Abstract
PURPOSE
This study aimed to examine the effects of treadmill training on anxious-depressive-like behaviors of transgenic Alzheimer rats in the early stage of Alzheimer’s disease (AD) and provided evidence of exercise in alleviating fear-avoidance behavior deficits.
METHODS
Male 2-month-old TgF344-AD and wild-type (WT) rats were divided into WT (n = 9), AD (n = 8), and AD + treadmill exercise (Exe) groups (n = 12). After 8 months of exercise, the passive avoidance test, Barnes maze task, novel object recognition test, and object location test were used to measure learning and memory function. The open field test, elevated plus maze, sucrose preference test and forced swim test were conducted to determine the anxious-depressive-like behavior of AD rats. Immunofluorescence staining, Western blot analysis, enzyme-linked immunosorbent assay (ELISA) analysis, and related assay kits were used to measure inflammatory cytokines, oxidative stress, amyloid-beta production, and tau hyperphosphorylation.
RESULTS
Behavioral tests revealed that 12-month old animals did not show any spatial learning and memory deficits but did display anxious-depressive-like behavior (open field, Center time: P = 0.008; Center entries: P = 0.009; Line crossings: P = 0.001). However, long-term exercise significantly inhibited anxious-depressive-like behavior in AD rats (Center time: P = 0.016; Center entries: P = 0.004; Line crossings: P = 0.033). In addition, these animals displayed increased Aβ deposition, Tau hyperphosphorylation, microgliosis, inflammatory cytokines release, and oxidative damage, which were attenuated significantly by long-term exercise training.
CONCLUSION
Long-term exercise training alleviated anxious-depressive-like behavior and improved fear-avoidance behavior in transgenic AD rats, supporting exercise training as an effective approach to prevent anxiety, depression and fear-avoidance behavior deficits in the early stages of AD pathogenesis.
Keywords: Treadmill exercise, Anxiety, Depression, Learning and memory, TgF344-AD rats
Introduction
Alzheimer’s disease (AD) is an irreversible, progressive brain disorder characterized by memory loss and cognitive decline (1). AD is the most common form of dementia, responsible for about 60–70% of cases of dementia (2). Extracellular amyloid plaques and intracellular neurofibrillary tangles are considered the main hallmarks of AD, contributing to and exacerbated by neuroinflammation, gliosis, oxidative damage, and metabolic energy failure, which culminate in the degradation and death of neurons (1). In addition to these well-known features, AD also causes other symptoms that do not attract enough attention during the treatment or prevention of AD, including anxiety and depression during the early and middle stages of the disease (3–5).
A small number of studies have implicated anxious-depressive-like behavior as both predictors and a causal factor accelerating AD progression. Recently, clinical studies demonstrated increased anxious-depressive symptoms in preclinical AD patients (6, 7). These studies reported a high occurrence of anxiety symptoms in AD patients compared with age-matched controls, and, in an epidemiological study, individuals with psychological distress or anxiety were found more likely to be diagnosed with AD in the future (8–10). Consistent with the studies on patients, experiments using AD mouse models also demonstrated a close relationship between anxiety-like behaviors and extensive cerebral plaque formation and neurofibrillary tangle burden (11–13). Additionally, a recent study using the novel TgF344-AD rat model confirmed the presence of increased anxiety-like behavior at the early stages of AD and suggests that anxiety-like behavior is an early endophenotype in the TgF344-AD rat model of Alzheimer’s disease (14). Although a large body of studies has recognized anxiety or depression as early predictors of future AD (15, 16), to the best of our knowledge, the effect of exercise training on anxious-depressive symptoms in the progression of AD is largely unknown.
As of now, the Food and Drug Administration has not approved any drugs for Alzheimer’s-related anxiety and depression. General anti-anxiety and depression medications are short-term approaches with many unwanted side effects (17, 18). Compared with the high pharmaceutical research costs and heavy medical and financial burden on both patients and society, exercise has received increasing attention as a non-pharmacological intervention in recent years (1). The beneficial role of exercise in the treatment and the prevention of AD has been reported by way of improving cerebral blood flow, increasing hippocampal volume, and decreasing both neuroinflammation and oxidative stress (1, 19). In addition, numerous studies have demonstrated the effects of exercise on depression and anxiety. For instance, supervised aerobic exercise with moderate intensity was confirmed as an effective method in the treatment of depression (20). Recently, a cross-sectional study including 269 individuals aged 18–45 years found that individuals performing regular exercise had a lower frequency of depression than those without exercise interventions (21). Although there is lack of data from rigorous, methodologically randomized, and controlled clinical trials, evidence from 12 randomized control trials (RCTs) revealed exercise may be a useful treatment for anxiety (22). The HUNT study, a longitudinal population study in Norway, reported that regular leisure-time exercise with any intensity conferred a beneficial effect on depression but not anxiety (23).
Using a battery of behavioral tests, we conducted this current study to analyze the effects of long-term treadmill exercise training on learning and memory changes and anxious-depressive-like behavior during the early stages of AD pathogenesis using a novel TgF344-AD rat model.
Methods
Animals and Experimental Design
Male TgF344-AD rats were used for the purposes of this study. Rats were maintained in propylene cages in an environment with an ambient temperature of 22–24°C, moisture of 50–60% under 12-h light/dark cycle. As shown in Fig. 1A, three groups of experimental animals (2-month old male rats) were included in the present study: (1) WT animals (n = 9), (2) TgF344-AD rats (n = 8) and (3) TgF344-AD rats with exercise (n = 12). TgF344-AD rats were originally generated on a Fischer 344 background. The Fischer 344 background animals were co-injected rat pronuclei with two human transgenes driven by the mouse prion promoter (Prp): Swedish mutation (K595N/M596L) with human amyloid beta (A4) protein (hAPP) gene and exon 9 mutions with human presenilin 1 gene (PS1) (24). To distinguish the transgene positive animals from transgene negative animals, we performed genotyping using the animal’s tail. As shown in Fig. 1C, a portion of the animal’s tail tip, not exceeding 5mm in length, was removed using a sterile sharp scalpel while wearing clean gloves. Tail tips were prepared with a mixture of tissue preparation solution and extraction solution at room temperature for 10 minutes. After adding neutralization solution, genomic DNA PCR was conducted using the following primers: R699 mPrp forward: 5’-CCTCTTTGTGACTATGTGGACTGATGTCGG-3’, R699 mPrp reverse: sequence:5’-GTGGATACCCCCTCCCCCAGCCTAGACC-3’ and R699 APP forward sequence: 5’-CCGAGATCTCTGAAGTGAAGATGGATG-3’. The genotyping process was repeated 2 times for each animal and the representative result of a TgF344-AD rat was shown in Fig. 1C. All animal surgery protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Augusta University and were carried out in compliance with National Institutes of Health guidelines. All efforts were made to minimize the pain or distress and reduce the number of animals used in the experiments. A single behavioral task was performed per day during behavioral testing to avoid the confounding effect of stress on the animals.
Figure 1. Schematic diagram of the experimental design and treadmill exercise protocol.
(A) Animals were subjected to genotyping on postnatal day 10 (P10) followed by separation into three groups: WT, sedentary AD group, and AD animals undergoing 8 months of treadmill exercise (n = 8–12). For the exercise group, treadmill exercise training was initiated at 2 months of age, continuing to 10 months. Behavioral tests were conducted at 12 months. (B) Weights of the rats were measured prior to exercise training, the end of training, and 2 months after the cessation of training. (C) Overview of the genotyping and representative results of PCR genotyping. Samples from AD animals show a 400bp APP Tg product. (D) Two weeks of adaptive training was carried out at the beginning of the exercise program with an initial intensity of 15 min of continuous running at 4 m/min on Monday (Mon, first week), 6 m/min for 30 min on Wednesday (Wed, first week), and 8 m/min for 45 min on Friday (Fri, first week). On the second week, exercise was performed at 8 m/min on Mon, 12 m/min on Wed, and 18 m/min on Fri for 45 min each day. After the adaptive training stage, treadmill exercise was performed at 18 m/min for 45 min, 3 days/week for the duration of the exercise training stage.
Treadmill Exercise
As shown in Fig. 1D, treadmill exercise training was initiated at 2 months of age and ended when the animals were 10 months old. Training was divided into two stages: the adaptive training stage (first two weeks) and exercise training stage (from the third week to animals 10th month). Exercise was carried out 3 times/week (Monday, Wednesday and Friday) in both the adaptive training stage and exercise training stage. During the adaptive training stage, the treadmill exercise consisted of 15 min of continuous running at 4 m/min on day 1 (Monday, first week), 6 m/min for 30 min on day 3 (Wednesday, first week), and 8 m/min for 45 min on day 5 (Friday, first week). On the second week, exercise was performed at 8 m/min on Monday, 12 min/min on Wednesday, and 18 m/min on Friday for 45 min followed by constant intensity for 3 days/week at 18 m/min during exercise training stage. Exercise was performed at night between 7: 00 p.m. and 10: 00 p.m. under dark room conditions to avoid the confounding stress of interrupting the natural circadian rhythm of the rats. As a measure of efficacy of the training regimen, animal weight was measured at the beginning of training, the end of training, and 2-months after training cessation. Weight reduction was confirmed in 10-month Exe rats, compared with AD group, and was sustained up to the 12-month time point, as expected (Fig. 1B).
Passive Avoidance Task
The passive avoidance task is a fear-motivated test applied to evaluate fear-based conditioned avoidance learning and memory/behavior in rodents, based upon the relationship formed between a specific aversive event and a specific environmental context. In this task, the normal animals learn to avoid an aversive event by not entering the specific environment (25). This test is performed in two equal size compartments: lighted (white and illuminated by a 24 V-10 W bulb) and dark (black and dark) boxes with a gate between the two chambers. Testing was divided into 2 phases: acquisition (training) phase and test phase. During the first training phase, animals were placed in the lighted box and allowed them a maximum of 5 min to cross the gate and enter into the dark box. After entering the dark box, animals in the dark box received a 2 s mild foot shock with the gate closed. Each animal was allowed 20 s in the dark box for habituation after the foot shock (0.5 mA). After 20 s, animals were removed from the apparatus and returned to their home cage. On day 2 (test phase), tests were performed similarly to the acquisition (training) without administering a foot shock in the dark box. An overhead camera recorded the latency to enter dark box. The data was analyzed using ANY-maze video tracking software (Stoelting; Wood Dale, IL, USA).
Barnes Maze Task
The Barnes maze is a widely accepted test of hippocampal dependent spatial learning and memory in animals (1, 26). The Barnes Maze consists of a circular platform with 18 holes serially around its perimeter and a black escape box (20 ×15 × 12 cm) hidden under one of these holes in which the animal can hide. The 122 cm diameter platform we used in this experiment was elevated 1 m above the floor with an overhead incandescent light (500 W, 1000 lux) shining down on the platform surface. In this test, animals instinctively seek out the hole above the black escape box to escape aversive stimuli, including a loud (60 dB) repetitive tone. The Barnes maze task included 3 days of once daily 3 min training trials, followed by a single 90 s probe test on day 4. During training trials, the researcher allowed the rats up to 3 min to find and enter the black escape box. The time taken for the animal to locate and enter the escape box is defined as the escape latency. If an animal cannot find the escape box within 3 min, the researcher gently directed it to find the location of the box. When the animal entered the escape box, the hole was covered and the animal remained in the box for 30 s to habituate. Researchers trained the animals once a day for three continuous training days. On the probe test day, after 3 days of training, the escape box was removed and the corresponding hole was blocked. The escape latency and time spent in the target quadrant where the target box located were recorded and analyzed by ANY-maze video tracking software (Stoelting; Wood Dale, IL, USA).
Novel Object Recognition (NOR) Test
The novel object recognition test is a widely used behavioral test to assess hippocampus-dependent recognition memory based on the tendency for animals to spend more time investigating a novel object than a familiar one (1). To test recognition, rats were placed in an empty box (40 × 50 × 50 cm) for habituation one day before the test. On the first day of the test (familiarization phase), the rats received 5 min to explore the two identical objects in the recognition box. On the second day (24 hours later, test phase), the researcher returned the rats to the open-field recognition box with two objects, the initial object explored during the familiarization phase and a newly introduced novel object. In this phase, each animal received 5 min to explore the two objects freely. ANY-maze video tracking software recorded the time spent exploring the objects. The discrimination index was calculated according to the following formula: Discrimination index (time spent on novel objects / total time spent on both exploring objects) × 100%. Exploration of an object was defined as the animal placing its nose within 2 cm of the zone where the object located.
Object Location Test (OLT)
To measure spatial cognitive ability, the object location test was conducted, as described previously by our laboratory (27). On the familiarization phase, the animals received 5 min to explore two identical objects fixed in the recognition box as described above. In the test phase, animals were allowed to explore one object in the original location and the other in a novel location for another 5 min. The time spent on objects exploration was recorded, analyzed and calculated in the same way as described in the NOR test.
Open Field Test and Defecations in Open Field
The open field test is a classical experimental test performed to assess general locomotor and anxiety-related behavior (28). The apparatus is a square-shaped enclosure consisting of a black wooden floor (56 cm × 56 cm) surrounded by 50 cm-tall walls. At the start of the test, animals were placed at the corner of the open field and allowed 5 min to explore freely. During this 5 min in exploration, the ANY-maze video tracking software recorded the number of line crossings, percentage of time spent in the center zone (26 × 26 cm), and number of entries in the central zone. In addition, the researcher also counted the number of defecations after removing the animal.
Elevated Plus Maze
The Elevated Plus maze was used to detect anxiety-like behavior (29). The apparatus consists of two opposing closed arms (50 cm × 10 cm) with 50 cm high walls of the same dimensions, two open arms and a central platform (10 cm × 10 cm) at the intersection of the open arms and closed arms. The plus maze was elevated 50 cm above the floor. During the 5-min test, the animal was first placed on the central platform. The time spent in the open arms and the numbers of open arms entries were recorded. Entry to the open arms was defined as all four paws within the open arms area. ANY- maze video tracking system was used to control the camera and record the data.
Sucrose Preference Test
The sucrose preference test was performed to investigate depression-like behavior based on the animal’s natural preference for sweets (30). Prior to testing, animals were first housed for 2 days with two bottles of plain water, followed by the 2% sucrose solution for another 2 days. Following this acclimation, rats were then deprived of water for 24 h, followed by free choice of either 2% sucrose solution or plain water for 8 h. Sucrose preference was calculated as the volume of sucrose consumed/total volume of water and sucrose intake.
Forced Swim Test
The forced swim test is a widely used test to study depressive-like behavior in rodents (28). The rat was gently lowered into a plastic cylinder filled with 30 cm deep, room temperature (25°C) water for 6 min. Immobility is defined as when the rat adopts an immobile posture, stays in one place, and treads water at the minimum pace necessary to keep its nose and eyes above water. The animals did not bob and push off the bottom of the cylinder when immobile (as the water is 30 cm deep). Longer immobile time is proposed to be related to increased “behavioral despair”. ANY-maze video tracking system recorded immobility time.
Brain Collection and Tissue Preparation
As described previously (27), animals were sacrificed under deep isoflurane anesthesia and the brains were quickly removed following perfusion with ice-cold saline. One side of the brains were quickly separated into hippocampal and cortical samples and frozen in liquid nitrogen. For tissue homogenization, a motor-driven Teflon homogenizer was applied with 400 μl ice-cold homogenization buffer (50-mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [HEPES], pH 7.4, 150-mM NaCl, 12-mM β-glycerophosphate, 1% Triton X-100), protease and phosphatase inhibitors. The homogenates were then vigorously mixed for 20 min on a rotator and centrifuged at 15,000 × g for 30 min at 4 °C to get total protein samples from the cortex and hippocampus. The remaining hemisphere was post-fixed in 4% paraformaldehyde at 4 °C for 48 h followed by cryoprotection with 30% sucrose solution. Leica Rm 2155 microtome was used for frozen sections (25 μm each) collection. The collected brain sections were stored in stock solution (FD NeuroTechnologies, Inc., Columbia, MD, USA; Catalog number: PC101) for subsequent immunofluorescence staining.
Immunofluorescence Staining and Confocal Microscopy
Immunofluorescence staining and confocal microscopy were performed using a protocol described in previous study (1). In brief, coronal brain sections (n= 5–6 per group) were permeabilized with 0.4% Trition X-100 and blocked with 10% normal donkey serum, followed by incubation with appropriate primary antibodies overnight at 4 °C. In this study, we used the following primary antibodies: β-Amyloid, 17–24 antibody (clone 4G8) antibody (1: 300, BioLegend; San Diego, CA, USA; Catalog number: 800703), PHF1 (1: 500, Thermo Scientific; Rockford, IL, USA; Catalog number: PA5–56621), Iba-1 (1: 200; Wako Chemicals; Richmond, VA, USA; Catalog number: 019–19741), 8-OHdG (1: 200; Abcam; Cambridge, MA, USA; Catalog number: ab48508). Sections were then washed 3 times in 0.4% Trition X-100 for 30 min each, followed by incubation with appropriate Alexa Fluor donkey secondary antibodies (594/647/488, Thermo Fisher) for 1 h at room temperature. Thereafter, brain sections were washed, coverslipped and sealed in DAPI Fluoromount-G (SouthernBiotech; Birmingham, AL, USA).
For amyloid deposition, brain sections were directly stained with Thioflavin S (ThioS) (1 mg/mL, Sigma-Aldrich; St. Louis, MO, USA) for 8 min at room temperature and then washed, coverslipped and sealed with clear nail polish. For detection of reactive oxygen species (ROS), dihydroethidium (DHE) staining (Anaspec; Fremont, CA, USA) was performed as described in previous study (26). Briefly, the nonfixed brain sections were stained with DHE (10 μM) in the dark for 10 min at room temperature. After 3 washes, sections were mounted and sealed for confocal microscopy. All the fluorescence images were captured and analyzed by LSM700 Meta confocal laser microscope (Carl Zeiss). For quantitative analysis of the number of plaques, plaques in the cortex and hippocampus of one hemisphere were counted in 5–6 animals from each group.
Western Blotting Analysis
Western blotting was performed as previously described (19). Proteins (50 μg/lane, n=4–5) were separated on 4–20% sodium dodecyl sulfate-polyacrylamide gel (SDS) and transferred onto polyvinylidene difluoride (PVDF) membrane. The PVDF membranes were then blocked with 3% blocking buffer (1X TBS-T with 3% Bovine Serum Albumin (BSA) and 0.05% Tween20) and incubated at 4 °C overnight with the following antibodies: Iba-1 (1: 200, Proteintech group; Rosemont, IL, USA; Catalog number: 10904–1-AP), PHF1 (1: 200, Thermo Scientific; Rockford, IL, USA; Catalog number: PA5–56621), 4-Hydroxynonenal (4-HNE) (1: 200; Abcam; Cambridge, MA, USA; Catalog number: ab46545) or 5-HTR6 (1:300; Novus Biologicals; Centennial, Colorado, USA; Catalog number: NBP 1–46557). After 3 rinses, the membrane was then incubated with appropriate HRP-conjugated secondary antibodies for 1 h at room temperature. A cold CCD digital imaging system was used to capture the images of bound protein, and Image J software (NIH, USA) was applied for semi-quantitative analyses of the bands. Band densities for the indicated proteins were normalized to loading control. Ponceau S staining was used as loading control for Western blots. In brief, the membrane was transferred to 5 ml ponceau S staining solution for 5 min, followed by 3 washes lasting 5 minutes each to remove the background staining.
Inflammtatory Cytokines Assay and Proteome Profiler Rat Cytokine Analysis
The levels of inflammatory cytokines were measured via enzyme-linked immunosorbent assay (ELISA). ELISA was performed to measure the levels of interleukin-1β (IL-1β), Tumor necrosis factor-alpha (TNF-α), and nuclear factor-kappaB (NF-κB) as described in detail by a previous study (1). In brief, samples were diluted to 50 μl containing the same amount of proteins (20 μg) from the cortex and hippocampus. The samples from different groups, in duplicate, were then loaded into polyvinyl chloride ELISA microplate (Corning) and incubated at 4 °C overnight. Thereafter, the plate wells were washed and then blocked with 200 μl blocking buffer (1% bovine serum albumin in PBS, 0.3% solution of H2O2) for 1 h. Afterwards, the specific antibodies were added and incubated for 4 h at 37 °C. Following 3 washes, the plates were then incubated with HRP-conjugated secondary antibodies for 1 h at room temperature. Finally, the plate was washed and incubated with TMB (3, 3’, 5, 5’-tetramethylbenzidine) solution (Thermo Fisher) for 30 min. Absorbance were read at 450 nm using spectrophotometer (Bio-Rad; Hercules, CA, USA) after adding 50 μl stop solution (sulfuric acid). Data were calculated and expressed as percent changes versus WT group.
Expressions of inflammatory cytokines were also investigated using a Proteome Profiler Rat Cytokine Array Kit (#ARY008, R&D Systems, Inc., MN, USA) following manufacturer’s instructions. Briefly, the membranes provided in the kit were blocked for 1 h, and 800 μg of protein from per group were mixed with a reconstituted detection antibody cocktail followed by incubation with the membrane. Inflammatory cytokines were captured in duplicate using capture antibodies targeting specific inflammatory cytokines, and the duplicated dots (captured proteins) in the membrane were visualized using chemiluminescence system described above. The density of each pairs of dots were analyzed with Image J software and expressed as percent changes versus WT groups.
Total antioxidant capacity assay
Total antioxidant capacity was measured using an antioxidant assay kit (709001; Cayman Chemical; Ann Arbor, MI, USA) as described (1). Briefly, samples (20 μg) were mixed with 150 μl of chromogen and 10 μl of metmyoglobin in the designated wells from the assay kit. After the addition of working solution (40 μl of hydrogen peroxide) to the wells, the plates were then sealed and incubated for 5 min. The absorbance was measured at 750 nm on a spectrophotometer. The antioxidant capacity of each sample was calculated using trolox standard curve which was calculated by Trolox concentration and absorbance. The values were expressed as percentage changes compared to the control group.
Protein Carbonyls Determination
The level of protein carbonyls was measured by the Protein Carbonyl Colorimetric Assay Kit (Cayman chemical, Ann Arbor, Michigan, USA; Item No. 10005020) following the manufacturer’s instruction. In brief, samples from each group (20 μg) were denatured by 5 μl of 12% SDS followed by addition of 1X DNPH Solution (10 μl). After incubation for 15 min at room temperature, samples were mixed with 7.5 μl of neutralization solution and then loaded in PVC ELISA microtiter plate and the levels of protein carbonyls were measured by ELISA analysis.
Measurement of GSH level
Samples (20 μg) from different groups were mixed with 10 μl of O-phthalaldehyde (1 mg/ml) and 180 μl Potassium phosphate EDTA (KPE) Buffer (0.1 M potassium phosphate buffer containing EDTA (5 mM), pH 7.5) in a black 96 well plate followed by incubation in the dark at room temperature for 10 min. The fluorescence of each samples was detected at excitation wavelengths (λex) = 335 nm and emission wavelengths (λem) = 420 nm in a microplate reader.
Measurement of serotonin (5-HT) levels
The levels of serotonin (5-HT) were measured via double-antibody sandwich ELISA method. In brief, the samples (20 μg) were incubated in a microplate pre-coated with 5-HT antibodies at 37 °C for 40 min. After 5 washes, biotinylated-5-HT antibodies (50 μl) were added and incubated at 37 °C for 20 min. Thereafter, the HRP-Strepavidine enzyme conjugate were added into wells and incubated at 37 °C for 10 min. Finally, substrate solutions were then incubated at 37 °C for 15 min in dark. After adding 100 μl stop solution, the absorbances were read at 450 nm using a spectrophotometer (Bio-Rad)
Statistical analysis
Statistical analyses were performed using ANY-maze video tracking software (Stoelting; Wood Dale, IL, USA) and SigmaStat (Systat Software; San Jose, CA, USA). All dependent variables were analyzed by one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls (S-N-K) post hoc tests. For the data of escape latency from the Barnes maze training trials, multiple groups comparisons were conducted with both mixed one-way ANOVA and two-way ANOVA followed by Tukey’s all pairwise comparisons test to determine differences among different groups, different days, and the differences between each group individually. All data were expressed as the mean ± SEM. P < 0.05 indicated significant difference between groups.
Results
Treadmill Exercise Improved Fear-avoidance Behavior
To examine the effect of exercise on cognitive function of 12-month old TgF344-AD rats, we conducted the passive avoidance task and Barnes maze task, which test fear-avoidance behavior and hippocampus-dependent contextual memory, respectively. As shown in Fig. 2A (a), rats received a training trial of the passive avoidance test in the acquisition (training) phase and a testing trial in the test phase. There were no significant differences in latency of entrance to the dark box among the three groups during the training trial (WT vs AD: P = 0.906; AD vs Exe: P = 0.252), as depicted in Fig. 2A (b), suggesting that there were no differences among groups before the electrical shock. However, AD animals displayed remarkable fear-avoidance behavior deficits compared with WT animals (P < 0.001), as evidenced by AD animals exhibiting a significant decrease in latency to enter into the dark box on the testing trial compared with WT animals (Fig. 2A (b)). In addition, results show that exercise training significantly improved these fear-avoidance behavior deficits, with exercise-treated animals exhibiting a latency time significantly longer than that of AD animals (Fig. 2A (b), P = 0.002). Animals underwent the Barnes maze task to investigate their hippocampus-dependent spatial reference learning and memory (Fig. 2B). Data demonstrated that there were no significant differences among three groups in escape latency (Day 1, WT vs AD: P = 0.483; AD vs Exe: P = 0.448; Day 2, WT vs AD: P = 0.913; AD vs Exe: P =0.655; Day 3, WT vs AD: P = 0.806; AD vs Exe: P = 0.711) and the quadrant occupancy (WT vs AD: P = 0.420; AD vs Exe: P = 0.504), suggesting animals from these three groups have comparable spatial learning and memory abilities in the Barnes maze test. However, animals from WT and AD exercise groups displayed able learning ability across the training trials, with steady reduction in latency between day 1 and day 3 (WT: Day 1 vs Day 3, P = 0.004; AD: Day 1 vs Day 3, P < 0.001; Exe: Day 1 vs Day 3, P < 0.001). Furthermore, the novel object recognition test and the object location test were applied to assess hippocampus-dependent recognition memory in the rodents (Fig. 2C). There were no significant differences in time spent exploring the novel object in the original location (WT vs AD: P = 0.590; AD vs Exe: P = 0.902) and the original object in new location (WT vs AD: P = 0.850; AD vs Exe: P = 0.730) among the three groups, suggesting 12-month-old AD animals have normal recognition memory.
Figure 2. Treadmill exercise training improve fear-avoidance behavior in TgF344-AD rats.
(A) The passive avoidance task was performed to test fear-avoidance behavior. (a) Schematic diagram of the passive avoidance test apparatus. (b) Latency to enter the dark box during the training phase and test phase was recorded and analyzed. (B) Representative tracking plots of rats on the training days (a-c) and probe trial day (e-g). Escape latency (d) and quadrant occupancy (h) were recorded and statistically analyzed. (C) The novel object test and object location test were performed to assess recognition memory and spatial cognitive ability. (a-c) Representative occupancy plots of animals’ exploration of the novel object (red) and the familiar object (yellow) in the novel object test (a-c). The time spent on exploring each object and the discrimination index percentage were analyzed (d). The representative occupancy plots and discrimination index of animals in object location test are shown in (e-h). All data are presented as mean ± SEM (n= 8–12). *P < 0.05 vs WT group, #P < 0.05 vs AD group. N.S., no significant difference.
Treadmill Exercise Alleviated Anxious-Depressive-Like Behavior of TgF344-AD Rats
To investigate the effect of treadmill exercise on anxiety-like behaviors in AD rats, animals from all groups were subjected to the open field test, a widely used test for anxiety. As shown in Fig. 3A, AD rats exhibited significantly decreased time spent in the center zone (P = 0.008), entries into the center zone (P = 0.009), and number of line crossings (P =0.001) compared with animals from WT group, features which were significantly alleviated in rats undergoing treadmill exercise training (Exe vs AD: P = 0.016 for time in the center zone; P = 0.004 for entries in the zone; P = 0.033 for number of line crossing). The results of the open field test indicate AD animals had attenuated exploratory behavior and preferred to stay close to the walls of the box compared with animals from WT and treadmill exercise groups. These differences were not present at baseline in the 2-month-old rats (Fig. 3A). In addition, we counted the number of defecations in the maze after each test once the rat was removed from the open field. AD rats exhibited significant increases in the number of defecations compared with WT rats (P = 0.002), while treadmill exercise prevented this increase in AD rats (P = 0.001, as depicted in Fig. 3A (f)). We also tested anxiety-like behavior using the elevated plus maze. As demonstrated by representative traces in Fig. 3B, AD animals were markedly less active in the open arms of the maze compared with the WT animals and exercise AD animals, as evidenced by decreased total entries (AD vs WT: P = 0.001; Exe vs AD: P = 0.015) and total time spent in the open arms ((AD vs WT: P = 0.045; Exe vs AD: P = 0.021) (Fig. 3B (b, c and d)). These differences were also not present at baseline in the 2-month-old rats (Fig. 3B (a, c and d)).
Figure 3. Treadmill exercise training significantly ameliorates anxious-depressive-like behavior in TgF344-AD rats.
(A) Schematic of zones in the open field arena and the representative traces of rats’ movement tested on 2-month-old (Baseline, a) and 12-month-old rats (b) during an open field test. Number of entries to the central area (c), time in center (d), number of line crossings (e), and defecations in field (f) were determined in the open field test. (B) Representative activity traces of animals in the elevated plus maze at baseline and 12-months were shown in (a&b). The time spent in the open arms and the numbers of entries to the open arms were analyzed in (c&d). (C) Schematic diagram of the sucrose preference test (a) and the percentage of the sucrose intake volume over the total fluid intake volume (b). (D) Schematic representation of mobility and immobility in the forced swim test (a). The total time of immobility was statistically analyzed (b). All data are presented as mean ± SEM (n = 8–12). *P < 0.05 vs WT group, #P < 0.05 vs AD group. N.S., no significant difference.
We next examined whether TgF344-AD rats aged 12 months exhibit depression-like behaviors using the sucrose preference test, a widely used reward-based test for depression. As shown in Fig. 3C, both WT and exercise rats had a significant preference for sucrose compared to AD rats (WT vs AD: P = 0.019; Exe vs AD: P = 0.027), suggesting 12-month-old AD animals exhibit depression-like behaviors which could be effectively prevented by exercise training. We did not observe these differences during baseline testing at 2 months of age (Fig. 3C (b)). In addition, we tested the animals using the forced swim test, which measures perseverance to identify a depressive phenotype, to confirm this observation. The results of the forced swim test, depicted in Fig. 3D, revealed that AD rats exhibited increased total immobility time compared with WT animals (P = 0.013). The immobility time of exercise-trained AD rats, however, was significantly reduced compared to AD non-exercised animals (P = 0.006), further confirming the beneficial effect of treadmill exercise in alleviating the depression-like behaviors present in the TgF344-AD rats. Taken together, these data indicate that treadmill exercise alleviated the anxious-depressive-like behavior in TgF344-AD rats.
Treadmill Exercise Attenuated β-Amyloid Pathology and Tau Hyperphosphorylation in TgF344-AD Rats
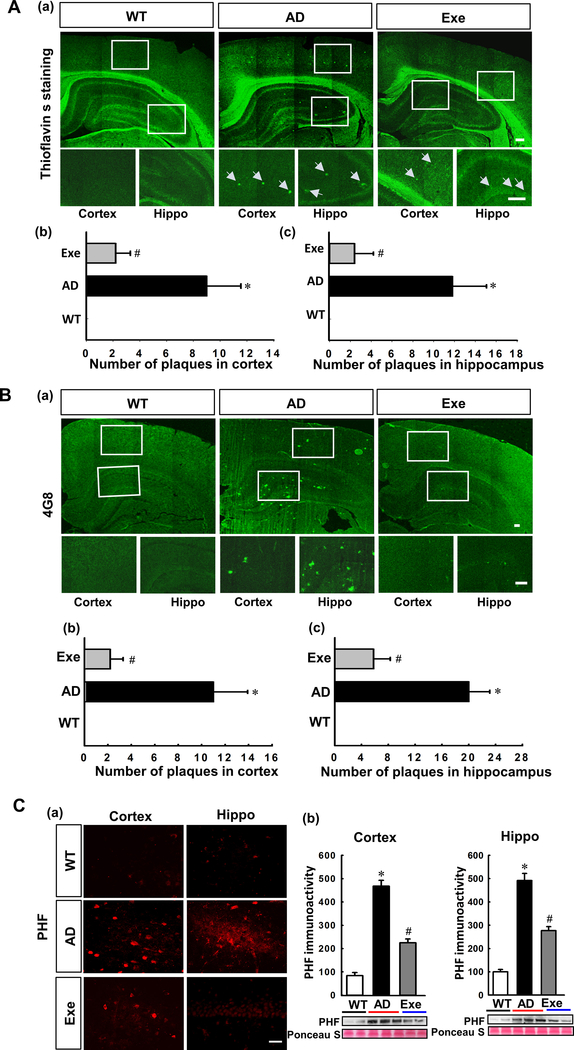
We next examined the effect of treadmill exercise training on several well-established AD biomarkers. Using ThioS staining and confocal microscopy Fig. 4A (a, b, and c), the number of plaques in the cortex and hippocampus were then counted and analyzed, revealing substantial amyloid deposition in AD rats compared with WT group (Cortex: P < 0.001; Hippocampus: P < 0.001) that was prevented by exercise training (Cortex: P < 0.001; Hippocampus: P < 0.001). In addition, we detected levels of Aβ (17–24) using 4G8 antibodies, and representative confocal microscopy images (Fig. 4B (a)) indicate significantly elevated levels of Aβ (17–24) in AD rats, as compared to the WT group (Cortex: P < 0.001; Hippocampus: P < 0.001). In contrast, exercise training significant alleviated this increase (Exe vs. AD, Cortex: P < 0.001; Hippocampus: P < 0.001) (Fig. 4B (b and c)). Hyperphosphorylated tau (PHF) is another hallmark of AD, and plays an important role in AD pathology. As shown in Fig. 4C, the results of immunofluorescence staining and Western blotting showed that the cortex and hippocampus presented remarkably increased PHF signal in AD group rats (AD vs. WT, Cortex: P < 0.001; AD vs. Hippocampus: P < 0.001) that were abated by treadmill exercise training (Exe vs. AD, Cortex: P < 0.001, Hippocampus: P < 0.001).
Figure. 4. Treadmill exercise training attenuates β-amyloid deposition and tau hyperphosphorylation in the cortex and hippocampus of TgF344-AD rats.
(A) Representative confocal microscopy images of ThioS staining (a) for WT, AD and AD+Exe groups in the cortex and hippocampus. Number of plaques in the cortex and hippocampus were counted and analyzed (b&c). Scale bar denotes 200 μm. (B) Sections from WT, AD and AD+Exe were subjected to immunofluorescent staining of Aβ (17–24) (clone 4G8). Representative confocal microscopy images were shown in (a). Number of Aβ plaques in the cortex and hippocampus were counted and analyzed (b&c). Scale bar denotes 20 μm. (C) Phosphorylation of tau protein (PHF) was examined by immunofluorescent staining (a) Scale bar represents 20 μm. Results of Western blot analysis of PHF were shown in (b) to further confirm the results of immunofluorescent staining. Ponceau S staining was used as loading control for Western blots, as shown in (b). All values are expressed as mean ± SEM. *P < 0.05 vs WT group, #P < 0.05 vs AD group. Hippo, Hippocampus. Areas enclosed in white boxes were enlarged below their respective image.
Treadmill Exercise Training Reduced Microgliosis and Suppressed Pro-inflammatory Cytokines Production in Hippocampus and Cortex of TgF344-AD Rats
Neuroinflammation is a characteristic pathology of AD in AD patients and animal models, presenting as increased microgliosis and elevated pro-inflammatory cytokine levels (1, 24). As shown in Fig. 5A, representative confocal microscopy of Iba1 show remarkably increased Iba1 signal in AD rats and a reduced signal in exercise animals, which was confirmed by Western blotting results (AD vs. WT, Cortex: P < 0.001; Hippocampus: P < 0.001; Exe vs. AD, Cortex: P < 0.001; Hippocampus: P < 0.001). In addition, we measured the effect of exercise training on the expression of pro-inflammatory cytokines in the cortex and hippocampus using ELISA. As shown in Fig. 5B, EILSA results demonstrated that the levels of the major proinflammatory transcription factor NF-κB (Fig. 5B (a)), pro-inflammatory cytokines TNFα (Fig. 5B (b)), and IL-1β (Fig. 5B (c)) in AD animals were significantly increased compared with WT animals (NF-κB, Cortex: P = 0.009, Hippocampus: P = 0.002; TNFα, Cortex: P < 0.001, Hippocampus: P < 0.001; IL-1β, Cortex: P = 0.02, Hippocampus: P <0.001), whereas exercise training was able to alleviate major proinflammatory transcription factor and pro-inflammatory cytokine release (NF-κB, Cortex: P < 0.001, Hippocampus: P < 0.001; TNFα, Cortex: P = 0.001, Hippocampus: P = 0.001; IL-1β, Cortex: P = 0.004, Hippocampus: P = 0.007). Furthermore, levels of pro-inflammatory cytokines in tissue proteins (include cortex and hippocampus) were measured using a Proteome Profiler Rat Cytokine Array Kit. Results shown in Fig. 5C indicate that 8 different pro-inflammation related cytokines (IL1-α, IL1-β, IL-1ra, IL-2, IL-3, IL-6, IL-17, TNFα) were markedly increased in AD group rats. In addition, the levels of 3 anti-inflammatory cytokines (IL-4, IL-10 and IL-13) were suppressed in AD animals compared with WT groups. This deleterious pattern of heightened pro-inflammatory signaling and decreased anti-inflammatory signaling was prevented in AD animals undergoing long-term exercise training.
Figure. 5. Treadmill exercise reduces microgliosis and suppresses pro-inflammatory cytokine production in the cortex and hippocampus of TgF344-AD Rats.
(A) Immunofluorescence staining and immunoactivity intensity analyses of IBA1 (a marker of microglia). Representative microscopy images of IBA1 in the cortex and hippocampal CA1 region (a). n=5–6 animals/group. Scale bar represents 30 μm. Western blotting and quantitative analyses of Iba-1 were performed using protein from the cortex and hippocampus (b, n=4). Ponceau S staining of the Western blots shown in (b) was used as loading control. (B) ELISA analyses of major pro-inflammatory transcription factor NF-κB (a), TNFα (b), and IL-1β (c) were performed to examine the effect of treadmill training on the release of inflammatory cytokines. (C) The levels of inflammatory cytokines in the tissue proteins (cortex and hippocampus) were measured using a Proteome Profiler Rat Cytokine Array Kit. All values are expressed as mean ± SEM. *P < 0.05 vs WT group, #P < 0.05 vs AD group.
Treadmill Exercise Training Inhibited Oxidative Stress and Enhances Total Antioxidant Capacity of TgF344-AD Rats
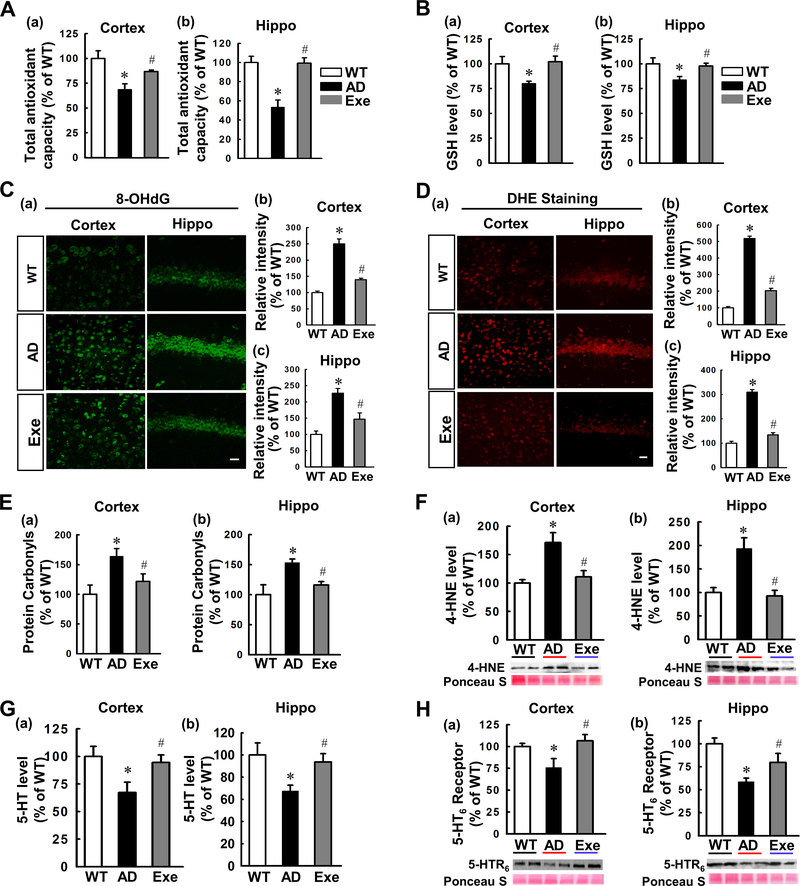
In addition to neuroinflammation, oxidative stress is a known feature of AD pathology (1). As shown in Fig. 6A (a&b), AD animals displayed diminished total antioxidant capacity in both the cortex (P = 0.011) and hippocampus (P = 0.001) compared with WT animals, which was significantly preserved by exercise training (Cortex: P = 0.017; Hippocampus: P = 0.001). Next, we measured GSH, a major antioxidant, in both the cortex (Fig. 6B (a)) and hippocampus (Fig. 6B (b). We found suppressed levels of GSH in both the cortex (P = 0.032) and hippocampus (P = 0.04) of AD animals that was abated by exercise training (Exe vs. AD, Cortex: P = 0.007; Hippocampus: P = 0.013). In addition, the immunoreactivity of 8-OHdG (a pivotal marker for measuring endogenous DNA oxidative damage) was significantly elevated in both the cortex (AD vs. WT, P < 0.001) and hippocampus (AD vs. WT, P < 0.001) of AD animals (Fig. 6C). Intriguingly, exercise profoundly attenuated 8-OHdG signals in both regions (Exe vs. AD, Cortex: P < 0.001; Hippocampus: P = 0.01). Reactive oxygen species (ROS) generation was detected by dihydroethidium (DHE) staining. As shown in Fig. 6D, AD animals exhibited markedly higher ROS levels compared to WT animals (Cortex: P < 0.001; Hippocampus: P < 0.001), whereas the increased levels of ROS were significantly mitigated by exercise training in both the cortex (P < 0.001) and hippocampus (P < 0.001). We further investigated the effect of exercise on protein carbonyls in AD animals (Fig. 6E). AD animals displayed increased protein carbonyl levels ((AD vs. WT, Cortex: P = 0.03; Hippocampus: P = 0.049) which were significantly alleviated by treadmill exercise ((Exe vs. AD, Cortex: P = 0.031; Hippocampus: P = 0.007). Furthermore, as shown in Fig. 6F, Western blotting results revealed that AD animals had a significantly increased 4-HNE content, indicating greater lipid peroxidation (AD vs. WT, Cortex: P = 0.008; Hippocampus: P = 0.009), which was significantly alleviated by treadmill exercise (Exe vs. AD, Cortex: P = 0.026; Hippocampus: P = 0.007).
Figure. 6. Treadmill exercise training reduces oxidative stress, enhances total antioxidant capacity, and improves the levels of 5-HT and its receptor in TgF344-AD.
(A) Total antioxidant capacity and (B) GSH levels in the cortex and hippocampus and were presented as percentage changes versus respective control group (n=5–8). (C) Representative 8-OHdG staining (a DNA damage marker) for WT, AD and Exe in the cortex and hippocampal CA1 region (a). Fluorescence intensity associated with 8-OHdG in the cortex (b) and CA1 (c) was calculated with Image J analysis software and expressed as percentage changes versus WT groups (n=4–5). (D) Representative images of dihydroethidium (DHE) staining in the cortex and CA1 were presented in (a). Fluorescence intensity was quantified and expressed as percentage changes versus control group (n=4–5). (E) Protein carbonyls were detected in the cortex and hippocampus and presented as percentage changes versus respective control group (n=5–8). (F) The levels of 4-HNE (an oxidative damage marker for lipid peroxidation) in the cortex (a) and hippocampus (b) were explored using Western blotting and quantitative analysis (n=4). (G) The levels of 5-HT in the cortex (a) and hippocampus (b) were measured using 5-HT assay kit as detailed in the method (n=5–6). (H) Western blot analysis was performed to measure the level of 5-HT6 receptor. Representative Western blotting and quantification of 5-HT6 receptor in the cortex and hippocampus were shown in (a&b) (n=5). Scale bar represents 20 μm. All values are expressed as mean ± SEM. *P < 0.05 vs WT group, #P < 0.05 vs AD group. Ponceau S staining of the Western blots shown in F and H was used as loading control.
Treadmill Exercise Preserved Levels of 5-HT and the Expression of 5-HT6 Receptor
Serotonin (5-HT) and its receptors are involved in the modulation of cognitive processes and play a role in the regulation of depression and anxiety (31). As shown in Fig. 6G, levels of 5-HT were significantly diminished in AD animals (Cortex: P = 0.033; Hippocampus: P = 0.032), while this decline was mitigated by treadmill exercise (Cortex: P = 0.045; Hippocampus: P = 0.022). Next, we tested expression of the 5-HT6 receptor using Western blot analysis. As shown in Fig. 6H, hippocampal and cortical expression of 5-HT6 receptor in AD group rats was significantly attenuated as compared to WT group (AD vs. WT, Cortex: P = 0.045; Hippocampus: P = 0.002). Intriguingly, the decreased expression of 5-HT6 receptor was inhibited by long-term treadmill exercise (Exe vs. AD, Cortex: P = 0.048; Hippocampus: P = 0.03).
Discussion
In the present study, using a novel rat AD model and numerous well-established behavioral tests, we contributed additional evidence to the growing body of work supporting anxious-depressive-like behavior as an early indicator of AD. As well, our results demonstrate that treadmill exercise training had efficacious effects on preventing fear-avoidance behavior deficits, the primary behavior deficits observed in the TgF344-AD rat model at 12 months of age. Importantly, the present study found that long-term treadmill exercise was able to ameliorate Aβ deposition, tau hyperphosphorylation, neuroinflammation, and oxidative stress, while elevating the levels of 5-HT and the 5-HT6 receptor demonstrating that the early intervention of exercise is crucial for people who are potentially susceptible to AD.
The ideal transgenic AD animal model should be able to mimic multiple aspects of human AD pathology, including its etiology and a time-dependent progression of pathophysiology. Tg AD mice are often considered as the “gold standard” of AD animal models, exhibiting high-level neuronal expression of Aβ. Tg AD mice, however, cannot manifest all the characteristic hallmarks of familial AD, including tauopathy and neuronal apoptosis, without the inclusion of human transgenes unrelated to familial AD (24). In the present study, the TgF344-AD rat model provided a more robust animal model for our investigations with a complete repertoire of AD pathological features (24). Previous studies have reported that both rats and humans have very high levels of 5-hydroxytryptamine 6 (5-HT6) receptor expressions and receptor binding in subcortical brain structures, which has been linked to depression in AD. However, the 5-HT6 expression and receptor binding was relatively low in mice (31). This suggests that rats are more fit for the study of depression than mice. In addition, rats are 4–5 million years closer to humans in biological evolution (24). Tg AD rats are morphologically, physiologically, and genetically more similar to humans than Tg AD mice (24). Taken together, this evidence supports the TgF344-AD rat as the better animal model for the investigation of anxious-depressive-like behavior in AD.
TgF344-AD rats present progressive age-dependent AD pathological features (24). Previous study have demonstrated that expression of two mutant human transgenes (APPsw and PS1△9 genes) was able to cause early-onset familial AD and precipitate the full array of AD pathologies in TgF344-AD rats (24). As a more robust animal model for AD, TgF344-AD rats are characterized by progressive Aβ deposition, tau hyperphosphorylation, neuroinflammation, oxidative stress, neuronal injury, and subsequent neuronal loss. Extracellular cerebral amyloid deposition (Aβ aggregation) and intracellular neurofibrillary tangles (tau phosphorylation) are well-known hallmarks of AD pathology (1). A previous study confirmed age-dependent Aβ accumulation in the TgF344-AD rat brain, although sex differences were not observed. Quantitative histological analysis of Tg AD rats using 4G8 antibodies (Aβ) and ThioS staining revealed significant age-dependent β-amyloid deposition (24). As reported previously (14, 24), 6-month-old AD animals did not show obvious learning and memory deficits, despite the presence of elevated Aβ1–42 levels in brain samples. 15-month-old animals presented significant learning deficits in Barnes maze test. In the current study, 12-month-old transgenic AD animals presented apparent amyloid deposition and tau phosphorylation that was strongly inhibited by treadmill exercise training. Importantly, our study demonstrated that long-term exercise training significantly protected against the typical pathology of these AD rats, contributing further evidence to support the beneficial effects of exercise training.
Our previous study demonstrated that exercise was able to exert cognitive preservation in Streptozotocin (STZ)-induced AD animal model (1). In the present study, the results of the Barnes maze, novel object recognition test, and object location test demonstrated that the AD behavioral phenotype in TgF344-AD rats had not yet progressed to the stage of spatial learning and memory impairments or spatial cognitive ability and recognition memory deficits, indicating that these animals are in the early stages of AD pathogenesis. These tests were performed 2 months after cessation of the training protocol to study the long-term sustained effects of exercise training, rather than acute effects of exercise bouts. Despite this, we found marked Aβ deposition and tau phosphorylation in 12-month-old AD rats and significant improvements in Exe rats. The passive avoidance task is a fear-motivated test applied to evaluate fear-avoidance behavior (25). In our study, the results of the passive avoidance test indicated deficits of fear-avoidance behavior in AD animals. Notably, long-term treadmill exercise training can prevent this impairment.
Symptoms of anxiety are frequently observed in patients with severe cognitive deficits (32). AD patients often show anxiety-like behavior, which is considered as an early indicator of AD (6). A longitudinal study of anxiety and Αβ connected elevated Αβ levels with worsening anxiety symptoms in AD patients (6). A follow-up study reported that anxiety was interrelated with memory deficits, and that the presence of anxiety-like behavior can be a strong predictor of future cognitive decline for elderly subjects (16). Recently, animal experiments on rats and mice have provided further support for this notion (12, 14). Although numerous studies have investigated the effects of exercise on depression, those examining the effect of exercise on anxiety-like behavior in AD are rare, and, to our best knowledge, we did not find any such research in the TgF344-AD rat model. Our results demonstrate that treadmill exercise training alleviates anxiety-like behaviors in AD animals before the occurrence of hippocampus-dependent learning and memory impairments.
Depression is another predictor for preclinical cognitive decline in AD (33). It is very common among patients with AD in the early and middle stages of the disease (34). Earlier-life depression or depressive symptoms are positively related to a 2-fold or greater increase in risk of dementia (35). Pathologically, patients with a lifetime history of depression display increased hippocampal plaque and tangle formation in neurons (4, 36). Considered as both a risk factor and prodrome of AD, depression often precedes clinical diagnosis of AD for several years (4). To manage the challenging behavioral changes in AD, non-pharmacological approaches have been suggested to be applied first in patients with mild to moderate depression and dementia (4). Physical exercise, a non-pharmacological approach, has been reported as a preventive or treatment method for AD, and numerous studies support the use of exercise as a treatment for depression (1, 37, 38). In our study, long-term treadmill exercise inhibited decreases in sucrose preference and significantly reduced the immobility time of AD animals, which demonstrates that treadmill exercise prevented depression-like behaviors in AD rats, which is consistent with a previous meta-analysis finding exercise was able to reduce depression-like symptoms of adult humans without clinical depression (39).
Neuroinflammation is a typical pathological feature of AD and widely accepted to play a critical role in the neurodegenerative disease (19). Elevated Aβ levels were associated with microglial activation and elevated secretion of inflammatory cytokines (19). As previously reported, microglia activate into a “pro-inflammatory” phenotype and release destructive inflammatory cytokines (e.g. NF-κB, TNFα, IL-1β) in several neurodegenerative diseases, including stroke, AD, and spinal cord injury (19). Treadmill exercise has suppressed activation of “pro-inflammatory” microglial phenotype in our previous study, and this action has been replicated different types of exercise training (1, 19). In agreement, we found that 12-month-old TgF344-AD rats presented significantly elevated activation of microglia and increased release of pro-inflammatory cytokines, which was strongly ameliorated in treadmill exercise trained animals. Importantly, our results found that the level of most pro-inflammatory cytokines were markedly suppressed by long-term training, while anti-inflammatory cytokines were elevated.
Oxidative stress is another pathological hallmark of AD. AD animal presented heightened ROS production and diminished antioxidant capacity, in line with previous studies (1, 19). Oxidative stress along the course of AD progression induces irreversible damage to biological molecules and occurs even at the early stage of AD progression, when significant senile plaques have not yet formed (1, 19). In agreement with these findings, we observed that TgF344 AD rats had significant increases in stress damage to proteins and DNA, as evidenced by higher levels of protein carbonyls and 8-OHdG signal, respectively. In addition, our study discovered elevated ROS production in the cortex and hippocampus. In agreement with previous studies (1, 19), we demonstrated that long-term exercise training can enhance total antioxidant capacity and GSH levels. Intriguingly, we found attenuated levels of 5-HT and expression of its receptor (5-HT6), which were preserved by exercise training, in line with increased anxious-depressive-like behavior in AD rats.
In conclusion, using a novel transgenic AD rat model, our study demonstrates that treadmill exercise training could ameliorate anxious-depressive-like behavior and attenuate fear- avoidance behavior deficits of TgF344-AD rats in the early stage of Alzheimer’s pathogenesis. The improvement of behavior in Tg AD rats was accompanied by a strong suppression of Aβ deposition, tau hyperphosphorylation, neuroinflammation, and oxidative stress after long-term exercise training. Importantly, our results demonstrate that early and chronic exercise intervention could delay the onset of AD symptoms by suppressing amyloidosis and tauopathy, as well as neuroinflammation, oxidative stress, and the expression of 5-HT and its receptor. In addition, our results indicated that accumulation of biomarkers (β-amyloid and tau hyperphosphorylation) at the early stage of AD could contribute to AD-induced anxiety and depression, without significantly affecting the cognitive function at this stage, as this accumulation might not reach a threshold where the amyloid deposition is able to induce the learning and memory deficits. Our results are consistent with previous work on humans that found individuals without any overt signs of dementia also presented significant cerebral amyloid deposits (40). In summary, our finding provides more information about the progression of AD pathology in the novel AD transgenic rat model and contributed more evidence to support anxious-depressive-like behavior as an early indicator of AD. Importantly, we provided additional information that early exercise intervention is very crucial for people who are potentially susceptible to AD. Future work should investigate the mechanisms underlying this preventative effect, as they may elucidate novel pathways capable of slowing or even halting the advance of AD-related dementia.
Acknowledgments
This study was supported by Research Grant AG058603 from National Institute on Aging,National Institutes of Health, and Innovation Project of Graduate School of South China Normal University (2018LKXM010).
Footnotes
Statement of interest
The authors declare that there is no conflict of interest in the current study. The results of the study are presented clearly, honestly, and without fabrication or inappropriate data manipulation. The results of the present study do not constitute endorsement by ACSM.
References
- 1.Wu C, Yang L, Tucker D et al. Beneficial Effects of Exercise Pretreatment in a Sporadic Alzheimer’s Rat Model. Medicine and science in sports and exercise. 2018;50(5):945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarawneh R, Holtzman DM. The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harbor perspectives in medicine. 2012;2(5):a006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breitve MH, Hynninen MJ, Bronnick K et al. A longitudinal study of anxiety and cognitive decline in dementia with Lewy bodies and Alzheimer’s disease. Alzheimer’s research & therapy. 2016;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutzmann H, Qazi A. Depression associated with dementia. Zeitschrift fur Gerontologie und Geriatrie. 2015;48(4):305–11. [DOI] [PubMed] [Google Scholar]
- 5.Bennett S, Thomas AJ. Depression and dementia: cause, consequence or coincidence? Maturitas. 2014;79(2):184–90. [DOI] [PubMed] [Google Scholar]
- 6.Donovan NJ, Locascio JJ, Marshall GA et al. Longitudinal Association of Amyloid Beta and Anxious-Depressive Symptoms in Cognitively Normal Older Adults. The American journal of psychiatry. 2018;175(6):530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsiao JJ, Teng E. Depressive Symptoms in Clinical and Incipient Alzheimer’s Disease. Neurodegenerative disease management. 2013;3(2):147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geda YE, Roberts RO, Knopman DS et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Archives of general psychiatry. 2008;65(10):1193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology. 2003;61(11):1479–85. [DOI] [PubMed] [Google Scholar]
- 10.Ferretti L, McCurry SM, Logsdon R, Gibbons L, Teri L. Anxiety and Alzheimer’s disease. Journal of geriatric psychiatry and neurology. 2001;14(1):52–8. [DOI] [PubMed] [Google Scholar]
- 11.Lok K, Zhao H, Zhang C et al. Effects of accelerated senescence on learning and memory, locomotion and anxiety-like behavior in APP/PS1 mouse model of Alzheimer’s disease. Journal of the neurological sciences. 2013;335(1–2):145–54. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YL, Xing RZ, Luo XB et al. Anxiety-like behavior and dysregulation of miR-34a in triple transgenic mice of Alzheimer’s disease. European review for medical and pharmacological sciences. 2016;20(13):2853–62. [PubMed] [Google Scholar]
- 13.Meng FT, Zhao J, Fang H, Liu YJ. The influence of chronic stress on anxiety-like behavior and cognitive function in different human GFAP-ApoE transgenic adult male mice. Stress. 2015;18(4):419–26. [DOI] [PubMed] [Google Scholar]
- 14.Pentkowski NS, Berkowitz LE, Thompson SM, Drake EN, Olguin CR, Clark BJ. Anxiety-like behavior as an early endophenotype in the TgF344-AD rat model of Alzheimer’s disease. Neurobiology of aging. 2018;61:169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banks SJ, Raman R, He F et al. The Alzheimer’s disease cooperative study prevention instrument project: longitudinal outcome of behavioral measures as predictors of cognitive decline. Dementia and geriatric cognitive disorders extra. 2014;4(3):509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinoff G, Werner P. Anxiety disorder and accompanying subjective memory loss in the elderly as a predictor of future cognitive decline. International journal of geriatric psychiatry. 2003;18(10):951–9. [DOI] [PubMed] [Google Scholar]
- 17.Brambilla P, Cipriani A, Hotopf M, Barbui C. Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: a meta-analysis of clinical trial data. Pharmacopsychiatry. 2005;38(2):69–77. [DOI] [PubMed] [Google Scholar]
- 18.Oyewumi LK. Abnormal involuntary movements: side-effect of neuroleptic drugs. Canadian family physician Medecin de famille canadien. 1982;28:105–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Y, Dong Y, Tucker D et al. Treadmill Exercise Exerts Neuroprotection and Regulates Microglial Polarization and Oxidative Stress in a Streptozotocin-Induced Rat Model of Sporadic Alzheimer’s Disease. Journal of Alzheimer’s disease : JAD. 2017;56(4):1469–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanton R, Reaburn P. Exercise and the treatment of depression: a review of the exercise program variables. Journal of science and medicine in sport. 2014;17(2):177–82. [DOI] [PubMed] [Google Scholar]
- 21.Khanzada FJ, Soomro N, Khan SZ. Association of Physical Exercise on Anxiety and Depression Amongst Adults. Journal of the College of Physicians and Surgeons--Pakistan : JCPSP. 2015;25(7):546–8. [PubMed] [Google Scholar]
- 22.Stonerock GL, Hoffman BM, Smith PJ, Blumenthal JA. Exercise as Treatment for Anxiety: Systematic Review and Analysis. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2015;49(4):542–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey SB, Overland S, Hatch SL, Wessely S, Mykletun A, Hotopf M. Exercise and the Prevention of Depression: Results of the HUNT Cohort Study. The American journal of psychiatry. 2018;175(1):28–36. [DOI] [PubMed] [Google Scholar]
- 24.Cohen RM, Rezai-Zadeh K, Weitz TM et al. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric abeta, and frank neuronal loss. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(15):6245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gimenez De Bejar V, Caballero Bleda M, Popovic N, Popovic M. Verapamil Blocks Scopolamine Enhancement Effect on Memory Consolidation in Passive Avoidance Task in Rats. Frontiers in pharmacology. 2017;8:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Tucker LD, Dong Yan et al. Tert-butylhydroquinone post-treatment attenuates neonatal hypoxic-ischemic brain damage in rats. Neurochemistry international. 2018;116:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L, Dong Y, Wu C et al. Photobiomodulation preconditioning prevents cognitive impairment in a neonatal rat model of hypoxia-ischemia. Journal of biophotonics. 2019:e201800359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Y, Sareddy GR, Wang J et al. Neuron-Derived Estrogen Regulates Synaptic Plasticity and Memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2019;39(15):2792–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Q, Tucker D, Dong Y, Zhao N, Zhang Q. Neuroprotective and Functional Improvement Effects of Methylene Blue in Global Cerebral Ischemia. Molecular neurobiology. 2016;53(8):5344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duca FA, Swartz TD, Covasa M. Effect of diet on preference and intake of sucrose in obese prone and resistant rats. PloS one. 2014;9(10):e111232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karila D, Freret T, Bouet V, Boulouard M, Dallemagne P, Rochais C. Therapeutic Potential of 5-HT6 Receptor Agonists. Journal of medicinal chemistry. 2015;58(20):7901–12. [DOI] [PubMed] [Google Scholar]
- 32.Bierman EJ, Comijs HC, Jonker C, Beekman AT. Symptoms of anxiety and depression in the course of cognitive decline. Dementia and geriatric cognitive disorders. 2007;24(3):213–9. [DOI] [PubMed] [Google Scholar]
- 33.Visser PJ, Verhey FR, Ponds RW, Kester A, Jolles J. Distinction between preclinical Alzheimer’s disease and depression. Journal of the American Geriatrics Society. 2000;48(5):479–84. [DOI] [PubMed] [Google Scholar]
- 34.Modrego PJ. Depression in Alzheimer’s disease. Pathophysiology, diagnosis, and treatment. Journal of Alzheimer’s disease : JAD. 2010;21(4):1077–87. [DOI] [PubMed] [Google Scholar]
- 35.Byers AL, Yaffe K. Depression and risk of developing dementia. Nature reviews. Neurology. 2011;7(6):323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rapp MA, Schnaider-Beeri M, Grossman HT et al. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Archives of general psychiatry. 2006;63(2):161–7. [DOI] [PubMed] [Google Scholar]
- 37.Alkadhi KA, Dao AT. Exercise decreases BACE and APP levels in the hippocampus of a rat model of Alzheimer’s disease. Molecular and cellular neurosciences. 2018;86:25–9. [DOI] [PubMed] [Google Scholar]
- 38.Carek PJ, Laibstain SE, Carek SM. Exercise for the treatment of depression and anxiety. International journal of psychiatry in medicine. 2011;41(1):15–28. [DOI] [PubMed] [Google Scholar]
- 39.Conn VS. Depressive symptom outcomes of physical activity interventions: meta-analysis findings. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2010;39(2):128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herrup K The case for rejecting the amyloid cascade hypothesis. Nature neuroscience. 2015;18(6):794–9. [DOI] [PubMed] [Google Scholar]