A 77-year-old man was admitted for severe PCR-confirmed COVID-19. The patient presented with severe hypoxemia and biological findings suggestive of a hyperinflammatory syndrome: severe lymphopenia in combination with signs of hypercytokinemia (elevated C-reactive protein), coagulopathy (elevated D-dimer levels), and hepatic injury (elevated lactate dehydrogenase) (Webb et al., 2020).
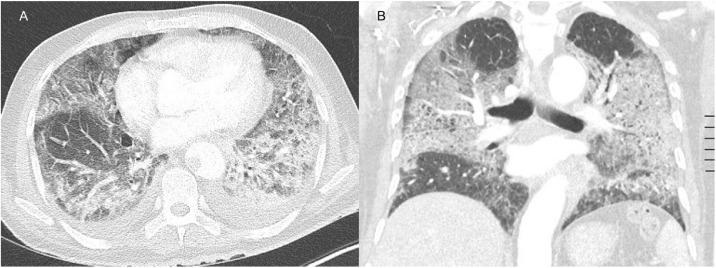
Computed tomography angiography (CTA) of the thorax showed ground glass opacities in the five lobes, but no signs of pulmonary embolism (Figure 2). The patient was treated with dexamethasone, a prophylactic dose of low molecular weight heparin (LMWH), high flow oxygen therapy, and a single infusion of tocilizumab within a clinical trial (Maes et al., 2020).
Figure 2.
Axial (A) and coronal (B) contrast-enhanced CT image in pulmonary window showing extensive ground glass opacities.
After 6 days of hospitalization, D-dimer levels had increased markedly to a level of 9210 ng/ml. CTA was repeated due to suspected pulmonary embolism. The images showed a partial thrombosis of the descending aorta (Figure 1 ). The patient was treated with therapeutic anticoagulation and made a full recovery.
Figure 1.
Axial (A) and coronal (B) contrast-enhanced CT image in mediastinal window showing a thrombus in the descending thoracic aorta. The red arrow indicates the thrombus.
Thromboembolic events are frequently described in COVID-19 patients and are the consequence of a hyperinflammatory response and endothelial dysfunction (Gu et al., 2021). The potential role of an antiphospholipid syndrome secondary to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been proposed (Roncati et al., 2020). An increase in D-dimer level has been shown to be associated with thromboembolic events, including arterial thrombosis.
An unexpected significant rise in D-dimer levels in the critically ill patient should prompt further investigation (Susen et al., 2020).
Funding source
None to declare.
Ethical approval
Patient consent was obtained.
Conflict of interest
The authors declare no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Gu S.X., Tyagi T., Jain K., Gu V.W., Lee S.H., Hwa J.M. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol. 2021;18(March (3)):194–209. doi: 10.1038/s41569-020-00469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes B., Bosteels C., De Leeuw E., Declercq J., Van Damme K., Delporte A. Treatment of severely ill COVID-19 patients with anti-interleukin drugs (COV-AID): a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(June (1)):468. doi: 10.1186/s13063-020-04453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncati L., Manenti A., Manco G., Farinetti A., Mattioli A. The COVID-19 arterial thromboembolic complications: from inflammation to immunothrombosis through antiphospholipid autoantibodies. Ann Vasc Surg. 2020;(December) doi: 10.1016/j.avsg.2020.12.006. S0890-5096(20)31103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susen S., Tacquard C.A., Godon A., Mansour A., Garrigue D., Nguyen P. Prevention of thrombotic risk in hospitalized patients with COVID-19 and hemostasis monitoring. Crit Care. 2020;24(June (1)):364. doi: 10.1186/s13054-020-03000-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb B.J., Peltan I.D., Jensen P., Hoda D., Hunter B., Silver A. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol. 2020;2(12):e754–e763. doi: 10.1016/S2665-9913(20)30343-X. [DOI] [PMC free article] [PubMed] [Google Scholar]