Graphical abstract
Keywords: LC–MS/MS, Favipiravir, Human plasma, Bioequivalence study, COVID-19, SARS-CoV-2
Abstract
A novel, fast and sensitive LC–MS/MS method was developed and validated for the bioanalysis of the antiviral agent favipiravir (FAV); a promising candidate for treatment of SARS-CoV-2 (COVID-19) in human plasma using pyrazinamide as an internal standard (IS). Simple protein precipitation was adopted for plasma sample preparation using methanol. Chromatographic separation was accomplished on Eclipse plus C18 column (50 × 4.6 mm, 3.5 μm) using a mobile phase composed of methanol-0.2 % acetic acid (20:80, v/v) pumped at a flow rate 0.6 mL/min in an isocratic elution mode. The API4500 triple quadrupole tandem mass spectrometer was operated with multiple-reaction monitoring (MRM) in negative electrospray ionization interface for FAV and positive for IS. The MRM function was used for quantification, with the transitions set at m/z 156.00→ 113.00 and m/z 124.80→ 81.00 for FAV and IS. The method was optimized and fully validated in accordance to US-FDA guidelines. Linearity was acquired over a concentration range of 100.0–20000.0 ng/mL by computing using weighted linear regression strategy (1/x2). The proposed method was effectively applied for the pharmacokinetic evaluation of FAV and to demonstrate the bioequivalence of a new FAV formulation (test) and reference product in healthy Egyptian human volunteers.
1. Introduction
An outbreak of SARS-CoV-2 infection has spread across the world. As of 1st of April 2020 nearly 874,151 cases have been diagnosed worldwide, and 43,804 have died from the pandemic [1]. However, no specific antiviral drugs have been approved for the treatment of SARS-CoV-2. The worldwide pandemic has driven the research area to work on developing medications or vaccines to stop or end its propagation. Recently, the management guidelines of many countries have included favipiravir (FAV) as a potential drug candidate in the treatment protocol.
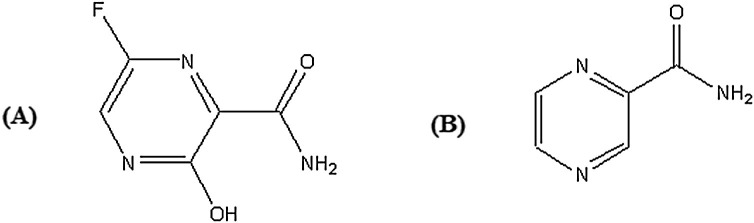
FAV is a new oral antiviral drug approved for management of evolving pandemic influenza infections in Japan. FAV is a prodrug that is ribosylated and phosphorylated intracellularly to form the active metabolite named ibofuranosyl-5-triphos-phate (T-705-RTP). It is chemically designated as 5-fluoro-2-oxo-1H-pyrazine-3-carboxamide, Fig. 1 A [2]. It is a modified purine nucleic acid analog that acts by selectively inhibit the viral RNA-dependent RNA polymerase (RdRp) enzymes, which are necessary for the transcription and replication of viral genomes. Not only does FAV inhibit replication of influenza A and B, but also the drug has shown promise in the treatment of avian influenza, and may be an alternate option for influenza strains that are resistant to neuramidase inhibitors. FAV has been further investigated for the treatment of life-threatening pathogens like Ebola virus, Lassa virus, and recently SARS-CoV-2 [[3], [4], [5]]. Following promising clinical studies on Japanese healthy volunteers, the Cmax of FAV reached within 2 h after a single oral dose, and then dramatically decreased with a short t1/2 of 2–5.5 h. Moreover, FAV percentage of plasma protein binding was found to be 54 % in humans [6,7].
Fig. 1.
Chemical structure of FAV (A) and the internal standard, pyrazinamide (B).
Upon surveying the literature, a spectroflorimetric method describing the determination of FAV in spiked human plasma is reported [8]. In addition to reported HPLC-UV methods for the determination of FAV in plasma of patients or non-human primates [9,10]. But no reported fully validated LC–MS/MS bioanalytical method was found for determination of FAV in human plasma of healthy volunteers. Accordingly, the main objective of this work was to develop and validate a novel LC–MS/MS bioanalytical method for determination of FAV in human plasma in accordance with US-FDA guidelines of bioanalytical method validation. In general, the LC–MS/MS methods are distinguished by their high senstivity and selectivity, which are crucial characteristics for bioanalysis, pharmacokinetic application and bioequivalence studies, compared to traditional HPLC methods. Furthermore, application of the developed method to estimate the phamacokinetic parameters of FAV and demonstrate the bioequivalence of Avigan® 200 mg tablet produced by Taisho Toyama Pharmaceutical Co., Ltd. Japan and Avipiravir 200 mg tablets, produced by Eva Pharma Pharmaceutical Industries, Egypt, on healthy, adult, human volunteers under fasting conditions.
2. Materials and methods
2.1. Chemicals and reagents
Favipiravir (FAV) (99.98 %, according to supplier certificate of analysis) was obtained from BDR Pharmaceuticals International Pvt. Ltd. (Mumbai, India). Pyrazinamide (IS) was obtained from Linaria Chemicals Ltd, (Bangkok, Thailand) with certified purity of 100.3 %. Methanol, acetic acid and ultra-pure water (HPLC grade) were obtained from Merck (Gernsheim, Germany). Acetonitrile (HPLC grade) was purchased from Chem-Lab (Zedelgem, Belgium). Fresh human plasma was obtained from holding company for biological products and vaccines VACSERA, Giza, Egypt; Batch No. 17123410, 17063573, 17122702, 17052939, 17052940, 17083880, Haemolyzed plasma; 17,041 and Lipemic plasma; 17041900.
2.2. Pharmaceutical formulations
Avigan® tablets (reference product), produced by Taisho Toyama Pharmaceutical Co., Ltd, Japan, Batch No.: FG1881. Avipiravir tablets (test product) manufactured by Eva Pharma Pharmaceutical Industries, Egypt, Batch No.: 2006499, labelled to contain 200 mg FAV per tablet.
2.3. Instrumentation
An Exion LC™ chromatographic system (ABSciex, Ontario, Canada) coupled with a triple quadrupole mass spectrometry API4500 (ABSciex, Ontario, Canada) was used for quantitative analysis. Data acquisition and processing was conducted using Analyst Software version 1.6.3 (Ontario, Canada) to control all parameters of the LC–MS/MS.
2.4. Liquid chromatographic and mass spectrometric conditions
Chromatographic elution was performed using Eclipse plus C18 column (50 × 4.6 mm, 3.5 μm; Agilent Technologies, CA, USA). The temperature was maintained at 25 °C inside the column oven with an injection volume 5 μL. A binary isocratic elution consisted of methanol-0.2 % acetic acid (20:80, v/v) was pumped at a flow rate 0.6 mL/min with a total run time of 3.0 min. Mobile phase components were ultrasonic degassed prior to use for 10 min. The multiple-reaction monitoring (MRM) mode was adjusted with negative electrospray ionization interface to detect FAV ions at transition pairs of m/z 156.00→ 113.00. Whereas, the detection of pyrazinamide (IS) ions was operated with positive electrospray ionization interface at transition pairs of m/z 124.80→ 81.00. The nebulizer gas is air (zero grade), while nitrogen is used as the auxiliary, curtain and collision gas. The source/gas-dependent parameters were set as follows: curtain gas, 30 psi; collision gas, 10 psi; medium temperature, 600 °C; ion source gas (GS1) at 30 psi and drying gas (GS2) at 30 psi; ion spray voltage, -4000 V and 4000 V for FAV and IS, respectively.
2.5. Standard solutions, calibrators and quality control samples
The primary stock solutions of 400.00 μg/mL of FAV and 200.00 μg/mL IS were prepared, separately, in a mixture of acetonitrile-water, 80:20, v/v. Appropriate dilutions were further performed in the same solvent mixture to obtain different working standard solutions of FAV. Calibration standards were prepared by spiking 450 μL blank plasma with 50 μL from each FAV working solution covering a concentration range of (100.0–20000.0 ng/mL). Quality control (QC) samples were prepared at LLOQ (100.0 ng/mL), Low QC (300.0 ng/mL), Mid QC-A (2000.0 ng/mL), Mid QC-B (6000.0 ng/mL) and High QC (16000.0 ng/mL), as illustrated in Table 1 .
Table 1.
Preparation of calibrators and quality control samples for FAV.
| Prepared samples | Working standard solution (μg/mL)a | Final volume | Final Concentration in plasma (ng/mL) |
|---|---|---|---|
| Calibrators | 1.00 | 500 μL | 100.00 |
| 5.00 | 500.00 | ||
| 10.00 | 1000.00 | ||
| 50.00 | 5000.00 | ||
| 100.00 | 10000.00 | ||
| 140.00 | 14000.00 | ||
| 180.00 | 18000.00 | ||
| 200.00 | 20000.00 | ||
| Low QC | 3.00 | 300.00 | |
| Mid QC-A | 20.00 | 2000.00 | |
| Mid QC-B | 60.00 | 6000.00 | |
| High QC | 160.00 | 16000.00 |
50 μL of each working standard solution was added to 450 μL plasma.
2.6. Sample preparation
Samples were thawed at ambient temperature earlier to analysis. Aliquot of 50 μL of IS (200.00 μg/mL) were added to each plasma sample (500 μL), vortexed for 30 s. Protein precipitation was carried out by adding 2 mL methanol, vortex mixed for 4 min, centrifuged at 5000 rpm for 5 min at 4 °C temperature, then 0.3 mL of supernatant was removed and diluted with 0.7 mL water, finally a volume of 5 μL was injected into the chromatographic system for further analysis.
2.7. Bioanalytical method validation
The developed bioanalytical method was fully validated covering all validation parameters listed in the US-FDA guidelines on bioanalytical method validation and then applied in the bioequivalence study of FAV tablets [11,12].
2.7.1. Selectivity
Selectivity of the proposed method was assessed by analyzing six different lots of blank human plasma chosen randomly from different sources in addition to haemolysed and lipemic plasma samples to illustrate the absence of chromatographic interfering from any endogenous plasma constituents and concomitant medications with the analytes and IS.
2.7.2. Linearity and range
Calibration plots were constructed using peak area ratio of FAV to that of pyrazinamide (IS) against the nominal concentration of calibration standards. The concentrations used for FAV were 100.0, 500.0, 1000.0, 5000.0, 10000.0, 14000.0, 18000.0, 20000.0 ng/mL and weighted linear regression method (1/x 2) was implemented. Concentrations of QC and study samples were calculated using the corresponding calibration curves. The acceptance criteria for calibrators at LLOQ should be ±20 % of the nominal standard concentrations, while other sets were ±15 %.
2.7.3. Carry-over
It was assessed by injecting blank samples after calibration standard at the upper limit of quantification (ULOQ), in order to confirm that the precision and accuracy of the suggested method is not influenced. The carry-over in the blank sample following high concentration standard should not be greater than 20 % of the peak response of the LLOQ.
2.7.4. Precision and accuracy
Spiked human plasma at concentrations of LLOQ, Low, Mid and High QC samples were analyzed. Repeatability and intermediate precision were assessed by injecting six replicates (n = 6) in the same day and eighteen replicates (n = 18) per concentration level on three consecutive days which expressed as (CV %). The accuracy of the proposed method was expressed as % Recovery should not exceed 15 % for the QC samples except for LLOQ, it was set as ± 20 % of the nominal values.
2.7.5. Extraction recovery and matrix effect
The extraction efficiency was assessed by comparing the peak areas of FAV spiked into blank plasma and extracted at four concentration levels of (300.0, 2000.0, 6000.0 and 16000.0 ng/mL) to those of corresponding un-extracted standard. Consistent and reproducible recoveries of both FAV and IS rather than 100 % recoveries are desirable. Matrix effect (ME) was assessed using drug-free human plasma samples obtained from six different sources in addition to hemolyzed and lipemic plasma. Matrix Factor (MF) was investigated by matching the response of FAV and IS from the post extracted plasma samples at Low QC and High QC levels to those prepared in pure standard (in absence of matrix) at equivalent concentration.
2.7.6. Dilution integrity
Dilution integrity was examined at two different dilutions. Spiked samples were prepared above the ULOQ, and processed in six replicates (n = 6) by two and four folds dilution with blank human plasma. Accuracy and precision should be within the acceptance criteria of 100 % ± 15 % and ≤ 15 %, respectively.
2.7.7. Stability
Low and high QC samples in six replicates (n = 6) were used to assess the stability of FAV in human plasma matrix during every step of sample preparation, analysis as well as the storage conditions against freshly prepared QCs. Evaluating short term stability of FAV in human plasma was performed at room temperature at 25 h. The processed QCs were stored in auto-sampler at 15 °C and assessed at 27 h. All samples were collected to be frozen and stored at -70 ± 15 °C for successive 42 days for evaluation of long-term stability. Moreover, the QC samples were estimated after 5 cycles of freeze and thaw, stored at -70 ± 15 °C for 12 h and then thawed independently at room temperature, The accuracy (%nominal) at each level should be ± 15 %.
2.7.8. Incurred plasma samples reanalysis
A subset of volunteer samples were re-analyzed for a second time to critically support the precision and accuracy measurements established with spiked QCs. The percentage difference of the results between the original analysis and the repeat analysis should be ±20 % of the mean for at least 67 % of the repeated samples.
2.8. Bioequivalence study and statistical analysis
This study was performed to investigate the bioequivalence of Avipiravir 200 mg tablets (test product) produced by Eva Pharma Pharmaceutical Industries Co. Egypt and Avigan® 200 mg tablets (reference product) produced by Taisho Toyama Pharmaceutical Co., Ltd. Japan following single oral dose administration to healthy adult male volunteers under fasting conditions. A randomized, single-dose, open-label, crossover bioequivalence study was designed on adult Egyptian volunteers, with a washout period of one week between dosing, under fasting conditions. Twenty-six volunteers were screened, randomized, and completed the two periods of the study. The study participants were caucasian, healthy males and meet the selection criteria specified for the study. The mean age was 27.5 years ranged from 20 to 49 years whereas the mean body mass index was 24.05 Kg/m2 ranged from 18.6–29.1. The demographic data of subjects is presented in Supplementary Table S.1. There was no protocol deviation through the clinical period.
Volunteers were examined thoroughly for medical history, BMI, physical examinations, vital signs, drug abuse test, alcohol in urine and laboratory tests (hematology, biochemistry, urinalysis, lipid profile and serology (Hepatitis B and C, HIV), coagulation function and ECG. All laboratory tests were carried out in a certified local laboratory, no clinically significant abnormalities were found.
Exclusion criteria included history or presence of significant medical condition or disease. History or presence of bleeding tendency, significant renal or hepatobiliary problems, significant asthma, urticaria or other allergic reactions, significant gastric and/or duodenal ulcers, participation in other trials conducted in the previous 3 months or previous hospitalization during the last 3 months. The administration of any drug within last 14 days and the ingestion of alcohol, caffeine, or xanthine-containing foods or beverages within 48 h prior to drug administration were not allowed.
The study was conducted according to the principles of good clinical practice (GCP) that have their origins in the declaration of Helsinki. The study protocol was revised and approved by the independent ethics committee of Zi Diligence biocenter, Egypt number 05/2020 (Approval Date: June 30, 2020) and Egyptian Drug Authority (Approval date: July 06, 2020).
Primary end points were the following pharmacokinetic parameters for FAV: Cmax, AUC0–t, AUC0–∞. Secondary end points were the following pharmacokinetic parameters: Tmax, Thalf and Kelimination (λz) [13]. In addition to, safety in terms of the overall proportion of volunteers with treatment adverse events was assessed.
Volunteers had overnight fasting for 10 h before the two periods of the study but the water was freely available. Each volunteer received a single oral dose of FAV 200 mg of either formulation (test or reference). The drug was administered orally in randomized fashion with 240 mL water. Volunteers continued to fast for 4.5 h post-dose. Drinking water was restricted from one-hour pre-dose till 2 h post-dose. During each study period, twenty-three (23) blood samples were collected via an indwelling catheter and transferred into tubes containing EDTA at pre-dose (-0.25 h) and 0.08, 0.16, 0.25, 0.50, 0.75, 1.00, 1.33, 1.67, 2.00, 2.33, 2.67, 3.00, 3.50, 4.00, 5.00, 6.00, 8.00, 10.00, 12.00, 16.00, 24.00 and 36.00 h post-dose after oral administration of test and reference formulations. The samples were immediately centrifuged after collection at 3500 rpm for 10 min. The plasma samples were frozen and stored at -70 ± 15 °C for further analysis.
The pharmacokinetic (PK) parameters for FAV were assessed using Kinetica®5.1 SP1 software and statistical analysis was performed using SAS® analytics Pro 9.4 software.
3. Results and discussion
3.1. Method development and optimization
Sample preparation in bioanalysis is considered an important and a key step to achieve the maximum extraction efficiency of the studied drug with negligible matrix effects and thus improve the sensitivity and accuracy of mass spectrometric determination. Two extraction approaches were tried, the first was plasma protein precipitation using methanol or acetonitrile and the other one was liquid-liquid extraction using different solvents such as diethyl ether, tertiary butyl methyl ether, ethyl acetate and n-hexane. Owing to the high polarity of FAV and for the ease, cost and avoid time consuming procedures, protein precipitation by methanol was found to be the optimum approach yielding efficient recovery results with easy and minimal manipulation steps of plasma samples. A structurally related pyrazinamide showing similar physicochemical properties and comparable extraction recovery as FAV was used as IS, Fig. 1 B.
Several stationary phases were tested to optimize the proposed chromatographic method such as Eclipse plus XDB-C18 column (100 × 4.6 mm, 3.5 μm), Zorbax C8 column (50 × 4.6 mm, 3.5 μm) and Eclipse plus C18 column (50 × 4.6 mm, 3.5 μm). The use of C8 columns resulted in poor separation between FAV peak and endogenous matrix components. Trials by Eclipse plus XDB-C18 column (4.6 × 100 mm, 3.5 μm) exhibited long analysis time. Optimum performance was displayed using Eclipse plus C18 column (4.6 × 50 mm, 3.5 μm) in terms of high resolution, short run time and symmetrical peaks. Also, various compositions in different ratios of acetonitrile or methanol with ammonium acetate buffer, ammonium formate buffer, 0.1 % aqueous formic acid and 0.2 % aqueous acetic acid in an isocratic elution mode were tried as mobile phase. Good peak shapes and high sensitivity were obtained by using a mixture composed of methanol-0.2 % acetic acid (20:80, v/v) pumped at a flow rate 0.6 mL/min. Under the optimum chromatographic conditions, the retention times of FAV and IS were found to be 2.10 and 1.40 min, respectively, Fig. 2 .
Fig. 2.
Multiple reaction monitoring (MRM) Chromatograms of: (A) blank plasma, (B) blank plasma spiked at LLOQ, (C) plasma samples of subject at 0.5 h after oral administration of one tablet containing FAV 200 mg.
Optimization of the mass spectrometric operating parameters was performed to obtain the highest signal for the analyzed drug and IS, Table 2 . The MRM was selected in the negative ion mode for FAV and positive mode for IS using the following transitions: m/z 156.00→ 113.00 and m/z 124.80→ 81.00, respectively, Fig. 3 .
Table 2.
LC–MS/MS parameters selected for the quantification of FAV and pyrazinamide (IS).
| Analyte | Q1a (m/z) | Q3b (m/z) | DPc (V) | EPd (V) | CEe (V) | CXPf (V) |
|---|---|---|---|---|---|---|
| FAV | 156.00 | 113.00 | −10.00 | −10.00 | −23.00 | −10.00 |
| Pyrazinamide (IS) | 124.80 | 81.00 | 50.00 | 10.00 | 20.00 | 15.00 |
Q1, precursor ion.
Q3, product ion.
DP, declustering potential.
EP, entrance potential.
CE, collision energy.
CXP, cell exit potential.
Fig. 3.
Representative spectra for FAV (A) and the internal standard, pyrazinamide (B).
3.2. Method validation
3.2.1. Selectivity
No significant interference was observed from endogenous constituents in all the lots of blank plasma. Moreover, none of the frequently co-administered medications for supportive care of viral infections (paracetamol, diclofenac sodium and ibuprofen) showed interference at the retention times of the analyte and IS. Representative chromatograms are shown in Fig. 2,
3.2.2. Calibration curve and quantitation range
Linearity was evaluated by preparing six calibration curves in human plasma, each consists of a blank, zero sample and calibration standards covering the range of 100–20000.0 ng/mL for FAV. Calibration curves were found to be steadily accurate and precise by fitting the peak-area ratio of the analyte to the IS versus concentrations over the calibration range of 100–20000.0 ng/mL. Weighted (1/x2) least-squares linear regression method was applied. Results of precision and accuracy assessment for LLOQ were within the tolerable limits, with CV% < 4.52 % and accuracy ranging from 98.30 to 107.42%.
3.2.3. Carry-over
Carry over was addressed by injecting blank plasma samples after a high concentration sample (ULOQ, 20000.0 ng/mL) for FAV. It was found that carry over in the blank samples not exceeded 20 % of LLOQ.
3.2.4. Accuracy and precision
Accuracy of the developed method expressed as % Recovery was found to be ranged from 95.55 to 108.15% for intra-day accuracy and within 99.57–106.02 % for inter-day accuracy. Intra-day and inter-day assay precision was assessed by using six replicates (n = 6) and eighteen replicates (n = 18) for intra-day and inter-day; respectively. The CV% for both type of precision was ranged from 2.06 to 7.11%, all results are summarized in Table 3 .
Table 3.
Intra- and Inter-day accuracy and precision results for FAV.
| Analyte | Concentration (ng mL−1) | Intra-day |
Inter-day |
|||
|---|---|---|---|---|---|---|
| RE (%) | CV (%) | RE (%) | CV (%) | |||
| FAV | LLOQ | 100.00 | −4.45 | 4.46 | −0.43 | 7.11 |
| Low QC | 300.00 | 7.56 | 3.79 | 6.02 | 6.15 | |
| Mid QC-A | 2000.00 | 8.15 | 2.06 | 5.38 | 4.22 | |
| Mid QC-B | 6000.00 | 7.79 | 3.27 | 4.96 | 6.48 | |
| High QC | 16000.00 | 1.92 | 4.24 | −0.03 | 5.57 | |
| n | 6 | 18 | ||||
3.2.5. Extraction recovery and matrix effect
Results of percentage recovery of FAV obtained from the three QC levels (Low QC, Mid QC and High QC) in 6 replicates were ranged from 85.12 to 99.04 %while the mean percentage recovery of IS was 87.46 %. The matrix effect (ME) for each batch was calculated at Low QC and High QC concentration levels by comparing the peak area of FAV over IS prepared in extracted plasma samples to the other prepared in the pure standard solution of equivalent concentration ranging from 0.91 to 1.10 and 0.93 for IS. Moreover, the IS normalized matrix factor was computed and it was found that CV% less than 3 % which indicated any ion suppression or enhancement from the human plasma was nearly negligible.
3.2.6. Dilution integrity
The integrity of dilution was monitored by diluting spiked human plasma samples at concentration of 16,000 ng/mL, 2 and 4 folds with blank matrix to bring within the quantitation range by performing six determination per dilution. Precision was expressed as CV% was found to be within 3.17–3.79 % and accuracy results were ranging from 103.29 to 105.32%.
3.2.7. Stability
FAV was found to be stable in human plasma at 25−30 °C for 25 h. The extracted plasma samples were processed, the results indicated that samples were stable when stored in auto-sampler at 15 °C for 27 h. Long term stability was checked for stored frozen QC samples at -70 ± 15 °C after 42 days. Samples were found to be stable after subjected to five freeze and thaw cycles. As presented in Table 4 , it was noticed that insignificant loss of the tested drug during sample storage during repeated thawing and freezing conditions.
Table 4.
Stability results for FAV in plasma at different conditions.
| Analyte | Concentration (ng mL−1) | Short term stability at room temperature (25 h) |
Freeze and thaw stability at -70°C (5 cycles) |
Long term stability at -70°C (42 days) |
Auto-sampler stability at 15°C (27 h) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accuracy (%) | CV (%) | Stability (%) | Accuracy (%) | CV (%) | Stability (%) | Accuracy (%) | CV (%) | Stability (%) | Accuracy (%) | CV (%) | Stability (%) | |||
| FAV | Low QC | 300.00 | 99.98 | 2.37 | 89.59 | 99.68 | 7.12 | 96.09 | 100.38 | 4.96 | 96.84 | 93.20 | 2.81 | 89.84 |
| High QC | 16000.00 | 96.55 | 4.44 | 93.82 | 92.56 | 4.84 | 99.05 | 106.35 | 4.55 | 106.20 | 91.58 | 4.39 | 98.01 | |
| n | 6 | 6 | 6 | 6 | ||||||||||
3.2.8. Incurred plasma samples reanalysis
The difference in percentage recoveries between the initial and measured concentrations during the repeated analysis of study samples was ranged from 0.11–18.42%.
3.3. Application to bioequivalence study
The developed LC–MS/MS method was successfully utilized to estimate FAV concentration in human plasma from a bioequivalence study. An open-label, randomized, single-dose study with cross-over design was conducted on healthy male volunteers, after single oral administration of Avipiravir 200 mg (test product) and Avigan® 200 mg (reference product) under fasting conditions. The proposed method exhibits sufficient sensitivity allowing accurate calculation of the investigated pharmacokinetic parameters of FAV in human plasma samples as demonstrated in Table 5 . The displayed results were not so far from the earlier reported in Avigan® Tablets data sheet [6], where minor variation in pharmacokinetic performance may be attributed to different sample size and sampling schedule. The mean plasma concentration curve with standard deviation bars that indicates the standard deviations at individual time points for FAV in the bioequivalence study is shown in Fig. 4 .
Table 5.
Pharmacokinetic parameters of FAV following administration of one tablet of Avipiravir (test product) and one tablet of Avigan® (reference product) under fasting conditions.
| Parameters | Test product | Reference product |
|---|---|---|
| Cmax (ng/mL) | ||
| Mean ± SD | 5225.63 ± 1572.41 | 5486.56 ± 1662.00 |
| Range | (2944.47–10066.90) | (3066.71–10560.50) |
| Tmax (h) | ||
| Median | 0.50 | 0.50 |
| Range | (0.16−1.67) | (0.25−2.33) |
| AUC0-t (ng h/mL) | ||
| Mean ± SD | 11755.94 ± 10538.78 | 11521.34 ± 8919.89 |
| Range | (6043.04–61474.30) | (5373.05–52544.30) |
| AUC0-∞ (ng h/mL) | ||
| Mean ± SD | 12136.88 ± 10791.03 | 11893.18 ± 9056.48 |
| Range | (6317.52–63221.60) | (5529.75–53595.80) |
| Ke (h−1) | ||
| Mean ± SD | 0.54 ± 0.12 | 0.55 ± 0.13 |
| Range | (0.15−0.78) | (0.17−0.75) |
| T1/2 (h) | ||
| Mean ± SD | 1.42 ± 0.69 | 1.37 ± 0.61 |
| Range | (0.89–4.68) | (0.92–4.15) |
Fig. 4.
Mean plasma concentration (± SD) following administration of single oral dose of FAV 200 mg tablets; Avipiravir (test product) and Avigan® (reference product) to 26 healthy subjects.
The point estimate and 90 % confidence intervals for the difference means between test and reference formulations for Cmax, AUC0-t and AUC0-∞ were found to be 93.46 % (86.34 %–101.17 %), 99.93 % (95.72 %–104.34 %) and 99.98 % (95.83 %–104.32 %), respectively. The parametric 90 % confidence intervals of the mean values for the test and reference ratio were within the acceptance limits of 80 % and 125 % for the pharmacokinetic parameters Cmax, AUC0-t and AUC0-∞. The difference between means of Tmax is not significant (P > 0.05) with respect to Wilcoxon Signed-Rank Test.
The results of the bioequivalence study confirmed that the two drug products are bioequivalent. Moreover, based on clinical observations, both products were well tolerated by the study volunteers and all left the study without reporting any adverse drug reactions or serious adverse events.
4. Conclusion
A novel, sensitive and reliable LC–MS/MS bioanalytical method was developed and fully validated according to FDA guidelines for the determination of FAV in human plasma. The implemented assay was found to be accurate and precise over a concentration range that permits assessment of pharmacokinetic parameters after oral administration of one tablet containing 200 mg FAV. The high throughput of the method permits the analysis of vast number of human plasma samples per day. The method was successfully employed to a bioequivalence study with cross over design in healthy Egyptian volunteers.
CRediT authorship contribution statement
Mosaad I. Morsy: substantial contribution to conception and design, substantial contribution to acquisition of data, final approval of the version to be published. Eman G. Nouman: substantial contribution to conception and design, substantial contribution to acquisition of data, final approval of the version to be published. Youmna M. Abdallah: substantial contribution to conception and design, substantial contribution to acquisition of data, final approval of the version to be published. Mourd A. Zainelabdeen: substantial contribution to conception and design, substantial contribution to acquisition of data. Mohamed M. Darwish: substantial contribution to conception and design, substantial contribution to acquisition of data, substantial contribution to analysis and interpretation of data, critically revising the article for important intellectual content. Amira S. Gouda: substantial contribution to conception and design, substantial contribution to analysis and interpretation of data, critically revising the article for important intellectual content. Ahmed Y. Hassan: substantial contribution to conception and design, substantial contribution to analysis and interpretation of data, drafting the article, critically revising the article for important intellectual content. Mamdouh R. Rezk: substantial contribution to conception and design, substantial contribution to analysis and interpretation of data, drafting the article, critically revising the article for important intellectual content. Ahmed M. Abdel-Megied: substantial contribution to conception and design, substantial contribution to analysis and interpretation of data, drafting the article, critically revising the article for important intellectual content. Hoda M. Marzouk: substantial contribution to conception and design, substantial contribution to acquisition of data, substantial contribution to analysis and interpretation of data.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Authors would like to acknowledge Eva pharma Pharmaceutical Industries, Egypt, for sponsoring this study and providing the facilities (e.g. test product, reference product and standard materials) for completing the work. Also, we would like to acknowledge Zi Diligence biocenter for their participation to finish this work with high dedication and sincerity.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jpba.2021.114057.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.World Health Organization . 2020. Coronavirus Disease 2019 (COVID-19) Situation Report – 85.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200414-sitrep-85-covid-19.pdf?sfvrsn=7b8629bb_4 (accessed Dec 11th.2020) [Google Scholar]
- 2.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal U., Raju R., Udwadia Z.F. Favipiravir: A new and emerging antiviral option in COVID-19. Med. J. Armed Forces India. 2020 doi: 10.1016/j.mjafi.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Q., Yang Y., Shen C., Li X., Peng L., Huang D., Zhang J., Zhang S., Wang F., Liu J., Chen L., Chen S., Wang Z., Zhang Z., Cao R., Zhong W., Liu Y., Liu L. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (Beijing) 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coomes E.A., Haghbayan H. Favipiravir, an antiviral for COVID-19? J. Antimicrob. Chemother. 2020;75(7):2013–2014. doi: 10.1093/jac/dkaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.2014. Report on the Deliberation Results, Avigan Tablet 200 Mg.https://www.pmda.go.jp/files/000210319.pdf (accessed Dec 11th.2020) [Google Scholar]
- 7.Agrawal U., Raju R., Udwadia Z.F. Favipiravir: A new and emerging antiviral option in COVID-19. Med. J. Armed Forces India. 2020;76(4):370–376. doi: 10.1016/j.mjafi.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Megahed S.M., Habib A.A., Hammad S.F., Kamal A.H. Experimental design approach for development of spectrofluorimetric method for determination of favipiravir; a potential therapeutic agent against COVID-19 virus: Application to spiked human plasma. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2021;249 doi: 10.1016/j.saa.2020.119241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen T.H.T., Guedj J., Anglaret X., Laouénan C., Madelain V., Taburet A.-M., Baize S., Sissoko D., Pastorino B., Rodallec A., Piorkowski G., Carazo S., Conde M.N., Gala J.-L., Bore J.A., Carbonnelle C., Jacquot F., Raoul H., Malvy D., Lamballerie Xd., Mentré F. Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted. PLoS Negl. Trop. Dis. 2017;11(2):1–18. doi: 10.1371/journal.pntd.0005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madelain V., Guedj J., Mentre F., Nguyen T.H., Jacquot F., Oestereich L., Kadota T., Yamada K., Taburet A.M., de Lamballerie X., Raoul H. Favipiravir pharmacokinetics in nonhuman Primates and insights for future efficacy studies of hemorrhagic fever viruses. Antimicrob. Agents Chemother. 2017;61(1) doi: 10.1128/AAC.01305-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.F.D.A. US Food and Drug Administration, Guidance for Industry . 2018. Bioanalytical Method Validation. US Department of Health and Human Services, Center for Drug Evaluation and Research and Center for Veterinary Medicine. 2018. [Google Scholar]
- 12.Zimmer D. New US FDA draft guidance on bioanalytical method validation versus current FDA and EMA guidelines: chromatographic methods and ISR. Bioanalysis. 2014;6(1):13–19. doi: 10.4155/bio.13.298. [DOI] [PubMed] [Google Scholar]
- 13.F.D.A. US Food and Drug Administration, Guidance for Industry . 2001. Statistical Approaches to Establishing Bioequivalence. US Department of Health and Human Services, Center for Drug Evaluation and Research (CDER) 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.