Abstract
Nematodes in South Africa have mainly been studied for their diversity and agricultural importance. However, the ecological status of nematodes and the effect of seasonal variation in local grasslands remain unknown. For this reason, a nematode study was conducted in the Telperion Nature Reserve and represented the first ecological study in a natural grassland area in South Africa. In total, 104 soil samples were collected during four consecutive seasons from 2015 until 2016 in three habitats, viz. (i) open grassland, (ii) shrubland with rocky outcrops, and (iii) riparian zone. From these the nematode community structure and soil ecosystem status were studied. In total, 93 genera from 50 families were recorded with herbivores and bacterivores being the most abundant trophic groups in all three habitats. Linear mixed models revealed that season had an overwhelmingly dominant impact on the condition, food web status, and functioning of the soil ecosystems with pairwise comparisons indicating that significantly higher values were recorded during winter. Interestingly, this seasonal shift can largely be attributed to fluctuations in the populations of only a few nematode groups (namely Aporcelaimellus, Dorylaimidae, Iotonchus, and Mononchus) with high colonizer-persister values. Although the reason for the higher abundance of specific nematode groups recorded during the winter is not explicitly clear, it is possibly linked to reduced competition from other soil fauna. This study clearly shows that further investigations are required to better understand the dynamics of grassland ecosystems.
Keywords: Ecosystem functioning, Food web status, Grassland habitats, Seasonal variation, Soil ecology
Nematodes occupy most terrestrial habitats on earth (Liu et al., 2019), even the furthest reaches of caves (Du Preez et al., 2017) and deep underground mines (Borgonie et al., 2011). Estimates also indicate that nematodes represent 80% of all multicellular organisms (Eisenhauer and Guerra, 2019; Van Den Hoogen et al., 2019). However, despite their omnipresent distribution and dominating abundance, many nematode communities are poorly studied with the majority of species remaining undescribed (Archidona-Yuste et al., 2016). This is especially true for many habitats in South Africa since most nematode-related studies are focused on agricultural systems and nematodes of economic importance (i.e. crop pests) (Procter, 2012; Fourie et al., 2017).
Grasslands, for example, host diverse soil ecosystems, represent more than 40% of Earth’s terrestrial surface area and provide essential ecosystem services including the provision of food, storage of carbon, mitigating droughts and floods, and erosion control (Hewins et al., 2018; Bengtsson et al., 2019; Gibson and Newman, 2019). However, although the structure and ecological status of grassland nematode communities have been investigated in some parts of the world (Ekschmitt et al., 2001; Biederman and Boutton, 2009; Kergunteuil et al., 2016), a literature survey revealed no ecological reports on the nematode communities of South African grasslands.
Studying the nematode communities of grasslands is not only relevant from an ecological perspective, but also from a conservation perspective. Nematodes are valuable indicators of soil ecosystem disturbance affected by, for example, agricultural activities and climate change (Ferris and Bongers, 2009; Zhong et al., 2017; Zhang et al., 2020). Therefore, studying the nematode communities of natural, undisturbed grasslands will generate valuable baseline data for monitoring disturbance and implementing conservation policies. This would be especially valuable for South Africa as the country’s grasslands are under continuous threat from overgrazing, cultivation, and urban expansion (Haddad and Butler, 2018). Woody (or bush) encroachment, driven by rising temperatures and increasing atmospheric carbon dioxide levels, also presents a major threat (Skowno et al., 2017). This while only 2.0 to 2.8% of South Africa’s grasslands are protected within conserved areas (Carbutt et al., 2011; Haddad and Butler, 2018).
When studying terrestrial ecosystems, it is also important to consider seasonal variation as water availability, temperature, and vegetative growth can have an important effect on the structure and functioning of soil ecosystems (Bongers and Ferris, 1999; Cardoso et al., 2016; Song et al., 2016; Siebert et al., 2020). Song et al. (2016), for example, showed that higher precipitation rates significantly increased nematode abundance and richness in a grassland ecosystem. Bakonyi et al. (2007), in turn, found that the effects of soil drying and warming on nematode communities were dependant on the presence of vegetation with the smallest effect recorded in grasses. Ultimately, understanding how seasonal variation affects soil ecosystems is necessary in order to accurately assess and monitor the potential threats posed by anthropogenic activities and climate change.
For these reasons, this study was undertaken and aimed at (i) studying the nematode community structure in the open grassland, shrubland with rocky outcrops and riparian zone habitats of the Telperion Nature Reserve and (ii) investigating the effect of seasonal-induced changes on the condition, food web status and functioning of the soil ecosystems.
Materials and methods
Site description
The study was conducted in the Telperion Nature Reserve (Mpumalanga province, South Africa) (Fig. 1) that has a total surface area of 9,061.3 ha. This reserve is located in the Rand Highveld Grassland (of which only 1% is conserved) and is dominated by grassy plains interspersed with rocky outcrops and woody plant species (Grobler, 1999). The greater region is characterized by strong summer rainfall (570-730 mm per annum) and dry and cold winters (frost may occur frequently). This is a fire prone ecosystem with high lightning flash densities making lightning-induced fires common. The lithology of Telperion Nature Reserve is dominated by Arenite-Conglomerate, producing dystrophic or mesotrophic soils with some red soils, as well as rocky areas with miscellaneous soils (Grobler, 1999). Three streams flow through the reserve that originates from higher lying wetlands and sponge areas.
Figure 1:
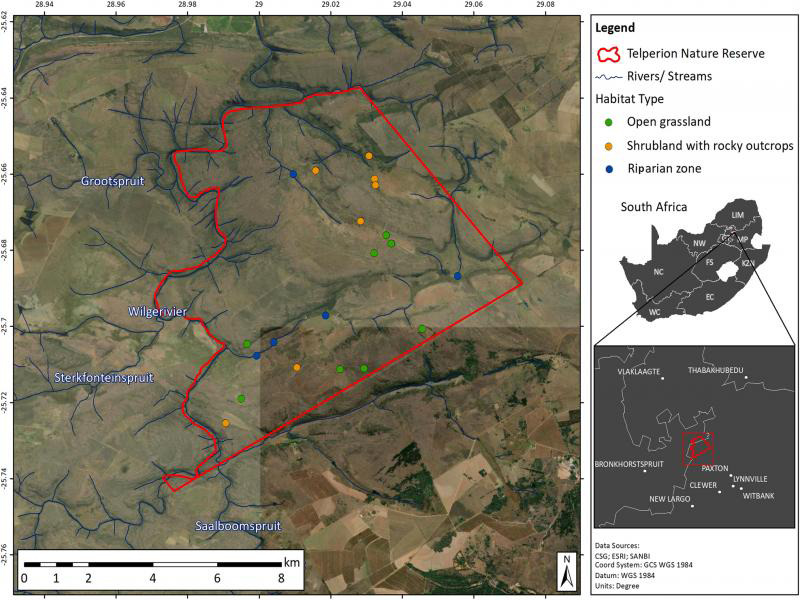
Satellite image of Telperion Nature Reserve (South Africa) indicating the sampling locations associated with the open grassland, shrubland with rocky outcrops and riparian zone habitats.
Rainfall and temperature data
Climatic data (rainfall and temperature) from January 2014 to December 2016 were obtained from the South African Weather Service (www.weathersa.co.za). The total amount of rain for 90 days prior to each sampling event (as listed in the following section) was calculated, while the mean, minimum, and maximum daily temperatures were extracted for the same period. Since sampling was based on climatic factors (as listed in the following section) and thus not evenly dated over 1 year, this 90-day period was considered before each sampling event.
Site selection and sample collections
Within Telperion Nature Reserve three main terrestrial habitats, namely, (i) open grassland (OG), (ii) shrubland with rocky outcrops (SRO), and (iii) riparian zone (RZ) were identified for investigation. Sites within each habitat were selected based on accessibility as some areas were restricted and/or not accessible by vehicle or foot. The elevation of the sites in the each habitat ranged as follows: OG: 1333 to 1482 m, SRO: 1361 to 1480 m, and RZ: 1304 to 1383 m above sea level. Ultimately, 26 sampling sites (Fig. 1) were selected and included: 11 OG, 10 SRO sites, and 5 RZ sites.
In total, 104 soil samples (one per site per season) were collected from the three terrestrial habitats during four consecutive seasons [Winter (11 June 2015), Spring (30 November 2015), Summer (24 February 2016), and Autumn (19 April 2016)]. These sampling dates were selected based on climatic patterns through consultation with the reserve manager. Winter samples were the first to be collected when frost was at its peak. Spring sampling followed the first rains, which due to a drought in South Africa came later than normally expected. Summer sampling was undertaken when grasses reached maturity and produced seeds, while Autumn samples were collected before the first frost occurred.
Multiple discrete soil samples (500 cm3 each) for the analysis of nematode community structure were collected from each site up to a depth of 20 cm using a garden trowel, which was cleaned between sampling sites to prevent contamination. Samples were collected beneath plants (if present). For the analysis of selected soil properties (soil texture and total organic carbon), a sub-sample was collected from each site. The latter was performed only during the summer (February 2016) sampling interval as the selected soil properties were unlikely to change significantly during the sampling period. All the collected samples were labeled and transported in cool boxes to the Nematology Unit of the Agricultural Research Council – Plant Health and Protection (Roodeplaat, South Africa). Samples were stored at 10°C until further analysis.
Nematode extraction, preservation, and identification
Nematodes were extracted from 250 cm3 soil samples using an adapted decanting and sieving method, followed by a sugar flotation method (Marais et al., 2017). The nematodes were then fixed in a heated 4% formaldehyde and 1% propionic acid (FPG) solution (Netscher and Seinhorst, 1969), dehydrated in a glycerin solution (Seinhorst, 1962), and mounted in glycerin on permanent glass slides using a wax ring (Marais et al., 2017). Nematodes were counted using a De Grisse counting dish (De Grisse, 1963) and identified to genus level using an Olympus BX53F microscope. Taxonomic classification was based on Maggenti et al. (1988) and Geraert (2019) for Tylenchina; Kleynhans et al. (1996) for Heteroderidae; Hunt (1993) for Aphelenchida; Decraemer (1995) and Duarte et al. (2010) for Trichodoridae; Andrássy (2009) for Dorylaimidae; and Andrássy (2005, 2007) for other free-living nematodes. Feeding groups were assigned, based on Yeates et al. (1993), as herbivores (He), bacterivores (Ba), fungivores (Fu), omnivores (Om), or predators (Pr). Nematodes were also assigned a cp-value based on Bongers and Bongers (1998).
Physical and biological properties of soil samples
The selected physical and biological properties were analyzed by Eco-Analytica (North-West University, South Africa) as follows: total organic carbon (C) content of the soil samples were determined using the loss-on-ignition method (Donkin, 1991) and the soil texture as described by Laker and Dupreez (1982).
Statistical analysis
Rarefaction curves were used to compare taxa richness between habitats. This was achieved by calculating the sample-based Mao Tau estimator (with inter- and extrapolation) using Estimate S 9.1 Software Package. Furthermore, differences in the abundance of nematode trophic groups between habitats were investigated by pooling samples from across seasons. Abundance values were log10(x + 1) transformed and visualized, while statistical significance between trophic groups and habitats was inferred using two-way analysis of variance (ANOVA) and Tukey’s multiple comparisons tests. These analyses were performed using Graphpad Prism 6 Software Package.
Selected nematode-based indices (i.e. maturity index 2-5, enrichment index, structure index, channel index, basal index, and metabolic footprints) were used to quantify the condition, food web status, and functioning of soil ecosystems (Ferris et al., 2001; Ferris and Bongers, 2009; Ferris, 2010). These indices were calculated using the Nematode Indicator Joint Analyses (NINJA) online tool (Sieriebriennikov et al., 2014). The Graphpad Prism 6 Software Package was used to create a violin plot of the maturity index 2-5, a measure of soil ecosystem condition. Also, values of the structure and enrichment indices were used to plot a faunal analysis and characterize the food web status of the studied soil ecosystems (Ferris et al., 2001). Furthermore, linear mixed models with pairwise comparisons were used to determine if the independent variables, i.e. season (Winter, Spring, Summer, and Autumn) and habitat (OG, SRO, and RZ) significantly affected (singularly and interactively) the calculated nematode-based indices. Prior to this analysis the nematode-based indices were log transformed (where needed). All linear mixed models were performed using SPSS Statistics 25 Software Package.
Lastly, a redundancy analysis (RDA) was used to investigate the relationship between the response (nematode-based indices) and explanatory variables [(factors (seasons and habitats) and numeric variables (soil texture and total organic carbon)]. Predictor effects of the listed explanatory variables were calculated using a Monte-Carlo permutation test (Šmilauer and Lepš, 2014). These multivariate analyses were performed and illustrated on a biplot using Canoco 5 Software Package. Significance for all univariate and multivariate analyses was regarded at p < 0.05.
Results
Environmental conditions
The total rainfall for 90 days prior to each sampling event is illustrated in Figure 2A. This shows a general increase over time with the lowest and highest rainfall recorded prior to the Winter and Autumn sampling events, respectively. However, it is important to note that South Africa experienced a drought in 2015. According to the South African Weather Service (www.weathersa.co.za), the Telperion Nature Reserve region received only 388 mm from January to December 2015. By contrast, the recorded annual rainfall in 2014 and 2016 were 794 and 748 mm, respectively.
Figure 2:
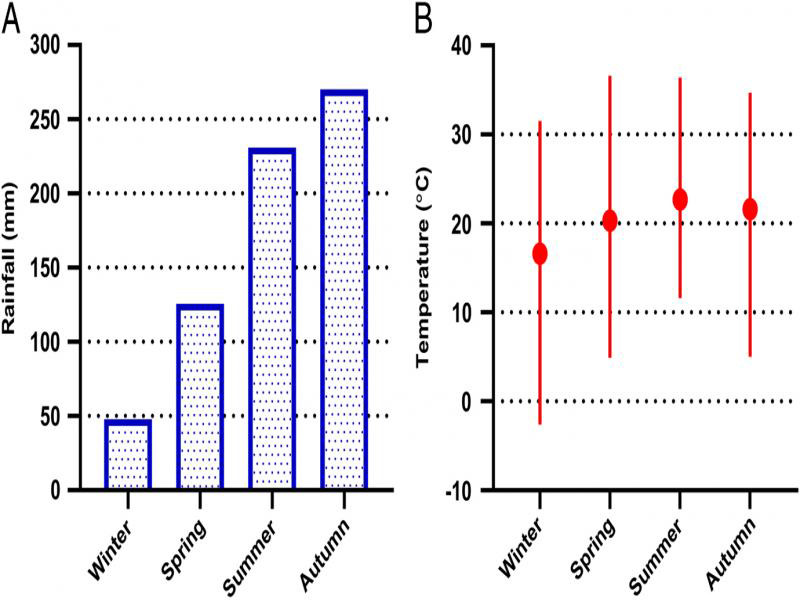
The (A) total rainfall and (B) temperature (mean, minimum, and maximum) for the 90 days prior to each sampling event at Telperion Nature Reserve (South Africa).
Temperature (mean, minimum, and maximum) values are illustrated in Figure 2B with the lowest and highest mean temperatures recorded, as expected, prior to the Winter and Summer sampling events, respectively. The largest range between minimum (−2.6°C) and maximum (31.5°C) values were recorded before Winter sampling. Finally, the soil texture and organic carbon content of the collected and analyzed soil samples are provided as Supplementary material (Table S1).
Table S1.
Investigated soil properties measured at the open grassland, shrubland with rocky outcrops and riparian zone habitats in the Telperion Nature Reserve (Mpumalanga province, South Africa).
| Habitat | Sand (%) | Silt (%) | Clay (%) | Organic carbon (%) |
|---|---|---|---|---|
| Open grassland | 91.77 ± 3.03 | 3.75 ± 2.33 | 4.47 ± 0.94 | 0.78 ± 0.24 |
| Shrubland with rocky outcrops | 86.45 ± 4.44 | 6.99 ± 3.13 | 6.54 ± 2.08 | 1.65 ± 0.84 |
| Riparian zone | 86.48 ± 7.18 | 8.18 ± 5.16 | 5.33 ± 2.24 | 1.59 ± 1.26 |
Note: Mean ± standard deviation values are reported.
Nematode community structure
Nematode communities associated with the studied habitats were investigated in terms of their taxonomic composition and richness, as well as their trophic abundance. In total, 93 genera, representing 50 families, were recorded in samples collected from Telperion Nature Reserve (Supplementary material Tables S2–S6) and were representative of the five major nematode trophic groups, namely, herbivores (Table S2), bacterivores (Table S3), fungivores (Table S4), omnivores (Table S5), and predators (Table S6). The largest number of taxa were found in the shrubland with rocky outcrops (SRO) habitat (77 genera), followed by the riparian zone (RZ) (74 genera) and open grasslands (OG) (68 genera) habitats. In terms of the number of recorded genera per trophic group, bacterivores dominated with 30 genera recorded in both the SRO and RZ habitats, while 24 bacterivore genera were found in the OG habitat. This was followed by herbivores (OG: 23; SRO: 23; RZ: 20), predators (OG: 11; SRO: 14; RZ: 13), fungivores (OG: 8; SRO: 9; RZ: 8), and omnivores (OG: 2; SRO: 2; RZ: 3). The results also showed that some nematode genera were present in all three habitats during all four seasons. This included Helicotylenchus, Rotylenchulus, Scutellonema, Tylenchus, Xiphinema (herbivores, Table S2); Acrobeles, Acrobeloides, Panagrolaimus, Prismatolaimus, Zeldia (bacterivores; Table S3); Aphelenchoides, Diphtherophora (fungivores; Table S4), and Dorylaimidae (omnivores, Table S5).
Table S2.
Occurrence and abundance of herbivores in 250 cm3 soil samples obtained from open grassland, shrubland with rocky outcrops and riparian zone sites in the Telperion Nature Reserve (Mpumalanga province, South Africa) as part of an ecological study that was conducted over four seasons from 2015 to 2016.
| Open grassland | Shrubland with rocky outcrops | Riparian zone | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family | Genus or Family | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn |
| Anguinidae (He1) | Subanguina | 1.36 ± 4.52 | 0 | 2.73 ± 9.05 | 0.45 ± 1.51 | 0 | 0 | 0 | 0 | 0 | 1.00 ± 2.24 | 0 | 1.00 ± 2.24 |
| Belondiridae (He5) | Axonchium | 0.91 ± 3.02 | 3.64 ± 4.52 | 0 | 0.91 ± 3.02 | 0 | 5.00 ± 12.69 | 3.00 ± 7.89 | 0.50 ± 1.58 | 0 | 0 | 1.00 ± 2.24 | 0 |
| Belonolaimidae (He3) | Tylenchorhynchus | 1.82 ± 6.03 | 0 | 0 | 30.91 ± 65.91 | 18.00 ± 56.92 | 0.50 ± 1.58 | 25.00 ± 36.82 | 8.00 ± 13.98 | 0 | 0 | 0 | 0 |
| Criconematidae (He3) | Criconematidaea | 1.82 ± 4.62 | 5.00 ± 13.42 | 0.91 ± 3.02 | 0.91 ± 3.02 | 3.00 ± 3.50 | 3.00 ± 6.32 | 1.00 ± 3.16 | 4.50 ± 9.56 | 1.00 ± 2.24 | 0 | 1.00 ± 2.24 | 0 |
| Criconema | 0.91 ± 3.02 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.00 ± 4.47 | |
| Criconemoides | 6.36 ± 15.67 | 10.45 ± 17.24 | 7.27 ± 11.91 | 8.18 ± 22.39 | 0.50 ± 1.58 | 9.00 ± 21.19 | 5.00 ± 14.14 | 0 | 5.00 ± 11.18 | 5.00 ± 11.18 | 1.00 ± 2.24 | 0 | |
| Hemicriconemoides | 0 | 1.82 ± 6.03 | 0 | 0 | 0 | 1.00 ± 3.16 | 0 | 0 | 0 | 1.00 ± 2.24 | 0 | 0 | |
| Hemicycliophora | 5.91 ± 19.60 | 2.27 ± 7.54 | 0.45 ± 1.51 | 1.82 ± 6.03 | 1.50 ± 4.74 | 0.50 ± 1.58 | 0 | 1.00 ± 3.16 | 165.00 ± 301.58 | 13.00 ± 29.07 | 69.00 ± 154.29 | 1.00 ± 2.24 | |
| Dorylaimellidae (He5) | Dorylaimellus | 58.18 ± 98.82 | 6.36 ± 13.80 | 8.64 ± 18.04 | 11.36 ± 31.23 | 21.50 ± 25.28 | 12.50 ± 20.85 | 8.50 ± 16.51 | 12.00 ± 25.19 | 0 | 5.00 ± 11.18 | 1.00 ± 2.24 | 55.00 ± 122.98 |
| Heteroderidae (He3) | Meloidogyne | 4.09 ± 10.68 | 2.27 ± 7.54 | 1.82 ± 4.05 | 25.45 ± 71.40 | 154.50 ± 350.55 | 13.00 ± 19.32 | 11.50 ± 16.67 | 8.00 ± 17.03 | 0 | 162.00 ± 342.90 | 45.00 ± 81.70 | 54.00 ± 120.98 |
| Hoplolaimidae (He3) | Hoplolaimidaea | 6.36 ± 10.51 | 7.27 ± 16.94 | 0 | 2.27 ± 4.10 | 15.50 ± 26.29 | 14.00 ± 26.01 | 10.50 ± 31.49 | 0 | 2.00 ± 4.47 | 0 | 1.00 ± 2.24 | 0 |
| Helicotylenchus | 38.64 ± 43.25 | 139.09 ± 246.41 | 42.27 ± 112.37 | 40.45 ± 64.67 | 52.00 ± 82.27 | 67.50 ± 102.29 | 31.50 ± 57.40 | 61.50 ± 12.83 | 6.00 ± 13.42 | 83.00 ± 185.59 | 3.00 ± 6.71 | 73.00 ± 139.45 | |
| Rotylenchulus | 34.55 ± 71.57 | 50.91 ± 128.78 | 31.82 ± 47.29 | 55.91 ± 125.89 | 34.50 ± 92.87 | 46.00 ± 143.72 | 53.00 ± 139.92 | 96.00 ± 275.77 | 2.00 ± 4.47 | 28.00 ± 59.85 | 3.00 ± 6.71 | 1.00 ± 2.24 | |
| Rotylenchus | 29.55 ± 42.51 | 21.36 ± 38.61 | 0 | 16.36 ± 22.59 | 18.50 ± 35.04 | 5.50 ± 11.65 | 0.50 ± 1.58 | 7.00 ± 10.59 | 15.00 ± 28.28 | 0 | 0 | 8.00 ± 7.89 | |
| Scutellonema | 110.45 ± 131.25 | 172.73 ± 231.38 | 38.64 ± 71.91 | 35.45 ± 36.77 | 44.00 ± 51.47 | 64.00 ± 92.04 | 131.00 ± 343.24 | 21.00 ± 23.78 | 19.00 ± 26.55 | 40.00 ± 58.74 | 35.00 ± 57.77 | 137.00 ± 275.83 | |
| Leptonchidae (He4) | Xiphinemella | 6.82 ± 22.61 | 0 | 1.36 ± 3.23 | 5.45 ± 9.34 | 4.50 ± 14.23 | 2.50 ± 7.91 | 0.50 ± 1.58 | 5.50 ± 12.57 | 0 | 0 | 1.00 ± 2.24 | 2.00 ± 4.47 |
| Longidoridae (He5) | Longidorus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.00 ± 6.71 | 0 | 0 |
| Xiphinema | 6.82 ± 13.65 | 2.27 ± 4.10 | 7.27 ± 10.81 | 5.91 ± 10.68 | 20.22 ± 31.09 | 3.00 ± 5.37 | 11.50 ± 16.17 | 11.00 ± 16.96 | 3.00 ± 4.47 | 2.00 ± 2.74 | 6.00 ± 13.42 | 1.00 ± 2.24 | |
| Pratylenchidae (He3) | Pratylenchus | 10.45 ± 19.42 | 22.73 ± 44.74 | 2.27 ± 3.44 | 5.91 ± 13.38 | 27.00 ± 34.50 | 16.00 ± 35.81 | 15.50 ± 29.67 | 5.50 ± 10.66 | 0 | 0 | 0 | 0 |
| Psilenchidae (He2) | Psilenchus | 0.91 ± 2.02 | 0 | 0 | 0 | 0 | 0 | 3.00 ± 9.49 | 0 | 31.00 ± 69.32 | 7.00 ± 15.65 | 15.00 ± 33.54 | 0 |
| Trichodoridae (He4) | Nanidorus | 0 | 2.73 ± 9.05 | 0 | 0 | 0 | 2.50 ± 5.40 | 1.50 ± 4.74 | 0.50 ± 1.58 | 5.00 ± 7.07 | 18.00 ± 28.42 | 313.00 ± 683.17 | 28.00 ± 49.32 |
| Paratrichodorus | 0 | 0 | 0 | 0 | 2.00 ± 6.32 | 0 | 0 | 0 | 0 | 6.00 ± 13.42 | 0 | 0 | |
| Trichodoridaea | 0 | 0.45 ± 1.51 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Trichodorus | 0 | 0 | 0 | 0 | 0 | 0.50 ± 1.58 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tylenchidae (He2) | Filenchus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 41.00 ± 80.96 | 0 | 0 |
| Coslenchus | 0 | 2.73 ± 9.05 | 0.45 ± 1.51 | 0 | 0 | 0.50 ± 1.58 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tylenchidaea | 0.45 ± 1.51 | 1.36 ± 3.23 | 0 | 0 | 0 | 2.50 ± 7.91 | 0 | 0.50 ± 1.58 | 1.00 ± 2.24 | 16.00 ± 30.50 | 0 | 0 | |
| Tylenchus | 77.73 ± 73.33 | 33.64 ± 40.38 | 15.91 ± 36.53 | 21.82 ± 24.21 | 121.50 ± 65.75 | 18.00 ± 18.59 | 14.00 ± 11.01 | 26.00 ± 27.37 | 139.00 ± 144.80 | 85.00 ± 100.93 | 246.00 ± 222.64 | 189.00 ± 263.71 | |
| Tylencholaimidae (He4) | Chitwoodius | 0 | 0 | 0 | 0.45 ± 1.51 | 0.50 ± 1.58 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tylenchulidae (He3) | Meloidoderita | 3.64 ± 12.06 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tylenchulus | 0 | 0 | 0.45 ± 1.51 | 0 | 0 | 2.00 ± 6.32 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total herbivores | 407.73 ± 194.43 | 489.09 ± 476.05 | 162.27 ± 214.97 | 270.00 ± 206.95 | 539.00 ± 383.70 | 289.00 ± 240.71 | 326.50 ± 363.48 | 268.50 ± 289.86 | 394.00 ± 353.77 | 516.00 ± 496.61 | 741.00 ± 922.38 | 552.00 ± 734.72 | |
Notes: Mean ± standard deviation values are reported. aGenus could not be identified.
Table S3.
Occurrence and abundance of bacterivores in 250 cm3 soil samples obtained from open grassland, shrubland with rocky outcrops and riparian zone sites in the Telperion Nature Reserve (Mpumalanga province, South Africa) as part of an ecological study that was conducted over four seasons from 2015 to 2016.
| Open grassland | Shrubland with rocky outcrops | Riparian zone | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family (functional guild) | Genus or Family | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn |
| Alaimidae (Ba4) | Alaimus | 98.64 ± 91.33 | 17.73 ± 19.15 | 2.27 ± 11.07 | 12.73 ± 19.02 | 92.00 ± 57.07 | 21.50 ± 22.74 | 18.50 ± 22.61 | 57.50 ± 63.56 | 10.00 ± 14.14 | 22.00 ± 49.19 | 1.00 ± 2.24 | 0 |
| Amphidelus | 0 | 0 | 0 | 0 | 0 | 0.51 ± 1.58 | 5.50 ± 7.62 | 5.00 ± 11.55 | 0 | 0 | 0 | 1.00 ± 2.24 | |
| Aphanolaimidae (Ba3) | Aphanolaimus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 ± 2.24 | 0 | 0 | 0 |
| Cephalobidae (Ba2) | Cephalobidaea | 0 | 13.18 ± 18.07 | 87.73 ± 192.33 | 113.64 ± 198.92 | 0 | 19.00 ± 45.26 | 32.00 ± 38.82 | 36.50 ± 45.03 | 0 | 0 | 3.00 ± 2.74 | 10.00 ± 14.14 |
| Acrobeles | 122.27 ± 112.48 | 69.09 ± 103.46 | 36.82 ± 55.51 | 160.45 ± 192.57 | 51.50 ± 69.76 | 52.50 ± 123.52 | 18.00 ± 39.24 | 24.50 ± 36.40 | 3.00 ± 6.71 | 47.00 ± 64.58 | 32.00 ± 54.15 | 13.00 ± 16.43 | |
| Acrobeloides | 60.45 ± 41.44 | 20.45 ± 21.62 | 25.00 ± 53.06 | 23.64 ± 23.67 | 24.00 ± 43.83 | 66.50 ± 152.19 | 43.00 ± 103.98 | 21.50 ± 39.86 | 25.00 ± 30.00 | 52.00 ± 52.51 | 62.00 ± 107.62 | 63.00 ± 105.69 | |
| Cephalobus | 3.18 ± 9.02 | 42.27 ± 59.22 | 54.09 ± 47.21 | 97.73 ± 171.37 | 18.00 ± 50.01 | 64.50 ± 118.75 | 116.00 ± 272.01 | 63.50 ± 76.52 | 0 | 75.00 ± 148.66 | 58.00 ± 57.07 | 26.00 ± 28.59 | |
| Cervidellus | 0 | 8.64 ± 15.51 | 7.27 ± 15.55 | 7.73 ± 10.09 | 0 | 8.00 ± 15.67 | 3.50 ± 5.30 | 4.50 ± 6.43 | 0 | 50.00 ± 111.80 | 1.00 ± 2.24 | 5.00 ± 8.66 | |
| Chiloplacus | 20.45 ± 22.52 | 4.09 ± 6.25 | 3.18 ± 10.55 | 0.91 ± 3.02 | 13.00 ± 15.49 | 13.50 ± 17.49 | 2.00 ± 6.32 | 16.00 ± 44.02 | 60.00 ± 128.65 | 4.00 ± 8.94 | 1.00 ± 2.24 | 0 | |
| Eucephalobus | 0 | 0.91 ± 3.02 | 1.82 ± 4.62 | 0 | 0 | 121.00 ± 380.88 | 8.50 ± 16.34 | 5.50 ± 11.17 | 0 | 9.00 ± 20.12 | 167.00 ± 365.10 | 40.00 ± 61.64 | |
| Heterocephalo-bellus | 0 | 0 | 0 | 0.45 ± 1.51 | 0 | 0 | 0 | 0.50 ± 1.58 | 0 | 0 | 0 | 0 | |
| Seleborca | 53.64 ± 90.11 | 57.73 ± 84.42 | 89.55 ± 186.83 | 57.73 ± 71.39 | 8.00 ± 22.01 | 18.50 ± 46.19 | 7.50 ± 6.35 | 14.50 ± 16.24 | 0 | 17.00 ± 30.33 | 6.00 ± 10.84 | 1.00 ± 2.24 | |
| Zeldia | 28.18 ± 32.96 | 35.45 ± 77.05 | 2.73 ± 5.18 | 13.18 ± 16.17 | 6.00 ± 7.38 | 8.00 ± 12.74 | 8.50 ± 13.75 | 4.00 ± 6.58 | 3.00 ± 6.71 | 22.00 ± 49.19 | 2.00 ± 4.47 | 4.00 ± 8.94 | |
| Chromadoridae (Ba3) | Punctodora | 0 | 0 | 0 | 0 | 0 | 1.00 ± 3.16 | 0 | 0 | 9.00 ± 12.45 | 0 | 0 | 0 |
| Chronogasteridae (Ba3) | Chronogaster | 0 | 0 | 0 | 0.45 ± 1.51 | 0.50 ± 1.58 | 0 | 2.00 ± 4.83 | 0.50 ± 1.58 | 279.00 ± 509.20 | 12.00 ± 14.40 | 95.00 ± 212.43 | 28.00 ± 57.18 |
| Cyatholaimidae (Ba4) | Achromadora | 0 | 0 | 4.55 ± 15.08 | 0 | 0 | 0 | 0 | 0 | 9.00 ± 15.17 | 2.00 ± 2.74 | 18.00 ± 40.25 | 0 |
| Diplogasteridae (Ba1) | Butlerius | 0 | 0 | 0 | 0 | 1.50 ± 3.37 | 4.50 ± 14.23 | 0 | 0 | 3.00 ± 6.71 | 2.00 ± 4.47 | 0 | 0 |
| Diplogasteroididae (Ba1) | Diplogasteroididaea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14.00 ± 31.30 | 1.00 ± 2.24 |
| Elaphonematidae (Ba3) | Elaphonema | 8.64 ± 17.76 | 1.82 ± 4.62 | 8.64 ± 15.51 | 12.73 ± 42.21 | 6.50 ± 11.56 | 0 | 7.00 ± 22.14 | 1.50 ± 3.37 | 0 | 0 | 0 | 0 |
| Mesorhabditidae (Ba1) | Mesorhabditis | 0 | 3.18 ± 10.55 | 0 | 8.18 ± 27.14 | 0 | 12.50 ± 32.68 | 1.50 ± 3.37 | 2.00 ± 6.32 | 0 | 14.00 ± 21.91 | 2.00 ± 4.47 | 0 |
| Metateratocepha-lidae (Ba3) | Euteratocephalus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5.00 ± 8.66 | 0 | 0 | 0 |
| Monhysteridae (Ba2) | Monhystera | 1.82 ± 6.03 | 0 | 0 | 0 | 2.50 ± 4.86 | 2.00 ± 6.32 | 0 | 0 | 8.00 ± 13.04 | 4.00 ± 6.52 | 3.00 ± 4.47 | 6.00 ± 10.84 |
| Monhystrella | 0 | 0 | 0 | 0 | 00 | 0 | 0 | 0 | 0 | 0 | 2.00 ± 4.47 | ||
| Neodiplogastridae (Ba1) | Fictor | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.50 ± 1.58 | 2.00 ± 4.47 | 0 | 0 | 0 |
| Koerneria | 0 | 0 | 0 | 0 | 2.00 ± 6.32 | 0 | 0 | 0 | 0 | 1.00 ± 2.24 | 3.00 ± 6.71 | 0 | |
| Odontolaimidae (Ba3) | Odontolaimus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.00 ± 4.47 | 0 | 0 | 0 |
| Osstellidae (Ba2) | Drilocephalobus | 0 | 0 | 18.18 ± 50.71 | 37.73 ± 71.46 | 0 | 0 | 3.50 ± 4.74 | 12.00 ± 14.57 | 0 | 0 | 0 | 1.00 ± 2.24 |
| Panagrolaimidae (Ba1) | Panagrolaimus | 26.36 ± 25.31 | 15.00 ± 23.02 | 0.91 ± 3.02 | 17.27 ± 35.31 | 17.50 ± 14.19 | 17.50 ± 39.32 | 12.50 ± 23.83 | 4.00 ± 7.38 | 39.00 ± 39.12 | 15.00 ± 21.21 | 19.00 ± 21.33 | 6.00 ± 6.52 |
| Plectidae (Ba2) | Anaplectus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.50 ± 1.58 | 0 | 0 | 0 | 0 |
| Ceratoplectus | 0 | 0 | 0 | 0.91 ± 2.02 | 10.50 ± 33.20 | 4.50 ± 11.17 | 2.50 ± 7.91 | 11.00 ± 25.03 | 0 | 2.00 ± 4.47 | 0 | 0 | |
| Plectus | 5.45 ± 12.34 | 0 | 0 | 0 | 4.00 ± 12.65 | 0 | 1.50 ± 2.42 | 3.50 ± 11.07 | 4.00 ± 4.18 | 1.00 ± 2.24 | 2.00 ± 4.47 | 1.00 ± 2.24 | |
| Wilsonema | 3.18 ± 7.83 | 0.45 ± 1.51 | 0 | 1.36 ± 2.34 | 6.50 ± 10.55 | 1.50 ± 2.42 | 2.50 ± 5.40 | 6.50 ± 17.17 | 0 | 6.00 ± 13.42 | 1.00 ± 2.24 | 1.00 ± 2.24 | |
| Prismatolaimidae (Ba3) | Prismatolaimus | 0.91 ± 2.02 | 0.91 ± 2.02 | 0.91 ± 3.02 | 3.18 ± 10.55 | 6.50 ± 11.80 | 7.00 ± 11.35 | 7.50 ± 13.99 | 7.00 ± 12.52 | 3.00 ± 2.74 | 5.00 ± 11.18 | 6.00 ± 13.42 | 3.00 ± 4.47 |
| Rhabditidae (Ba1) | Cruznema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.50 ± 1.58 | 0 | 0 | 0 | 0 |
| Rhabditidaea | 0.45 ± 1.51 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Rhabditis | 1.36 ± 3.23 | 2.27 ± 4.67 | 0 | 3.18 ± 5.13 | 18.00 ± 31.55 | 12.50 ± 34.42 | 2.00 ± 4.22 | 5.00 ± 11.55 | 7.00 ± 13.04 | 6.00 ± 8.94 | 0 | 0 | |
| Rhabdolaimidae (Ba3) | Rhabdolaimus | 0 | 0 | 0.45 ± 1.51 | 0 | 0 | 0 | 0.50 ± 1.58 | 0 | 0 | 0 | 0 | 0 |
| Teratocephalidae (Ba3) | Teratocephalus | 0.45 ± 1.51 | 0.45 ± 1.51 | 0 | 0 | 0 | 4.00 ± 11.01 | 0 | 0 | 4.00 ± 6.52 | 0 | 0 | 0 |
| Total bacterivores | 435.45 ± 275.30 | 293.64 ± 291.33 | 349.09 ± 507.99 | 573.18 ± 517.51 | 288.50 ± 147.82 | 460.50 ± 719.66 | 306.00 ± 435.38 | 308.00 ± 126.34 | 476.00 ± 506.13 | 368.00 ± 364.02 | 496.00 ± 603.15 | 212.00 ± 129.79 | |
Notes: Mean ± standard deviation values are reported. aGenus could not be identified.
Table S4.
Occurrence and abundance of fungivores in 250 cm3 soil samples obtained from open grassland, shrubland with rocky outcrops and riparian zone sites in the Telperion Nature Reserve (Mpumalanga province, South Africa) as part of an ecological study that was conducted over four seasons from 2015 to 2016.
| Open grassland | Shrubland with rocky outcrops | Riparian zone | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family | Genus or Family | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn |
| Anguinidae (Fu2) | Ditylenchus | 8.64 ± 13.98 | 29.55 ± 40.28 | 28.18 ± 33.86 | 10.91 ± 13.75 | 1.50 ± 4.74 | 20.50 ± 38.18 | 19.00 ± 19.97 | 23.50 ± 30.56 | 0 | 25.00 ± 34.64 | 130.00 ± 144.27 | 110.00 ± 186.38 |
| Aphelenchidae (Fu2) | Aphelenchus | 15.91 ± 16.71 | 133.18 ± 190.56 | 86.36 ± 246.80 | 24.55 ± 35.67 | 12.50 ± 13.59 | 51.50 ± 63.20 | 80.00 ± 161.57 | 9.00 ± 9.66 | 0 | 19.00 ± 26.08 | 72.00 ± 102.02 | 68.00 ± 141.01 |
| Aphelenchoididae (Fu2) | Aphelenchoides | 8.64 ± 15.34 | 10.45 ± 15.72 | 21.36 ± 61.08 | 5.91 ± 7.01 | 13.00 ± 23.12 | 284.50 ± 887.40 | 16.00 ± 30.26 | 4.00 ± 6.58 | 15.00 ± 23.98 | 13.00 ± 13.04 | 21.00 ± 20.74 | 12.00 ± 21.39 |
| Diphtherophoridae (Fu3) | Diphtherophora | 23.18 ± 22.28 | 8.64 ± 13.62 | 5.00 ± 7.42 | 0.91 ± 2.02 | 24.00 ± 19.55 | 3.00 ± 6.32 | 23.50 ± 67.37 | 32.50 ± 86.35 | 9.00 ± 15.17 | 6.00 ± 13.42 | 34.00 ± 73.26 | 32.00 ± 35.81 |
| Leptonchidae (Fu4) | Leptonchus | 0 | 1.36 ± 3.23 | 0.91 ± 3.02 | 0.91 ± 3.02 | 0 | 1.00 ± 2.11 | 0 | 0.50 ± 1.58 | 0 | 1.00 ± 2.24 | 0 | 0 |
| Proleptonchus | 0 | 0 | 3.18 ± 10.55 | 0 | 0 | 0 | 0 | 0.50 ± 1.58 | 0 | 0 | 0 | 0 | |
| Neotylenchidae (Fu2) | Neotylenchidaea | 5.91 ± 10.20 | 3.64 ± 5.05 | 8.64 ± 15.02 | 10.00 ± 13.04 | 5.00 ± 15.81 | 11.50 ± 20.15 | 12.50 ± 18.45 | 18.50 ± 19.87 | 0 | 17.00 ± 28.20 | 23.00 ± 51.43 | 10.00 ± 17.32 |
| Paraphelenchidae (Fu2) | Paraphelenchus | 0 | 0 | 0 | 0 | 2.00 ± 6.32 | 0 | 0 | 0 | 1.00 ± 2.24 | 0 | 9.00 ± 20.12 | 0 |
| Tylencholaimellidae (Fu4) | Tylencholaimellus | 6.36 ± 11.85 | 0 | 0.45 ± 1.51 | 0.45 ± 1.15 | 3.50 ± 8.18 | 0.50 ± 1.58 | 2.00 ± 6.32 | 0 | 0 | 0 | 0 | 1.00 ± 2.24 |
| Tylencholaimidae (Fu4) | Tylencholaimidaea | 2.73 ± 9.05 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 ± 2.24 | 0 | 0 | 0 |
| Tylencholaimus | 7.27 ± 24.12 | 1.82 ± 4.05 | 24.09 ± 65.83 | 1.36 ± 4.52 | 7.00 ± 14.76 | 0 | 9.00 ± 28.46 | 0 | 2.00 ± 4.47 | 0 | 8.00 ± 15.25 | 0 | |
| Total fungivores | 78.64 ± 54.13 | 188.64 ± 229.54 | 178.18 ± 342.99 | 55.00 ± 41.23 | 68.50 ± 35.83 | 372.50 ± 863.64 | 162.00 ± 179.20 | 88.50 ± 120.21 | 28.00 ± 22.53 | 81.00 ± 93.10 | 297.00 ± 341.31 | 233.00 ± 360.60 | |
Notes: Mean ± standard deviation values are reported. aGenus could not be identified.
Table S5.
Occurrence and abundance of omnivores in 250 cm3 soil samples obtained from open grassland, shrubland with rocky outcrops and riparian zone sites in the Telperion Nature Reserve (Mpumalanga province, South Africa) as part of an ecological study that was conducted over four seasons from 2015 to 2016.
| Open grassland | Shrubland with rocky outcrops | Riparian zone | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family | Genus or Family | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn |
| Dorylaimidae (Om4) | Dorylaimidaea | 55.91 ± 22.89 | 20.45 ± 23.07 | 11.36 ± 19.25 | 17.27 ± 21.14 | 49.00 ± 29.23 | 14.00 ± 14.10 | 17.50 ± 29.65 | 16.00 ± 16.96 | 15.00 ± 12.75 | 15.00 ± 14.14 | 6.00 ± 6.52 | 18.00 ± 40.25 |
| Dorylaimus | 0.45 ± 1.51 | 0.45 ± 1.51 | 0 | 0 | 0.50 ± 1.58 | 0 | 1.00 ± 3.16 | 0 | 3.00 ± 6.71 | 0 | 0 | 0 | |
| Mesodorylaimus | 0 | 0 | 0 | 0 | 3.00 ± 9.49 | 1.50 ± 4.74 | 0 | 2.50 ± 7.91 | 1.00 ± 2.24 | 3.00 ± 4.47 | 1.00 ± 2.24 | 2.00 ± 4.47 | |
| Tylencholaimidae (Om4) | Discomyctus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6.00 ± 13.42 |
| Mydonomidae (Om4) | Dorylaimoides | 0.91 ± 3.02 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total omnivores | 57.27 ± 23.60 | 20.91 ± 23.00 | 11.36 ± 19.25 | 17.27 ± 21.14 | 52.50 ± 31.38 | 15.50 ± 14.990 | 18.50 ± 29.160 | 18.50 ± 20.010 | 19.00 ± 9.62 | 18.00 ± 16.81 | 7.00 ± 5.70 | 26.00 ± 52.73 | |
Notes: Mean ± standard deviation values are reported. aGenus not identified.
Table S6.
Occurrence and abundance of predators in 250 cm3 soil samples obtained from open grassland, shrubland with rocky outcrops and riparian zone sites in the Telperion Nature Reserve (Mpumalanga province, South Africa) as part of an ecological study that was conducted over four seasons from 2015 to 2016.
| Open grassland | Shrubland with rocky outcrops | Riparian zone | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family | Genus or Family | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn |
| Actinolaimidae (Pr5) | Neoactinolaimus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.00 ± 4.47 | 0 |
| Anatonchidae (Pr4) | Iotonchus | 4.09 ± 7.01 | 0.45 ± 1.51 | 0.45 ± 1.51 | 0 | 9.50 ± 12.79 | 1.50 ± 2.42 | 3.00 ± 4.22 | 1.00 ± 3.16 | 2.00 ± 4.47 | 0 | 14.00 ± 31.30 | 4.00 ± 8.94 |
| Aphelenchoididae (Pr2) | Seinura | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 44.00 ± 98.39 |
| Aporcelaimidae (Pr5) | Aporcelaimidaea | 1.36 ± 4.52 | 0 | 0 | 1.82 ± 4.62 | 0 | 1.00 ± 3.16 | 0 | 0.50 ± 1.58 | 0 | 0 | 0 | 3.00 ± 6.71 |
| Aporcelaimellus | 16.36 ± 16.75 | 0.91 ± 3.02 | 5.91 ± 9.95 | 1.36 ± 4.52 | 3.50 ± 11.07 | 3.50 ± 4.12 | 9.50 ± 13.83 | 4.50 ± 9.56 | 2.00 ± 4.47 | 0 | 0 | 0 | |
| Aporcelaimus | 1.36 ± 3.23 | 5.00 ± 9.49 | 5.00 ± 9.22 | 4.09 ± 12.00 | 0 | 3.50 ± 5.80 | 3.50 ± 11.07 | 0 | 1.00 ± 2.24 | 2.00 ± 2.74 | 6.00 ± 13.42 | 19.00 ± 39.75 | |
| Makatinus | 0 | 0.91 ± 3.02 | 0 | 0 | 0 | 0 | 1.50 ± 4.74 | 0.50 ± 1.58 | 0 | 0 | 0 | 0 | |
| Ironidae (Pr4) | Ironus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4.00 ± 8.94 | 0 | 0 | 0 |
| Mononchidae (Pr4) | Mononchidaea | 0.45 ± 1.51 | 0.45 ± 1.51 | 0 | 0 | 0 | 0 | 1.00 ± 3.16 | 0 | 0 | 2.00 ± 2.74 | 0 | 0 |
| Cobbonchus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.00 ± 4.47 | 0 | 0 | 0 | |
| Granonchulus | 0 | 0 | 0 | 0 | 0 | 1.50 ± 4.74 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mononchus | 0.45 ± 1.51 | 0.45 ± 1.51 | 0 | 1.82 ± 6.03 | 14.00 ± 29.04 | 0.50 ± 1.58 | 0 | 0 | 2.00 ± 2.74 | 0 | 0 | 0 | |
| Mylonchulidae (Pr4) | Mylonchulus | 0 | 0 | 0.91 ± 3.02 | 0 | 2.00 ± 4.83 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Paraxonchiidae (Pr5) | Paraxhonchium | 4.55 ± 10.60 | 1.36 ± 3.23 | 3.64 ± 12.06 | 0 | 1.50 ± 4.74 | 0 | 3.00 ± 7.89 | 6.00 ± 14.49 | 0 | 0 | 0 | 0 |
| Qudsianematidae (Pr4) | Discolaiminaea | 1.36 ± 4.52 | 0 | 0 | 0 | 2.00 ± 4.83 | 0 | 0 | 4.00 ± 8.76 | 0 | 0 | 0 | 0 |
| Discolaimoides | 9.09 ± 14.80 | 2.73 ± 5.18 | 12.73 ± 25.43 | 20.91 ± 27.28 | 1.50 ± 3.37 | 0 | 1.00 ± 3.16 | 9.00 ± 12.87 | 2.00 ± 2.74 | 0 | 0 | 3.00 ± 6.71 | |
| Discolaimus | 0.91 ± 3.02 | 0.45 ± 1.51 | 0 | 1.36 ± 4.52 | 0.50 ± 1.58 | 2.50 ± 5.40 | 0.50 ± 1.58 | 0.50 ± 1.58 | 0 | 1.00 ± 2.24 | 1.00 ± 2.24 | 2.00 ± 2.74 | |
| Eudorylaimus | 0 | 0 | 0 | 0 | 0 | 0.50 ± 1.58 | 0 | 0 | 0 | 0 | 2.00 ± 4.47 | 0 | |
| Labronema | 0 | 1.82 ± 6.03 | 0 | 0 | 0.50 ± 1.58 | 0.50 ± 1.58 | 1.00 ± 3.16 | 0.50 ± 1.58 | 0 | 0 | 6.00 ± 8.94 | 0 | |
| Tobrilidae (Pr4) | Tobrilus | 0 | 0 | 0 | 0 | 1.00 ± 3.16 | 0 | 0 | 0 | 2.00 ± 2.74 | 1.00 ± 2.24 | 1.00 ± 2.24 | 1.00 ± 2.24 |
| Tripylidae (Pr3) | Tripyla | 0 | 0 | 0 | 0.45 ± 1.51 | 1.50 ± 4.74 | 0.50 ± 1.58 | 1.00 ± 3.16 | 0 | 0 | 0 | 0 | 0 |
| Total predators | 40.00 ± 28.81 | 14.55 ± 20.30 | 28.64 ± 24.40 | 31.82 ± 27.04 | 37.5 ± 34.56 | 15.5 ± 11.20 | 24.5 ± 21.67 | 26.5 ± 24.23 | 18.00 ± 13.51 | 6.00 ± 4.18 | 32.00 ± 39.15 | 76.00 ± 131.79 | |
Notes: Mean ± standard deviation values are reported. aGenus could not be identified.
A comparison of taxa richness between habitats was made using rarefaction curves (Fig. 3). These curves illustrate the sample-based observed (solid line) and extrapolated (dotted line) Mau Tau richness, while 95% confidence intervals are indicated as shaded bands. When comparing richness at the 20 samples mark (total number of samples collected at the RZ habitat), it is clear that RZ presented the greatest richness with 84 genera. This was followed by SRO and OG, which presented 73 and 65 genera, respectively. Nonetheless, it is important to note that these curves did not reach an asymptote (leveling), suggesting that additional sampling might be required to record the complete nematode community. To this end, extrapolation revealed that more than 50 samples per habitat are likely required at which point an estimated 98, 91, and 83 genera would be predicted to be collected at the RZ, SRO, and OG habitats, respectively.
Figure 3:
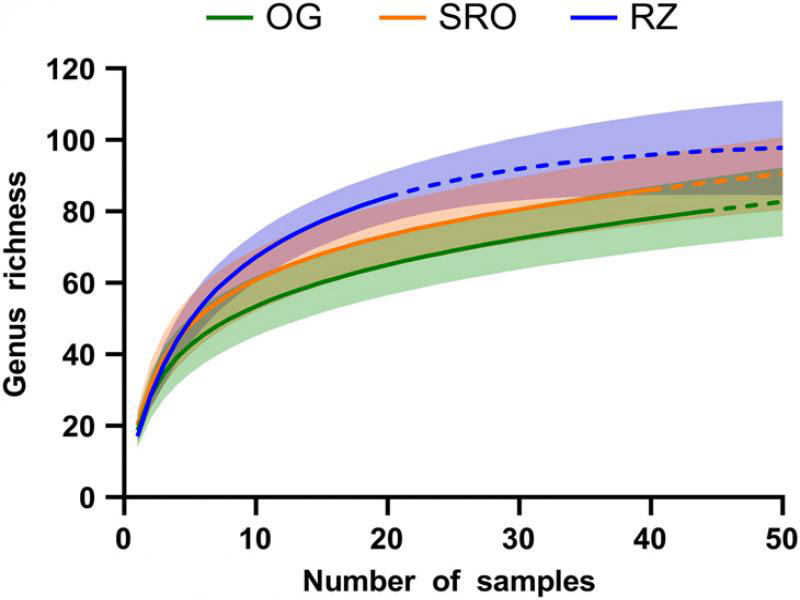
Rarefaction curves illustrate the sample-based observed (solid line) and extrapolated (dotted line) Mau Tau richness of nematode genera at the open grassland (OG), shrubland with rocky outcrops (SRO), and riparian zone (RZ) habitats in Telperion Nature Reserve (South Africa). The shaded bands represent the 95% confidence intervals.
Lastly, the nematode trophic abundances per habitat were considered. Figure 4 shows that herbivores and bacterivores were the most abundant trophic groups, followed by fungivores, omnivores, and predators. The multiple comparisons tests revealed that although no significant differences occurred between herbivores and bacterivores, both these trophic groups differed significantly from fungivores, omnivores, and predators at all three habitats. Also, significant differences between fungivore, omnivore, and predator abundances were recorded at all three habitats with the exception of no significant difference between fungivores and omnivores/predators at the RZ habitat. No significant differences in nematode trophic abundances were recorded between habitats.
Figure 4:
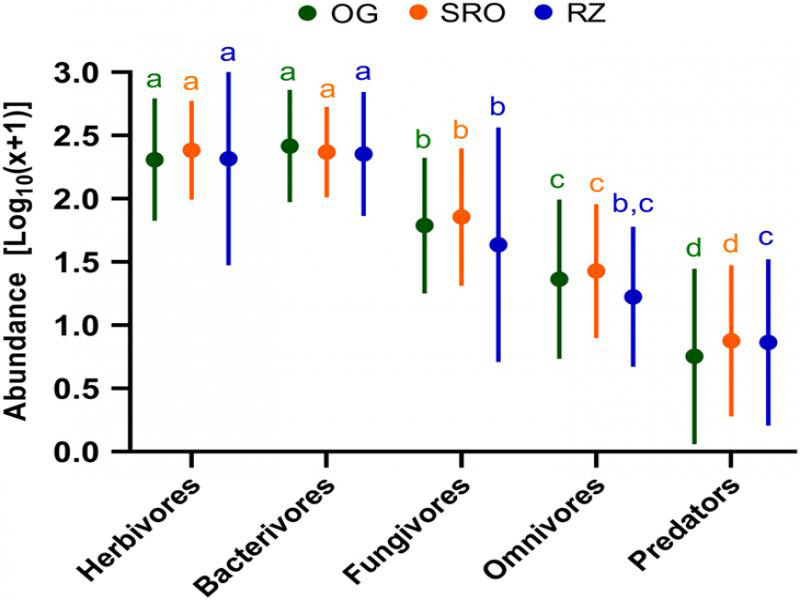
Log10(x + 1)-transformed nematode abundances (mean, minimum, and maximum) per trophic group at the open grassland (OG), shrubland with rocky outcrops (SRO), and riparian zone (RZ) habitats in Telperion Nature Reserve (South Africa). Bars with common superscript do not differ significantly (p > 0.05).
Soil ecosystem condition and food web status
The maturity index 2-5 was used to investigate the soil ecosystem condition of the studied grassland habitats. A violin plot (Fig. 5) illustrates the median, quartiles, minimum, maximum, and data distribution (shape outline) of this index per habitat per season. Minimum and maximum values ranged from 2 (RZ in Spring) to 3.7 (SRO in Winter), respectively, while the highest and lowest median values for all three habitats were recorded during the Winter and Spring, respectively. Also worth noting is that the SRO habitat had the highest median values during all four seasons. This habitat was thus classified as having the most stable soil ecosystem. The results from the linear mixed models (Table 1) showed that habitat and season had a significant effect on the maturity index 2-5. Yet, pairwise comparisons revealed that these effects were only evident between seasons, which showed significantly higher maturity index 2-5 values in Winter compared to the rest of the seasons. Differences between Spring, Summer, and Autumn were not significant.
Figure 5:
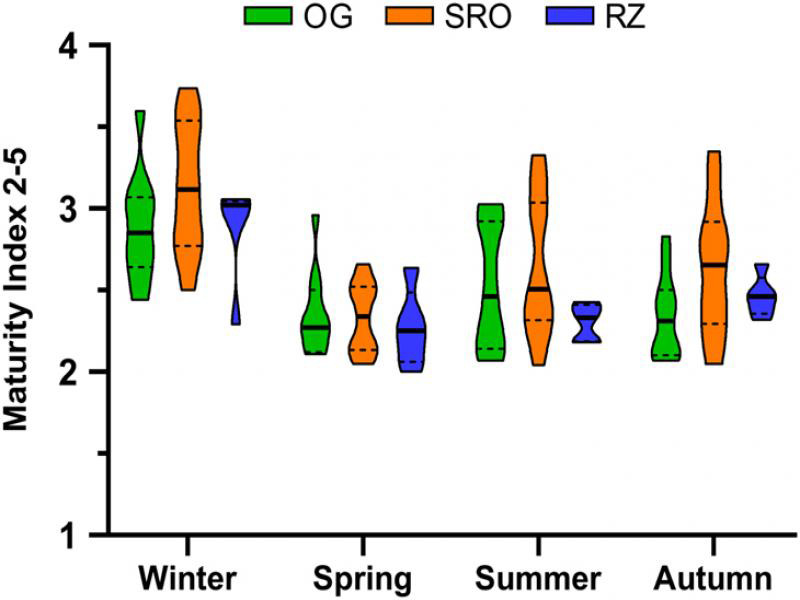
Maturity index 2-5 violin plot of values recorded during the studied seasons at the open grassland (OG), shrubland with rocky outcrops (SRO), and riparian zone (RZ) habitats in Telperion Nature Reserve (South Africa). The bold line within each plot indicates the median value, dashed lines are quartiles, minimum, and maximum are the bounds of the plot and data distribution is shown by plot shape outline.
Table 1.
Mixed linear models reveal the effect (singularly and interactively) of the independent variables, season and habitat, on the calculated nematode-specific indices.
| Variable | Source | F ratio | p value | Variable | Source | F ratio | p value |
|---|---|---|---|---|---|---|---|
| Maturity index 2–5 | Habitat | 3.66 | 0.04 | Bacterivore footprint | Habitat | 0.04 | 0.96 |
| Season | 14.73 | <0.001 | Season | 2.46 | 0.07 | ||
| Habitat × Season | 0.42 | 0.86 | Habitat × Season | 0.58 | 0.75 | ||
| Channel index | Habitat | 2.49 | 0.11 | Fungivore footprint | Habitat | 0.35 | 0.71 |
| Season | 11.67 | <0.001 | Season | 1.52 | 0.22 | ||
| Habitat × Season | 1.38 | 0.24 | Habitat × Season | 1.01 | 0.42 | ||
| Basal index | Habitat | 4.96 | 0.01 | Omnivore footprint | Habitat | 0.76 | 0.48 |
| Season | 11.08 | <0.001 | Season | 2.54 | 0.06 | ||
| Habitat × Season | 0.35 | 0.91 | Habitat × Season | 0.29 | 0.94 | ||
| Enrichment index | Habitat | 8.77 | <0.001 | Predator footprint | Habitat | 1.45 | 0.26 |
| Season | 7.65 | <0.001 | Season | 4.53 | <0.01 | ||
| Habitat × Season | 1.08 | 0.38 | Habitat × Season | 0.71 | 0.65 | ||
| Structure index | Habitat | 2.00 | 0.16 | Enrichment footprint | Habitat | 1.49 | 0.25 |
| Season | 13.33 | <0.001 | Season | 0.29 | 0.83 | ||
| Habitat × Season | 0.64 | 0.70 | Habitat × Season | 0.84 | 0.54 | ||
| Herbivore footprint | Habitat | 0.56 | 0.58 | Structure footprint | Habitat | 0.11 | 0.90 |
| Season | 1.60 | 0.20 | Season | 4.73 | <0.01 | ||
| Habitat × Season | 1.34 | 0.25 | Habitat × Season | 0.52 | 0.79 |
The status of the soil food webs were investigated using the nematode faunal analysis (Fig. 6). This analysis showed that all three habitats had the highest structure (complexity) in the Winter with the SRO and RZ habitats classified as maturing and enriched with balanced (bacterial vs. fungal) decomposition pathways. The OG habitat was classified as mature and fertile characterized by fungal dominated decomposition. In Spring, however, nematode community composition in all three habitats represented degraded and depleted food webs. During Summer and Autumn, the communities represented either degraded and depleted or mature and fertile food webs. The faunal analysis also revealed the clustering of habitats (i.e. low variability between habitats) especially during Winter and Spring. The effect of season and habitat on the two indices used to determine the food web status, namely the structure index and enrichment index (Fig. 6), were further investigated using linear mixed models. This revealed that while both season and habitat had a significant effect on the enrichment index, only season had a significant effect on the structure index. Pairwise comparisons showed, as with the maturity index 2-5, that communities in Winter had significantly higher enrichment and structure compared to the other seasons, while the OG habitat had significantly lower enrichment compared to the SRO and RZ habitats.
Figure 6:
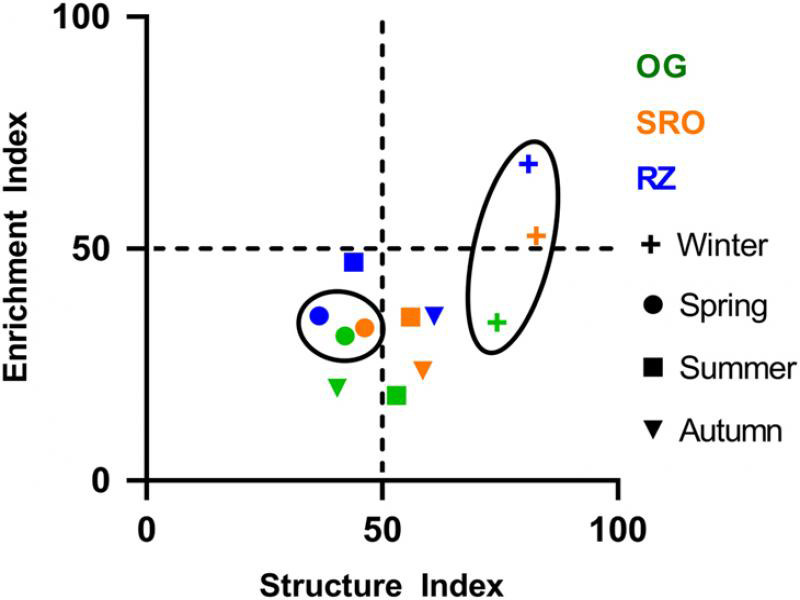
Nematode faunal analysis indicating the food web status during the studied seasons at the open grassland (green), shrubland with rocky outcrops (orange), and riparian zone (blue) habitats in Telperion Nature Reserve (South Africa). Winter and Spring seasonal clusters are circled.
Finally, a redundancy analysis illustrated on a biplot (Fig. 7) was used to investigate the relationship between the explanatory and response variables. The explanatory variables accounted for 42% (p < 0.001) of the observed variation in the response variables with axes 1 and 2 representing 35.3% (p < 0.001) and 5.5% (p < 0.001), respectively. This analysis revealed that the three habitats could be differentiated based on the predominant soil fraction, i.e. the OG, RZ, and SRO habitats consisted of more sandy, silty, or clayey soils, respectively. Nonetheless, only clay presented a significant effect of 4.3% on the response variables. In addition, organic carbon levels were positively correlated with the RZ habitat and enrichment index, but had only a minor effect (< 4%) on the response variables. Furthermore, the redundancy analysis biplot showed that a positive correlation existed between the OG habitat and the channel and basal indices. The SRO habitat, in turn, presented a positive correlation to the maturity 2-5 and structure indices. Finally, the Monte-Carlo permutation test revealed that 30% of the variation in response variables can be attributed to seasonality.
Figure 7:
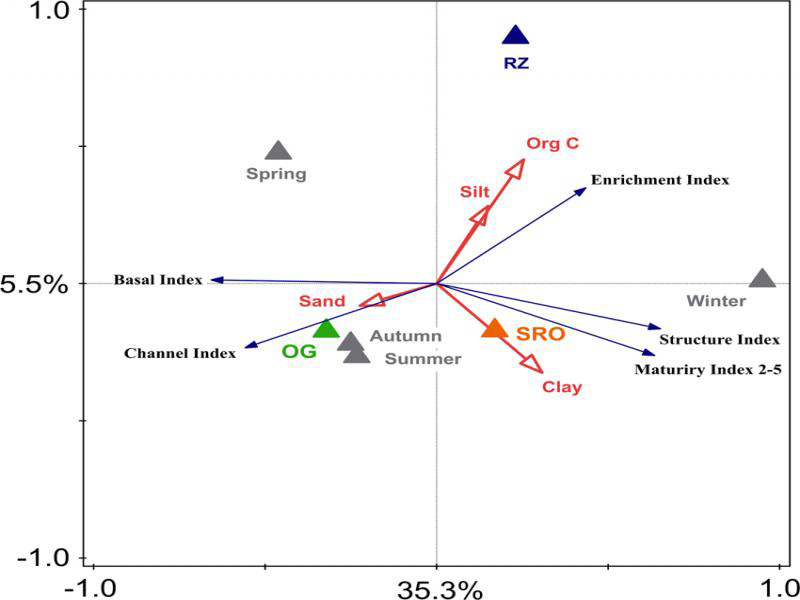
Redundancy analysis of the relationship between the response (nematode-based indices) and explanatory variables (factors: seasons and habitats; numeric variables: soil texture and total organic carbon). Measurements were taken during four consecutive seasons at open grassland (OG), shrubland with rocky outcrops (SRO), and riparian zone (RZ) habitats in Telperion Nature Reserve (South Africa).
Soil ecosystem functioning
Metabolic footprints (Table 2) were used to assess the soil ecosystem functioning, i.e. the magnitude of functions and services delivered by the soil ecosystems. Generally, relatively high herbivore footprints were recorded, while especially high herbivore footprints were observed in Winter and Spring at the SRO and RZ habitats, respectively. However, even though clear differences were recorded in the herbivore footprint between seasons and habitats, the linear mixed models (Table 1) revealed that none of these differences were significant. Of the remaining footprints, significant seasonal effects were only recorded for the predator and structure footprints. Pairwise comparisons revealed that the predator footprints were significantly higher in the Winter compared to Spring and Summer, while the structure footprints were significantly higher in the Winter compared to Spring and Autumn. No significant habitat effects were recorded for any of the footprints.
Table 2.
Metabolic footprints calculated from 250 cm3 soil samples obtained from riparian zone, shrubland with rocky outcrops and open grassland sites in the Telperion Nature Reserve (Mpumalanga province, South Africa) as part of an ecological study that was conducted over four seasons from 2015 to 2016.
| Open grassland (OG) | Shrubland with rocky outcrops (SRO) | Riparian zone (RZ) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | |
| Herbivore footprint | 120 ± 70 | 118 ± 119 | 53 ± 63 | 168 ± 265 | 682 ± 1,326 | 116 ± 111 | 124 ± 117 | 116.11 ± 134 | 97 ± 81 | 683 ± 1,300 | 331 ± 326 | 289 ± 434 |
| Bacterivore footprint | 84 ± 49 | 56 ± 62 | 50 ± 74 | 99 ± 87 | 85 ± 63 | 91 ± 156 | 47 ± 52 | 57.21 ± 30 | 89 ± 62 | 66 ± 58 | 65 ± 67 | 29 ± 12 |
| Fungivore footprint | 14 ± 11 | 23 ± 26 | 25 ± 39 | 9 ± 7 | 11 ± 6 | 34 ± 60 | 23 ± 23 | 17.56 ± 22 | 4 ± 4 | 16 ± 18 | 49 ± 58 | 37 ± 55 |
| Omnivore footprint | 179 ± 97 | 103 ± 110 | 89 ± 94 | 91 ± 155 | 123 ± 67 | 78 ± 69 | 102 ± 124 | 58 ± 50 | 63 ± 34 | 54 ± 53 | 89 ± 150 | 241 ± 477 |
| Predator footprint | 12 ± 11 | 3 ± 4 | 6 ± 10 | 11 ± 11 | 31 ± 26 | 7 ± 9 | 7 ± 8 | 8 ± 8 | 15 ± 14 | 4 ± 3 | 22 ± 42 | 15 ± 13 |
| Enrichment footprint | 16 ± 8 | 30 ± 40 | 19 ± 38 | 21 ± 18 | 46 ± 65 | 68 ± 147 | 24 ± 21 | 23.46 ± 25 | 28 ± 26 | 35 ± 34 | 51 ± 44 | 33 ± 55 |
| Structure footprint | 217 ± 109 | 114 ± 112 | 103 ± 94 | 107 ± 153 | 178 ± 62 | 91 ± 78 | 122 ± 140 | 86 ± 62 | 123 ± 47 | 66 ± 61 | 132 ± 140 | 266 ± 481 |
Note: Mean ± standard deviation values are reported.
Discussion
The nematode community structure
Grasslands typically support a high richness of nematode taxa (Song et al., 2017). Yeates and Lee (1997) and Bell et al. (2005) reported 32 and 70 genera, respectively, from tussock grasslands in New Zeeland. Popovici and Ciobanu (2000), in turn, listed between 33 and 67 genera from different grassland regions in Romania, while Čerevková (2006) reported 64 genera in grasslands from the Slovakia Republic. The present study, however, reported a substantially higher number of nematode genera of 93 in total. Rarefaction curves suggest that increased sampling efforts at these sites would result in further taxa being added to that total. At the RZ habitat, for example, extrapolation of the Mau Tao richness indicator suggests that close to a 100 nematode genera could be recovered with increased sampling efforts. The high number of recorded and estimated genera is important as it is commonly accepted that a high richness in nematode taxa are indicative of healthy and functioning soil ecosystems (Ekschmitt et al., 2001; Ferris, 2010; Sánchez-Moreno and Ferris, 2018). A high richness of herbivore taxa in natural environments are often the result of a high diversity in plant species (Hodda et al., 2009; Cortois et al., 2017). According to Hodda et al. (2009), some evidence suggests that a potential reason for this is the lack of competition resulting from root systems creating very defined niches.
Another important consideration when looking at nematode community structure is the abundance distribution between trophic groups (Ferris et al., 2001; Ferris, 2010; Van Den Hoogen et al., 2019). A recent study by Van Den Hoogen et al. (2019) investigated the abundance of nematode trophic groups across all major terrestrial biomes and showed that, on average, bacterivores dominated, followed by herbivores, fungivores, omnivores, and predators. The same trend was observed for temperate grasslands, which is the classification under which the South African grassland biome is listed (Van Den Hoogen et al., 2019). However, even though bacterivores are reportedly the most abundant trophic group on a global and biome scale, studies have reported regional grassland nematode communities with large and even dominating herbivore populations (Porazinska et al., 2003; Ayres et al., 2008). Similarly, during the present study, no significant difference was recorded between the two most abundant groups, namely herbivores and bacterivores, at any of the habitats.
It is worth noting that herbivores of economic importance (e.g. Meloidogyne) occurred in relatively high numbers at some sampling sites. Other herbivores (e.g. Helicotylenchus, Rotylenchulus, and Scutellonema) were present in all the habitats during all four seasons. The presence of these herbivore taxa, sometimes in high numbers, poses a threat to crop production in this region. According to various studies conducted in production areas where major grain crops (e.g. maize and soybean) are produced, Meloidogyne is generally the most predominant nematode pest. Substantial yield losses in maize (up to 60%) (Riekert and Henshaw, 1998) and soybean (up to 100%) (Smit and De Beer, 1998; Fourie et al., 2013) due to infection by this genus demonstrate its adverse impact on crop production. Moreover, Helicotylenchus, Rotylenchulus, and Scutellonema are also abundant in local crop fields, indicating their potential impact on crop production (Fourie et al., 2001; Bekker et al., 2016; Mbatyoti et al., 2018). Therefore, grasslands may act as reservoirs for herbivores that pose a threat to crop production should such areas be converted into agricultural fields.
Seasonal and habitat induced changes in grassland ecosystems
Seasonality had a large and significant effect on the condition, food web status and functioning of the studied grassland soil ecosystems. This effect was most evident progressing from Winter to Spring. During Winter the habitats were enriched with stable ecosystems and structured food webs, while depletion with reduced stability and structure were evident in Spring. Furthermore, during the Winter increased ecosystem functioning were evident with significantly higher predator and structure footprints. This even though low temperatures and reduced precipitation (as recorded during the 90 days period prior to Winter sampling) typically exert a negative influence on nematode communities (Verschoor et al., 2001; Song et al., 2017; Andriuzzi et al., 2020). According to Song et al. (2017), the optimal temperature for the survival and reproduction of soil nematodes ranges from 20 to 25°C, while extremes below 5 and above 30°C significantly inhibit development. Similarly, water availability influences primary production and thus energy flow into the soil food web, while also regulating organic carbon decomposition (Andriuzzi et al., 2020). It should be noted that the especially high herbivore footprints can be attributed to the large number of Meloidogyne that were present in some of the samples. Metabolic footprints are calculated using the biomass of adult females (Ferris, 2010), which can substantially increase footprint values if large numbers of, for example, some sedentary endoparasitic nematodes are recorded.
In contrast to seasonal effects, habitats and the measured soil properties had minimal influence on the studied soil ecosystems. This despite habitat type, soil texture and organic carbon levels being known to substantially influence soil faunal communities (Du Preez et al., 2018; Sánchez-Moreno and Ferris, 2018; Sprunger et al., 2019). Nonetheless, the positive correlation (as evidenced by the redundancy analysis) between organic carbon and the enrichment index is in line with the general understanding that higher organic carbon levels support opportunistic bacterial-feeding nematodes (Sánchez-Moreno and Ferris, 2018). Increased levels of soil organic carbon at the RZ habitat is therefore likely the reason for this habitat also having the highest nematode community enrichment levels during all four seasons in the soil food web analysis. Conversely, the basal and channel indices were positively correlated with the OG habitat, indicating that soil ecosystems associated with this habitat were likely more degraded, but with greater fungal decomposition (Ferris et al., 2001; Ferris, 2010; Sánchez-Moreno and Ferris, 2018).
Interestingly, the abundance of nematode taxa (Supplementary material: Tables S2–S6) made it clear that the dramatic shift in nematode community in Winter can largely be attributed to fluctuations in the populations of only a few predacious nematodes (namely Aporcelaimellus, Iotonchus, and Mononchus), as well as one omnivorous nematode group, the Dorylaimidae. These taxa are assigned a colonizer-persister (c-p) value of 4 (Dorylaimidae and Iotonchus) and 5 (Aporcelaimellus and Mononchus) (Sieriebriennikov et al., 2014), which means that they are regarded as persisters (or k-strategists). Their presence in large numbers therefore infers greater ecosystem stability and food web structure (Ferris et al., 2001; Sánchez-Moreno and Ferris, 2018), as was recorded during Winter at the SRO and OG habitats. The reason for the increased number of members of these nematode groups recorded during Winter is not explicitly clear, however, findings from previous studies provide some potential answers. According to De Ley (1992), desert and dune sands often contain a remarkably high proportion of dorylaims, while Arpin (1969) repeatedly found dorylaims (predators and omnivores) in desiccation experiments. De Ley and Mundo-Ocampo (2004), in turn, stated that the ecological sensitivity of these nematodes may have been overestimated and that certain species may be capable of surviving desiccation and freezing. Omnivorous nematodes typically also have versatile feeding habits and can probably interact at various levels of the soil food web (Hanel, 2003). It is therefore possible that dorylaims in the studied Rand Highveld Grasslands of South Africa are not adversely affected by the conditions present in Winter and may even increase in numbers following reduced competition for resources from other faunal groups. The possibility that certain nematode families and genera are favored by Winter conditions (low temperature and rainfall), infers that nematode community responses to altered conditions are taxa dependent (see also Papatheodorou et al., 2004).
Conclusion
The grassland habitats of Telperion Nature Reserve host a diversity of nematode taxa, many of which are likely still to be observed or described. Although the studied nematode communities were dominated by herbivores and bacterivores, it was the less abundant nematode groups, i.e. omnivores and predators, which had a significant influence on the condition, food web status, and functioning of the soil ecosystems. Furthermore, evidence suggests that only a few taxa are largely responsible for seasonal shifts in measurable soil ecosystem parameters.
This study also recorded a high abundance in nematodes of economic importance, which has important implications for crop production in this grassland biome.
Acknowledgments
The authors thank Oppenheimer Generations, South Africa for access to their property, Telperion Nature Reserve, and the opportunity to collect samples. The following people are also thanked: Duncan MacFadyen, manager of research and conservation at Oppenheimer Generations, Elsabe Bosch, manager at Telperion Nature Reserve, Adoration Shubane and Elsa van Niekerk at ARC-PHP for technical assistance, Christel du Preez for constructive comments, Pieter Holtzhausen for help with creating maps, the biometrician Suria Ellis of the NWU for her assistance with the analyses of the data and Robert Colwell for help with rarefaction curves. We thank the Professional Development Program of the Agricultural Research Council for funding this project.
References
- Andrássy, I. 2005. Free-living nematodes of Hungary, (Nematoda Errantia) Vol. 1 Hungarian Natural History Museum and Systematic Zoology Research Group of the Hungarian Academy of Sciences, Budapest. [Google Scholar]
- Andrássy, I. 2007. Free-living nematodes of Hungary, (Nematoda Errantia) Vol. 2 Hungarian Natural History Museum and Systematic Zoology Research Group of the Hungarian Academy of Sciences, Budapest. [Google Scholar]
- Andrássy, I. 2009. Free-living nematodes of Hungary, (Nematoda Errantia) Vol. 3 Hungarian Natural History Museum and Systematic Zoology Research Group of the Hungarian Academy of Sciences, Budapest. [Google Scholar]
- Andriuzzi, W. S. , Franco, A. L. C. , Ankrom, K. E. , Cui, S. , De Tomasel, C. M. , Guan, P. , Gherardi, L. A. , Sala, O. E. and Wall, D. H. . 2020. Body size structure of soil fauna along geographic and temporal gradients of precipitation in grasslands. Soil Biology and Biochemistry 140:107638, available at: 10.1016/j.soilbio.2019.107638. [DOI] [Google Scholar]
- Archidona-Yuste, A. , Navas-Cortés, J. A. , Cantalapiedra-Navarrete, C. , Palomares-Rius, J. E. and Castillo, P. . 2016. Unravelling the biodiversity and molecular phylogeny of needle nematodes of the genus Longidorus (Lematoda: Longidoridae) in olive and a description of six new species. PLoS ONE 11: e0147689, available at: 10.1371/journal.pone.0147689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpin, P. 1969. Etude préliminaire d’un facteur écologique important pour les nématodes: L’humidité actuelle du sol. Revue d’Ecologie et de Biologie du Sol 6:429–435. [Google Scholar]
- Ayres, E. , Wall, D. H. , Simmons, B. L. , Field, C. B. , Milchunas, D. G. , Morgan, J. A. and Roy, J. . 2008. Belowground nematode herbivores are resistant to elevated atmospheric CO2 concentrations in grassland ecosystems. Soil Biology and Biochemistry 40:978–985, available at: 10.1016/j.soilbio.2007.11.018. [DOI] [Google Scholar]
- Bakonyi, G. , Nagy, P. , Kovács-Láng, E. , Kovács, E. , Barabás, S. , Répási, V. and Seres, A. . 2007. Soil nematode community structure as affected by temperature and moisture in a temperate semiarid shrubland. Applied Soil Ecology 37:31–40, available at: 10.1016/j.apsoil.2007.03.008. [DOI] [Google Scholar]
- Bekker, S. , Fourie, H. , Rashidi, M. , Daneel, M. , Shokoohi, E. and Nel, A. . 2016. Discriminating between the eggs of two egg-mass-producing nematode genera using morphometric and molecular techniques. Nematology 18:1119–1123, available at: 10.1163/15685411-00003022. [DOI] [Google Scholar]
- Bell, N. L. , Davis, L. T. , Sarathchandra, S. U. , Barratt, B. I. P. , Ferguson, C. M. and Townsend, R. J. . 2005. Biodiversity of indigenous tussock grassland sites in Otago, Canterbury and the central North Island of New Zealand II. Nematodes. Journal of the Royal Society of New Zealand 35:303–319, available at: 10.1080/03014223.2005.9517786. [DOI] [Google Scholar]
- Bengtsson, J. , Bullock, J. M. , Egoh, B. , Everson, C. , Everson, T. , O’connor, T. , O’farrell, P. J. , Smith, H. G. and Lindborg, R. . 2019. Grasslands – more important for ecosystem services than you might think. Ecosphere 10: e02582, available at: 10.1002/ecs2.2582. [DOI] [Google Scholar]
- Biederman, L. A. and Boutton, T. W. . 2009. Biodiversity and trophic structure of soil nematode communities are altered following woody plant invasion of grassland. Soil Biology and Biochemistry 41:1943–1950, available at: 10.1016/j.soilbio.2009.06.019. [DOI] [Google Scholar]
- Bongers, T. and Bongers, M. . 1998. Functional diversity of nematodes. Applied Soil Ecology 10: 239–251. [Google Scholar]
- Bongers, T. and Ferris, H. . 1999. Nematode community structure as a bioindicator in environmental monitoring. Trends in Ecology & Evolution 14:224–228. [DOI] [PubMed] [Google Scholar]
- Borgonie, G. , García-Moyano, A. , Litthauer, D. , Bert, W. , Bester, A. , Van Heerden, E. , Möller, C. , Erasmus, M. and Onstott, T. C. . 2011. Nematoda from the terrestrial deep subsurface of South Africa. Nature 474:79–82, available at: 10.1038/nature09974. [DOI] [PubMed] [Google Scholar]
- Carbutt, C. , Tau, M. , Stephens, A. and Escott, B. . 2011. The conservation status of temperate grasslands in southern Africa. Grassroots 11:17–23. [Google Scholar]
- Cardoso, M. S. O. , Pedrosa, E. M. R. , Ferris, H. , Rolim, M. M. and Oliveira, L. S. C. . 2016. Nematode fauna of tropical rainforest in Brazil: a descriptive and seasonal approach. Journal of Nematology 48:116–125, available at: 10.21307/jofnem-2017-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čerevková, A. 2006. Nematode communities in three types of grassland in the Slovak Republic. Helminthologia 43:171–176, available at: 10.2478/s11687-006-0032-y. [DOI] [Google Scholar]
- Cortois, R. , Veen, G. F. , Duyts, H. , Abbas, M. , Strecker, T. , Kostenko, O. , Eisenhauer, N. , Scheu, S. , Gleixner, G. , De Deyn, G. B. and Van Der Putten, W. H. . 2017. Possible mechanisms underlying abundance and diversity responses of nematode communities to plant diversity. Ecosphere 8: e01719, available at: 10.1002/ecs2.1719. [DOI] [Google Scholar]
- De Grisse, A. 1963. A counting dish for nematodes excluding border effect. Nematologica 9:162–162. [Google Scholar]
- De Ley, P. 1992. The nematode community of a marginal soil at Camberene, Senegal, with special attention to functional morphology and niche partitioning in the family Cephalobidae. Mededelingen van de Koninklijke Academie voor Wetenschappen, Letteren en Schone Kunsten van België – Klasse der Wetenschappen 53:107–153. [Google Scholar]
- De Ley, P. and Mundo-Ocampo, M. . 2004. “Cultivation of nematodes”, In Chen, Z. X. , Chen, S. Y. and Dickson, D. W. (Eds), Nematology: advances and perspectives, Vol. 1, Nematode morphology, physiology and ecology CAB International, Wallingford, 541–619. [Google Scholar]
- Decraemer, W. 1995. The family Trichodoridae: stubby root and virus vector nematodes Springer, Dordrecht. [Google Scholar]
- Donkin, M. 1991. Loss-on-ignition as an estimator of soil organic carbon in A-horizon forestry soils. Communications in Soil Science and Plant Analysis 22:233–241. [Google Scholar]
- Du Preez, G. , Majdi, N. , Swart, A. , Traunspurger, W. and Fourie, H. . 2017. Nematodes in caves: a historical perspective on their occurrence, distribution and ecological relevance. Nematology 19:627–644, available at: 10.1163/15685411-00003068. [DOI] [Google Scholar]
- Du Preez, G. C. , Daneel, M. S. , Wepener, V. and Fourie, H. . 2018. Beneficial nematodes as bioindicators of ecosystem health in irrigated soils. Applied Soil Ecology 132:155–168, available at: 10.1016/j.apsoil.2018.08.008. [DOI] [Google Scholar]
- Duarte, I. , De Almeida, M. T. M. , Brown, D. , Marques, I. , Neilson, R. and Decraemer, W. . 2010. Phylogenetic relationships, based on SSU rDNA sequences, among the didelphic genera of the family Trichodoridae from Portugal. Nematology 12: 171–180. [Google Scholar]
- Eisenhauer, N. and Guerra, C. A. . 2019. Global maps of soil-dwelling nematode worms. Nature 572:187–188, available at: 10.1038/d41586-019-02197-0. [DOI] [PubMed] [Google Scholar]
- Ekschmitt, K. , Bakonyi, G. , Bongers, M. , Bongers, T. , Boström, S. , Dogan, H. , Harrison, A. , Nagy, P. , O’Donnell, A. G. , Papatheodorou, E. M. , Sohlenius, B. , Stamou, G. P. and Wolters, V. . 2001. Nematode community structure as indicator of soil functioning in European grassland soils. European Journal of Soil Biology 37:263–268, available at: 10.1016/S1164-5563(01)01095-0. [DOI] [Google Scholar]
- Ferris, H. 2010. Form and function: metabolic footprints of nematodes in the soil food web. European Journal of Soil Biology 46:97–104, available at: 10.1016/j.ejsobi.2010.01.003. [DOI] [Google Scholar]
- Ferris, H. and Bongers, T. . 2009. “Indices developed specifically for analysis of nematode assemblages”, In Wilson, M. J. and Kakouli-Duarte, T. (Eds), Nematodes as environmental indicators CABI Publishing, Wallingford, 124–145. [Google Scholar]
- Ferris, H. , Bongers, T. and De Goede, R. G. M. . 2001. A framework for soil food web diagnostics: extension of the nematode faunal analysis concept. Applied Soil Ecology 18:13–29, available at: 10.1016/S0929-1393(01)00152-4. [DOI] [Google Scholar]
- Fourie, H. , Mc Donald, A. H. and De Waele, D. . 2013. Host and yield responses of soybean genotypes resistant or susceptible to Meloidogyne incognita in vivo. International Journal of Pest Management 59:111–121, available at: 10.1080/09670874.2013.772261. [DOI] [Google Scholar]
- Fourie, H. , Mcdonald, A. H. and Loots, G. C. . 2001. Plant-parasitic nematodes in field crops in South Africa. 6. Soybean. Nematology 3:447–454. [Google Scholar]
- Fourie, H. , Spaull, V. W. , Jones, R. , Daneel, M. S. and De Waele, D. . 2017. “Introduction”, In Fourie, H. , Spaull, V. W. , Jones, R. , Daneel, M. S. and De Waele, D. (Eds), Nematology in South Africa: a view from the 21st century Springer International Publishing, Cham, 1–12. [Google Scholar]
- Geraert, E. 2019. The Dolichodoridae of the world: identification of the family Dolichodoridae Academia Press, Gent. [Google Scholar]
- Gibson, D. J. and Newman, J. A. . 2019. “Grasslands and climate change: an overview”, In Gibson, D. J. and Newman, J. A. (Eds), Grasslands and climate change Cambridge University Press, Cambridge, 3-18. [Google Scholar]
- Grobler, A. 1999. Phytosociology and veld condition assessment of Ezemvelo Game Reserve University of Pretoria, Pretoria. [Google Scholar]
- Haddad, C. R. and Butler, V. P. . 2018. Ground-dwelling spider assemblages in contrasting habitats in the central South African Grassland Biome. Koedoe 60:1–12, available at: 10.4102/koedoe.v60i1.1482. [DOI] [Google Scholar]
- Hanel, L. 2003. Soil nematodes in cambisol agroecosystems of the Czech Republic. Biólogia Bratislava 58:205–216. [Google Scholar]
- Hewins, D. B. , Lyseng, M. P. , Schoderbek, D. F. , Alexander, M. , Willms, W. D. , Carlyle, C. N. , Chang, S. X. and Bork, E. W. . 2018. Grazing and climate effects on soil organic carbon concentration and particle-size association in northern grasslands. Scientific reports 8: 1336, available at: 10.1038/s41598-018-19785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodda, M. , Peters, L. and Traunspurge, W. . 2009. “Nematode diversity in terrestrial, freshwater aquatic and marine systems”, In Wilson, M. J. and Kakouli-Duarte, T. (Eds), Nematodes as Environmental Indicators CABI Publishing, Wallingford, 45–93. [Google Scholar]
- Hunt, D. J. 1993. Aphelenchida, Longidoridae and Trichodoridae: their systematics and bionomics CAB International, Wallingford. [Google Scholar]
- Kergunteuil, A. , Campos-Herrera, R. , Sánchez-Moreno, S. , Vittoz, P. and Rasmann, S. . 2016. The abundance, diversity, and metabolic footprint of soil nematodes is highest in high elevation alpine grasslands. Frontiers in Ecology and Evolution 4:1–12, available at: 10.3389/fevo.2016.00084. [DOI] [Google Scholar]
- Kleynhans, K. , Berg, E. , Swart, A. , Marais, M. and Buckley, N. . 1996. Plant nematodes in South Africa ARC-Plant Protection Research Institute, Pretoria. [Google Scholar]
- Laker, M. C. and Dupreez, C. C. . 1982. An investigation into the accuracy of hydrometers for soil particle size determination. Agroplantae 14:17–22. [Google Scholar]
- Liu, T. , Hu, F. and Li, H. . 2019. Spatial ecology of soil nematodes: perspectives from global to micro scales. Soil Biology and Biochemistry 137: 107565, available at: 10.1016/j.soilbio.2019.107565. [DOI] [Google Scholar]
- Maggenti, A. R. , Luc, M. , Raski, D. J. , Fortuner, R. and Geraert, É. . 1988. A reappraisal of Tylenchina (Nemata). 11. List of generic and supra-generic taxa, with their junior synonyms. Revue Nématol 11:177–188. [Google Scholar]
- Marais, M. , Swart, A. , Fourie, H. , Berry, S. D. , Knoetze, R. and Malan, A. P. . 2017. “Techniques and procedures”, In Fourie, H. , Spaull, V. W. , Jones, R. K. , Daneel, M. S. and De Waele, D. (Eds), Nematology in South Africa: a view from the 21st century Springer, Cham, 73–118. [Google Scholar]
- Mbatyoti, A. , Daneel, M. S. , Swart, A. , De Waele, D. and Fourie, H. . 2018. Terrestrial non-parasitic nematode assemblages associated with glyphosate-tolerant and conventional soybean-based cropping systems. Journal of Nematology 50:243–260, available at: 10.21307/jofnem-2018-028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netscher, C. and Seinhorst, J. W. . 1969. “Propionic acid better than acetic acid in killing nematodes”, Nematologica, Vol. 15 p. 286. [Google Scholar]
- Papatheodorou, E. M. , Argyropoulou, M. D. and Stamou, G. P. . 2004. The effects of large- and small-scale differences in soil temperature and moisture on bacterial functional diversity and the community of bacterivorous nematodes. Applied Soil Ecology 25: 37–49, available at: 10.1016/s0929-1393(03)00100-8. [DOI] [Google Scholar]
- Popovici, I. and Ciobanu, M. . 2000. Diversity and distribution of nematode communities in grasslands from Romania in relation to vegetation and soil characteristics. Applied Soil Ecology 14:27–36, available at: 10.1016/S0929-1393(99)00048-7. [DOI] [Google Scholar]
- Porazinska, D. L. , Bardgett, R. D. , Blaauw, M. B. , Hunt, H. W. , Parsons, A. N. , Seastedt, T. R. and Wall, D. H. . 2003. Relationships at the aboveground–belowground interface: plants, soil biota, and soil processes. Ecological Monographs 73:377–395, available at: 10.1890/0012-9615(2003)073[0377:Rataip]2.0.Co;2. [DOI] [Google Scholar]
- Procter, D. L. C. 2012. Soil-living nematodes of a savanna community in Botswana: a preliminary report. Journal of Arid Environments 76:142–146, available at: 10.1016/j.jaridenv.2011.08.001. [DOI] [Google Scholar]
- Sánchez-Moreno, S. and Ferris, H. . 2018. “Nematode ecology and soil health”, In Sikora, R. , Coyne, D. , Hallmann, J. and Timper, P. (Eds), Plant parasitic nematodes in subtropical and tropical agriculture CAB International, Wallingford, 62–86. [Google Scholar]
- Seinhorst, J. W. 1962. On the killing, fixation and transferring to glycerine of nematodes. Nematologica 8:29–32. [Google Scholar]
- Siebert, J. , Ciobanu, M. , Schädler, M. and Eisenhauer, N. . 2020. Climate change and land use induce functional shifts in soil nematode communities. Oecologia 192:281–294, available at: 10.1007/s00442-019-04560-4. [DOI] [PubMed] [Google Scholar]
- Sieriebriennikov, B. , Ferris, H. and De Goede, R. . 2014. NINJA: an automated calculation system for nematode-based biological monitoring. European Journal of Soil Biology 61:90–93, available at: 10.1016/j.ejsobi.2014.02.004. [DOI] [Google Scholar]
- Skowno, A. L. , Thompson, M. W. , Hiestermann, J. , Ripley, B. , West, A. G. and Bond, W. J. . 2017. Woodland expansion in South African grassy biomes based on satellite observations (1990–2013): general patterns and potential drivers. Global Change Biology 23:2358–2369, available at: 10.1111/gcb.13529. [DOI] [PubMed] [Google Scholar]
- Šmilauer, P. and Lepš, J. . 2014. Multivariate analysis of ecological data using CANOCO 5. Cambridge: Cambridge University Press. [Google Scholar]
- Smit, M. A. and De Beer, G. P . 1998. Report of the national soybean cultivar trials 1998/99 Agricultural Research Council, Potchefstroom. [Google Scholar]
- Song, M. , Li, X. , Jing, S. , Lei, L. , Wang, J. and Wan, S. . 2016. Responses of soil nematodes to water and nitrogen additions in an old-field grassland. Applied Soil Ecology 102:53–60, available at: 10.1016/j.apsoil.2016.02.011. [DOI] [Google Scholar]
- Song, D. , Pan, K. , Tariq, A. , Sun, F. , Li, Z. , Sun, X. , Zhang, L. , Olusanya, O. A. and Wu, X. . 2017. Large-scale patterns of distribution and diversity of terrestrial nematodes. Applied Soil Ecology 114:161–169, available at: 10.1016/j.apsoil.2017.02.013. [DOI] [Google Scholar]
- Sprunger, C. D. , Culman, S. W. , Peralta, A. L. , Dupont, S. T. , Lennon, J. T. and Snapp, S. S. . 2019. Perennial grain crop roots and nitrogen management shape soil food webs and soil carbon dynamics. Soil Biology and Biochemistry 137:107573, available at: 10.1016/j.soilbio.2019.107573. [DOI] [Google Scholar]
- Van Den Hoogen, J. , Geisen, S. , Routh, D. , Ferris, H. , Traunspurger, W. , Wardle, D. A. , De Goede, R. G. M. , Adams, B. J. , Ahmad, W. , Andriuzzi, W. S. , Bardgett, R. D. , Bonkowski, M. , Campos-Herrera, R. , Cares, J. E. , Caruso, T. , De Brito Caixeta, L. , Chen, X. , Costa, S. R. , Creamer, R. , Mauro Da Cunha Castro, J. , Dam, M. , Djigal, D. , Escuer, M. , Griffiths, B. S. , Gutiérrez, C. , Hohberg, K. , Kalinkina, D. , Kardol, P. , Kergunteuil, A. , Korthals, G. , Krashevska, V. , Kudrin, A. A. , Li, Q. , Liang, W. , Magilton, M. , Marais, M. , Martín, J. A. R. , Matveeva, E. , Mayad, E. H. , Mulder, C. , Mullin, P. , Neilson, R. , Nguyen, T. A. D. , Nielsen, U. N. , Okada, H. , Rius, J. E. P. , Pan, K. , Peneva, V. , Pellissier, L. , Carlos Pereira Da Silva, J. , Pitteloud, C. , Powers, T. O. , Powers, K. , Quist, C. W. , Rasmann, S. , Moreno, S. S. , Scheu, S. , Setälä, H. , Sushchuk, A. , Tiunov, A. V. , Trap, J. , Van Der Putten, W. , Vestergård, M. , Villenave, C. , Waeyenberge, L. , Wall, D. H. , Wilschut, R. , Wright, D. G. , Yang, J. -I. and Crowther, T. W. . 2019. Soil nematode abundance and functional group composition at a global scale. Nature 572:194–198, available at: 10.1038/s41586-019-1418-6. [DOI] [PubMed] [Google Scholar]
- Verschoor, B. C. , De Goede, R. G. M. , De Hoop, J. -W. and De Vries, F. W. . 2001. Seasonal dynamics and vertical distribution of plant-feeding nematode communities in grasslands. Pedobiologia 45:213–233, available at: 10.1078/0031-4056-00081. [DOI] [Google Scholar]
- Yeates, G. , Bongers, T. , De Goede, R. , Freckman, D. and Georgieva, S. . 1993. Feeding habits in soil nematode families and genera – an outline for soil ecologists. Journal of Nematology 25:315–331. [PMC free article] [PubMed] [Google Scholar]
- Yeates, G. W. and Lee, W. G. . 1997. Burning in a New Zealand snow-tussock grassland: effects on vegetation and soil fauna. New Zealand Journal of Ecology 21:73–79. [Google Scholar]
- Zhang, G. , Sui, X. , Li, Y. , Jia, M. , Wang, Z. , Han, G. and Wang, L. . 2020. The response of soil nematode fauna to climate drying and warming in Stipa breviflora desert steppe in Inner Mongolia, China. Journal of Soils and Sediments 20:2166–2180, available at: 10.1007/s11368-019-02555-5. [DOI] [Google Scholar]
- Zhong, S. , Zeng, H. C. and Jin, Z. Q. . 2017. Influences of different tillage and residue management systems on soil nematode community composition and diversity in the tropics. Soil Biology and Biochemistry 107:234–243, available at: 10.1016/j.soilbio.2017.01.007. [DOI] [Google Scholar]


