Summary
Background
The clinical benefit of LDL cholesterol lowering treatment in older patients remains debated. We aimed to summarise the evidence of LDL cholesterol lowering therapies in older patients.
Methods
In this systematic review and meta-analysis, we searched MEDLINE and Embase for articles published between March 1, 2015, and Aug 14, 2020, without any language restrictions. We included randomised controlled trials of cardiovascular outcomes of an LDL cholesterol-lowering drug recommended by the 2018 American College of Cardiology and American Heart Association guidelines, with a median follow-up of at least 2 years and data on older patients (aged ≥75 years). We excluded trials that exclusively enrolled participants with heart failure or on dialysis because guidelines do not recommend lipid-lowering therapy in such patients who do not have another indication. We extracted data for older patients using a standardised data form for aggregated study-level data. We meta-analysed the risk ratio (RR) for major vascular events (a composite of cardiovascular death, myocardial infarction or other acute coronary syndrome, stroke, or coronary revascularisation) per 1 mmol/L reduction in LDL cholesterol.
Findings
Data from six articles were included in the systematic review and meta-analysis, which included 24 trials from the Cholesterol Treatment Trialists’ Collaboration meta-analysis plus five individual trials. Among 244 090 patients from 29 trials, 21 492 (8·8%) were aged at least 75 years, of whom 11 750 (54·7%) were from statin trials, 6209 (28·9%) from ezetimibe trials, and 3533 (16·4%) from PCSK9 inhibitor trials. Median follow-up ranged from 2·2 years to 6·0 years. LDL cholesterol lowering significantly reduced the risk of major vascular events (n=3519) in older patients by 26% per 1 mmol/L reduction in LDL cholesterol (RR 0·74 [95% CI 0·61–0·89]; p=0·0019), with no statistically significant difference with the risk reduction in patients younger than 75 years (0·85 [0·78–0·92]; pinteraction=0·37). Among older patients, RRs were not statistically different for statin (0·82 [0·73–0·91]) and non-statin treatment (0·67 [0·47–0·95]; pinteraction=0·64). The benefit of LDL cholesterol lowering in older patients was observed for each component of the composite, including cardiovascular death (0·85 [0·74–0·98]), myocardial infarction (0·80 [0·71–0·90]), stroke (0·73 [0·61–0·87]), and coronary revascularisation (0·80 [0·66–0·96]).
Interpretation
In patients aged 75 years and older, lipid lowering was as effective in reducing cardiovascular events as it was in patients younger than 75 years. These results should strengthen guideline recommendations for the use of lipid-lowering therapies, including non-statin treatment, in older patients.
Funding
None.
Introduction
Clinical trials of therapies lowering LDL cholesterol concentration have consistently shown a reduction in the risk of cardiovascular events.1,2 However, the clinical benefit from LDL cholesterol lowering in older patients remains debated because participants aged 75 years or older were not well represented in individual trials.3,4 In the Cholesterol Treatment Trialists’ Collaboration (CTTC),5 major vascular events were reduced by 21% per 1 mmol/L reduction in LDL cholesterol with statin treatment or a more intensive statin regimen, but with some possible attenuation in older patients.
The 2018 American College of Cardiology and American Heart Association (ACC/AHA) cholesterol guidelines have lower strength recommendations for older patients compared with those for younger patients.6 The 2019 European Society of Cardiology and European Atherosclerosis Society dyslipidaemia guidelines endorse treating older patients, but add specific considerations to assess comorbidities before initiating treatment.7 In clinical practice, studies show that the use of lipid lowering in older patients, an important demographic that accounts for almost 20% of the population,8 is lower than in younger patients.9,10
Several subgroup analyses from randomised controlled trials with statin and non-statin lipid-lowering therapies added new evidence regarding the efficacy and safety of lowering LDL cholesterol in older patients.5,11–15 Given these new data, we aimed to summarise the evidence of lipid-lowering therapies in the older population and readdress whether older patients should be treated less intensively than younger patients.
Methods
Search strategy and selection criteria
In this systematic review and meta-analysis, we followed PRISMA guidelines.16 BG and NAM searched MEDLINE and Embase for all randomised, controlled, cardiovascular outcome trials of LDL cholesterol-lowering therapy as recommended by the 2018 ACC/AHA guidelines (statin, ezetimibe, evolocumab, and alirocumab)6 published between March 1, 2015, and Aug 14, 2020, without any language restrictions. A complete list of search terms and inclusion and exclusion criteria are given in the appendix (p 4). The start date for our search was chosen because the CTTC provided data for statins in older patients in 2019,5 and the first outcome trial for these recommended non-statin therapies was published in 2015. BG and NAM screened titles, abstracts, and full text of papers identified in our search and assessed for risk of bias. BG and NAM extracted the data for patients aged at least 75 years using a standardised data form for aggregated study-level data and any discrepancies were resolved by consensus. The CTTC meta-analysis provided data for statin treatment.5 We extracted pooled data of older patients (aged >75 years) from 24 trials with statins, excluding four trials that exclusively enrolled participants with heart failure or on dialysis.5 This decision was based on the 2018 US and 2019 European guidelines, which do not recommend lipid-lowering treatment in patients with heart failure or advanced kidney disease who do not have another indication.6,7
Data analysis
Outcomes from each trial were selected to most closely approximate the target composite endpoint of major vascular events, which consisted of cardiovascular death, acute myocardial infarction or other acute coronary syndrome, coronary revascularisation, or stroke when available, because all these events have been shown to be reduced by therapies that lower LDL cholesterol. In some instances, the selected outcome that best matched the target composite was a secondary composite endpoint for the original trial. The specific composite outcome selected for each trial is listed in the appendix (p 5). We also examined the individual components of the composite outcome, as well as non-cardiovascular death and all-cause death. We extracted data of participants younger than 75 years to compare the treatment effect between older and younger patients. Since the younger data in the Treat Stroke to Target trial14 were presented by two age categories (<65 years and 65–75 years), we estimated the effect in younger patients using a fixed effect approach. Safety outcomes of interest that were available included cancer, haemorrhagic stroke, new-onset diabetes, and neurocognitive adverse events (appendix p 18).
The hazard ratio (HR) or rate ratio and 95% CI was extracted and normalised per 1 mmol/L (38·67 mg/dL) difference in LDL cholesterol for each trial, as has been done previously.2 When the HR or rate ratio were not available, a risk ratio (RR) was calculated (appendix p 6). When the results were pooled, RR was used to describe the effect estimate. In the CTTC, the rate ratios in age subgroups were presented with 99% CIs and therefore we calculated 95% CIs before pooling with other trials. A random-effects meta-analysis with a restricted maximum likelihood approach was used to account for heterogeneity between trials in lipid-lowering therapies, follow-up duration, and study populations. Patients were stratified by statin5,14 versus non-statin11–13,15 LDL cholesterol-lowering therapies for the primary and secondary endpoints, and by with versus without established atherosclerotic cardiovascular disease for the primary endpoint (stratified analyses by presence of baseline atherosclerotic cardiovascular disease were not uniformly available for individual outcomes). We assessed heterogeneity using Cochran’s Q statistic, and Higgins and Thompsons’ I2, as well as average dispersion in effect sizes τ2. The risk of bias was assessed according to the Cochrane tool for assessing risk of bias in randomised clinical trials (appendix p 9) and sensitivity analyses were done for the primary endpoint by excluding trials that were at risk of bias and by applying the Hartung-Knapp adjustment.17 Publication bias for the primary endpoint of major vascular events was assessed using Egger’s regression test and a funnel plot.18
For safety endpoints, HRs or rate ratios and 95% CIs were extracted from the original trials if available or an RR was calculated from raw counts for each trial and meta-analysed using a random effects model with a restricted maximum likelihood approach after normalisation of RR per 1 mmol/L reduction in LDL cholesterol. Statistical analyses were done using R (version 3.6.1) and the R package metafor (version 2.0-0).19
Role of the funding source
There was no funding source for this study. All authors had full access to all the data in the study and the corresponding author had final responsibility for the decision to submit for publication.
Results
We included data from six articles in the systematic review and meta-analysis, which included data from 24 trials from the CTTC meta-analysis plus five individual trials (appendix p 19). Among 244 090 patients from 29 trials, 21 492 (8·8%) were older (aged at least 75 years) at the time of randomisation, of whom 11 750 (54·7%) were from statin trials, 6209 (28·9%) from ezetimibe trials, and 3533 (16·4%) from PCSK9 inhibitor trials (table). Most trials met the criteria for low risk of bias according to the Cochrane tool for assessing risk of bias in randomised clinical trials (appendix p 9). Median follow-up ranged from 2·2 to 6·0 years.5,11–15 The statin trials consisted of 24 trials from the CTTC meta-analysis (statin or more intensive statin vs placebo or less intensive statin)5 and the Treat Stroke to Target trial (target LDL cholesterol <1·8 mmol/L [70 mg/dL] vs 2·3–2·8 mmol/L [90–110 mg/dL]).14 The non-statin trials were IMPROVE-IT (ezetimibe 10 mg vs placebo, in addition to simvastatin),11 EWTOPIA 75 (ezetimibe 10 mg vs usual care),12 FOURIER (evolocumab vs placebo, in addition to maximally tolerated statin therapy),15 and ODYSSEY OUTCOMES (alirocumab vs placebo, in addition to maximally tolerated statin therapy).13 Demographic data were available for all older patients in the non-statin trials, for whom the mean age was 79 years and 4792 (49·2%) were women and 4950 (50·8%) were men. The treatment effect reported in the trials in older and in younger (aged <75 years) patients are summarised in the appendix (pp 11–17).
Table:
Patient characteristics
| Older patients | Population | Age, years | Female patients | Male patients | Treatment | Achieved LDL cholesterol, mmol/L (mg/dL) | Median follow-up, years | Major vascular events | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental group | Control group | Experimental group | Control group | ||||||||
| Statin therapy | |||||||||||
| CTTC5 | 11108 | Primary prevention and secondary prevention | NA | NA | NA | Statin or more intensive statin | Placebo or less intensive statin | NA | NA | 4·9 | 1695 |
| Treat Stroke to Target14 | 642 | Secondary prevention | NA | NA | NA | Target LDL cholesterol <1·8 mmol/L (70 mg/dL) | Target LDL cholesterol 2·3–2·8 mmol/L (90·110 mg/dL) | 1·7 (65·0) | 2·5 (96·0) | 3·5 | 74 |
| Non-statin therapy | |||||||||||
| IMPROVE-IT11 | 2798 | Secondary prevention | 80 (4) | 947 (33·8%) | 1851 (66·2%) | Ezetimibe plus simvastatin | Placebo plus simvastatin | 1·4 (55·1) | 1·8 (68·7) | 6·0 | 1017 |
| EWTOPIA 7512 | 3411 | Primary prevention | 81 (5) | 2539 (74·4%) | 872 (25·6%) | Ezetimibe | Usual care | 3·2 (123·8) | 3·6 (139·7) | 4·1 | 222 |
| FOURIER15 | 2526 | Secondary prevention | 78 (3) | 848 (33·6%) | 1678 (66·4%) | Evolocumab on background of statin therapy | Placebo on background of statin therapy | 1·0 (40·0) | 2·4 (91·5) | 2·2 | 283 |
| ODYSSEY OUTCOMES13 | 1007 | Secondary prevention | 78 (3) | 458 (45·5%) | 549 (54·5%) | Alirocumab on background of statin therapy | Placebo on background of statin therapy | 1·5 (56·9) | 2·5 (97·0) | 2·8 | 228 |
| Total | 21 492 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | 3·3 (2·2–4·6) | 3519 |
Data are n, n (%), mean (SD), or median (IQR), unless otherwise indicated. The methods used for extraction of LDL cholesterol data in each study are summarised in the appendix (pp 5, 6). CTTC=Cholesterol Treatment Trialists’ Collaboration. EWTOPIA 75=Ezetimibe Lipid-Lowering Trial on Prevention of Atherosclerosis Disease in 75 or Older. FOURIER=Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Patients with Elevated Risk. IMPROVE-IT=Improved Reduction of Outcomes: Vytorin Efficacy International Trial. NA=not available in patients aged 75 years or older. ODYSSEY OUTCOMES=Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment with Alirocumab.
Baseline LDL cholesterol concentrations in the experimental groups ranged from 2·0 mmol/L (77·8 mg/dL) to 4·2 mmol/L (162·0 mg/dL), with a weighted mean of 2·8 mmol/L (107·6 mg/dL) and SD of 0·7 mmol/L (25·9 mg/dL; appendix p 10). The mean achieved LDL cholesterol concentrations after randomisation ranged from 1·0 mmol/L (40·0 mg/dL) to 3·2 mmol/L (123·8 mg/dL) in the experimental group (table). The mean reduction in LDL cholesterol ranged from 0·4 mmol/L (13·6 mg/dL) to 1·3 mmol/L (51·5 mg/dL), with a weighted mean of 0·9 mmol/L (36·2 mg/dL) and SD of 0·4 mmol/L (14·9 mg/dL; appendix p 10).
3519 (16·4%) of 21 492 patients had a major vascular event in the trials, of which 2736 (77·7%) occurred in secondary prevention patients and 783 (22·3%) in primary prevention patients. The weighted rate of major vascular events in the control groups was 5·7% per year in older patients versus 4·1% per year in younger patients. Overall, lipid-lowering therapies reduced the risk of major vascular events in older patients by 26% per 1 mmol/L reduction in LDL cholesterol (figure 1; appendix p 7). The magnitude of the risk reduction was not statistically different to that seen in younger patients (figure 2; appendix p 7), with no significant interaction (pinteraction=0·37). Among older patients, the RRs per 1 mmol/L reduction in LDL cholesterol were not statistically different for statin and non-statin treatment (figure 2; pinteraction=0·64). Likewise, we found no evidence of treatment difference in patients with established atherosclerotic cardiovascular disease at baseline and in those without (pinteraction=0·89; appendix p 20).
Figure 1: Effect of LDL cholesterol lowering on the risk of major vascular events with statin and non-statin treatment in older patients.
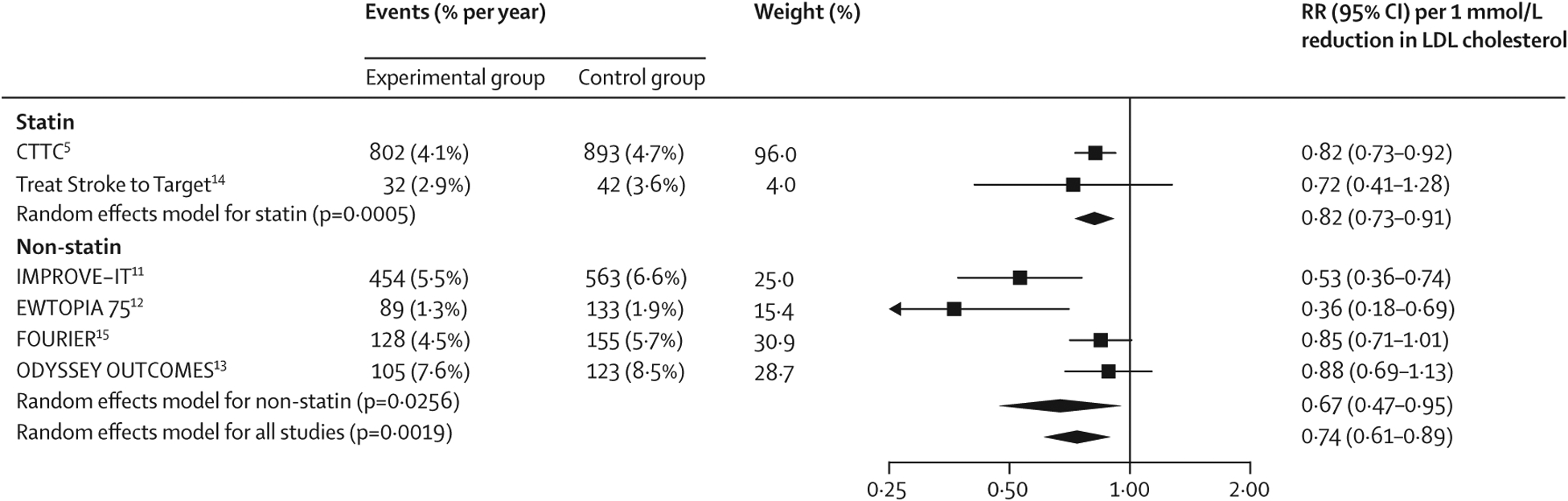
Older patients were aged 75 years or older. RRs per 1 mmol/L reduction in LDL cholesterol were generated from a random effects model. In the ODYSSEY OUTCOMES trial, the event numbers were provided at 4 years, whereas the RR is for the entire duration of trial. CTTC=Cholesterol Treatment Trialists’ Collaboration. EWTOPIA 75=Ezetimibe Lipid-Lowering Trial on Prevention of Atherosclerosclerotic Disease in 75 or Older. FOURIER=Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Patients with Elevated Risk. IMPROVE-IT=Improved Reduction of Outcomes: Vytorin Efficacy International Trial. ODYSSEY OUTCOMES=Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment with Alirocumab. RR=risk ratio.
Figure 2: Effect of LDL cholesterol lowering on the risk of major vascular events in older versus younger patients.
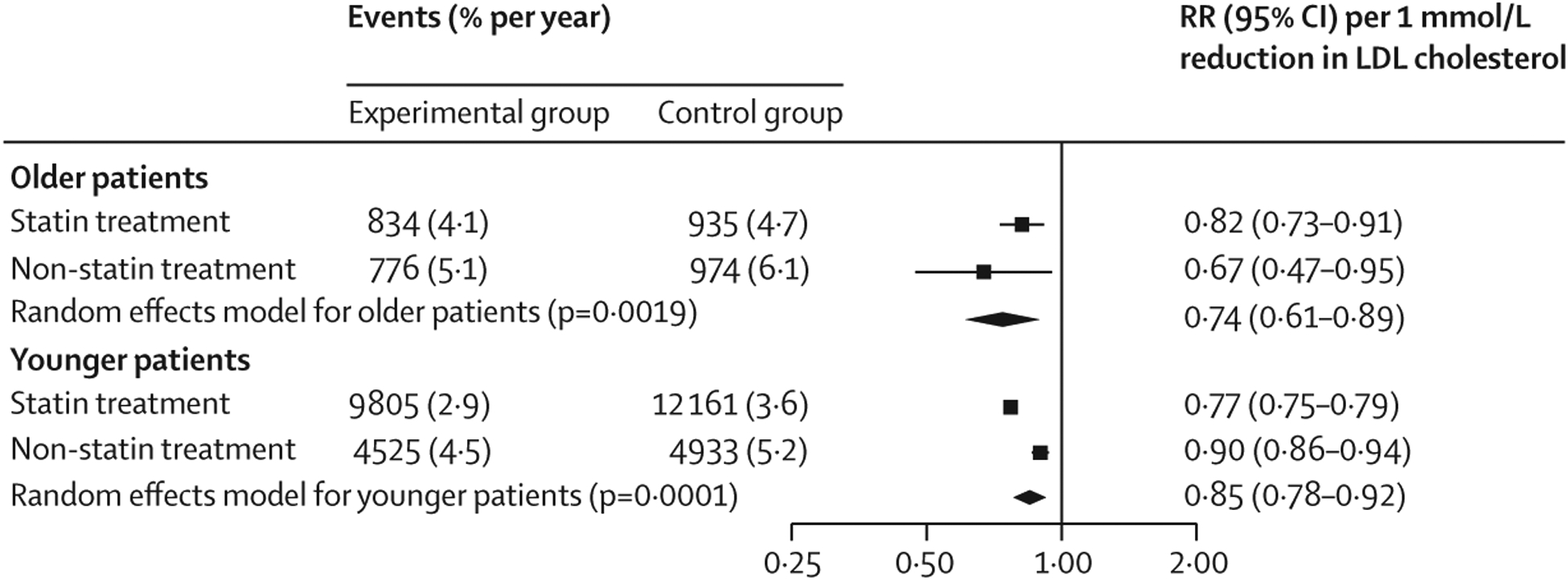
Older patients were aged 75 years or older and younger patients were younger than 75 years. RRs per 1 mmol/L reduction in LDL cholesterol were generated from a random effects model. RR=risk ratio.
Lipid-lowering therapies reduced cardiovascular death in older patients by 15% per 1 mmol/L reduction in LDL cholesterol, myocardial infarction by 20%, stroke by 27%, and coronary revascularisation by 20% (figure 3; appendix p 7). The magnitudes of treatment effect in older patients were not statistically different for statin and non-statin trials (appendix pp 21–24), except for stroke, for which the risk reduction was slightly greater with non-statin compared with statin (pinteraction=0·034). As expected, we found no effect of lipid lowering on non-cardiovascular death (appendix p 25). The RR for all-cause death was 0·93 (95% CI 0·84–1·02; p=0·13; appendix p 26).
Figure 3: Effect of LDL cholesterol lowering on the risk of individual efficacy endpoints in older patients.
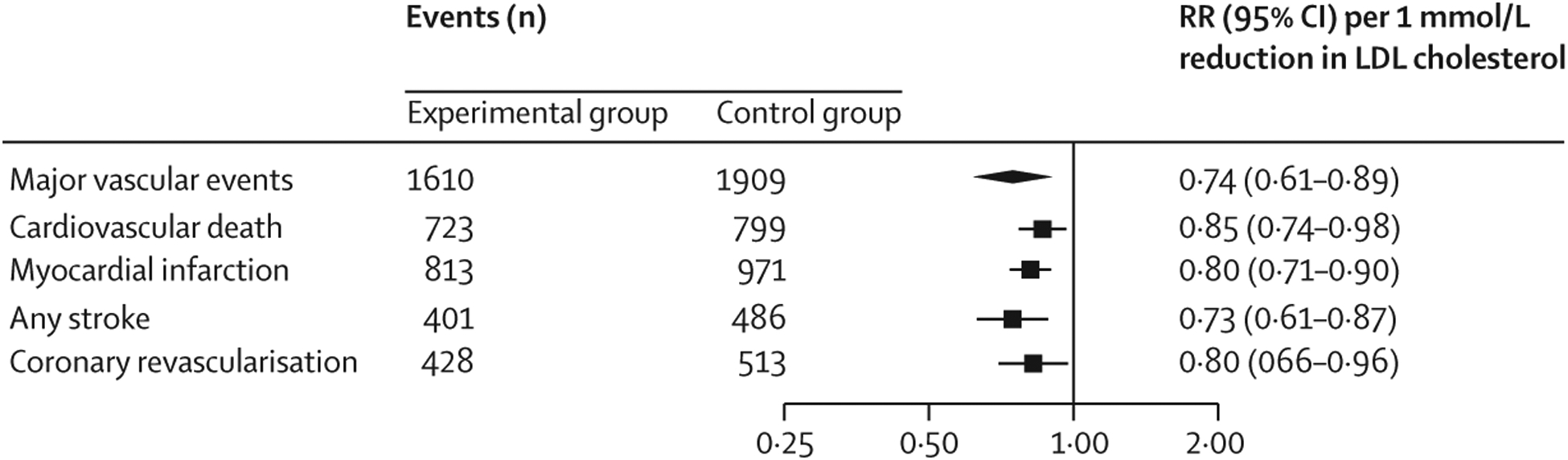
Older patients were aged 75 years or older. RRs per 1 mmol/L reduction in LDL cholesterol were generated from a random effects model. RR=risk ratio.
Using all the trials but applying the Hartung-Knapp method for adjustment, yielded a similar overall treatment effect for the major vascular events endpoint among older patients (RR 0·74 [95% CI 0·55–0·98]). We found moderate heterogeneity across the studies (I2=67·61%). However, when the smallest trial with an open-label design (EWTOPIA 75) was excluded, the heterogeneity decreased substantially (I²<0·01%) and the effect estimate continued to show a substantial benefit for lipid lowering (RR 0·81 [95% CI 0·74–0·88]). Sensitivity analyses excluding the studies at risk of bias (Treat Stroke to Target and EWTOPIA 75) yielded similar results for major vascular events (0·81 [0·74–0·89]). A funnel plot suggested a slight presence of publication bias (appendix p 27). This asymmetry was mostly due to the inclusion of the EWTOPIA 75 trial. However, the EWTOPIA 75 trial’s weight was only 6·6% for the overall pooled result and a sensitivity analysis excluding the EWTOPIA 75 trial still showed a significant benefit for lipid lowering in older patients.
Each 1 mmol/L reduction of cholesterol in statin and non-statin trials was not associated with an increased risk of cancer in older patients (figure 4; appendix pp 8, 28). For the other safety outcomes, the available data were limited to non-statin trials and did not show an increased risk of haemorrhagic stroke, new-onset diabetes, or neurocognitive adverse events (appendix pp 8, 29–31).
Figure 4: Effect of LDL cholesterol lowering on the risk of safety endpoints in older patients.
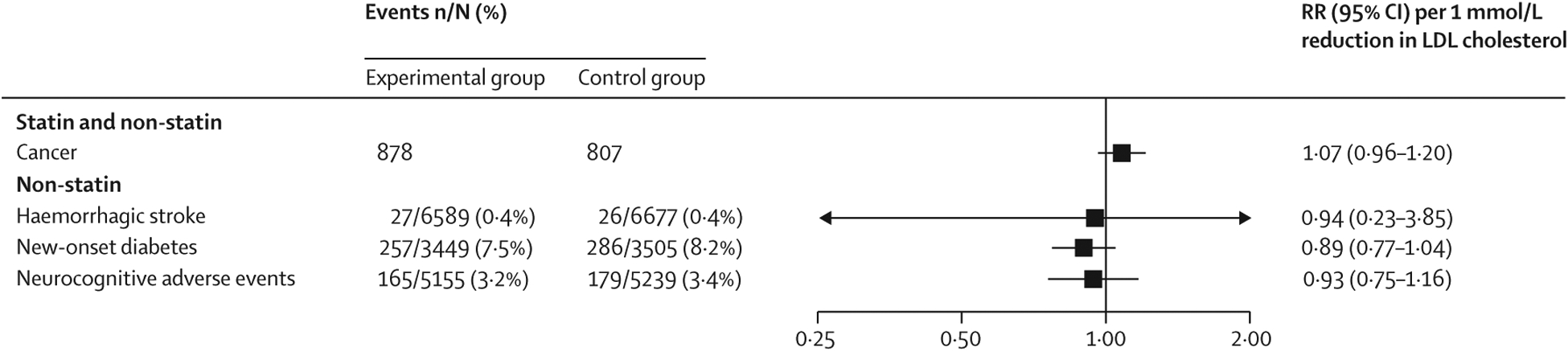
Older patients were aged 75 years or older. Data are n or n/N (%), unless otherwise indicated. RRs per 1 mmol/L reduction in LDL cholesterol were generated from a random effects model. The numbers of patients without cancer events per treatment group were not available in the Cholesterol Treatment Trialists’ Collaboration meta-analysis. The definitions of reported safety data in each trial are given in the appendix (p 18). RR=risk ratio.
Discussion
Individuals aged 75 years and older account for almost 20% of the population in high-income countries.8 Concerns for lesser relative risk reductions, briefer duration to affect risk of cardiovascular outcomes, and increased incidence of adverse events have led to lower usage rates of lipid lowering treatments in this important segment of the population, compared with usage in younger patients.4 Indeed, the ACC/AHA cholesterol guidelines give different recommendations to manage lipid lowering in older compared with younger patients.6 Specifically, in patients with atherosclerotic cardiovascular disease not at very high risk, for patients aged 75 years or younger the guidelines give a class I recommendation for high-intensity statin and a class IIb recommendation for the addition of ezetimibe if the LDL cholesterol remains 1·8 mmol/L (70 mg/dL) or more. By contrast, in patients older than 75 years, only a class IIa recommendation is given for a statin, which can be either moderate or high intensity, and no recommendation exists for the addition of a non-statin. Additionally, the recommendations for the use of statin treatment in high-risk groups, such as patients with severe hypercholesterolaemia, diabetes, or as primary prevention, were all made for patients aged between 40 and 75 years, whereas no specific guidance was given for individuals aged 75 years or older. In general, the level of evidence in older patients was considered low.
This meta-analysis adds new evidence regarding the efficacy and safety of lowering LDL cholesterol in older patients. We found an unequivocal reduction in the risk of major vascular events with statin and non-statin LDL cholesterol-lowering therapy that was at least as good as that seen in younger patients. Moreover, significant reductions were seen for all the individual endpoints, including cardiovascular death, myocardial infarction, stroke, and coronary revascularisation.
Older individuals have higher rates of major vascular events. In our meta-analysis the rates for major vascular events per annum were almost 40% higher in those aged 75 years and older compared with those younger than 75 years. Thus, given comparable relative risk reductions emerging over just a few years of treatment, the absolute risk reductions expected from treating older patients should be higher than those in younger patients. At age 65 years, life expectancy is approximately 20 years for men and greater than that for women in high-income countries,20 which corresponds to an average time window of at least 10 years to reduce cardiovascular disease in patients aged 75 years. Moreover, coronary heart disease still remains the leading cause of death in older people.21 Although we have shown clear efficacy for lipid lowering in older patients, we also note that the data support keeping LDL cholesterol well controlled as early as possible in individuals to prevent the development of atherosclerosis.22,23 Indeed, coronary imaging studies show plaque regression when LDL cholesterol is below approximately 1·8 mmol/L, and epidemiological studies show very low rates of coronary heart disease in societies in which the average LDL cholesterol is at or below 1·8 mmol/L.24,25 Thus, our ability to treat patients with atherosclerosis and the disease’s complications in older patients should not preclude the initiation of lipid-lowering therapies earlier in life to help prevent the development of atherosclerosis.23,26
Our meta-analysis substantially expands on previous meta-analyses in older patients using an older age cut-off (≥75 years instead of ≥65 years),27 and extends the CTTC meta-analysis of 24 statin trials with an additional statin trial and four trials with non-statin lipid-lowering drugs.5 Our findings are also supported by an observational analysis from the US Veterans Health Administration that showed initiation of statin among 320 000 patients aged 75 years or older without cardiovascular disease was associated with a 20% lower risk of cardiovascular death compared with patients who were not prescribed a statin.28 The ongoing randomised controlled trial in 18 000 patients older than 70 years and without cardiovascular disease (STAREE; NCT02099123) will clarify the efficacy of statin compared with placebo to reduce major vascular events in an older primary prevention population.
In addition to showing that lipid-lowering therapies reduce mortality and morbidity in older patients, we also found no offsetting safety concerns. Some concerns related to haemorrhagic stroke were considered as a potential barrier for LDL cholesterol lowering in older patients with statins. In the CTTC meta-analysis, no data were presented for statin treatment with respect to haemorrhagic stroke in older patients. However, in the non-statin trials no signal has been observed, nor was an excess of either new-onset diabetes or neurocognitive adverse events shown in older patients with non-statin treatment.
Our meta-analysis has some limitations. First, slight differences exist in the outcome definitions and included events between trials. However, lipid lowering has been shown to be roughly equally effective on a per mmol/L reduction in LDL cholesterol for all the elements of the composite, and in our meta-analysis we found similar efficacy for all the components. Also, in the CTTC meta-analysis and in the Treat Stroke to Target trial, older patients were defined as those older than 75 years and not age 75 years or older; however, this minor difference should not affect the clinical implications of this meta-analysis for older patients. Second, the trials were of different durations. The benefit of lipid lowering is less in the first year than it is in subsequent years.29 Thus, the PCSK9 inhibitor trials might have underestimated the long-term benefit of such treatment.30 Third, the data for the benefit of lipid lowering for cardiovascular risk reduction for primary prevention in older patients are sparse, with slightly less than a quarter of the major vascular events in primary prevention patients and with the open-label EWTOPIA 75 trial having considerable weight. Fourth, little safety data are available from the CTTC meta-analysis for statin trials in older patients and in the ODYSSEY OUTCOMES trial, safety data were presented only in those aged 65 years or older (and not ≥75 years). Fifth, the data for the treatment effect estimates by statin intensity were not provided by the CTTC for the older versus younger populations separately. Finally, older patients who are included in clinical trials might not be representative of everyday practice because individuals with extensive non-cardiovascular comorbidities or those unable to attend follow-up visits would typically not be enrolled, although the enrolled patients appeared to have the typical cardiovascular comorbidities. Notably, because the rate of major vascular events tends to be lower in clinical trials than in registries, the absolute risk reduction of the number of major vascular events with lipid lowering in clinical practice might be even larger than that observed in clinical trials.
In conclusion, in patients aged 75 years and older, lipid lowering is as effective in reducing cardiovascular events as it is in younger adults and reduces cardiovascular death. These results should strengthen guideline recommendations for the use of lipid-lowering therapies, including non-statin therapy, in older patients.
Supplementary Material
Research in context.
Evidence before this study
Clinical trials of treatments lowering LDL cholesterol have consistently shown a reduction in the risk of cardiovascular events. However, the clinical benefit from LDL cholesterol lowering in older patients remains debated because participants aged 75 years or older were not well represented in individual trials. In the Cholesterol Treatment Trialists’ Collaboration meta-analysis, major vascular events were reduced by 21% per 1 mmol/L reduction in LDL cholesterol with statin therapy, but with some possible attenuation in older patients. Practice guidelines have noted that the level of evidence in older patients is low and some have lower strength recommendations for older patients than for younger patients. We searched MEDLINE and Embase for articles published between March 1, 2015, and Aug 14, 2020, without any language restrictions, including randomised controlled trials of cardiovascular outcomes of an LDL cholesterol-lowering drug recommended by the 2018 American College of Cardiology and American Heart Association guidelines, with a median follow-up of at least 2 years and data on older patients (aged ≥75 years).
Added value of this study
This meta-analysis involving 21 492 older patients from statin and non-statin trials of lipid-lowering treatments adds new evidence regarding the efficacy and safety of lowering LDL cholesterol in older patients. We found an unequivocal reduction in the risk of major vascular events with both statin and non-statin LDL cholesterol-lowering treatments, which was similar to that seen in younger patients. Moreover, significant reductions were seen for all of the individual components of the composite endpoint, including cardiovascular death, myocardial infarction, stroke, and coronary revascularisation.
Implications of all the available evidence
Life expectancy for patients aged 75 years in high-income countries is expected to be at least 10 years. Older individuals have high rates of major vascular events and, given comparable relative risk reductions with lipid-lowering treatments, should therefore have high absolute risk reductions. These results should strengthen guideline recommendations for the use of lipid-lowering treatments, including non-statin therapy, in older patients.
Declaration of interests
BG’s activities in the TIMI Study Group, Harvard Medical School, are supported by grants from the Geneva University Hospitals, Eugenio Litta and Athemis Foundations. NAM reports grant support from the US National Institutes of Health. KI is a member of the TIMI Study Group, which has received institutional research grant support through Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA, from Abbott, Amgen, Aralez, AstraZeneca, Bayer, BRAHMS, Daiichi Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Roche, Takeda, The Medicines Company, and Zora Biosciences. CPC reports research grants from Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Janssen, Merck, and Pfizer; and consulting fees from Aegerion, Alnylam, Amarin, Amgen, Applied Therapeutics, Ascendia, Boehringer Ingelheim, Bristol Myers Squibb, Corvidia, Eli Lilly, HLS Therapeutics, Innovent, Janssen, Kowa, Merck, Pfizer, Rhoshan, and Sanofi. PS reports research grant support (through Imperial College London, London, UK) from Pfizer and Amgen and honoraria for consulting from Pfizer and Amgen. AK reports grants and personal fees from Abbott and Mylan; personal fees from Amgen and AstraZeneca, Pfizer, and Bayer; and grants from Sanofi and Novartis, outside of the submitted work. EB reports research grants through Brigham and Women’s Hospital from AstraZeneca, Daiichi Sankyo, Merck, and Novartis; and consultant fees from Amgen, Cardurion, MyoKardia, NovoNordiak, and Verve. RPG has received grants from Amgen and Anthos Therapeutics; honoraria for CME lectures from Amgen, Daiichi Sankyo, and Servier; and consultant fees from Amgen, American College of Cardiology, AstraZeneca, CryoLife, CVS Caremark, Daiichi Sankyo, Esperion, Gilead, GlaxoSmithKline, SAJA Pharmaceuticals, Samsung, and Servier. MSS reports research grant support through Brigham and Women’s Hospital from Abbott Laboratories, Amgen, AstraZeneca, Critical Diagnostics, Daiichi Sankyo, Eisai, Genzyme, Gilead, GlaxoSmithKline, Intarcia, Janssen Research and Development, The Medicines Company, MedImmune, Merck, Novartis, Poxel, Pfizer, Roche Diagnostics, and Takeda; and personal fees from Alnylam, Bristol Myers Squibb, CVS Caremark, Dynamix, Esperion, Ionis, and MyoKardia.
Footnotes
Data sharing
Because this meta-analysis was based on data extracted from previously published research, most of the data and study materials are available in the public domain. Additional data for the secondary and safety endpoints were retrieved from the original FOURIER and IMPROVE-IT databases. Data from this additional analysis will not be made publicly available; however, we encourage interested parties to contact the corresponding author for further discussions.
See Online for appendix
References
- 1.Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet 2010; 376: 1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabatine MS, Wiviott SD, Im K, Murphy SA, Giugliano RP. Efficacy and safety of further lowering of low-density lipoprotein cholesterol in patients starting with very low levels: a meta-analysis. JAMA Cardiol 2018; 3: 823–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US preventive services task force. JAMA 2016; 316: 2008–24. [DOI] [PubMed] [Google Scholar]
- 4.Mortensen MB, Falk E. Primary prevention with statins in the elderly. J Am Coll Cardiol 2018; 71: 85–94. [DOI] [PubMed] [Google Scholar]
- 5.Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 2019; 393: 407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2019; 139: e1082–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020; 41: 111–88. [DOI] [PubMed] [Google Scholar]
- 8.Fuster V Changing demographics: a new approach to global health care due to the aging population. J Am Coll Cardiol 2017; 69: 3002–05. [DOI] [PubMed] [Google Scholar]
- 9.Nanna MG, Navar AM, Wang TY, et al. Statin use and adverse effects among adults >75 years of age: insights from the patient and provider assessment of lipid management (PALM) registry. J Am Heart Assoc 2018; 7: e008546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko DT, Mamdani M, Alter DA. Lipid-lowering therapy with statins in high-risk elderly patients: the treatment-risk paradox. JAMA 2004; 291: 1864–70. [DOI] [PubMed] [Google Scholar]
- 11.Bach RG, Cannon CP, Giugliano RP, et al. Effect of simvastatin-ezetimibe compared with simvastatin monotherapy after acute coronary syndrome among patients 75 years or older: a secondary analysis of a randomized clinical trial. JAMA Cardiol 2019; 4: 846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation 2019; 140: 992–1003. [DOI] [PubMed] [Google Scholar]
- 13.Sinnaeve PR, Schwartz GG, Wojdyla DM, et al. Effect of alirocumab on cardiovascular outcomes after acute coronary syndromes according to age: an ODYSSEY OUTCOMES trial analysis. Eur Heart J 2020; 41: 2248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amarenco P, Kim JS, Labreuche J, et al. A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med 2020; 382: 9–19. [DOI] [PubMed] [Google Scholar]
- 15.Sever P, Gouni-Berthold I, Keech A, et al. LDL-cholesterol lowering with evolocumab, and outcomes according to age and sex in patients in the FOURIER trial. Eur J Prev Cardiol 2020; published online Feb 4. 10.1177/2047487320902750. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–69. [DOI] [PubMed] [Google Scholar]
- 17.Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med 2001; 20: 3875–89. [DOI] [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viechtbauer W Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36: 1–48. [Google Scholar]
- 20.Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet 2017; 389: 1323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odden MC, Coxson PG, Moran A, Lightwood JM, Goldman L, Bibbins-Domingo K. The impact of the aging population on coronary heart disease in the United States. Am J Med 2011; 124: 827–33.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luirink IK, Wiegman A, Kusters DM, et al. 20-year follow-up of statins in children with familial hypercholesterolemia. N Engl J Med 2019; 381: 1547–56. [DOI] [PubMed] [Google Scholar]
- 23.Pencina MJ, Pencina KM, Lloyd-Jones D, Catapano AL, Thanassoulis G, Sniderman AD. The expected 30-year benefits of early versus delayed primary prevention of cardiovascular disease by lipid lowering. Circulation 2020; 142: 827–37. [DOI] [PubMed] [Google Scholar]
- 24.Nicholls SJ, Tuzcu EM, Sipahi I, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 2007; 297: 499–508. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan H, Thompson RC, Trumble BC, et al. Coronary atherosclerosis in indigenous South American Tsimane: a cross-sectional cohort study. Lancet 2017; 389: 1730–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ference BA, Bhatt DL, Catapano AL, et al. Association of genetic variants related to combined exposure to lower low-density lipoproteins and lower systolic blood pressure with lifetime risk of cardiovascular disease. JAMA 2019; 322: 1381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afilalo J, Duque G, Steele R, Jukema JW, de Craen AJ, Eisenberg MJ. Statins for secondary prevention in elderly patients: a hierarchical bayesian meta-analysis. J Am Coll Cardiol 2008; 51: 37–45. [DOI] [PubMed] [Google Scholar]
- 28.Orkaby AR, Driver JA, Ho YL, et al. Association of statin use with all-cause and cardiovascular mortality in US veterans 75 years and older. JAMA 2020; 324: 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016; 388: 2532–61. [DOI] [PubMed] [Google Scholar]
- 30.Ference BA, Cannon CP, Landmesser U, Luscher TF, Catapano AL, Ray KK. Reduction of low density lipoprotein-cholesterol and cardiovascular events with proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors and statins: an analysis of FOURIER, SPIRE, and the Cholesterol Treatment Trialists Collaboration. Eur Heart J 2018; 39: 2540–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


