Abstract
Background:
We previously reported a prospective study showing axillary lymph node dissection (ALND) is associated with increased breast skin thickening during and 6 weeks post-radiation therapy (RT), and now report ALND’s long-term impact at 1 year.
Methods:
Among 66 women who received whole breast RT after lumpectomy, objective ultrasound measurements of epidermal thickness over four quadrants of the treated breast were measured at five time points: before RT, week 6 of RT, and 6 weeks, 6 months, and 1 year post-RT. Skin thickness ratio (STRA) was generated by normalizing for corresponding measurements of the contralateral breast.
Results:
A total of 2,436 ultrasound images were obtained. Among 63 women with evaluable data at 1 year, mean STRA significantly increased at 6 months (absolute mean increase of 65%, SD 0.054), and remained elevated at 1 year post-RT (absolute mean increase of 44%, SD 0.048). In multivariable analysis, ALND compared to sentinel lymph node biopsy, longer interval between surgery and RT, increased baseline STRA, and Caucasian race predicted for more severe changes in STRA at 1 year compared to baseline (all P < .05).
Conclusions:
In the setting of whole breast RT, our findings suggest that ALND has long-term repercussions on breast skin thickening.
Keywords: breast cancer, lymph node dissection, radiation therapy, skin thickening, toxicity
1 |. BACKGROUND
Adjuvant whole breast radiation therapy (RT) following breast conserving surgery for breast cancer promotes breast conservation by reducing in-breast recurrence and the need for mastectomy.1 Whole breast RT is typically well-tolerated, but cutaneous toxicity during and after RT is common with as many as 31% to 90% of patients experiencing erythema, edema, moist desquamation, pain, cutaneous thickening and/or breast hardening.2–5
Many previous studies have found both patient and treatment factors that are associated with acute changes in the breast during and shortly after RT. Patient characteristics, such as large breast volume, smoking, and high body mass index (>25 kg/m2), have been associated with increased risk of acute dermatitis.5,6 Treatment-related factors, such as prior chemotherapy treatment, concurrent hormonal therapy, RT fraction size (standard fractionation versus hypofractionated RT), intensity modulated RT (IMRT) vs conventional 3D conformal RT, use of lumpectomy boost, and overall maximum radiotherapy dose within the breast, have also been associated with increased acute cutaneous toxicity.5–7
However, fewer studies have examined factors contributing to long-term breast changes. Among these publications, large breast size and RT boost administration appear to be associated with increased risk of late adverse side effects after whole breast RT.8,9 RT treatment objectives limiting the breast volume receiving 108% of the prescription dose to 1 mL or less have been associated with low rates of patient reported adverse cosmetic outcomes.10 IMRT is typically associated with more uniform dose distributions and lower maximum RT doses.11 However, a randomized controlled trial comparing standard wedge RT to IMRT demonstrated no significant differences in the primary endpoint of chronic pain or in the secondary endpoints of cosmesis and quality of life were noted 10 years after RT administration; nevertheless, among all patients, acute moist desquamation was associated with late subcutaneous fibrosis and telangiectasia.12 Indeed, increased breast skin thickness, a hallmark of fibrosis, may impact a patient’s long-term quality of life, as permanent changes in breast texture and appearance can impact a woman’s self-image and be a constant reminder of a patient’s breast cancer journey.
In our previous cross-sectional and prospective studies, we found that objective skin thickness measurements using ultrasound images strongly correlate with physician assessments of cutaneous toxicity grading using the Radiation Therapy Oncology Group (RTOG) criteria.13,14 In our longitudinal study using ultrasound tissue characterization of acute RT-induced changes to the skin, larger breast volume and surgical evaluation of the axilla with full lymph node dissection vs sentinel lymph node biopsy alone were both associated with increased skin thickening 6 weeks after RT.13 We established that axillary surgery may impact the breast during and shortly after RT. The purpose of this study was to report predictors of long-term breast skin thickening 1 year after RT using ultrasound to objectively measure epidermal thickness in an overlapping cohort of breast patients including some women (77%) described in our previous study plus additional patients who agreed to participate and undergo baseline and long-term assessments.13 Based on our previous findings, we hypothesized that a full axillary lymph node dissection would put patients at risk for more severe changes in breast skin thickening 1 year after RT.
2 |. PATIENTS AND METHODS
Stage 0-III breast cancer patients between the ages of 18 and 75 years were eligible for this Institutional Review Board-approved study of skin thickening during and after whole breast RT treatment following breast conserving surgery (BCS). Sixty-six eligible patients enrolled and provided informed consent. Among the 31 patients who received chemotherapy, 19 had neoadjuvant and 12 had adjuvant chemotherapy. At the time of BCS, patients with invasive breast cancer had the axilla assessed with sentinel lymph node biopsy alone as defined as fewer than six lymph nodes removed (n = 26) or full axillary lymph node dissection as defined as six or more lymph nodes removed (n = 22). Patients with breast augmentation implants and those treated with mastectomy, hypofractionated RT, partial breast irradiation, and electrons matched to shallow tangents were excluded from this study.
In brief, participants were treated with definitive whole breast RT to a dose of 50 Gy at 2 Gy per fraction with 6 and/or 18 MV photons followed by a 10 to 16 Gy sequential boost to the lumpectomy cavity with heterogeneity corrections. The dose and fractionation scheme were relatively common during the study period from 2010 to 2015. Tissue equivalent bolus was not used during any part of RT. Boost dose was based on surgical margin width: 10 Gy for ≥2 mm margins, 14 Gy for <2 mm margins, or 16 Gy for positive margins. The boost was delivered to lumpectomy cavity and scar plus a 2.5 cm margin with electrons. If the lumpectomy cavity depth precluded adequate coverage with electrons, a mini photon tangent boost was used. Patients with positive lymph nodes and/or large primary tumor (n = 17) were also treated with supraclavicular irradiation to a dose of 50 Gy at 2 Gy per fraction. Patients who had tumors with extracapsular nodal extension, incomplete nodal dissection, or greater than 20% of positive nodes received intentional axillary nodal (level I/II) irradiation (n = 9). Internal mammary lymph node basins were intentionally targeted in six patients (Table 2).
Table 2.
Treatment characteristics
| Characteristic | N = 66 |
|---|---|
| Axillary surgery | |
| Sentinel node biopsy alone | 24 (36%) |
| Axillary lymph node dissection | 22 (33%) |
| Axillary lymph node dissection alone | 6 |
| Sentinel biopsy that converted to axillary dissection | 16 |
| None | 20 (30%) |
| Chemotherapy | |
| No | 35 (53%) |
| Yes | |
| Neoadjuvant | 19 (29%) |
| Adjuvant | 12 (18%) |
| Endocrine therapy | |
| No | 19 (29%) |
| Yes | 47 (71%) |
| Oncoplastic reduction mammoplasty at time of partial mastectomy | |
| No | 54 (82%) |
| Yes | 12 (18%) |
| Drains placed at time of partial mastectomy | |
| No | 52 (79%) |
| Yes | 14 (21%) |
| Antibiotics given before radiation therapy | |
| No | 57 (86%) |
| Yes | 9 (14%) |
| Radiotherapy | |
| Maximum dose (%) | |
| Mean (range) | 107.8 (105.1‐115.6) |
| Breast volume receiving >107% of whole breast dose (cm3) | |
| Mean (range) | 86.9 (0‐1697.9) |
| ≥2 cm3 of breast tissue receiving >107% of whole breast dose | |
| No | 40 (61%) |
| Yes | 26 (39%) |
| Supraclavicular Nodal Irradiation | |
| No | 49 (74%) |
| Yes | 17 (26%) |
| Intent to treat level 1 of axilla | |
| No | 57 (86%) |
| Yes | 9 (14%) |
| Intent to Treat Internal Mammary Lymph Nodes | |
| No | 60 (91%) |
| Yes | 6 (9%) |
| Boost | |
| Electron | 41 (62%) |
| Photon | 17 (26%) |
| Boost dose, Gy | |
| 10 | 54 (82%) |
| 14 | 11 (17%) |
| 16 | 1 (1%) |
Our methods for measuring skin thickness have been described in a previous study.13 Ultrasound was used to obtain objective measurements of epidermal thickness over all four quadrants of the treated breast, as well as all four quadrants of the untreated breast. On each image set, the epidermis was delineated consistent with our previous studies.14,15 A skin thickness ratio (STRA) was generated by dividing the mean epidermal thickness value of the treated breast by mean epidermal thickness value of the contralateral breast. We previously confirmed the reproducibility of the STRA measurements by three different operators and found no significant inter- or intra-rater operator differences between measurements of the unaffected breast acquired at different time points (all P > .05); our prior work also showed that the STRA correlates with RTOG grading criteria of RT-induced skin toxicity in breast cancer patients.14,16 Therefore, we again used STRA as an objective measure of RT-induced skin toxicity in this study where STRA was measured at baseline (within 1 week before beginning RT), during week 6 of RT, and 6 weeks, 6 months, and 1 year post-RT. Elevated STRA at 1 year post-RT from baseline demonstrates persistent skin thickening of the breast.
2.1 |. Statistical analysis
Descriptive statistics were conducted on demographic data and study outcomes. Variables deviating from a normal distribution were log-transformed for analyses, as necessary. Repeated measures analysis of variance was used to determine changes in mean STRA over time following RT. Simple linear regression analyses were performed to assess significant patient, tumor, and treatment variable predictors of STRA at 1 year post-RT, as well as changes (1 year relative to baseline) in STRA measurements. Potential predictors included patient age, race, history of diabetes, body mass index, smoking status (current vs previous or none), postmenopausal status (Y/N), baseline STRA, breast volume, tumor size and stage (0/I vs II/III), time between surgery and RT, prior chemotherapy treatment (Y/N), antibiotics given Before starting RT (Y/N), sentinel biopsy that converted to axillary lymph node dissection (Y/N), breast volume receiving more than 107% of the prescribed RT dose, intent to treat level I (Y/N), oncoplastic reduction mammoplasty at time of partial mastectomy (Y/N), axillary drain(s) placed at time of surgery (Y/N), boost dose (10 Gy vs > 10 Gy), boost technique (photon vs electron), and boost volume. Next, multivariable linear regression analyses were performed to assess relationships between relevant variables and STRA. Starting with significant predictors, backward model selection based on Akaike information criterion (AIC) was conducted to identify the final model. A P value less than .2 was used as a cutoff for significant predictors to be included in model selection. Data are presented as mean ± standard deviation, and P values less than .05 were considered statistically significant. Because this was a secondary data analysis, no adjustments for multiple comparisons were made.17
3 |. RESULTS
Relevant patient, tumor and treatment characteristics including dosimetric data were collected (Tables 1 and 2). Twelve women received oncoplastic reductions at the time of breast conserving surgery. The median number of lymph nodes removed from patients with sentinel lymph node biopsy alone was 3 (range, 1–5), while median number of lymph nodes removed from patients with full lymph node dissection was 13 (range, 6–23). Among the 22 patients who received axillary lymph node dissection, 16 patients initially had sentinel biopsy that had to be converted to axillary lymph node dissection. Axillary drains were placed in 14 patients at time of partial mastectomy and all of them received axillary dissection at the time of surgery (Table 2). Some patients received neoadjuvant chemotherapy (n = 19), while others received adjuvant chemotherapy (n = 12) based on advanced cancer stage, triple negative receptor status, Her2 positive receptor status, or high 21 Gene Recurrence Score; none received chemotherapy concurrently with RT. Among patients who did not receive adjuvant chemotherapy (n = 54), the mean number of days from surgery to initial baseline assessment was 53 days (range, 29–97 days), whereas among women who received adjuvant chemotherapy, the mean number of days from surgery to initial baseline STRA measurement was 153 days (range, 129–208 days). For the duration of the study, a total of 2,436 ultrasound images were obtained.
Table 1.
Patient and tumor characteristics
| Characteristic | N = 66 |
|---|---|
| Age | |
| Mean (range) | 56.3 (26‐75) |
| Body mass index, kg/m2 Mean (range) | 29.9 (19.1‐60.2) |
| Breast volume, cm3 Mean (range) | 1919.9 (223.3‐6988.3) |
| Diabetes | |
| No | 57 (86%) |
| Yes | 9 (14%) |
| Race | |
| Caucasian | 37 (56%) |
| African American | 29 (44%) |
| Smoking | |
| None | 54 (82%) |
| Previous | 8 (12%) |
| Current | 4 (6%) |
| Cancer stage | |
| 0 | 17 (26%) |
| I | 18 (27%) |
| IIA | 20 (30%) |
| IIB | 11 (17%) |
| Tumor size, cm Mean (range) | 1.8 (0.1‐6.0) |
| Node positive | |
| Yes | 20 (30%) |
| No | 46 (70%) |
Before simulation, all surgical drains were pulled and patients were without obvious signs of infection. Notably, nine women were given oral antibiotics before starting RT because of suspicion for cellulitis and/or infection.
3.1 |. Predictors of STRA 1 year after completion of RT
Among the 63 women with 1 year STRA measurements, mean STRA was 1.28 ± 0.31 at baseline. The mean STRA 6 months (1.65 ± 0.41) and 1 year post-RT (1.44 ± 0.38) was significantly higher than baseline measures, (P < .001 for all comparisons Figure 1). In univariate analysis, predictors of higher STRA at 1 year post-RT were STRA measures at all time points leading up to 1 year (P < .001, for all time points). STRA measures were highly correlated through-out the study period. Supraclavicular nodal irradiation treatment (P < .001), increased time between surgery and RT (P < .001), full axillary lymph node dissection (P = .03), and receipt of chemotherapy (P = .015) were also predictors of increased STRA in univariate analysis. Notably, Caucasian race was not a statistically significant predictor on univariate analysis. Interestingly, among participants who had axillary lymph node dissection, drain placement was a statistically significant predictor for increased STRA at 1 year (P = .002). In MVA, predictors of more severe STRA at 1 year included time interval between surgery and RT (P = .054), baseline STRA (P = .009), and STRA at 6 months post-RT (P < .001).
Figure 1.
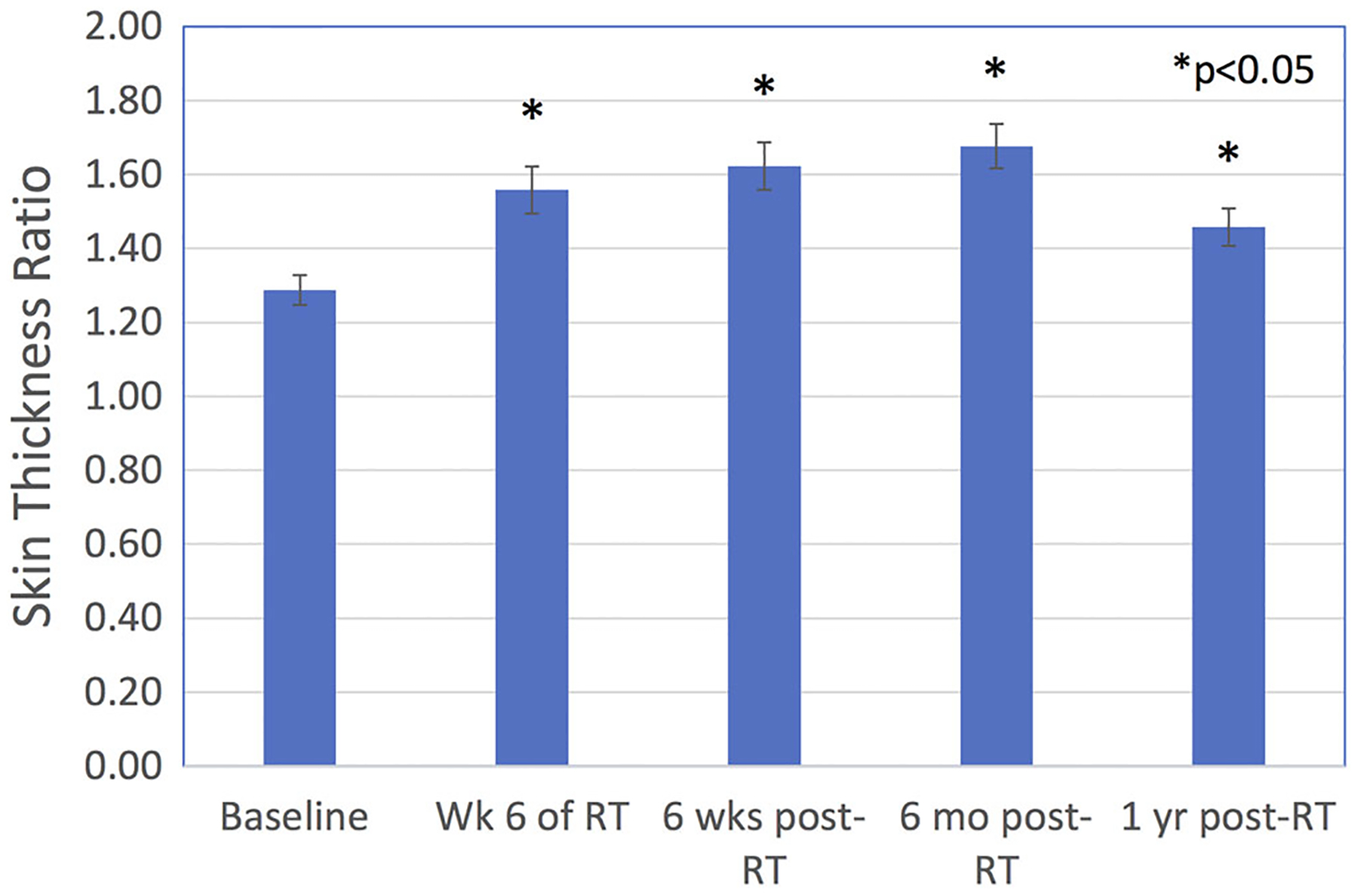
Skin thickness ratio (STRA) before, during, and after whole breast radiotherapy. *P < .05 for all comparisons relative to baseline. Mean STRA 6 months and 1 year after radiotherapy was significantly larger than baseline mean STRA. RT, radiation therapy
Given that STRA at 6 months post-RT was significantly associated with baseline STRA, we performed MVA without the intermediate time point. When the variable of STRA at 6 months post-RT was removed from the model, four factors predicted for more severe STRA at 1 year post-RT: increased time interval between surgery and RT (P = .010), high baseline STRA (P < .001), full axillary lymph node dissection (P < .016), and Caucasian race (P = .036). Axillary lymph node dissection was associated with a higher baseline STRA than sentinel lymph node biopsy alone or no surgical axillary evaluation, P < .03 for both comparisons; at every measured time point, the mean STRA of patients with axillary lymph node dissection remained persistently elevated compared to patients with sentinel lymph node biopsy alone or to patients with no axillary surgery (Figure 2). The receipt of adjuvant chemotherapy did not predict for more severe STRA at 1 year post-RT although adjuvant chemotherapy increases the time interval between surgery and RT. When controlling for adjuvant chemotherapy vs no chemotherapy or neoadjuvant chemotherapy, we found that the time interval between surgery and RT remained a predictor for STRA at 1 year (P = .007).
Figure 2.
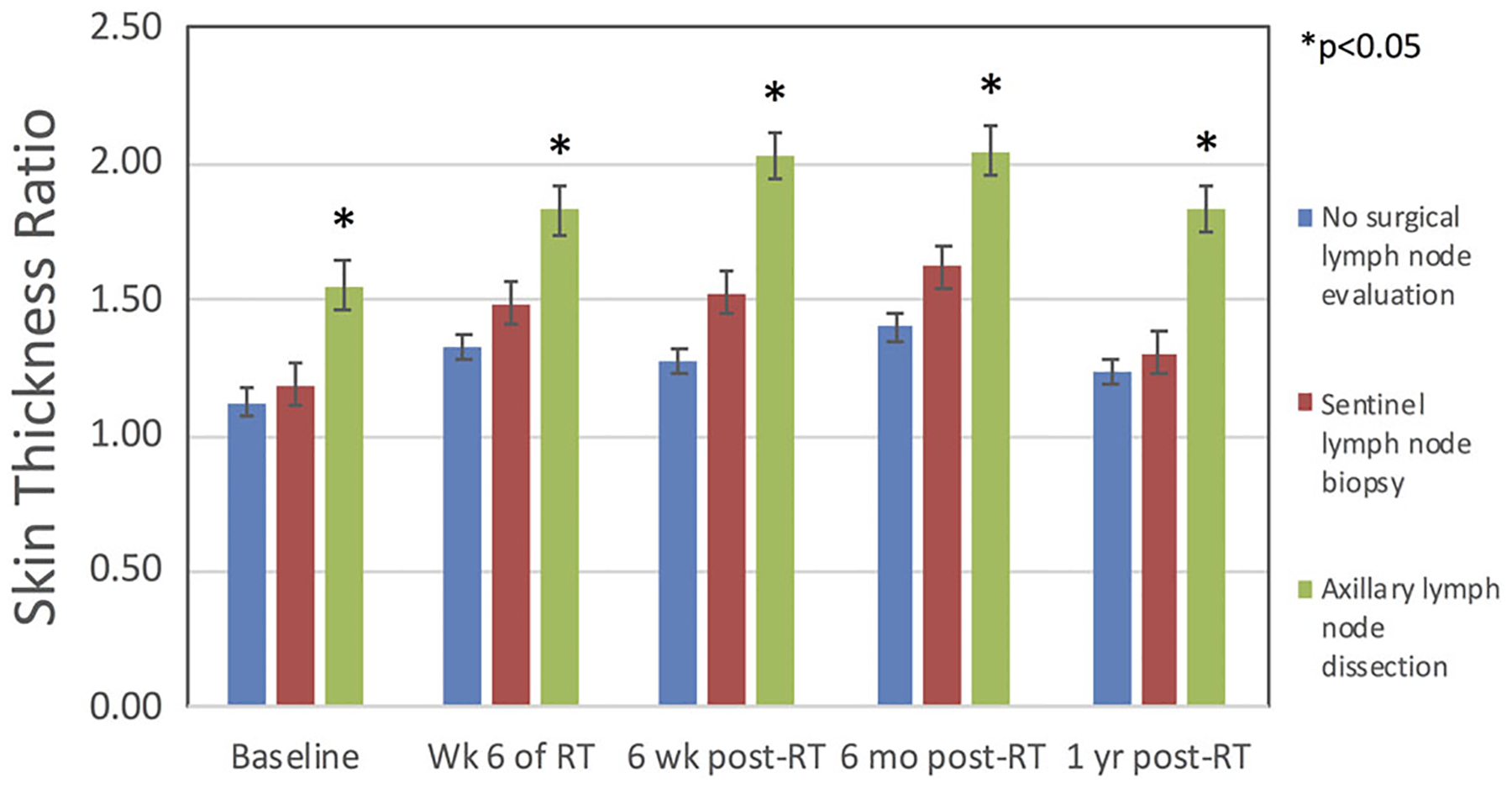
The influence of axillary surgery on skin thickness ratio (STRA) before, during and after breast radiotherapy (RT). Mean STRA among women who received full axillary lymph node dissection was significantly higher at every timepoint up to 1 year relative to baseline than in women without full axillary lymph node dissection (*P < .05 for all comparisons)
3.2 |. Changes in STRA 1 year after RT compared to baseline STRA
In our cohort, 23 participants (39%) had an STRA at 1 year post-RT that was the same or better than baseline STRA. However, the majority developed increased skin thickening 1 year after completing RT. In univariate analysis, predictors of larger changes in STRA included elevated STRA 6 months post-RT (P < .001), full axillary lymph node dissection (P = .019), and increased time between surgery and RT (P = .037). Sub-analysis among participants with lymph node dissection did not reveal that drain placement was a statistically significant predictor of change in STRA at 1 year. In MVA, predictors of change in STRA at 1 year compared to STRA at baseline included longer time interval between surgery and RT (P = .03), increased STRA at baseline (P < .001), increased STRA at 6 months post-RT (P < .001), and full axillary lymph node dissection (P = .028). When the variable of increased STRA at 6 months post-RT was removed from the model due to its high correlation with baseline STRA, the four factors which predicted for larger STRA at 1 year also predicted for larger changes in STRA at 1 year relative to baseline: longer time interval between surgery and RT (P = .010), increased STRA at baseline (P < .001), full axillary lymph node dissection (P = .016), and Caucasian race (P = .036). Other factors, such as receipt of chemotherapy, electron boost, and supraclavicular nodal irradiation did not predict for larger increases of STRA at 1 year post-RT compared to baseline.
4 |. DISCUSSION
In our study of skin thickening up to 1 year after RT, the highest mean STRA was 6 months post-RT; while the mean STRA at 1 year decreased relative to the 6 month post-RT time point, the decline in STRA did not reach the mean pre-RT value. Notably, 61% of the patients had persistently higher mean STRA at 1 year. Normal tissue injury from RT contributes to persistent breast edema,18 but our data also indicates that axillary dissection surgery leads to increased breast skin thickening before RT as well as long after RT is complete. We previously reported the early results of our prospective, longitudinal study,13 which determined that full lymph node dissection impacts breast skin thickness during and up to 6 weeks post-RT. The current study, which now includes 1 year follow up, documents the persistent effect of axillary surgery on the breast in BCS patients treated with whole breast RT. Cutaneous skin toxicity is a common side effect during RT and may continue to persist as breast edema, fibrosis, and/or breast hardening after completion of treatment. While the majority of patients who receive whole breast RT have no to mild skin and subcutaneous late toxicity, a small but variable percentage, ranging from <10% to 25%, of patients have persistent grade 2 to 3 late toxicities.8,19,20
In our initial report, active smoking predicted for worse baseline STRA.13 While active smoking did not predict for increased STRA at 1 year post-RT, higher baseline STRA significantly predicted for increased STRA at 1 year. Other variables that also predicted for increased STRA at 1 year included axillary lymph node dissection, longer time interval from surgery to RT, and Caucasian race. Patients who received a full axillary dissection appeared to have higher mean STRA at all time points compared to patients without surgical lymph node evaluation or sentinel lymph node biopsy alone (Figure 2). In addition, axillary dissection was a predictor of more severe change in STRA at 1 year, suggesting less recovery of skin thickness at 1 year. Our findings indicate that full axillary lymph node dissection has a durable impact on the breast compared to sentinel lymph node biopsy alone or no axillary surgery. The effect of extensive axillary manipulation is also reflected by the finding that patients who had axillary drain placed at time of partial mastectomy also appeared to be at risk of long-term breast skin thickening at 1 year. Indeed, the trauma from axillary lymph node dissection may lead to lymphatic congestion from disrupted lymphatics and result in increased breast skin thickening or edema after RT. While several randomized studies have demonstrated equivalent axillary control and disease outcomes in patients with clinically node negative disease treated with full lymph node dissection or sentinel lymph node biopsy or axillary RT,21,22 rates of lymphedema are significantly higher with an aggressive surgical approach to the axilla.21 Our study also indicates that long-term breast skin thickening and edema is another unintended consequence of a full lymph node dissection. A current prospective, randomized study, Alliance for Clinical Trials in Oncology A011202, is comparing axillary lymph node dissection to axillary radiation therapy in patients with persistent node-positive breast cancer after neoadjuvant chemotherapy. Axillary RT in this study may not only improve lymphedema rates, but also breast edema and thickening long term.23 We encourage multi-disciplinary discussion regarding axillary lymph node dissection as there are many clinical scenarios where sentinel lymph node biopsy and full axillary lymph node dissection result in equivalent cancer outcomes; however, our findings suggest that axillary lymph node dissection may increase the likelihood of persistent breast skin thickening.
Interestingly, we also found that a longer time interval between surgery and RT is associated with increased breast skin thickening long term. Both injury from surgery and RT incite an acute inflammatory response with fibroblast recruitment and extracellular matrix deposition and activation.24 It is possible that a longer period of time between surgery and RT may result in more pronounced breast skin thickening due to surgical-induced fibrosis that has had more time to develop before starting RT. Additional ongoing investigations with super-high resolution ultrasound may clarify the mechanism by which surgical treatment of the axilla may alter in-breast lymphatic drainage during and after RT.
Our study had several strengths and limitations. First, we prospectively measured breast skin thickening using ultrasound to obtain objective measurements with high intra-rater and inter-rater reliability. Second, our study included many African American patients (44%) reflecting the community that we serve. Notably, Caucasian race, and not African American race, was a significant predictor of both higher STRA at 1 year post-RT and greater change in STRA at 1 year compared to baseline. Nevertheless, our overall study was relatively small with only 63 evaluable patients at 1 year. Additional investigation is needed to better understand the relationship between race and breast skin thickening.
Our study exclusively permitted patients with conventional fractionation as opposed to hypofractionation due to the time period of patient enrollment. During this period, hypofractionation was not widely adopted, as many clinicians were awaiting long-term results of randomized, phase III clinical trials.19,20 The relationship between breast skin thickening and hypofractionated RT is the focus of another clinical study that has recently completed accrual at our institution. Nevertheless, many breast cancer patients treated to the breast or chest wall and regional nodes with radiation continue to receive conventionally fractionated therapy, and therefore, our findings still have clinical relevance in the modern era.
Our study did not prospectively measure breast or arm lymphedema. However, the epidermal thickening of the breast may be an early indicator of breast and/or arm lymphedema, and additional investigations to establish the associations are warranted. Early interventions with breast massage, physical therapy, as well as pentoxifylline and vitamin E,25 may be important treatments in helping decrease epidermal thickness and relieve symptoms in patients who have higher risk for developing breast skin thickening.
Furthermore, follow-up studies are ongoing at our institution to determine if these results remain true in a group of women who are treated with increasingly common hypofractionated radiation regimens.
5 |. CONCLUSIONS
Our results from this prospective, longitudinal study of BCS patients treated with subsequent whole breast RT suggests that full axillary lymph node dissection has a long-term impact on breast skin thickening up to 1 year post-RT. These results are consistent with those from our previous study of epidermal thickness during and shortly after whole breast RT. Many studies are focused on de-escalating the surgical approach to the axilla to decrease rates of lymphedema and long-term side effects to the upper extremities while not altering breast cancer outcomes. Based on our results, less axillary surgery is also likely to improve breast cosmesis. While treatment-related factors play an important role in long-term side effects to the upper extremity and breast, additional studies are needed to determine biological predictors of these toxicities in breast cancer survivors.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health (NIH), National Cancer Institute (NCI) grants R21 CA155511, R03CA173770, R03CA183006, and P30CA138292. A seed grant also provided by the Radiation Therapy Oncology Group Community Clinical Oncology and Symptom Management Group. Funding was also provided by the Fred Cooper Family Foundation Breast Cancer Initiative and Robbins Scholar Award from the Winship Cancer Institute of Emory University. Elements of this study were presented at the ASTRO 2017 Annual Meeting in San Diego, CA.
Footnotes
CONFLICT OF INTEREST NOTIFICATION
The authors state that an actual or potential conflict of interest does not exist with the submitted manuscript.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. Citation for the data of last entry – not applicable.
REFERENCES
- 1.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. [DOI] [PubMed] [Google Scholar]
- 2.Harsolia A, Kestin L, Grills I, et al. Intensity-modulated radiotherapy results in significant decrease in clinical toxicities compared with conventional wedge-based breast radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68(5):1375–1380. [DOI] [PubMed] [Google Scholar]
- 3.Fisher J, Scott C, Stevens R, et al. Randomized phase III study comparing best supportive care to biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97–13. Int J Radiat Oncol Biol Phys. 2000;48(5):1307–1310. [DOI] [PubMed] [Google Scholar]
- 4.Freedman GM, Li T, Nicolaou N, Chen Y, Ma CC, Anderson PR. Breast intensity-modulated radiation therapy reduces time spent with acute dermatitis for women of all breast sizes during radiation. Int J Radiat Oncol Biol Phys. 2009;74(3):689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26(13):2085–2092. [DOI] [PubMed] [Google Scholar]
- 6.De Langhe S, Mulliez T, Veldeman L, et al. Factors modifying the risk for developing acute skin toxicity after whole-breast intensity modulated radiotherapy. BMC Cancer. 2014;14:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukesh MB, Harris E, Collette S, et al. Normal tissue complication probability (NTCP) parameters for breast fibrosis: pooled results from two randomised trials. Radiother Oncol. 2013;108(2): 293–298. [DOI] [PubMed] [Google Scholar]
- 8.Goldsmith C, Haviland J, Tsang Y, Sydenham M, Yarnold J, FAST Trialists’ Group. Large breast size as a risk factor for late adverse effects of breast radiotherapy: is residual dose inhomogeneity, despite 3D treatment planning and delivery, the main explanation? Radiother Oncol. 2011;100(2):236–240. [DOI] [PubMed] [Google Scholar]
- 9.De Santis MC, Bonfantini F, Di Salvo F, et al. Factors influencing acute and late toxicity in the era of adjuvant hypofractionated breast radiotherapy. Breast. 2016;29:90–95. [DOI] [PubMed] [Google Scholar]
- 10.Shaitelman SF, Lei X, Thompson A, et al. Three-year outcomes with hypofractionated versus conventionally fractionated whole-breast irradiation: results of a randomized, noninferiority clinical trial. J Clin Oncol. 2018:1800317. JCO1800317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vicini FA, Sharpe M, Kestin L, et al. Optimizing breast cancer treatment efficacy with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2002;54(5):1336–1344. [DOI] [PubMed] [Google Scholar]
- 12.Pignol JP, Truong P, Rakovitch E, Sattler MG, Whelan TJ, Olivotto IA. Ten years results of the Canadian breast intensity modulated radiation therapy (IMRT) randomized controlled trial. Radiother Oncol. 2016;121(3):414–419. [DOI] [PubMed] [Google Scholar]
- 13.Torres MA, Yang X, Noreen S, et al. The impact of axillary lymph node surgery on breast skin thickening during and after radiation therapy for breast cancer. Int J Radiat Oncol Biol Phys. 2016;95(2):590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida EJ, Chen H, Torres M, et al. Reliability of quantitative ultrasonic assessment of normal-tissue toxicity in breast cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82(2):724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Y, Tannenbaum A, Chen H, et al. Automated skin segmentation in ultrasonic evaluation of skin toxicity in breast cancer radiotherapy. Ultrasound Med Biol. 2013;39(11):2166–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu T, Zhou J, Yoshida EJ, et al. Quantitative ultrasonic evaluation of radiation-induced late tissue toxicity: pilot study of breast cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(3):811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li G, Taljaard M, Van den Heuvel ER, et al. An introduction to multiplicity issues in clinical trials: the what, why, when and how. Int J Epidemiol. 2017;46(2):746–755. [DOI] [PubMed] [Google Scholar]
- 18.Verbelen H, Gebruers N, Beyers T, De Monie AC, Tjalma W. Breast edema in breast cancer patients following breast-conserving surgery and radiotherapy: a systematic review. Breast Cancer Res Treat. 2014;147(3):463–471. [DOI] [PubMed] [Google Scholar]
- 19.Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11): 1086–1094. [DOI] [PubMed] [Google Scholar]
- 20.Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513–520. [DOI] [PubMed] [Google Scholar]
- 21.Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981–22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louis-Sylvestre C, Clough K, Asselain B, et al. Axillary treatment in conservative management of operable breast cancer: dissection or radiotherapy? Results of a randomized study with 15 years of follow-up. J Clin Oncol. 2004;22(1):97–101. [DOI] [PubMed] [Google Scholar]
- 23.Joint Clinical Trial Office (JCTO). Comparison of axillary lymph node dissection with axillary radiation for patients with node-positive breast cancer treated with chemotherapy. Weill Cornell Medicine, New York: Presbyterian. [Google Scholar]
- 24.Straub JM, New J, Hamilton CD, Lominska C, Shnayder Y, Thomas SM. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol. 2015;141(11):1985–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobson G, Bhatia S, Smith BJ, Button AM, Bodeker K, Buatti J. Randomized trial of pentoxifylline and vitamin E vs standard follow-up after breast irradiation to prevent breast fibrosis, evaluated by tissue compliance meter. Int J Radiat Oncol Biol Phys. 2013;85(3):604–608. [DOI] [PubMed] [Google Scholar]


