Abstract
Aim:
Our objective was to assess whether increased duration of metformin therapy is associated with incident peripheral neuropathy (PN) in older Veterans with diabetes.
Methods:
Using national Veterans Affairs registry data from 2002 to 2015, we examined Veterans (50 + years) with diabetes. Long-term metformin therapy was defined as prescription ≥ 500 mg/day, filled for ≥ 6 consecutive months. Metformin therapy duration was examined both as continuous and categorical measures. Incident PN was defined by medical chart review. We estimated unadjusted and adjusted (variables selected a priori) odds ratios (OR) and 95% confidence intervals (CI) using logistic regression.
Results:
The study included n = 210,004 individuals (mean ± SD: age: 66.2 ± 8.4 yrs, 96% male) prescribed metformin for 47.0 ± 34.0 months. Nineteen percent developed PN during follow-up. After adjusting for age, body mass index, duration of time receiving health care within the VA, smoking status, alcohol abuse, and vitamin B12 testing and treatment, the number of months of metformin treatment was associated with elevated odds for incident PN (aOR (metformin treatment - continuous) = 1.009 (95% CI = 1.009, 1.010); aOR (metformin treatment – categorical (ref: 6–<18 months): 18–<44.1 months = 1.57 (1.51–1.63), 44.1–<61 months = 2.05 (1.97–2.14), 61 + months = 2.69 (2.58–2.79), all p-values < 0.0001).
Conclusion:
Our study suggests that Veterans treated for at least 18 months with metformin are approximately 2–3 times more likely to develop PN than those treated at least six, but<18 months. Future studies are needed to determine whether the association we found may be due to a decline in vitamin B12 status following metformin initiation.
Keywords: Aging, Metformin, Type 2 diabetes, Veteran, Vitamin B12
1. Introduction
The prevalence of type 2 diabetes mellitus (T2DM) in aging adults in the U.S. has risen dramatically, particularly among U.S. Veterans, where it currently affects over 20% of the Veteran population [1]. Metformin is recommended as a first line treatment for individuals with T2DM and normal kidney function, resulting in nearly 70% of all U.S. Veterans receiving metformin to treat their T2DM [2].
Older adults are at greater risk of vitamin B12 deficiency than their younger counterparts [3], but this risk may be magnified in older adults with T2DM. We [4] and others [5–7] have shown that long-term metformin therapy is associated with lower serum vitamin B12 concentration. Though the clinical impact of long-term metformin therapy and low B12 is currently unknown, it could have implications for exacerbation of peripheral neuropathy since prolonged vitamin B12 deficiency may have deleterious effects on peripheral nerves, leading to irreversible nervous system damage [8]. Peripheral neuropathy is among the most common complication of T2DM, affecting approximately 50% of patients [9]. Despite these links, to date, few studies have assessed the impact of metformin directly on peripheral neuropathy [10–12].
The present study aimed to examine whether long-term (six or more months) metformin therapy was associated with the development of peripheral neuropathy in older Veterans. We hypothesized that a longer duration of metformin treatment in older Veterans with T2DM would be associated with a greater incidence of peripheral neuropathy. The findings from this study may be useful to formulate best practices for diabetes management among older adults.
2. Subjects
The study was approved by the Emory University Institutional Review Board and the participating VA Research and Development Committee. We conducted a retrospective cohort study using existing national Veterans Affairs (VA) data for Veterans receiving care at any VA medical center (VAMC) between January 2002 and December 2015. Veterans aged 50 years or older receiving long-term metformin only drug therapy for their T2DM management were identified from outpatient records. Those using other antihyperglycemic agents, including insulin, were excluded due to their associations with more advanced T2DM and the potential for co-existing peripheral neuropathy. Those without complete age, sex, and race/ethnicity data or having an International Classification of Diseases code from the ninth revision (ICD-9) code diagnosis of pernicious anemia (ICD 9: 281.0) or end-stage renal disease (ICD-9: 585.6) at baseline also were excluded (Fig. 1).
Fig. 1 –
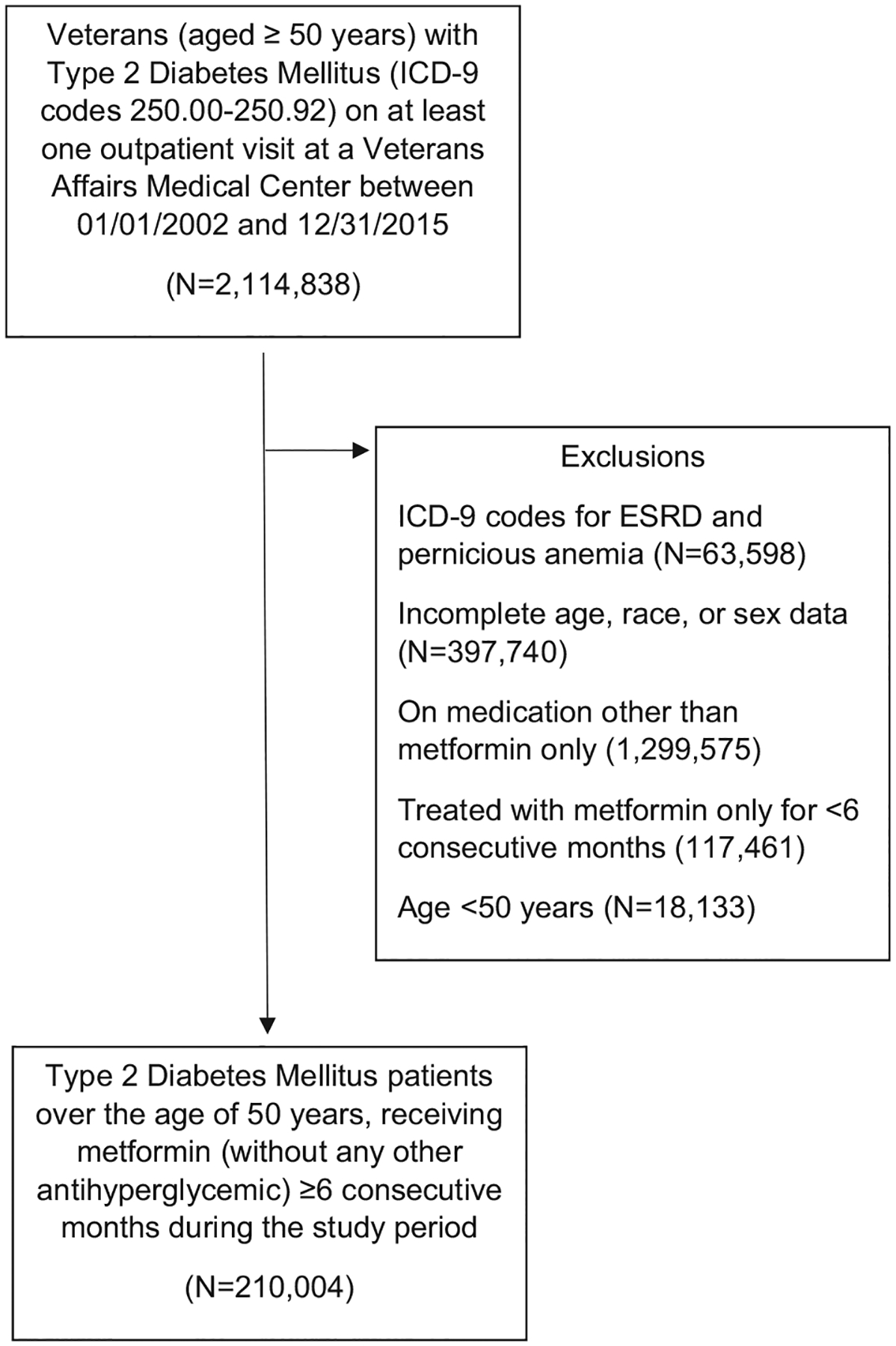
Subject Selection Criteria.
T2DM was defined as use of at least one T2DM code (ICD-9: 250.00–250.92) at a primary care location or use of at least two T2DM codes at a non-primary care location and at least one outpatient prescription of diabetes medication during the study period. Long-term metformin therapy was defined as a dose of ≥ 500 mg/day and refilled for at least six consecutive months during the study period.
3. Materials and methods
3.1. Study variables
We examined the following demographic variables as co-factors: age, race/ethnicity, and sex. We also examined body mass index (BMI), the number of years treated at a VAMC during the study period, and clinical factors, including estimated glomerular filtration rate (eGFR) and hemoglobin A1c. Serum vitamin B12 concentrations measured any time after six consecutive months of metformin prescription and hemoglobin A1c and creatinine concentrations from the test closest to the vitamin B12 test date or closest to metformin initiation date when vitamin B12 testing was not available using VAMC laboratory records were examined. Creatinine was used to calculate estimated glomerular filtration rate eGFR [13]. Outliers (vitamin B12 values > 2,000 pg/dL and creatinine values > 25 mg/dL) were recoded as missing. Serum B12 concentrations were classified as deficient (<170 pg/dL), borderline low (170–<300 pg/dL), or adequate (≥300 pg/dL) based on previously established cut-points among older Veterans [14]. Peripheral neuropathy was defined as receiving any of the following (ICD)-9 diagnosis codes during the study period: 356.8, 356.9 and 250.6. Smoking history was identified using previously validated methods [15] and patients were classified as “ever” having smoked (past or present) vs. “never” having smoked. The presence of alcohol abuse was defined as receiving any of the following ICD-9 diagnosis codes related to alcohol use disorder or alcohol-related end organ damage during the study period: 291.0–291.8, 291.82–291.9, 303.90–303.93, 305.00–305.03, 357.5, 425.5, 535.30–535.31, 571.0, and 571.2–571.3.
3.2. Statistical analysis
Data were analyzed using SAS version 9.3 (SAS Institute Inc., Cary, NC). Descriptive statistics including means and standard deviations, or frequency and percents, were calculated for selected variables stratified by those who developed peripheral neuropathy and those who did not and compared using t-tests for continuous variables and chi-squared tests for categorical variables. Significance was set at P < 0.05. We used unadjusted and adjusted logistic regression to examine the association between duration of metformin therapy and the development of peripheral neuropathy. Duration of metformin therapy was examined as both continuous and categorical variable. For categorical measure of metformin therapy duration, we created quartiles of months of metformin use from the distribution of those who did not develop peripheral neuropathy. Based on this distribution, the cut-offs for categorical metformin use durations were: quartile 1: 6–<18 months, quartile 2: 18–<44.1 months, quartile 3: 44,1–<61 months, and quartile 4: 61 + months. Quartile 1 was used as a reference level in logistic regression analysis. Unadjusted and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using multivariable logistic regression models. Co-variables in multivariable models were selected using backward logistic regression and a 10% change-in-estimate criterion. Covariates included age, BMI, duration of time treated within the VA Health Care System, eGFR, race/ethnicity, sex, smoking status, alcohol abuse, vitamin B12 concentration, testing, and treatment status.
4. Results
4.1. Subject Selection
A total of 2,114,838 Veterans had ICD-9 codes indicating T2DM on at least one outpatient visit at the VAMC during the study period. Of these, 63,598 were excluded due to ICD-9 codes for ESRD and pernicious anemia and an additional 397,740 were excluded due to incomplete age, race, or sex data. Among the remaining patients, 228,137 had a prescription filled for metformin for at least six consecutive months, and received no other antihyperglycemic agents. Of these patients, 210,004 were over the age of 50 years and included in the final analyses (Fig. 1).
4.2. Demographic data
Overall, patients (mean age: 66.2 years) were majority male (96%), non-Hispanic white (84%), and past or current smokers (79%). Alcohol abuse was reported in 9% of patients. On average, they were treated at the VAMC for 11.4 years and had been on metformin therapy for 47.0 months. During the study duration, 58.8% received a serum vitamin B12 assessment, of which, 3.0% were deficient, 20.5% borderline low, and 76.5% adequate. After a duration of at least six months of metformin prescription, only 27.8% of patients received vitamin B12 treatment and 19.3% developed peripheral neuropathy during the study period. Comparison of demographic and clinical variables between the two groups, those with and without the development of peripheral neuropathy during the study period, are presented in Table 1. The average time to develop peripheral neuropathy after metformin initiation was 28.1 ± 30 months. Of those developing peripheral neuropathy, 26.9% did not have a vitamin B12 assessment and only 39.3% received vitamin B12 treatment.
Table 1 –
Subject characteristics, total and stratified by the development of peripheral neuropathy, among older adults with Type 2 Diabetes Mellitus seeking outpatient care at U.S. Veterans Affairs Medical Centers, 2002–2015.
| Total | Developed Peripheral Neuropathy | Did Not Develop Peripheral Neuropathy | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 210,004 | N = 40,534 | N = 169,470 | ||||||||
| Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | ||
| Age (years) | 66.2 | ± | 8.4 | 65.8 | ± | 8.1 | 66.3 | ± | 8.5 | <0.0001 |
| BMI (kg/m2) | 29.6 | ± | 9.7 | 31.3 | ± | 8.0 | 29.2 | ± | 10.0 | <0.0001 |
| Duration of metformin treatment (months) | 47.0 | ± | 34.0 | 59.2 | ± | 36.7 | 44.1 | ± | 32.7 | <0.0001 |
| Duration treated at the VAMC (years) | 11.4 | ± | 4.0 | 12.3 | ± | 3.6 | 11.2 | ± | 4.1 | <0.0001 |
| eGFR (mL/min/1.73 m2) | 77.1 | ± | 20.8 | 78.7 | ± | 17.2 | 76.7 | ± | 21.5 | <0.0001 |
| Hemoglobin A1c (%) | 5.8 | ± | 2.0 | 6.0 | ± | 1.7 | 5.8 | ± | 2.1 | <0.0001 |
| Hemoglobin A1c (mmol/mol) | 40 | ± | 13 | 42 | ± | 12 | 40 | ± | 14 | <0.0001 |
| N | , | % | N | , | % | N | , | % | ||
| Race and Ethnicity | <0.0001 | |||||||||
| Non-Hispanic White | 175,646 | , | 84 | 34,747 | , | 86 | 140,899 | , | 83 | |
| Non-Hispanic Black | 29,316 | , | 14 | 5,047 | , | 12 | 24,269 | , | 14 | |
| Other | 5,042 | , | 2 | 740 | , | 2 | 4,302 | , | 3 | |
| Sex (N, % male) | 201,357 | , | 96 | 39,248 | , | 96.8 | 162,109 | , | 95.7 | <0.0001 |
| Smoker (N, % yes) | 165,705 | , | 78.9 | 32,906 | , | 81.2 | 132,799 | , | 78.4 | <0.0001 |
| Alcohol abuse (N, % yes) | 19,665 | , | 9.4 | 5,329 | , | 13.1 | 14,336 | , | 8.5 | <0.0001 |
| Received vitamin B12 assessment (N, % yes) | 123,463 | , | 58.8 | 29,641 | , | 73.1 | 93,822 | , | 55.4 | <0.0001 |
| Serum vitamin B12 (pg/dL) | <0.0001 | |||||||||
| <170 | 3,750 | , | 3.0 | 872 | , | 2.2 | 2,878 | , | 1.7 | |
| 170-<300 | 25,262 | , | 20.5 | 5,970 | , | 14.7 | 19,292 | , | 11.4 | |
| ≥300 | 94,451 | , | 76.5 | 22,799 | , | 56.3 | 71,652 | , | 42.3 | |
| Received vitamin B12 treatment (N, % yes) | 58,429 | , | 27.8 | 15,943 | , | 39.3 | 42,486 | , | 25.1 | <0.0001 |
BMI: body mass index; VAMC: U.S. Veterans Affairs Medical Centers; eGFR: estimated glomerular filtration rate
Duration of metformin use among those who developed peripheral neuropathy was on average 59.2 ± 36.7 months and for those who did not develop peripheral neuropathy was 44.1 ± 32.7 months. Those who developed peripheral neuropathy were, on average, younger compared to those who did not (65.8 ± 8.1 vs. 66.3 ± 8.5 years).
4.3. Regression analyses
Our unadjusted analysis showed a significant association between duration of metformin use (both continuous and categorical measures) and peripheral neuropathy (Table 2). For the continuous measure of duration of metformin use, there was 1% increase in the odds for each month increment in use. For categorical measure, unadjusted ORs showed a positive association with peripheral neuropathy, with estimates 1.78, 2.45, and 3.99 for quartiles 2, 3, and 4 of duration of metformin use, respectively, compared to the lowest quartile. These associations remained significant after controlling for potential confounders. In our univariate analysis, the development of peripheral neuropathy does not appear to be influence by hemoglobin A1c; therefore, we did not include it in the multivariate model (OR (95% CI): 0.997 (0.973–1.020), p-value = 0.78). For continuous measure of duration of metformin use, adjusted estimate was 1.009 (95% CI = 1.009–1.01); and for categorical measure, the adjusted estimate was 1.57, 2.05, and 2.69 for quartiles 2, 3, and 4 of duration of metformin use, respectively, compared to the lowest quartile. These results show that as the duration of metformin use increases, there is an increase in the odds of developing peripheral neuropathy in the target population.
Table 2 –
Unadjusted and adjusted analysis to examine the association between duration of metformin use and development of peripheral neuropathy among older adults with Type 2 Diabetes Mellitus seeking outpatient care at U.S. Veterans Affairs Medical Centers, 2002–2015.
| Unadjusted Analysis | Adjusted Analysis* | |||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Months of metformin use | ||||
| Continuous | 1.01 (1.01–1.01) | <0.0001 | 1.009 (1.009–1.01) | <0.0001 |
| Categorical | ||||
| 6–<18 | Ref | Ref | ||
| 18 –<44.1 | 1.78 (1.73–1.83) | <0.0001 | 1.57 (1.51–1.63) | <0.0001 |
| 44.1–< 61 | 2.45 (2.37–2.54) | <0.0001 | 2.05 (1.97–2.14) | <0.0001 |
| 61+ | 3.19 (3.09–3.29) | <0.0001 | 2.69 (2.58–2.79) | <0.0001 |
| Age (years) | 0.99 (0.99–0.99) | <0.0001 | 1.00 (0.99–1.00) | 0.4218 |
| BMI (kg/m2) | 1.02 (1.02–1.03) | <0.0001 | 1.02 (1.017–1.02) | <0.0001 |
| Duration treated at the VAMC (years) | 1.07 (1.07–1.08) | <0.0001 | 1.02 (1.01–1.02) | <0.0001 |
| eGFR (mL/min/1.73 m2) | 1.005 (1.004–1.005) | <0.0001 | 1.00 (0.99–1.00) | 0.3540 |
| Race and Ethnicity | ||||
| Non-Hispanic White | Ref | Ref | ||
| Non-Hispanic Black | 0.84 (0.82–0.87) | <0.0001 | 0.82 (0.79–0.85) | <0.0001 |
| Other | 0.69 (0.64–0.76) | <0.0001 | 0.73 (0.67–0.79) | <0.0001 |
| Sex | ||||
| Female | Ref | Ref | ||
| Male | 1.38 (1.30–1.47) | <0.0001 | 1.34 (1.26–1.43) | <0.0001 |
| Smoking | ||||
| Never Smoker | Ref | Ref | ||
| Ever Smoker | 1.17 (1.14–1.21) | <0.0001 | 1.02 (0.99–1.05) | 0.1931 |
| Unknown smoking status | 0.90 (0.84–0.97) | 0.004 | 1.07 (1.00–1.15) | 0.0605 |
| Alcohol abuse | ||||
| Absent | Ref | Ref | ||
| Present | 1.64 (1.58–1.69) | <0.0001 | 1.34 (1.29 – 1.39) | <0.0001 |
| Received vitamin B12 assessment | ||||
| No | Ref | Ref | ||
| Yes | 2.19 (2.14–2.25) | <0.0001 | 1.69 (1.64–1.73) | <0.0001 |
| Serum vitamin B12 (pg/dL) | ||||
| Unknown | 0.45 (0.44–0.46) | <0.0001 | – | – |
| <170 | 0.95 (0.88–1.03) | 0.21 | 0.72 (0.67–0.78) | <0.0001 |
| 170-<300 | 0.97 (0.94–1.01) | 0.09 | 0.86 (0.83–0.89) | <0.0001 |
| ≥300 | Ref | Ref | ||
| Received vitamin B12 treatment | ||||
| No | Ref | Ref | ||
| Yes | 1.94 (1.89–1.98) | <0.0001 | 1.53 (1.49–1.57) | <0.0001 |
BMI: body mass index; VAMC: U.S. Veterans Affairs Medical Centers; eGFR: estimated glomerular filtration rate.
Each variable is adjusted for all other variables in the model.
5. Discussion
We find that Veterans treated for at least 18 months with metformin are approximately 2–3 times more likely to develop peripheral neuropathy than those treated at least six, but <18 months. Our data support the need to monitor serum vitamin B12 concentrations in older patients with T2DM treated long-term with metformin by demonstrating that the duration of metformin therapy is a risk factor for the development of new peripheral neuropathy at a rate of approximately 1% per month and slightly >10% per year of treatment. However, studies suggest that older persons prescribed metformin are two to three times less likely to receive vitamin B12 testing compared to those without metformin treatment [4]. In line with other studies of long-term metformin treatment [4,16,17], we find that despite being on metformin treatment for at least six months, only slightly more than half of the Veterans had a serum vitamin B12 assessment indicating that healthcare providers often fail to order a vitamin B12 assessment for their patients on long-term metformin therapy. Though it may take years to deplete physiologic reserves of vitamin B12, declines in circulating vitamin B12 concentrations have been observed within four weeks after initiation of metformin [18], signifying the need for early monitoring.
Since diabetic neuropathy is the most common complication associated with diabetes mellitus [19], healthcare providers may overlook other contributing factors for peripheral neuropathy resulting in unnecessary and potentially harmful pharmacological treatments. Similar to other studies [5,20], we find that ~ 25% of long-term metformin users have serum vitamin B12 concentrations that are deficient or borderline low. The mechanism by which vitamin B12 deficiency may lead to and/or exacerbate peripheral neuropathy in patients with T2DM treated with long-term metformin therapy remains to be explored. It is believed that metformin interferes with vitamin B12 absorption in the gastrointestinal tract [21]. Vitamin B12 is involved in the conversion of methylmalonyl-CoA to succinyl-CoA and homocysteine to methionine; therefore, metformin-induced vitamin B12 deficiency may have a deleterious impact on the macrovascular system, including hematological and neurological manifestations, via increased concentrations of methylmalonic acid and homocysteine [22].
Only ~ 30% of Veterans on long-term metformin received vitamin B12 treatment during the duration of this study. It remains to be seen whether repletion or supplementation of vitamin B12 will prevent or slow the progression of diabetic peripheral neuropathy, with the majority of current research finding no evidence that oral vitamin B12 supplements improve the clinical symptoms of neuropathy [23]. While vitamin B12 deficiency is reversible, the progress of the neuropathy may not be reversible with initiation of vitamin B12 therapy, highlighting the need for early vitamin B12 monitoring and intervention.
Strengths of this analysis include the large sample size, which allowed for adjustment of several confounders [24], and the ability for long-term (over 10 years) follow-up using nationally representative, longitudinal data from the VA clinical data warehouse. Our study also has certain practical limitations. Data to quantify alcohol use were not available in the VA database. A limited number of women were included in the analyses, limiting generalizability. Since prior studies have suggested that men are more susceptible to vitamin B12 deficiency [25,26], similar studies in women are needed. Further, vitamin B12 prescribed from outside of the VA or the use of over-the-counter vitamin supplements was not collected during the study. Lastly, the use of clinical encounter data did not allow us to systematically examine the decline in B12 status following metformin initiation because nearly half of the study population did not receive serum B12 assessment and differential testing rates between those with and without peripheral neuropathy were present..
In summary, our findings show that there is an elevated risk for the development of new peripheral neuropathy with longer duration metformin treatment in older Veterans with T2DM. Despite this finding, only about half of the Veterans receiving long-term metformin were evaluated for serum vitamin B12 concentrations; therefore, more research is needed to determine factors affecting vitamin B12 monitoring among older adults who are prescribed metformin. Further, future studies are needed to determine whether the risk for peripheral neuropathy may be altered with concomitant B12 supplementation following metformin initiation. As first line therapy for T2DM management, these results may inform clinical guidelines regarding serum vitamin B12 testing among the growing number of older adults with T2DM who are treated with metformin.
Acknowledgement
This study was supported by the Department of Veterans Affairs Birmingham/Atlanta Geriatric Research, Education, and Clinical Center and awards to Dr. Vaughan (IK2 RX000747) and Dr. Phillips (CSP #2008, IK2 CX001907, I01 CX001899, I01 CX001737, and HSR&D IIR 07-138) and the National Institutes of Health awards to Dr. Phillips (R21 DK099716, R18 DK066204, R03 AI133172, U01 DK091958, U01 DK098246, UL1 TR002378). The sponsors had no role in the design, methods, data collection, or analysis of the study and had no role in the preparation of the manuscript.
Footnotes
Disclosure statement
Dr. Phillips declares that there is no duality of interest associated with this manuscript. With regard to potential conflicts of interest, Dr. Phillips has served on Scientific Advisory Boards for Boehringer Ingelheim and Janssen, and has or had research support from Merck, Amylin, Eli Lilly, Novo Nordisk, Sanofi, PhaseBio, Roche, Abbvie, Vascular Pharmaceuticals, Janssen, Glaxo SmithKline, and the Cystic Fibrosis Foundation. Dr. Phillips is also a cofounder and Officer and Board member and stockholder of a company, Diasyst, Inc., which is developing software aimed to help improve diabetes management. The other authors have nothing to disclose.
References
- [1].Liu Y, Sayam S, Shao X, Wang K, Zheng S, Li Y, Wang L. Prevalence of and Trends in Diabetes Among Veterans, United States, 2005–2014. Prev. Chronic Dis 2017;14. 10.5888/pcd14.170230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wheeler S, Moore K, Forsberg CW, Riley K, Floyd JS, Smith NL, Boyko EJ. Mortality among veterans with type 2 diabetes initiating metformin, sulfonylurea or rosiglitazone monotherapy. Diabetologia 2013;56(9):1934–43. [DOI] [PubMed] [Google Scholar]
- [3].Stover PJ. Vitamin B12 and older adults:. Current Opin Clin Nutrit Metabolic Care 2010;13(1):24–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kancherla V, Elliott JL Jr, Patel BB, Holland NW, Johnson II TM, Khakharia A, Phillips LS, Oakley GP Jr, Vaughan CP. Long-term Metformin Therapy and Monitoring for Vitamin B12 Deficiency Among Older Veterans. J Am Geriatr Soc 2017;65 (5):1061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Reinstatler L, Qi YP, Williamson RS, Garn JV, Oakley GP. Association of Biochemical B12 Deficiency With Metformin Therapy and Vitamin B12 Supplements: The National Health and Nutrition Examination Survey, 1999–2006. Diabetes Care 2012;35(2):327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pflipsen MC, Oh RC, Saguil A, Seehusen DA, Seaquist D, Topolski R. The Prevalence of Vitamin B12 Deficiency in Patients with Type 2 Diabetes: A Cross-Sectional Study. J American Board Family Med 2009;22(5):528–34. [DOI] [PubMed] [Google Scholar]
- [7].Leung S, Mattman A, Snyder F, Kassam R, Meneilly G, Nexo E. Metformin induces reductions in plasma cobalamin and haptocorrin bound cobalamin levels in elderly diabetic patients. Clin Biochem 2010;43(9):759–60. [DOI] [PubMed] [Google Scholar]
- [8].Staff NP, Windebank AJ. Peripheral neuropathy due to vitamin deficiency, toxins, and medications. Continuum (Minneap Minn). 2014;20:1293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Iqbal Z, Azmi S, Yadav R, Ferdousi M, Kumar M, Cuthbertson DJ, Lim J, Malik RA, Alam U. Diabetic Peripheral Neuropathy: Epidemiology, Diagnosis, and Pharmacotherapy. Clin Ther 2018;40(6):828–49. [DOI] [PubMed] [Google Scholar]
- [10].Gupta K, Jain A, Rohatgi A. An observational study of vitamin b12 levels and peripheral neuropathy profile in patients of diabetes mellitus on metformin therapy. Diabet Metabolic Syndrome Clin Res Rev 2018;12(1):51–8. [DOI] [PubMed] [Google Scholar]
- [11].Bell DSH. Metformin-Induced Vitamin B12 Deficiency Presenting as a Peripheral Neuropathy. South Med J 2010;103 (3):265–7. [DOI] [PubMed] [Google Scholar]
- [12].Wile DJ, Toth C. Association of Metformin, Elevated Homocysteine, and Methylmalonic Acid Levels and Clinically Worsened Diabetic Peripheral Neuropathy. Diabet Care 2010;33(1):156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Levey AS, Stevens LA, Schmid CH, Zhang Y(, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med 2009;150(9):604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bernard MA, Nakonezny PA, Kashner TM. The Effect of Vitamin B 12 Deficiency on Older Veterans and Its Relationship to Health. J Am Geriatr Soc. 1998;46 (10):1199–206. [DOI] [PubMed] [Google Scholar]
- [15].McGinnis KA, Brandt CA, Skanderson M, Justice AC, Shahrir S, Butt AA, Brown ST, Freiberg MS, Gibert CL, Goetz MB, Kim JW, Pisani MA, Rimland D, Rodriguez-Barradas MC, Sico JJ, Tindle HA, Crothers K. Validating Smoking Data From the Veteran’s Affairs Health Factors Dataset, an Electronic Data Source. Nicotine Tob Res 2011;13(12):1233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pierce SA, Chung AH, Black KK. Evaluation of Vitamin B 12 Monitoring in a Veteran Population on Long-Term, High-Dose Metformin Therapy. Ann Pharmacother 2012;46(11):1470–6. [DOI] [PubMed] [Google Scholar]
- [17].Fogelman Y, Kitai E, Blumberg G, Golan-Cohen A, Rapoport M, Carmeli E. Vitamin B12 screening in metformin-treated diabetics in primary care: were elderly patients less likely to be tested?. Aging Clin Exp Res 2017;29(2):135–9. [DOI] [PubMed] [Google Scholar]
- [18].de Jager J, Kooy A, Lehert P, Wulffele MG, van der Kolk J, Bets D, Verburg J, Donker AJM, Stehouwer CDA. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ 2010;340(may19 4). c2181–c2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Juster-Switlyk K, Smith AG. Updates in diabetic peripheral neuropathy. F1000Res 2016;5:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Aroda VR, Edelstein SL, Goldberg RB, Knowler WC, Marcovina SM, Orchard TJ, Bray GA, Schade DS, Temprosa MG, White NH, Crandall JP. Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab. 2016;101(4):1754–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McCreight LJ, Bailey CJ, Pearson ER. Metformin and the gastrointestinal tract. Diabetologia 2016;59(3):426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ahmed MA, Muntingh GL, Rheeder P. Perspectives on Peripheral Neuropathy as a Consequence of Metformin-Induced Vitamin B12 Deficiency in T2DM. Int J Endocrinol 2017;2017:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jayabalan B, Low LL. Vitamin B supplementation for diabetic peripheral neuropathy. smedj 2016;57(02):55–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Grisold A, Callaghan BC, Feldman EL. Mediators of diabetic neuropathy: is hyperglycemia the only culprit?. Current Opin Endocrinol Diabet Obes 2017;24(2):103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Margalit I, Cohen E, Goldberg E, Krause I. Vitamin B12 Deficiency and the Role of Gender: A Cross-Sectional Study of a Large Cohort. Ann Nutr Metab 2018;72(4):265–71. [DOI] [PubMed] [Google Scholar]
- [26].Loikas S, Koskinen P, Irjala K, Lopponen M, Isoaho R, Kivela SL, Pelliniemi T-T. Vitamin B12 deficiency in the aged: a population-based study. Age Ageing 2007;36(2):177–83. 10.1093/ageing/afl150. [DOI] [PubMed] [Google Scholar]


