Abstract
Background
Fabry disease is an X-linked recessive lysosomal storage disease resulting in the cellular accumulation of globotriaosylceramide particularly globotriaosylceramide. The disease is characterized by a dilated vasculopathy with arterial ectasia in muscular arteries and arterioles. Previous venous plethysomographic studies suggest enhanced endothelium-dependent vasodilation in Fabry disease indicating a functional abnormality of resistance vessels.
Methods
We examined the mechanical properties of the radial artery in Fabry disease, a typical fibro-muscular artery. Eight control subjects and seven patients with Fabry disease had a right brachial arterial line placed allowing real time recording of intra-arterial blood pressure. Real time B-mode ultrasound recordings of the right radial artery were obtained simultaneously allowing calculation of the vessel wall internal and external diameter, the incremental Young's modulus and arterial wall thickness. By simultaneously measurement of the distal index finger-pulse oximetry the pulse wave speed was calculated. From the wave speed and the internal radial artery diameter the volume flow was calculated by Womersley analysis following truncation of the late diastolic phase.
Results
No significant difference was found between Fabry patients and controls for internal or external arterial diameters, the incremental Young's modulus, the arterial wall thickness, the pulse wave speed and the basal radial artery blood flow. Further, no significant difference was found for the radial artery blood flow in response to intra-arterial acetylcholine or sodium nitroprusside. Both drugs however, elevated the mean arterial flow.
Conclusions
The current study suggests that no structural or mechanical abnormality exists in the vessel wall of fibro-muscular arteries in Fabry disease. This may indicate that a functional abnormality downstream to the conductance vessels is the dominant feature in development Fabry vasculopathy.
Background
Fabry disease is an X-linked recessive lysosomal disorder resulting in the cellular accumulation of glycosphingolipid particularly globotriaosylceramide (Gb3) due decreased levels of the lysosomal enzyme α-galactosidase A [1,2]. The condition primarily affects the kidneys, heart and central nervous system (CNS) resulting in organ damage and failure. In the CNS, the condition results in small and large vessel stroke. The etiology of the enhanced stroke risk is unclear and may be due to altered arterial wall mechanical properties. Such an alteration could account for the vascular ectasia seen in this condition in large fibro-muscular arteries [3]. Arterial wall properties are defined by inner and outer arterial diameters, the arterial wall thickness and the incremental Young's elastic modulus [4]. The volume flow (Q) in an artery can be estimated by application of Womersley analysis [5]. Such an analysis allows comparison of the effect of pharmacological agents in the vascular bed under study. We examined the arterial wall and flow properties in the radial artery in Fabry disease at rest and following intra-arterial infusion of acetylcholine (ACh) and sodium nitroprusside (SNP). ACh induces vasodilation by an obligatory endothelium mechanism while SNP induces arterial vasodilation by a direct action on arterial smooth muscle allowing examination of endothelial and smooth muscle function in Fabry disease [6].
Materials and Method
Seven hemizygote Fabry patients (age range 22 – 42 years) and eight male control volunteers (age range 23 × 46 years) were enrolled in accordance with institutional guidelines. All volunteers and patients gave informed consent. All study participants were normotensive with serum cholesterols in the normal range. A right intra-brachial arterial line was placed in each subject followed by attachment to an analog disposable pressure transducer kit with a dynamic frequency response of 40 Hz for the fluid filled tubing and > 200 Hz for the pressure transducer alone (TruWave Disposable Pressure Transducer, Edwards Lifesciences, Irvine CA). The brachial artery pressure signal was digitized over a time interval of thirty seconds (National Instruments DAQ Card-AI-16E-4 digitizing frequency 2000 Hz, graphical user interface constructed in LabVIEW (version 5.1, 11500 Mopac Expwy, Austin, TX 78759-3504)). The real time signal was stored simultaneously with the right index finger pulse oximetry waveform right wrist radial artery external tonometry and EKG. The pulse wave speed was calculated by taking the difference between the upstroke of the brachial artery, radial artery external tonometer and the index finger pulse ensemble average waveforms. A total of three pulse wave speeds were measured as follows:- (a) between the intra-arterial brachial line and the external radial tonometer (b) between the intra-arterial brachial line and the index finger and (c) between the radial artery external tonometer and the index finger. The wave speed used in the Womersley flow calculation was taken as the average of the three measured wave speeds across the whole of the forearm vascular bed.
A representative pressure time waveform was calculated by taking the ensemble average of the sampled intra-arterial pulse pressure waveforms using the initial systolic upstroke as the reference point. The intra-brachial arterial line was used for infusion of acetylcholine (ACh) (dosage range 7.5 mg/min × 30 mg/min) and sodium nitroprusside (SNP) (dosage range 0.8 mg/min × 3.2 mg/min). Intra-brachial drugs were allowed to reach a steady state by continuous infusion over at least two minutes prior to recording the pressure waveform. At baseline and peak infusion dose of ACh and SNP the radial artery was imaged in real time using B-mode ultrasound (Cl-10 Mhz-5 MHz Probe, HDI 3000 ATL Lab, Bothell, WA) with the images stored on optical disk. Subsequently, the B-mode images were downloaded and converted from AVI video clips to single frame TIFF images followed by calculation of the spatial cross correlation allowing estimation of the peak systolic and diastolic frames. From the peak systolic and diastolic frames the incremental Young's elastic modulus (E) was calculated according to equation below
![]()
where ΔP is the pulse pressure range, ΔR is the change in the internal vessel radius over the cardiac-cycle, Ri is the internal vessel radius and Ro is the external vessel radius [4].
The pulse pressure for each subject was derived from the ensemble pressure waveform while estimates of the vessel radii were obtained directly from the TIFF images using NIH Image 1.62 in a blinded fashion. The peak systolic and peak diastolic images were measured separately but in an identical fashion by defining the perimeter of the arterial lumen and the external vessel wall perimeter defined by the medial-adventitial plane with a piece-wise polygon. Using NIH Image the respective internal and external areas were calculated. The caliper line placement along the intimal-medial border and the medial-adventitial interface was judged by review of the video records, and by calculation of a moving edge image by the logical .AND. combination of gradient images, and sequential time difference images. The individual internal and external vessel lengths were also measured and the internal and external vessel radii calculated by dividing the internal and external vessel areas by their respective lengths. This method allowed use of the whole vessel image in estimation of the arterial radii (Figure 1). Subtraction of the internal and external vessel diameters allowed estimation of the vessel wall thickness. The Womersley solution is the only analytical solution for time varying flow in a rigid infinitely long pipe. Validation of the equation for physiological flows has been performed with a high correlation between the calculated mean and observed mean flow [4]. The harmonic volume flow (Q) was calculated by using the following
Figure 1.
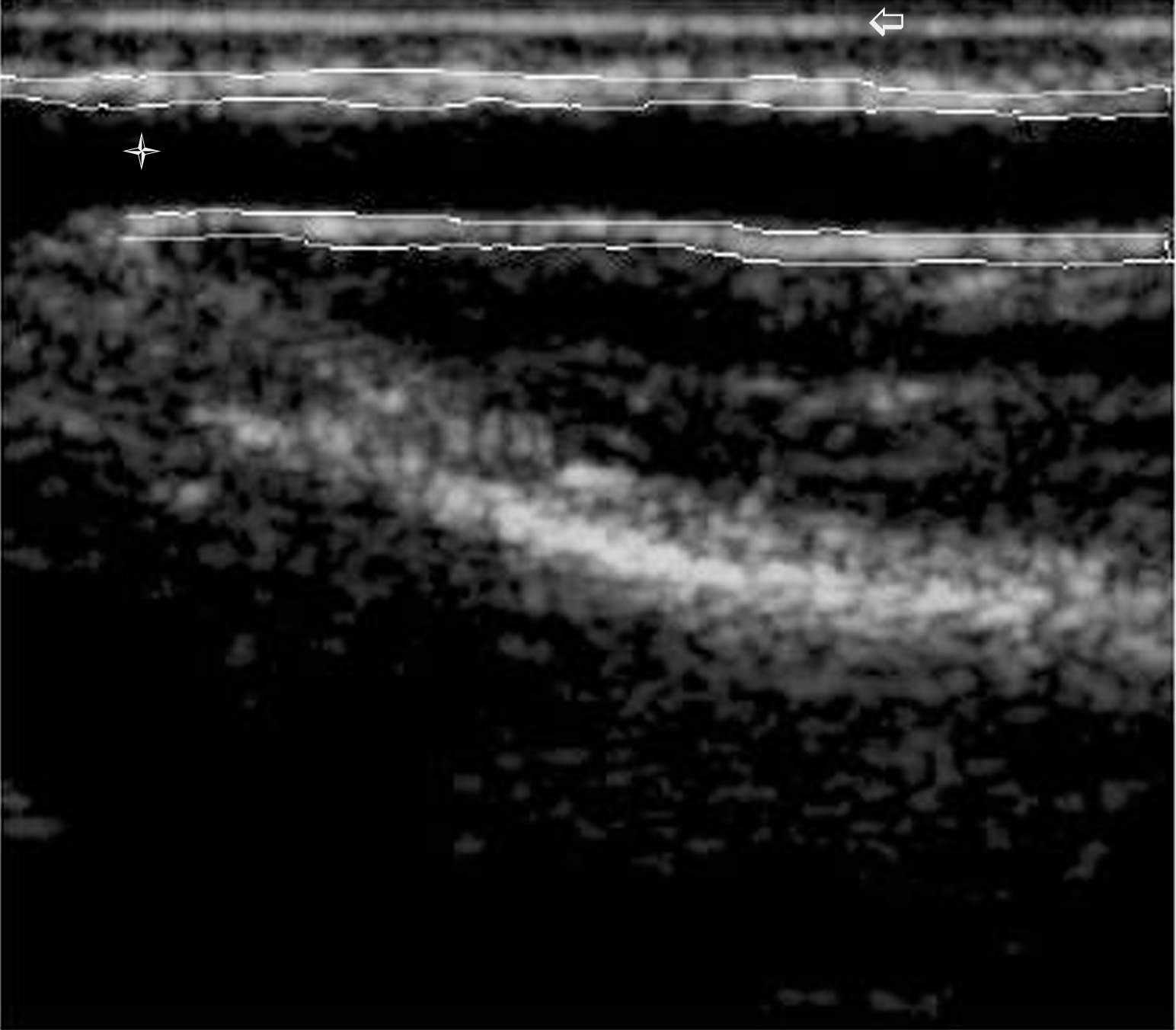
B-mode ultrasound image of the radial artery. The arterial lumen is indicated by  while the skin surface is indicated by
while the skin surface is indicated by  . Image consists 256 × 239 pixels (isotropic scale 0.1 mm/pixel).
. Image consists 256 × 239 pixels (isotropic scale 0.1 mm/pixel).
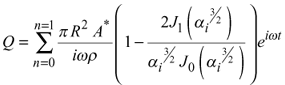
where R is the internal vessel radius, A* is the complex conjugate of the pressure gradient, α is the Womersley parameter 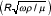 , ω is the frequency, ρ is the fluid density, μ the fluid viscosity and J1 and J0 are first and zero order complex Bessel functions [7] and i = (-1)½. The calculation included the zeroth harmonic or mean flow up to the sixteenth harmonic. The pressure gradient for each individual measurement was determined by calculating the time derivative of the ensemble pulse pressure waveform
, ω is the frequency, ρ is the fluid density, μ the fluid viscosity and J1 and J0 are first and zero order complex Bessel functions [7] and i = (-1)½. The calculation included the zeroth harmonic or mean flow up to the sixteenth harmonic. The pressure gradient for each individual measurement was determined by calculating the time derivative of the ensemble pulse pressure waveform 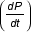 , interpolated with a natural cubic spline of 4096 points, by forward differencing. This derived function was then multiplied by the reciprocal of the wave speed (c) to give the spatial pressure gradient
, interpolated with a natural cubic spline of 4096 points, by forward differencing. This derived function was then multiplied by the reciprocal of the wave speed (c) to give the spatial pressure gradient 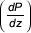 as in the following equation [4].
as in the following equation [4].
![]()
An example of an ensemble pressure gradient waveform together with the associated derived Womersley volume flow is shown in Figure 2. The Womersley flow curves were integrated over the systolic and initial diastolic flow to give the estimated mean volume flow for each subject and pharmacological intervention after truncation of late diastolic flow. This was necessary since the integration of Womersley flow derived by estimation of the spatial pressure gradient from the arterial pressure time derivative over the complete time cycle is zero, and since the contribution of the late diastolic phase to volume flow is negligible. Using this model the subject's mean radial arterial Q was calculated at baseline and following the peak infusion of ACh (30 μg/minute) and SNP (3.2 μg /minute). Statistical analysis was performed in Matlab 6.0 (Natick, MA) using the Wilcoxon rank sum test.
Figure 2.
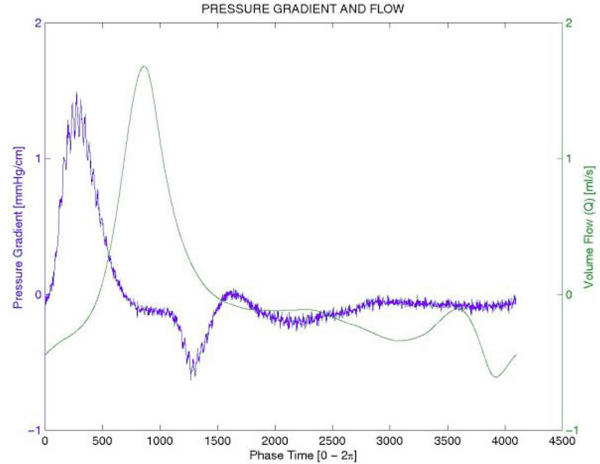
An illustrative graph of a calculated spatial pressure gradient from a corresponding ensemble time dependent pulse pressure waveform together with the associated Womersley model derived volume flow.
Results
The radial arterial wall parameters for the Fabry group and controls are presented in Table 1. The following baseline parameters were estimated: - the internal and external vessel diameters, the pulse pressure difference (maximum arterial pressure - minimum arterial pressure), arterial wall thickness, the arterial wall incremental Young's modulus, the pulse pressure wave speed and the maximum excursion of the radial artery wall during the pulse pressure cycle. No significant difference was found for any of these parameters between the Fabry group and the control group (Wilcoxon Rank Sum test). The radial artery mean volume flow was estimated at baseline to be 0.1723 ± 0.1128 ml/s (n = 7) in the Fabry group and 0.1147 ± 0.0769 ml/s (n = 7) in the control group. These values were not significantly different. No significant difference was found in the mean Q values after maximal infusion of ACh (Fabry group, 0.2046 ± 0.1588 ml/s, n = 4, Control group, 0.1707 ± 0.0783 ml/s, n = 6) or SNP (Fabry group, 0.2515 ± 0.0470 ml/s, n = 4, Control group, 0.2469 ± 0.1657 ml/s, n = 6). Both drugs, however, resulted in increased mean blood flow. For the other radial arterial parameters, no significant difference was found between Fabry disease compared to controls following infusion of ACh or SNP (Table 2).
Table 1.
Summary of baseline radial artery vessel wall parameters in Fabry disease and controls.
| Radial Artery Wall Properties | Fabry Disease | Controls | p Value |
| Internal Diameter (mm) | 1.99 ± 0.35 | 2.11 ± 0.56 | NS |
| n = 7 | n = 8 | ||
| External Diameter (mm) | 3.14 ± 0.21 | 3.10 ± 0.65 | NS |
| n = 7 | n = 8 | ||
| Pulse Pressure (mmHg) | 67.21 ± 7.65 | 57.52 ± 6.02 | NS |
| n = 7 | n = 8 | ||
| Wall Thickness (mm) | 1.51 ± 0.35 | 1.03 ± 0.27 | NS |
| n = 7 | n = 8 | ||
| Incremental Modulus (dyne/cm2) | 3.47 × 105 ± 2.55 × 105 | 3.21 × 105 ± 2.11 × 105 | NS |
| n = 7 | n = 8 | ||
| Pulse Wave Speed (cm/s) | 440.7 ± 130.5 | 433.3 ± 52.5 | NS |
| n = 7 | n = 8 | ||
| Wall Pulse Excursion (mm) | 0.20 ± 0.09 | 0.13 ± 0.07 | NS |
| n = 7 | n = 8 | ||
| Basal Volume Flow (ml/s) | 0.1723 ± 0.1128 | 0.1147 ± 0.0769 | NS |
| n = 7 | n = 7 | NS |
Table 2.
Summary of radial arterial vessel wall parameters in Fabry disease and controls following intra-brachial acetylcholine (ACh) and sodium nitroprusside (SNP).
| Radial Artery Wall Properties | Fabry Disease ACh | Fabry Disease SNP | Control ACh | Control SNP |
| Internal Diameter (mm) | 2.01 ± 0.31 | 2.23 ± 0.10 | 2.18 ± 0.11 | 2.44 ± 0.26 |
| n = 4 | n = 4 | n = 6 | n = 6 | |
| External Diameter (mm) | 3.18 ± 0.61 | 3.46 ± 0.27 | 3.26 ± 0.19 | 3.54 ± 0.16 |
| n = 4 | n = 4 | n = 6 | n = 6 | |
| Pulse Pressure (mmHg) | 64.47 ± 3.09 | 64.01 ± 5.23 | 57.71 ± 5.27 | 53.64 ± 6.59 |
| n = 4 | n = 4 | n = 6 | n = 6 | |
| Wall Thickness (mm) | 1.17 ± 0.36 | 1.43 ± 0.54 | 1.07 ± 0.19 | 1.09 ± 0.18 |
| n = 4 | n = 4 | n = 6 | n = 6 | |
| Incremental Modulus (dyne/cm2) | 2.99 × 105 ± 1.99 × 105 | 2.69 × 105 ± 7.67 × 104 | 2.87 × 105 ± 1.02 × 105 | 3.68 × 105 ± 2.38 × 105 |
| n = 4 | n = 4 | n = 6 | n = 6 | |
| Pulse Wave Speed (cm/s) | 503.42 ± 276.43 | 545.00 ± 223.58 | 480.75 ± 60.58 | 482.12 ± 103.24 |
| n = 7 | n = 7 | n = 8 | n = 8 | |
| Wall Pulse Excursion (mm) | 0.17 ± 0.12 | 0.22 ± 0.02 | 0.13 ± 0.06 | 0.20 ± 0.05 |
| n = 4 | n = 4 | n = 6 | n = 6 | |
| Volume Flow (ml/s) | 0.2046 ± 0.1588 | 0.2515 ± 0.0470 | 0.1707 ± 0.0783 | 0.2469 ± 0.1657 |
| n = 4 | n = 4 | n = 6 | n = 6 |
Discussion
We show that in the age range considered, no statistically significant difference was apparent in radial artery wall properties in Fabry disease compared to controls (Table 1). It is important to emphasize the subject's age range in relation to arterial wall properties since age related changes tend to increase arterial wall stiffness [4]. The calculation of Young's incremental elastic modulus (E) represents an isotropic simplification of the arterial wall elastic properties, however, in our study we calculated E using the true intra-arterial pressure measurement and not by an indirect techniques of arterial pressure estimation. Further, the estimation of the arterial internal and external diameters were derived from the complete vessel image available and not single point estimates of the arterial diameters. Such point estimation of arterial diameters allows observer bias resulting in error propagation into any dependent parameters such as the incremental modulus and mean flow calculation by Womersley analysis. Estimation of the mean vessel diameter was obtained by analyzing the peak systolic image and the peak diastolic image followed by taking the arithmetic mean. Estimation of the peak systolic and diastolic frames was performed by an unbiased method corresponding to the maximum and minimum of the spatial cross-correlation function. The lack of a significant difference in the incremental modulus in Fabry disease has important implications for management of these patients with cardiovascular or cerebrovascular disease either surgically or by interventional radiological techniques, since some of the inherent risks of these techniques such as angioplasty are related to the mechanical properties of the arterial wall. We found no discernible intimal-medial layer thickness in our patient or control population. This is contrary to other investigators who have found a significant increase in the intimal-medial thickness in the radial artery in Fabry disease by non-invasive B-mode imaging [8]. Unfortunately, these investigators did not confirm their radiological findings by arterial biopsy and histopathological examination. Further, in the current study, the tunica media principally determines the mechanical properties of the arterial wall, the arterial wall layer measured in our study. It is important to note that the lack of difference in the directly calculated arterial incremental Young's modulus was corroborated independently by the lack of significant difference between the experimentally derived pulse wave speeds in the Fabry patient and control groups. In the present study, only the radial artery was examined. It is not clear at present whether this fibro-muscular artery is representative of other fibro-muscular arteries such as the coronary, cerebrovascular and renal arteries. In our patient group, undergoing radial artery B-mode imaging, no evidence of cerebrovascular dolichoectasia was found by magnetic resonance angiography. It remains to be determined whether there is even a radiological correlation between cerebral dolichoectasia and potential abnormalities in the peripheral vasculature such as the radial artery. If this were the case, then direct measurements from the radial artery might suffice. However, in patients undergoing selective cerebral angiography, similar measurements to those presented here could be obtained. Despite caution about extrapolating the mechanical properties of the radial artery to cerebral vessels it should be noted that Fabry disease is typically a systemic disorder with selective tissue vulnerability determined more by patient characteristics than by tissue or organ system properties.
The lack of a significant difference in the radial artery mean volume flow in response to ACh in Fabry disease contrasts with findings from a previous study using forearm venous plethysmography to estimate the forearm vascular bed blood flow following intra-brachial ACh and SNP [9]. The reason for the apparent variance in the current study could be due to insufficient statistical power or may indicate a functional abnormality predominantly affecting the resistance vessels in Fabry disease. This is further suggested by the lack of significant change in radial artery wall properties between Fabry and control subjects with or without ACh or SNP infusion. Such a functional abnormality in the resistance arterioles could reduce the downstream vascular impedance allowing enhanced forearm vascular bed flow. This enhanced flow could over time result in conductance vessel ectasia.
Conclusions
The present study suggests that the mechanical and flow properties of fibro-muscular arteries in Fabry disease are not significantly different from similar vessels in age-matched controls suggesting that the localization of the functional abnormality is in the small resistance arterioles.
Competing Interests
None
Supplementary Material
B-mode ultrasound video data file (A VI format) of control subject.
B-mode ultrasound video data file (A VI format) of Fabry subject.
Acknowledgments
Acknowledgements
The intra-mural program of the National Institutes of Health supported this work
Contributor Information
David F Moore, Email: dfm1@helix.nih.gov.
Gheona Altarescu, Email: altaresg@ninds.nih.gov.
Randall Pursley, Email: pursley@exchange.nih.gov.
Umberto Campia, Email: umberto.campia@medstar.net.
Julio A Panza, Email: panzaj@nhlbi.nih.gov.
Emilios Dimitriadis, Email: dimitria@helix.nih.gov.
Raphael Schiffmann, Email: rs4e@nih.gov.
References
- Brady R, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. Enzymatic defect in Fabry disease: ceramide trihexosidase deficiency. N Engl J Med. 1967;276:1163–1167. doi: 10.1056/NEJM196705252762101. [DOI] [PubMed] [Google Scholar]
- Desnick R, Ioannou YA, Eng CM. α-Galactosidase A Deficiency: Fabry Disease. In: R.C. Scriver, A. L. Beaudet, W. S. Sly, D. Valle, editor. In The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill: New York.; 2001. pp. 3733–3774. [Google Scholar]
- Mitsias P, Levine SR. Cerebrovascular complications of Fabry's disease. Ann Neurol. 1996;40:8–17. doi: 10.1002/ana.410400105. [DOI] [PubMed] [Google Scholar]
- Nichols WW, O'Rourke MF. McDonald's Blood Flow in Arteries Theoretical, Experimental and Clinical Principles. Oxford University Press: New York. 1998.
- Womersley JR. Oscillatory motion of a viscous liquid in a thin-walled elastic tube: I. The linear approximation for long waves. Philosophical Magazine. 1955;46:199–221. [Google Scholar]
- Furchotts RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Moore DF, Pursley R, Altarescu G, Schiffmann R, Dimitriadis E. Real Time Peripheral Arterial Flow and Wall Properties derived by Pulse Waveform Analysis and B-mode Ultrasound Imaging 14th IEEE Conference on Computer-Based Medical Systems, Bethesda, MD. 2001. pp. 216–221.
- Boutouyrie P, Laurent S, Laloux B, Lidove O, Grunfeld JP, Germain DP. Non-invasive evaluation of arterial involvement in patients affected with Fabry disease. J Med Genet. 2001;38:629–631. doi: 10.1136/jmg.38.9.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarescu G, Moore DF, Pursley R, Campia U, Goldstein S, Bryant M, Panza JA, Schiffmann R. Enhanced endothelium-dependent vasodilation in Fabry disease. Stroke. 2001;32:1559–1562. doi: 10.1161/01.str.32.7.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
B-mode ultrasound video data file (A VI format) of control subject.
B-mode ultrasound video data file (A VI format) of Fabry subject.


