Abstract
Background
The emergence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic is posing a great threat to global health and economy. Due to the lack of broad diagnostic setup, consistent reagent supply lines, and access to laboratory instruments and equipment, it is undoubtedly an enormous burden for developing countries to face the crisis.
Objectives
To develop a cost-effective, reliable and sensitive multiplex assay for SARS-CoV-2 screening which would expand the testing capacities of a developing and low-income country like Bangladesh.
Study design
Initially a singleplex and then a multiplex real-time reverse-transcriptase PCR assays were developed targeting 2 nucleocapsid genes of SARS-CoV-2, and the human RNase P gene as an internal control using laboratory-made mastermixes. Three sets of primer- probes were designed for each of the target genes and one set was optimized for the final reaction set-up. Limit of detection, cross-reactivity and reproducibility were checked in order to assess the sensitivity and specificity of the assays, and validation was done using clinical specimens.
Results
Clinical evaluation of the new assays using 240 nasopharyngeal swabs showed 100 % sensitivity, specificity, and accuracy in detecting SARS-CoV-2 infection in human. Equal efficiency and concordant results were observed between the singleplex and multiplex approaches. Notably, the kit was able to detect SARS-CoV-2 RNA at very low concentration upto 5 copies/reaction.
Conclusion
This is the first locally developed multiplex rRT-PCR kit in Bangladesh providing rapid and low-cost screening of COVID-19 which would be valuable for infection prevention and clinical management in the perspective of Bangladesh.
Keywords: SARS-CoV-2, Singleplex rRT-PCR, Multiplex rRT-PCR, Cost effective assay, In-house kit, Developing country
1. Introduction
The coronavirus disease termed as COVID-19 is a highly pathogenic viral infection caused by novel severe acute respiratory syndrome coronavirus (SARS-CoV-2) and has been declared as a pandemic and an international public health emergency by the World Health Organization (WHO). Until December 2020, the virus has spread across 235 territories with about 36 million confirmed cases and more than 1 million deaths. Bangladesh is the second most affected country in South Asia and the country had reported 481,945 cases of COVID-19, and the death toll stood at 6,906 As of December 9, 2020 (Covid-19 Update, 2020).
As clinical features cannot alone define the diagnosis of COVID-19, early diagnosis is crucial for prevention and management of this pandemic. On the other hand, the exact number of infected people with SARS-CoV-2 cannot be known, as many asymptomatic cases go undetected (Kobayashi et al., 2020; Mizumoto et al., 2020). Such situation prioritizes the availability of specific and sensitive assays for the detection of the virus which is essential for accurate diagnosis of infected cases, assessment of the extent of outbreak, and monitoring of intervention strategies. Nucleic acid detection-based approach real-time reverse transcriptase-PCR (rRT-PCR) method is considered as the ‘gold standard’ for the detection of some viruses and as such is in great demand today for the detection of SARS-CoV-2 (Shen et al., 2020; Wan et al., 2016) throughout the world including Bangladesh. Initially, the US CDC developed singleplex SARS-CoV-2 assay targeting the viral nucleocapsid gene was adopted in several countries as well as in Bangladesh (Lu et al., 2020a). After that, many rRT-PCR assays targeting alternative viral genes have been developed and issued by the US Food and Drug Administration (Zhen et al., 2020; Ishige et al., 2020; Chan et al., 2020; Visseaux et al., 2020).
With such high demand for rRT-PCR based COVID-19 test kits, many regions around the world are experiencing a shortage of laboratory-based molecular-assay tests. The situation is more critical in a developing and low-income country like Bangladesh as scarcity of testing kits appears to be a major setback for preventing the spread of the deadly coronavirus. In addition, as all the rRT-PCR kits for COVID-19 detection in Bangladesh are imported, the quality of the test kits is questionable for lengthy and complicated transportation process. Furthermore, the imported kits are very expensive and it is apparent that government cannot afford the cost of COVID-19 testing kits for unlimited time. In such a case, development of locally produced sensitive and specific in-house assays would be cost saving and thus can reduce this enormous burden of the country. In this purpose, we primarily developed an in-house singleplex, and then a multiplex rRT-PCR assay to detect SARS-CoV-2 in human respiratory specimens targeting two regions of viral nucleocapsid (N) gene due to its relative abundance in sub-genomic mRNA during virus replication (Moreno et al., 2008). The newly developed kit has been named as RealDetect™ COVID-19 RT-PCR Kit.
2. Materials and methods
2.1. Kit development
2.1.1. Primers and probes
A total three sets of primer pairs for each target gene (N1, N2 and RP) were used where one pair was taken from the list of primers published by CDC (Lu et al., 2020a). The other two sets of primer pairs for N1, N2 and RP were designed using Primer3 plus, IDT oligoanalyzer and NCBI primer-blast tools. All of these primers and probes were purchased from Integrated DNA Technologies-IDT (Coralville, IA, USA). For multiplexing, the 5′ bases of N1, N2 and RP probes were labeled with FAM, ROX and VIC, respectively. In addition to the 3′ quenchers, an internal quencher was added to the N1 and RP probes.
2.1.2. Preparation of PCR master mix
An in-house master mix was prepared and optimized to perform one-step rRT-PCR to minimize the cost. The reagents used for the preparation of master mix were ultrapure Tris-buffer, 2 M KCl, 1x TE buffer and recombinant Taq DNA polymerase from Invitrogen (California, USA); 10 mM dNTPs, 25 mM MgCl2 and random hexamer primer (ThermoFisher Scientific, Massachusetts, USA); ArrayScript™ M-MLV reverse-transcriptase and RNase inhibitor (Applied Biosystems, California, USA); and Nuclease-free water (Integrated DNA Technologies, Iowa, USA).
2.1.3. Control samples
Known copies of 2019-nCoV_N positive control plasmids and Hs_RPP30 control containing human RNase P gene purchased from IDT were used as positive control and internal control, respectively. The main stock of the positive plasmid control for each targets contained 2lac copy/μl. The potency of each plasmid controls was checked by serially diluted solutions (2000, 200 and 20 copy/μl) using three replicates for each dilution. Finally, 200 copy/μl was used as positive control in the kit according to the general recommendation from IDT. These plasmid controls were directly added to the mastermix as templates. Samples were considered positive for SARS-CoV-2 infection when a signal was detected at Ct <38 and negative if the N-gene specific signals were undetermined or detected at Ct >38 along with the amplified RP signal of a Ct <40. A specimen was considered invalid when both N1/N2 signals and RP signal were undetermined. A no-template control (NTC) consisting of nuclease-free water was also used.
2.1.4. Optimization of singleplex and multiplex rRT-PCR
rRT-PCR reactions were run on different Real-Time PCR instruments including QuantStudio3, QuantStudio5, ABI-7500 Fast (Applied Biosystems, California, USA), and BioRad CFX96 (Bio-Rad Laboratories, California, USA). For singleplex, each 20 μL reaction mixture contained 10 μL master mix, 1.5 μL of primers/probes mix, and 8.5 μL extracted RNA. The detection channel was FAM.
To develop a multiplex rRT-PCR with N1, N2 and RP, a total of 3 sets of primer pairs were designed which were used in different combinations and concentrations to optimize the final reaction setup. The cycling program was also optimized using temperature gradient rRT-PCR for setting up the reverse-transcription step and PCR annealing temperature. The filter combinations were FAM (N1), ROX (N2) and VIC (RP). A final reaction volume of 25 μL consisting 13.5 μL master mix, 2 μL primer-probe mix, and 9.5 μL extracted RNA or a positive plasmid control containing all three target sequences was subjected to amplify together in a single-reaction tube.
2.1.5. Evaluation of specificity
The SARS-CoV-2 genome is significantly similar to other coronaviruses and human pathogenic respiratory viruses like Bat corona viruses (88 %), SARS-CoV (79 %) and MERS-CoV (50 %) (Wang et al., 2020; Lu et al., 2020b). To investigate whether our developed assays cross-react with other human-pathogenic coronaviruses namely SARS-CoV and MERS-CoV, and respiratory viruses like adenovirus, rhinovirus, human-metapneumovirus, influenza virus, parainfluenza virus and respiratory syncytial viruses, positive controls or clinical specimens positive for respiratory pathogens were tested to further evaluate the specificity of the assays.
2.1.6. Accelerated stability testing
Accelerated testing, commonly used to make stability predictions, was done following the formula derived from Arrhenius equation (Lu et al., 2020b). The test kits stored at 4 ± 2 °C were tested at predetermined time points to estimate the shelf life (Table S1), which was compared to the results obtained for the same lots stored at −20 °C for a 6-week time period. A panel of positive and negative samples which were stored as single separate aliquots was run at each time point to check the kit efficiency.
2.2. Kit validation
2.2.1. Place of study and ethical approval
The kit was validated at institute for developing Science and Health initiatives (ideSHi), a Contract Research Organization (CRO) facility. The SARS-CoV-2 nasopharyngeal (NP) swab specimens were provided by a national reference laboratory, Institute for Epidemiology, Disease Control, and Research (IEDCR). The study was registered and ethically approved by the National Research Ethics Committee (NREC) of Bangladesh Medical Research Council (BMRC).
2.2.2. Sample information
To make the kit validation process statistically significant, the appropriate number of sample to be tested was calculated applying a methodological approach (Waterman, 2011). Two reference kits namely xABT Multiple Real-time PCR kit (XABT, Beijing Applied Biological Technologies, China) and A* Star Fortitute kit 2.0 (Accelerate Technologies, Singapore) were used for validation. According to the statistical analysis, a total of 240 clinical specimens, 120 for each reference kit were considered to be evaluated. These samples included 60 COVID-19 positive (30 fresh and 30 stored) and 60 COVID-19 negative (30 fresh and 30 stored) specimens. The samples were deidentified and blinded by IEDCR before sending to the referred laboratory.
2.2.3. Viral RNA extraction and rRT-PCR
The deidentified COVID-19 positive and negative specimens in viral transport medium were subjected to RNA extraction using QIAamp Viral RNA Mini Kit (Qiagen, Germantown, USA) followed by rRT-PCR using optimized protocols for both singleplex and multiplex approaches. Assay performance was compared to the results obtained from two different reference kits in terms of Ct values and detection rate.
2.2.4. Statistical analysis
For validation of the assays, sensitivity, specificity, positive predictive value, negative predictive value, kappa analysis, and two-sided (upper/ lower) 95 % CI were calculated using open EPI tool (http://openepi.com/).
3. Results
3.1. Multiplexing of N1, N2 and RP genes using rRT-PCR for detection of SARS-CoV-2
Upon reactions with different combination and concentration of primers and probes, a primer-probe mix consisting of N1 primer sets from CDC, and N2 plus RP sets from our designed set P1 was finalized for the multiplex assay (Table 1 ).
Table 1.
Primers and probes used in singleplex and multiplex rRT-PCR.
| Target gene | Name | Oligonucleotide sequence (5′>3′) |
|---|---|---|
| N1 | N1_cdcF_Primer | 5′-GAC CCC AAA ATC AGC GAA AT-3′ |
| N1_cdcR_Primer | 5′-TCT GGT TAC TGC CAG TTG AAT CTG-3′ | |
| Probe_N1 | 5′-FAM/ACCCCGCAT/ZEN/TACGTTTGGTGGACC/3IABkFQ | |
| N2 | P1_Forward N2 | 5′-GAT TAC AAA CAT TGG CCG CAA ATT GC-3′ |
| P1_Reverse N2 | 5′-ATG CGC GAC ATT CCG AAG AAC G-3′ | |
| Probe_N2 | 5′-ROX/ACAATTTGCCCCCAGCGCTTCAG/3IAbRQSp | |
| RNase P | P1_Forward RP | 5′-GA TTT GGA CCT GCG AGC G-3′ |
| P1_Reverse RP | 5′-AG CGG CTG TCT CCA CAA GT-3′ | |
| Probe_RP | 5′-VIC/TTCTGACCT/ZEN/GAAGGCTCTGCGCG/3IABkFQ |
Abbreviations: P1, Primer set 1; RP, RNase P.
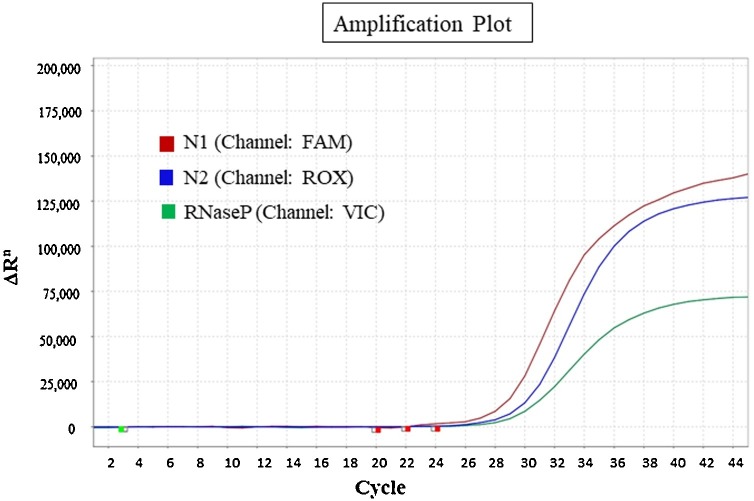
The optimized combination showed the best amplification for each of the target genes (Fig. 1 ) with ideal baseline, cycle of threshold and minimal background noise in the following reaction protocol- 48 °C (10 min), 95 °C (8 min), followed by 45 cycles of 95 °C (15 s) and 57 °C (45 s). Both the singleplex and multiplex reactions were found to be compatible on QuantStudio3, QuantStudio 5, ABI-7500 and BioRad CFX96 Real-time PCR system.
Fig. 1.
Amplification curves for each of the target genes (N1, N2 and RNase P) in multiplex assay with the optimized combination of primer-probes.
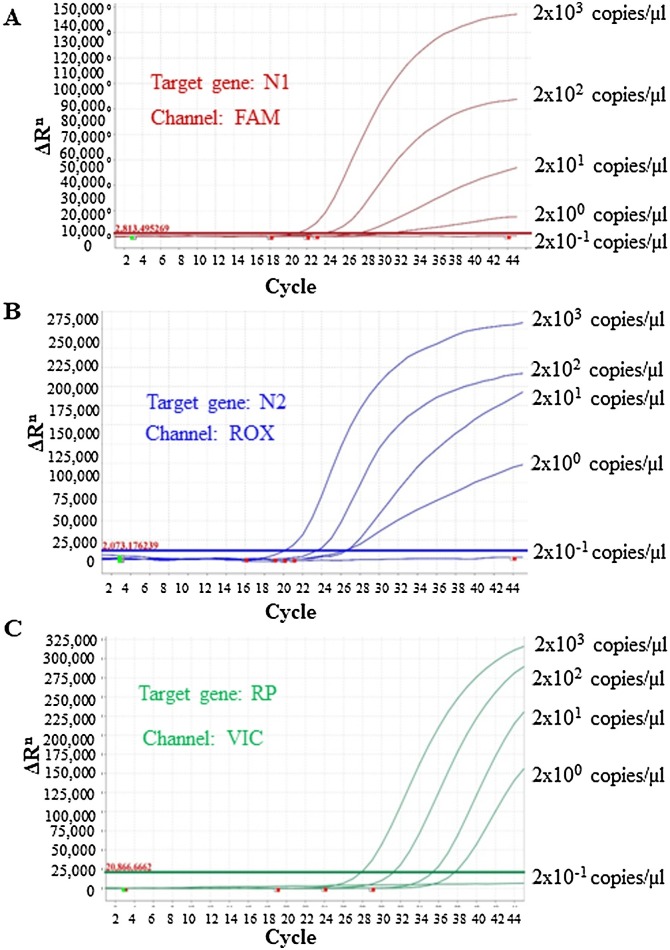
3.2. Limit of detection (LOD) of the in-house multiplex kit
For the LoD assessment, 10-fold serial dilutions of synthetic positive controls (2000, 200, 20, 2 and 0.2 copies/μL) using 5 replicates for each dilution were assessed. The amplification curves with detection rates for all three targets have been depicted in Fig. 2 . The detection rate was 100 % for up to 2 copies/μL having 5/5 replicates positive; whereas 1/5 were positive at 0.2 copies/μL. Thus the calculated LoD was found to be 5copies/reaction and 10copies/reaction with 95 % and 100 % detection probability, respectively.
Fig. 2.
Amplification curves for different copies (2000, 200, 20, 2 and 0.2 copies/μL) of target genes including internal control gene. A Limit of detection (LoD) for N1 target gene. B LoD for N2 target. C LoD for RNase P target gene.
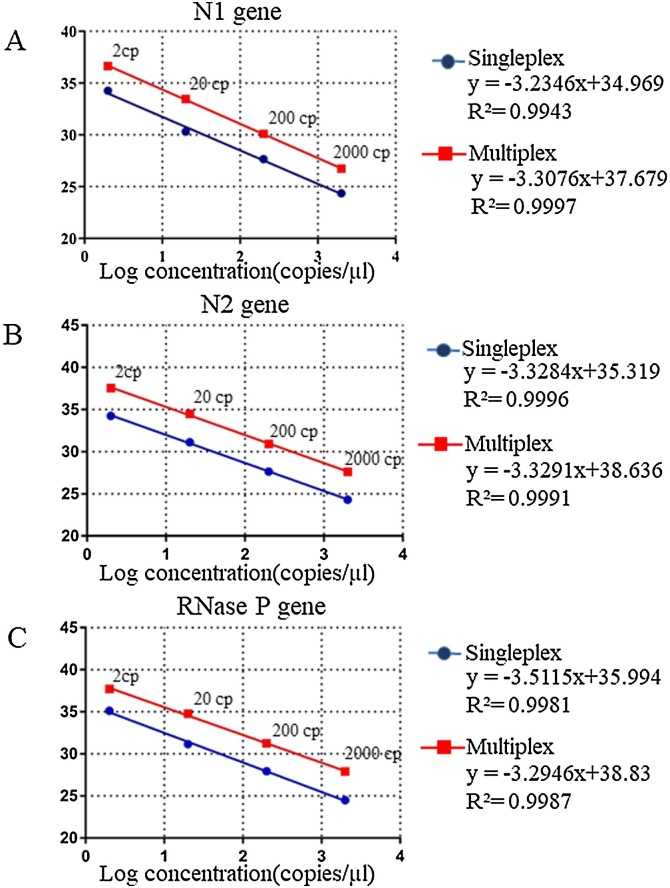
3.3. Efficiency of the multiplex rRT-PCR assays
Both singleplex and multiplex assays exhibited concordant results showing equal efficiency and linearity with regression correlation coefficient, R2 ≥0.99 for each of the targets- N1, N2 and RP genes (Fig. 3 ). CT values were slightly higher by 2–2.5 for each dilution in multiplex than singleplex reactions.
Fig. 3.
Assessment of calibration curves through singleplex and multiplex rRT-PCR for different copies of synthetic positive controls of N1, N2 and RNase P. A Standard curve for N1. B Standard curve for N2. C Standard curve for RNase P.
3.4. Cross-reactivity
The new assays showed no cross-reactivity with closely-related human coronavirus species like SARS-CoV and MERS-CoV or other respiratory viruses like adenovirus, rhinovirus, human-metapneumovirus, influenza virus, parainfluenza virus and respiratory syncytial viruses. The methods have thus been shown to be highly capable of detecting the novel SARS-CoV-2 with 100 % specificity.
3.5. Assay reproducibility and stability
Assay reproducibility and repeatability were evaluated on five negative and five positive specimens. Both singleplex and multiplex assays were repeated five times in five consecutive weeks. Each of the five experiments exhibited high precision and accuracy showing minimal deviation from the mean Ct values for N1, N2 and RP targets (Table 2 ). In addition, the coefficients of variation of the precision CT values were found to be ≤5 %.
Table 2.
Reproducibility of RealDetect™ Covid-19 singleplex and multiplex assays in a 5-week time period.
| Ct Values for singleplex PCR |
Ct Values for multiplex PCR |
|||||
|---|---|---|---|---|---|---|
| N1 | N2 | RP | N1 | N2 | RP | |
| Week 1 | 27.1 | 27.53 | 29.5 | 28.3 | 28.24 | 29.25 |
| Week 2 | 28.34 | 27.55 | 29.87 | 29.05 | 30.07 | 30.54 |
| Week 3 | 27.27 | 27.9 | 30.1 | 29.43 | 30.43 | 30.77 |
| Week 4 | 26.9 | 28.2 | 30.2 | 29.87 | 30.66 | 31.47 |
| Week 5 | 27.7 | 28.9 | 29.9 | 29.9 | 30.48 | 29.2 |
| Mean ± SD | 27.46 ± 0.57 | 28.01 ± 0.56 | 29.91 ± 0.26 | 29.31 ± 0.66 | 29.97 ± 0.99 | 30.24 ± 0.99 |
| CV (%) | 2.7 % | 1.9 % | 0.8 % | 2.2 % | 3.3 % | 3.2 % |
Abbreviations: SD, Standard deviation; CV, Coefficient of variation.
The kit was found to be stable for up to 6 weeks at 4 ± 2 °C and produced accurate results for each rRT-PCR experiment for both positive and negative specimens which were stored as single separate aliquots to avoid repeated freeze thawing. Thus, the estimated stability of the kits is at least 6months at −20 °C.
3.6. Kit validation using clinical specimens
Among the 240 clinical nasopharyngeal specimens (120 positive and 120 negative) tested with the new kit at the validation site against two reference kits, 38 samples were strongly positive for Covid-19 (Ct ≤26), while 53 and 29 specimens were moderately (Ct >26 to ≤32) and weakly (Ct>32 to ≤38) positive, respectively. With comparison to xABT Multiple Real-time PCR kit, the new singleplex kit had detected 119/120 samples where 1 positive sample with high Ct value (37.6) was not detected by our kit and thus exhibited 98.33 % sensitivity, 100 % specificity and 99.17 % accuracy. On the other hand, the multiplex assay could reveal 100 % similar result which were in perfect agreement (Table 3 ). Compared to the second reference kit A*Star Fortitute, 120/120 samples were correctly detected by both singleplex and multiplex methods indicating a diagnostic accuracy, sensitivity and specificity of 100 % with a kappa value of 1 (95 % CI, 0.8211–1.179).
Table 3.
Validation and performance evaluation of RealDetect™ Covid-19 RT-PCR kit.
| Singleplex method |
Multiplex method |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Result | Test by RealDetect Covid-19 RT-PCR kit | Test by xABT Multiple Real-time PCR kit | Test by RealDetect Covid-19 RT-PCR kit | Test by A* Star Fortitute kit | Test by RealDetect Covid-19 RT-PCR kit | Test by xABT Multiple Real-time PCR kit | Test by RealDetect Covid-19 RT-PCR kit | Test by A* Star Fortitute kit | |
|
Samples tested positive, N = 120 |
Strong (Ct ≤26) N=38 |
18 | 18 | 20 | 20 | 18 | 18 | 20 | 20 |
| Moderate (Ct >26 to ≤32) N = 53 |
27 | 27 | 26 | 26 | 27 | 27 | 26 | 26 | |
| Low (Ct>32 to ≤38) N= 29 |
14 | 15 | 14 | 14 | 15 | 15 | 14 | 14 | |
| Samples tested negative, N= 120 | 61 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | |
| Total sample, n | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 | |
| Sensitivity,% (95 % CI) | 98.33 (91.06–99.96) | 100 (93.98–100) | 100 (93.98–100) | 100 (93.98–100) | |||||
| Specificity, % (95 % CI) | 100 (94.04–100) | 100 (93.98–100) | 100 (93.98–100) | 100 (93.98–100) | |||||
| Positive predictive value (95 %CI) | 100 (93.94–100) | 100 (93.98–100) | 100 (93.98–100) | 100 (93.98–100) | |||||
| Negative predictive value (95 %CI) | 98.36 (91.2–99.96) | 100 (93.98–100) | 100 (93.98–100) | 100 (93.98–100) | |||||
| Accuracy, % (95 % CI) | 99.17 (95.43–99.85) | 100 (96.9–100) | 100 (96.9–100) | 100 (96.9–100) | |||||
| Kappa value (95 % CI) | 0.98 (0.8044–1.162) | 1 (0.8211–1.179) | 1 (0.8211–1.179) | 1 (0.8211–1.179) | |||||
Abbreviations: CI, Confidence Interval.
4. Discussion
The COVID-19 pandemic has affected more than 200 countries throughout the world, causing severe illness and deaths. Unless an effective vaccine is available, rapid and reliable laboratory diagnosis of SARS-CoV-2 infection and on basis of the diagnosis results, taking timely actions such as maintaining physical distance and going into quarantine are the ultimate solutions to suppress the transmission and a critical component of public health interventions. Therefore, it would be highly beneficial for our country if the COVID-19 diagnostic kits can be locally developed and made commercially available.
Here we have reported an in-house development of rRT-PCR-based method for routine specimen screening of SARS-CoV-2. Initially we started with the development of a singleplex assay based on US CDC RT-PCR kit (Lu et al., 2020a). As singleplex assays are time-consuming, we further designed multiple sets of primers and probes to develop a multiplex assay. The benefits of multiplex assay include (1) time-saving as two viral target sequences and also the human housekeeping gene in a clinical specimen can be amplified in a single tube, (2) simplified diagnosis of COVID-19 with fewer reactions for each patient specimen, (3) increased number of specimen testing, and (4) reduced reagent cost and thus can be extremely helpful to confront the ongoing pandemic emergency. Several multiplex RT-PCR kits have been developed targeting different 2019-nCoV virus specific genes, such as RNA-dependent RNA polymerase (RdRp), envelope (E), and nucleocapsid (N1/N2/N3), open reading frame (ORF1) and surface (S) genes etc. (Visseaux et al., 2020; Rosner, 2015; Corman et al., 2020). Among these targets, N-gene is more conserved and stable, with abundant expression during infection and fewer mutations over time (Zhen and Berry, 2020; Drosten et al., 2003; Marra et al., 2003). One study also showed that the N gene–based rRT-PCR assay was more sensitive than the ORF1, for detection of SARS-CoV-2 in clinical specimens (Cong et al., 2020). Therefore, rRT-PCR assays targeting the N-gene of SARS-CoV-2 could theoretically achieve enhanced diagnostic sensitivity. However, very few multiplex assays based on N gene-derived N1 and N2 markers have been established so far (Chu et al., 2020; Perchetti et al., 2020). This might be due to the fact that multiplexing of N1 and N2 genes can be difficult as the presence of closely related N genes sometimes lead to cross-hybridization with each other and there is a high possibility of mis-priming with other templates (Kudo et al., 2020). Hence, this in-house multiplex rRT-PCR kit has been developed targeting N1 and N2 markers of SARS-CoV-2 using optimized primer-probe sets selected from multiple sets that had been found to be the most efficient with no observed cross-talk between the primers.
In addition, using two COVID-19 virus specific target gene markers in the kit have reduced the possibility of false-negative results caused by any polymorphisms occurred within the primer binding sites of the target sequences. Notably, while most of the other available kits with similar composition used the same reporter dye FAM for both N1 and N2 targets (Petrillo et al., 2020) here we used two different dyes for different targets which would independently detect two regions of N gene of SARS-CoV-2 and thus enhance the efficiency of the multiplex approach.
In this assay, cut-off values for viral target genes were set to be as CT < 38, while a CT < 40 was considered as a positive signal for the internal control. There were two reasons behind using different cut-off values for target genes and human housekeeping gene (RNase P). Firstly, throughout the kit development as well as validation process, higher CT values (by ~ 2) were found for RNase P than N1 and N2 genes for the same dilution series (2000, 200, 20 and 2 copies/μl). Secondly, as the possibility of endogenous gene detection which is crucial for generating a valid result, depends on availability of human cells in the NP swab specimens, so setting a comparatively higher CT value for RNase P ultimately lessens the number of invalid results.
The study also demonstrated that both multiplex and singleplex amplification of N1 and N2 sequences using rRT-PCR was equally sensitive and specific. The LoD assessment showed that the kit was able to detect upto 2 RNA copies/μL with no observed false-positive reactivity, indicating that SARS-CoV-2 detection can be done when the viral RNA is present at low concentrations.
Validation for the new kit was done in order to confirm that these methods were reliably sensitive and specific for detection of COVID-19 viral RNA in clinical specimens. The kit validation tests against two different reference kits demonstrated that both assay results were concordant with the original results having 99.17 % and 100 % accuracy, respectively. Also, both assays proved to be sensitive and specific with high reproducibility. Hence, the kit can be considered as a valid diagnostic tool for COVID-19 viral RNA detection, having the advantage of a less complex and unambiguous results interpretation pattern.
Apparently, the SARS-CoV-2 pandemic has resulted in a shortage of molecular diagnostic reagents. Moreover, Bangladesh, being a developing country is completely dependent upon the imported kits where the quality of the test kits is most likely to be compromised during shipment and the exorbitantly high shipment charge and cost of the kits is difficult to be borne. Such locally developed cost-effective and sensitive test kits will significantly increase the testing capacities of the country averting the need for imported kits, which will assist to get a clearer picture of the actual number of infections to stop the spread of the disease.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Farjana Akther Noor: Conceptualization, Validation, Formal analysis, Investigation, Writing - original draft, Visualization, Supervision, Project administration. Kazi Sarjana Safain: Methodology, Validation, Formal analysis, Data curation, Writing - original draft, Visualization. Md. Walid Hossain: Methodology, Validation, Formal analysis, Data curation. Khalid Arafath: Methodology, Validation, Formal analysis, Data curation. Kaiissar Mannoor: Conceptualization, Investigation, Writing - review & editing. Mazbahul Kabir: Conceptualization, Investigation, Resources, Visualization, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
MK is the co-founder of OMC Healthcare (Pvt.) Ltd. The other authors have no conflict of interest to declare.
Acknowledgments
We specially thank Dr. Firdausi Qadri and her team at institute for developing Science and Health initiatives (ideSHi) for providing their laboratory facilities to validate the kit using clinical specimens of SARS-CoV-2.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jviromet.2021.114147.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Chan J.F.-W., Yip C.C.-Y., To K.K.-W., Tang T.H.-C., Wong S.C.-Y., Leung K.-H., Fung A.Y.-F., Ng A.C.-K., Zou Z., Tsoi H.-W. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J. Clin. Microbiol. 2020:58. doi: 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D., PaN Y., CheNg S., Hui K.P., KRiShNaN P., Liu Y., Ng D.Y., WaN C.K., YaNg P., WaNg Q., PeiRiS M., PooN L.L.M. Molecular diagnosis of a novel Coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin. Chem. 2020;66:549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y., Ulasli M., Schepers H., Mauthe M., V’kovski P., Kriegenburg F., Thiel V., de Haan C.A., Reggiori F. Nucleocapsid protein recruitment to replication-transcription complexes plays a crucial role in coronaviral life cycle. J. Virol. 2020:94. doi: 10.1128/JVI.01925-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covid-19 Bangladesh . Institute of Epidemiology, Disease Control and Research (IEDCR); 2020. Update. [Google Scholar]
- Drosten C., Günther S., Preiser W., Van Der Werf S., Brodt H.-R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Ishige T., Murata S., Taniguchi T., Miyabe A., Kitamura K., Kawasaki K., Nishimura M., Igari H., Matsushita K. Highly sensitive detection of SARS-CoV-2 RNA by multiplex rRT-PCR for molecular diagnosis of COVID-19 by clinical laboratories. Clin. Chim. Acta. 2020;507:139–142. doi: 10.1016/j.cca.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Jung S.-m., Linton N.M., Kinoshita R., Hayashi K., Miyama T., Anzai A., Yang Y., Yuan B., Akhmetzhanov A.R. Multidisciplinary Digital Publishing Institute; 2020. Communicating the Risk of Death from Novel Coronavirus Disease (COVID-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo E., Israelow B., Vogels C.B., Lu P., Wyllie A.L., Tokuyama M., Venkataraman A., Brackney D.E., Ott I.M., Petrone M.E. Detection of SARS-CoV-2 RNA by multiplex RT-qPCR. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Wang L., Sakthivel S.K., Whitaker B., Murray J., Kamili S., Lynch B., Malapati L., Burke S.A., Harcourt J. US CDC real-time reverse transcription PCR panel for detection of severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 2020;26:1654. doi: 10.3201/eid2608.201246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J.L., Zúñiga S., Enjuanes L., Sola I. Identification of a transcription enhancer in coronavirus. J. Virol. 2008;82(8):3882–3893. doi: 10.1128/JVI.02622-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchetti G.A., Nalla A.K., Huang M.-L., Jerome K.R., Greninger A.L. Multiplexing primer/probe sets for detection of SARS-CoV-2 by qRT-PCR. J. Clin. Virol. 2020 doi: 10.1016/j.jcv.2020.104499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrillo S., Carrà G., Bottino P., Zanotto E., De Santis M.C., Margaria J.P., Giorgio A., Mandili G., Martini M., Cavallo R. A novel multiplex qRT-PCR assay to detect SARS-CoV-2 infection: high sensitivity and increased testing capacity. Microorganisms. 2020;8:1064. doi: 10.3390/microorganisms8071064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B. Nelson Education; 2015. Fundamentals of Biostatistics. [Google Scholar]
- Shen M., Zhou Y., Ye J., Al-Maskri A.A.A., Kang Y., Zeng S., Cai S. Recent advances and perspectives of nucleic acid detection for coronavirus. J. Pharm. Anal. 2020;10(2):97–101. doi: 10.1016/j.jpha.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visseaux B., Le Hingrat Q., Collin G., Bouzid D., Lebourgeois S., Le Pluart D., Deconinck L., Lescure F.-X., Lucet J.-C., Bouadma L. Evaluation of the QIAstat-Dx Respiratory SARS-CoV-2 Panel, the first rapid multiplex PCR commercial assay for SARS-CoV-2 detection. J. Clin. Microbiol. 2020;58(8) doi: 10.1128/JCM.00630-20. e00630-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Z., Zhang Y.n., He Z., Liu J., Lan K., Hu Y., Zhang C. A melting curve-based multiplex RT-qPCR assay for simultaneous detection of four human coronaviruses. Int. J. Mol. Sci. 2016;17:1880. doi: 10.3390/ijms17111880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Li X., Li T., Zhang S., Wang L., Wu X., Liu J. The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur. J. Clin. Microbiol. Infect. Dis. 2020:1. doi: 10.1007/s10096-020-03899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman K.C. The application of the accelerated stability assessment program (ASAP) to quality by design (QbD) for drug product stability. AAPS PharmSciTech. 2011;12:932. doi: 10.1208/s12249-011-9657-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen W., Berry G.J. Development of a new multiplex real-time RT-PCR assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection. J. Mol. Diagn. 2020;22(12):1367–1372. doi: 10.1016/j.jmoldx.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen W., Manji R., Smith E., Berry G.J. Comparison of four molecular in vitro diagnostic assays for the detection of SARS-CoV-2 in nasopharyngeal specimens. J. Clin. Microbiol. 2020;58(8) doi: 10.1128/JCM.00743-20. e00743-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.