingly, 82% of symptomatic NRCD patients in our study appeared to have excellent or good GFD adherence. This is likely an overestimate due to the inherent challenges associated with assessing GFD adherence through dietary recalls and interviews compared to objective measures such as fecal gluten immunogenic peptides[17].
Fourteen (67%) of the 21 symptomatic NRCD patients with suspected ongoing gluten exposure experienced resolution of their symptoms with improved GFD adherence. It is unclear whether the remaining 7 patients were unsuccessful at fully excluding dietary gluten or if healthcare providers erroneously suspected continued gluten exposure as the cause of NRCD. This cohort was prior to the development of the technology allowing the identification of gluten immunogenic peptides in urine and stools. While these technologies appear promising, currently there is no validated measure of gluten exposure other than dietitian assessment. Although the second step in the management algorithm of NRCD in adults after excluding gluten exposure or food intolerance is to proceed to duodenal biopsies to assess for villous atrophy[2], most patients in this pediatric NRCD cohort did not undergo repeat endoscopy. Moreover, some degree of villous atrophy 6 mo to 12 mo after CeD diagnosis is not uncommon among patients in clinical remission[19].
The second most common cause of NRCD was constipation, which was also an initial manifestation of CeD for most of these patients. Constipation is a common presenting symptom among children with CeD[5,20]. 196 children included in this cohort were constipated at the time of diagnosis, but only 18 had persisting constipation that was deemed to be the cause of NRCD. The GFD is inherently low in fibers which may precipitate constipation in CeD patients[21]. Thus, some patients with constipation as a cause of NRCD may have had either initially treated constipation that recurred on the GFD after stopping laxative (n = 7), new constipation probably caused by the GFD (n = 3) or persisting constipation despite initial laxative therapy (n = 7) or noncompliance to laxative therapy (n = 1). The latter may have been suffering from functional constipation unrelated to CeD, as it is a quite common disorder in children[22]. Nevertheless, many of these patients subsequently improved, highlighting that early and intensive strategies in addition to the GFD can be effective and may avoid persistent symptoms in children.
Notably, constipation is not typically reported as an etiology of NRCD in adults, possibly because they are diagnosed with IBS. Functional abdominal pain and IBS were the cause of NRCD in 10% of our cohort. In comparison, 18% of adult NRCD patients may have IBS[6]. Other less common etiologies include lactose intolerance, present in 9% of our cohort and 7% of adults with NRCD[6]. Upper GI conditions, including gastroesophageal reflux, peptic ulcer disease, Helicobacter pylori infection and eosinophilic esophagitis were also responsible for persistent symptoms in pediatric NRCD. Finally, anxiety and eating disorders were responsible for NRCD and may be associated with gastrointestinal dysmotility. This underscores the need to consider the patient as a whole, and how conditions other than CeD may contribute to symptoms on a GFD.
While most children with NRCD had resolution of their symptoms over the follow-up period, symptoms persisted in 36%. Patients with constipation had higher rates of resolution than those with diagnoses such as functional abdominal pain or IBS. Functional abdominal pain is frequent among children with and without CeD, with a recent multicenter study finding a prevalence of 2.1% to 8.2%[23]. The prevalence of IBS is somewhat lower, from 1.2% to 2.9%[15,24].
Strengths and limitations
This large cohort of children with CeD provides epidemiologic data on the characteristics, causes and evolution of pediatric NRCD thereby addressing an important knowledge gap. Chart reviews conducted by either one of two authors (GV and MD) were reviewed by an expert clinical pediatric gastroenterologist; however, the retrospective nature of this study limited our ability to perform a standardized evaluation of each patient. The large number of clinicians at our institution contributed to variability in documentation and differences in approach to medical evaluation. Also, many etiologies of NCRD are diagnosed based primarily on clinical history. Although inclusion of only children who were diagnosed at BCH reduces referral bias, our cohort may differ from populations in other geographic areas.
CONCLUSION
In summary, NRCD after six months of GFD is frequent among children, especially females. Initial presentation with constipation, abdominal pain and absence of abdominal distension is associated with subsequent diagnosis of NRCD. Although gluten exposure was the most frequent cause, a wide variety of diagnoses are also found among NRCD patients. Our study highlights the importance of performing a diligent search for the above etiologies for NRCD in any celiac child with persistent clinical symptoms despite being on GFD and reinforces the need for close follow up in the first year of a CeD diagnosis.
ARTICLE HIGHLIGHTS
Research background
Non-responsive celiac disease (NRCD) is defined as the persistence of symptoms in individuals with celiac disease (CeD) despite being on a gluten-free diet (GFD). There is scant literature about NRCD in the pediatric population.
Research motivation
Addressing an important knowledge gap, this study examines a large cohort of children with CeD providing data on the characteristics, causes and evolution of pediatric NRCD. By characterizing this sub-population of individuals with CeD, we are better equipped to provide clinical guidance and follow-up in those with persistent symptoms.
Research objectives
Through this retrospective cohort study, we sought to determine the incidence, clinical characteristics, and underlying causes of NRCD in children. Additionally, symptom evolution was detailed and compared to identify any potential predictors for NRCD.
Research methods
Retrospective cohort study performed at Boston Children’s Hospital (BCH). Children < 18 years diagnosed with CeD by positive serology and duodenal biopsies compatible with Marsh III histology between 2008 and 2012 were identified in the BCH’s Celiac Disease Program database. Medical records were longitudinally reviewed from the time of diagnosis through September 2015. NRCD was defined as persistent symptoms at 6 mo after the initiation of a GFD, and causes of NRCD as well as symptom evolution were detailed and compared to identify any potential predictors for NRCD.
Research results
Six hundred and sixteen children were included in this retrospective study, of which 91 (15%) met criteria for NRCD, and of this, most were female (77%). Abdominal pain [odds ratio (OR) 1.8 95% confidence interval (CI) 1.1-2.9], constipation (OR 3.1 95%CI 1.9-4.9) and absence of abdominal distension (OR for abdominal distension 0.4 95%CI 0.1-0.98) at diagnosis were associated with NRCD. NRCD was attributed to a wide variety of diagnoses with gluten exposure (30%) and constipation (20%) being the most common causes. 64% of children with NRCD improved on follow-up.
Research conclusions
NRCD after ≥ 6 mo of GFD is frequent among children, especially females, and is associated with initial presenting symptoms of constipation and/or abdominal pain. Gluten exposure is the most frequent cause. Our study highlights the importance of performing a diligent search for the etiologies for NRCD in any celiac child with persistent clinical symptoms despite being on GFD and reinforces the need for close follow up in the first year of a CeD diagnosis.
Research perspectives
Although the use of a large pediatric cohort positively contributes to the breadth of knowledge surrounding NRCD, and inclusion of only children who were diagnosed at BCH reduces referral bias, our cohort may differ from populations in other geographic areas. As such, a future direction of note is to extend this project to include pediatric Celiac Disease Programs across the United States, to assess if geographic location is a factor in the manifestation and characterization of NRCD.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Institutional Review Board of Boston Children’s Hospital.
Informed consent statement: Patients were not required to give informed consent to the study because this study presented no more than minimal risk to patient privacy and confidentiality.
Conflict-of-interest statement: Leffler DA is employed by Takeda Pharmaceuticals International Co. Kelly CP has acted as a scientific advisor to companies attempting to develop new diagnostic and management approaches for Celiac disease including Cour Pharma, Glutenostics, Innovate, Immunogenx and Takeda. He also acts as Principal Investigator on a research grant on Celiac disease supported by Aptalis. Silvester JA has served on an advisory board for Takeda Pharmaceuticals and has received research funding from Biomedal S.L., Cour Pharma and Glutenostics.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Unsolicited manuscript
Peer-review started: December 3, 2020
First decision: December 21, 2020
Article in press: February 1, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Makovicky P, Pop TL S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ
Contributor Information
Gopal Veeraraghavan, Division of Gastro-enterology, Hepatology and Nutrition, Boston Children's Hospital, Boston, MA 02115, United States; The Celiac Center, Department of Medicine and Division of Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA 02215, United States; Celiac Research Program, Harvard Medical School, Boston, MA 02115, United States.
Amelie Therrien, Division of Gastroenterology, Hepatology and Nutrition, Boston Children's Hospital, Boston, MA 02115, United States; The Celiac Center, Department of Medicine and Division of Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA 02215, United States; Celiac Research Program, Harvard Medical School, Boston, MA 02115, United States.
Maya Degroote, Division of Gastroenterology, Hepatology and Nutrition, Boston Children's Hospital, Boston, MA 02115, United States.
Allison McKeown, Division of Gastroenterology, Hepatology and Nutrition, Boston Children's Hospital, Boston, MA 02115, United States.
Paul D Mitchell, Institutional Centers for Clinical and Translational Research, Boston Children's Hospital, Boston, MA 02115, United States.
Jocelyn A Silvester, Division of Gastroenterology, Hepatology and Nutrition, Boston Children's Hospital, Boston, MA 02115, United States; The Celiac Center, Department of Medicine and Division of Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA 02215, United States; Celiac Research Program, Harvard Medical School, Boston, MA 02115, United States.
Daniel A Leffler, The Celiac Center, Department of Medicine and Division of Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA 02215, United States; Celiac Research Program, Harvard Medical School, Boston, MA 02115, United States; Gastrointestinal Therapeutics, Takeda Pharmaceutical International Co, Cambridge, MA 02139, United States.
Alan M Leichtner, Division of Gastroenterology, Hepatology and Nutrition, Boston Children's Hospital, Boston, MA 02115, United States; Celiac Research Program, Harvard Medical School, Boston, MA 02115, United States.
Ciaran P Kelly, The Celiac Center, Department of Medicine and Division of Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA 02215, United States; Celiac Research Program, Harvard Medical School, Boston, MA 02115, United States.
Dascha C Weir, Division of Gastroenterology, Hepatology and Nutrition, Boston Children's Hospital, Boston, MA 02115, United States. dascha.weir@childrens.harvard.edu; Celiac Research Program, Harvard Medical School, Boston, MA 02115, United States.
Data sharing statement
No additional data are available.
References
- 1.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–1743. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 2.Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656–676; quiz 677. doi: 10.1038/ajg.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill ID, Fasano A, Guandalini S, Hoffenberg E, Levy J, Reilly N, Verma R. NASPGHAN Clinical Report on the Diagnosis and Treatment of Gluten-related Disorders. J Pediatr Gastroenterol Nutr. 2016;63:156–165. doi: 10.1097/MPG.0000000000001216. [DOI] [PubMed] [Google Scholar]
- 4.Murray JA, Watson T, Clearman B, Mitros F. Effect of a gluten-free diet on gastrointestinal symptoms in celiac disease. Am J Clin Nutr. 2004;79:669–673. doi: 10.1093/ajcn/79.4.669. [DOI] [PubMed] [Google Scholar]
- 5.Sansotta N, Amirikian K, Guandalini S, Jericho H. Celiac Disease Symptom Resolution: Effectiveness of the Gluten-free Diet. J Pediatr Gastroenterol Nutr. 2018;66:48–52. doi: 10.1097/MPG.0000000000001634. [DOI] [PubMed] [Google Scholar]
- 6.Leffler DA, Dennis M, Hyett B, Kelly E, Schuppan D, Kelly CP. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol. 2007;5:445–450. doi: 10.1016/j.cgh.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 7.O'Mahony S, Howdle PD, Losowsky MS. Review article: management of patients with non-responsive coeliac disease. Aliment Pharmacol Ther. 1996;10:671–680. doi: 10.1046/j.1365-2036.1996.66237000.x. [DOI] [PubMed] [Google Scholar]
- 8.Abdulkarim AS, Burgart LJ, See J, Murray JA. Etiology of nonresponsive celiac disease: results of a systematic approach. Am J Gastroenterol. 2002;97:2016–2021. doi: 10.1111/j.1572-0241.2002.05917.x. [DOI] [PubMed] [Google Scholar]
- 9.Leffler DA, Dennis M, Edwards George JB, Jamma S, Magge S, Cook EF, Schuppan D, Kelly CP. A simple validated gluten-free diet adherence survey for adults with celiac disease. Clin Gastroenterol Hepatol 2009; 7: 530-536, 536.e1-536. :e2. doi: 10.1016/j.cgh.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 10.Silvester JA, Kurada S, Szwajcer A, Kelly CP, Leffler DA, Duerksen DR. Tests for Serum Transglutaminase and Endomysial Antibodies Do Not Detect Most Patients With Celiac Disease and Persistent Villous Atrophy on Gluten-free Diets: a Meta-analysis. Gastroenterology 2017; 153: 689-701. :e1. doi: 10.1053/j.gastro.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gidrewicz D, Trevenen CL, Lyon M, Butzner JD. Normalization Time of Celiac Serology in Children on a Gluten-free Diet. J Pediatr Gastroenterol Nutr. 2017;64:362–367. doi: 10.1097/MPG.0000000000001270. [DOI] [PubMed] [Google Scholar]
- 12.Isaac DM, Rajani S, Yaskina M, Huynh HQ, Turner JM. Antitissue Transglutaminase Normalization Postdiagnosis in Children With Celiac Disease. J Pediatr Gastroenterol Nutr. 2017;65:195–199. doi: 10.1097/MPG.0000000000001480. [DOI] [PubMed] [Google Scholar]
- 13.Makovicky P, Rimarova K, Boor A, Makovicky P, Vodicka P, Samasca G, Kruzliak P. Correlation between antibodies and histology in celiac disease: incidence of celiac disease is higher than expected in the pediatric population. Mol Med Rep. 2013;8:1079–1083. doi: 10.3892/mmr.2013.1627. [DOI] [PubMed] [Google Scholar]
- 14.Korterink JJ, Diederen K, Benninga MA, Tabbers MM. Epidemiology of pediatric functional abdominal pain disorders: a meta-analysis. PLoS One. 2015;10:e0126982. doi: 10.1371/journal.pone.0126982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M. Functional Disorders: Children and Adolescents. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Jericho H, Sansotta N, Guandalini S. Extraintestinal Manifestations of Celiac Disease: Effectiveness of the Gluten-Free Diet. J Pediatr Gastroenterol Nutr. 2017;65:75–79. doi: 10.1097/MPG.0000000000001420. [DOI] [PubMed] [Google Scholar]
- 17.Comino I, Fernández-Bañares F, Esteve M, Ortigosa L, Castillejo G, Fambuena B, Ribes-Koninckx C, Sierra C, Rodríguez-Herrera A, Salazar JC, Caunedo Á, Marugán-Miguelsanz JM, Garrote JA, Vivas S, Lo Iacono O, Nuñez A, Vaquero L, Vegas AM, Crespo L, Fernández-Salazar L, Arranz E, Jiménez-García VA, Antonio Montes-Cano M, Espín B, Galera A, Valverde J, Girón FJ, Bolonio M, Millán A, Cerezo FM, Guajardo C, Alberto JR, Rosinach M, Segura V, León F, Marinich J, Muñoz-Suano A, Romero-Gómez M, Cebolla Á, Sousa C. Fecal Gluten Peptides Reveal Limitations of Serological Tests and Food Questionnaires for Monitoring Gluten-Free Diet in Celiac Disease Patients. Am J Gastroenterol. 2016;111:1456–1465. doi: 10.1038/ajg.2016.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stefanolo JP, Tálamo M, Dodds S, de la Paz Temprano M, Costa AF, Moreno ML, Pinto-Sánchez MI, Smecuol E, Vázquez H, Gonzalez A, Niveloni SI, Mauriño E, Verdu EF, Bai JC. Real-World Gluten Exposure in Patients With Celiac Disease on Gluten-Free Diets, Determined From Gliadin Immunogenic Peptides in Urine and Fecal Samples. Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.03.038. [DOI] [PubMed] [Google Scholar]
- 19.Belei O, Dobrescu A, Heredea R, Iacob ER, David V, Marginean O. Histologic recovery among children with celiac disease on a gluten-free diet. A long-term follow-up single-center experience. Arch Med Sci. 2018;14:94–100. doi: 10.5114/aoms.2018.72241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khatib M, Baker RD, Ly EK, Kozielski R, Baker SS. Presenting Pattern of Pediatric Celiac Disease. J Pediatr Gastroenterol Nutr. 2016;62:60–63. doi: 10.1097/MPG.0000000000000887. [DOI] [PubMed] [Google Scholar]
- 21.Vici G, Belli L, Biondi M, Polzonetti V. Gluten free diet and nutrient deficiencies: A review. Clin Nutr. 2016;35:1236–1241. doi: 10.1016/j.clnu.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Koppen IJN, Vriesman MH, Saps M, Rajindrajith S, Shi X, van Etten-Jamaludin FS, Di Lorenzo C, Benninga MA, Tabbers MM. Prevalence of Functional Defecation Disorders in Children: A Systematic Review and Meta-Analysis. J Pediatr 2018; 198: 121-130. :e6. doi: 10.1016/j.jpeds.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 23.Saps M, Sansotta N, Bingham S, Magazzu G, Grosso C, Romano S, Pusatcioglu C, Guandalini S. Abdominal Pain-Associated Functional Gastrointestinal Disorder Prevalence in Children and Adolescents with Celiac Disease on Gluten-Free Diet: A Multinational Study. J Pediatr. 2017;182:150–154. doi: 10.1016/j.jpeds.2016.11.049. [DOI] [PubMed] [Google Scholar]
- 24.Saps M, Adams P, Bonilla S, Chogle A, Nichols-Vinueza D. Parental report of abdominal pain and abdominal pain-related functional gastrointestinal disorders from a community survey. J Pediatr Gastroenterol Nutr. 2012;55:707–710. doi: 10.1097/MPG.0b013e3182662401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.