Abstract
A 79-year-old Japanese man with polymyalgia rheumatica was admitted to hospital with coronavirus disease (COVID-19). On admission, he was treated with ciclesonide inhalation, ivermectin, and meropenem. He was intubated 6 days after admission, and methylprednisolone therapy was initiated (1000 mg/day). Hypoxemia and chest radiographic findings temporarily improved. However, chest computed tomography showed bilateral ground-glass attenuations, multiple nodules, and consolidation. Aspergillus fumigatus was cultured from the tracheal aspirate and he was diagnosed with COVID-19-associated invasive pulmonary aspergillosis (CAPA) and treated with liposomal amphotericin B. However, he died 28 days after admission.
Keywords: COVID-19 associated invasive pulmonary aspergillosis, COVID-19, Acute respiratory distress syndrome
Abbreviations
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- ARDS
acute respiratory distress syndrome
- CAPA
COVID-19-associated invasive pulmonary aspergillosis
- BDG
(1, 3)-β-D-glucan
1. Introduction
Coronavirus disease 2019 (COVID-19) is a novel infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that was first reported in Wuhan, Hubei province, China, at the end of 2019 [1]. It has since been declared a global pandemic that resulted in more than 60 million confirmed COVID-19 cases and more than 1.4 million deaths globally; there were more than 20,000 confirmed cases and more than 2000 deaths in Japan by the end of November 2020.
A recent report showed an increased risk for invasive pulmonary aspergillosis with high mortality in patients with severe influenza and acute respiratory distress syndrome (ARDS) [2]. Furthermore, cases of COVID-19-associated invasive pulmonary aspergillosis (CAPA) have also been reported worldwide [3], but there have been a few reports among Japanese patients with CAPA. We hereby present a case of CAPA in Japan.
2. Case report
A 79-year-old Japanese man with diabetes mellitus and polymyalgia rheumatica, treated with betamethasone (0.5 mg/day), prednisolone (2 mg/day), and methotrexate (10 mg/week), was in contact with patients with COVID-19 three days before admission to our hospital. One day before admission, he developed high fever (38.5 °C), cough, and general fatigue; his nasal swab SARS-CoV-2 polymerase chain reaction (PCR) was positive, and he was diagnosed with COVID-19. Laboratory findings on the day of admission revealed normal peripheral white blood cell count (4600/μL) and slightly elevated C-reactive protein level (3.4 mg/dL) (Table 1 ). Chest computed tomography (CT) revealed no infiltrations (Fig. 1 ), and he was treated with inhaled ciclesonide (200 μg, twice daily) and oral ivermectin (9 mg, once daily). His general condition and oxygenation worsened, and on the 4th day, bilateral ground-glass attenuations appeared on his chest X-ray film. We then initiated dexamethasone (6 mg/day), meropenem (3 g/day), remdesivir (200 mg/day), and heparin therapy; he was transferred to the intensive care unit, intubated, and mechanically ventilated on the 6th day. Dexamethasone was substituted for methylprednisolone (1000 mg/day for 3 days), followed by 70 mg/day for one week and subsequently 60 mg/day for one week, resulting in temporary improvement of hypoxemia and chest radiographic findings. Chest CT showed bilateral ground-glass attenuations and multiple nodules and consolidations on the 23rd day (Fig. 2 ). A large amount of fungus with septate hyphae and an acute angle branching suggestive of Aspergillus fumigatus (A. fumigatus) was detected in the Papanicolaou stain (Fig. 3 ) of the tracheal aspirate. A. fumigatus was cultured from the tracheal aspirate, and significant elevation in the serum level of (1, 3)-β-D-glucan (BDG) (632.4 pg/mL) was observed on the 24th day, which was under 11.0 pg/mL (within normal limit) on the 8th and 14th days.
Table 1.
Results of peripheral blood analysis on admission.
| <Blood cell counts> | <Blood chemistry> | <Serology> | ||||||
|---|---|---|---|---|---|---|---|---|
| WBC | 4600 | /μL | TP | 5.5 | g/dL | CRP | 3.4 | mg/dL |
| Neutrophils | 70.6 | % | Alb | 3.0 | g/dL | |||
| Lymphocytes | 20.2 | % | T-bil | 0.8 | mg/dL | <Coagulation> | ||
| Eosinophils | 1.5 | % | AST | 29 | IU/L | PT | 14.1 | sec |
| Monocytes | 7.5 | /μL | ALT | 24 | IU/L | PT-% | 73.8 | % |
| Basophils | 0.2 | g/dL | LDH | 200 | IU/L | INR | 1.15 | |
| RBC | 408 × 104 | /μL | BUN | 17 | mg/dL | APTT | 31.7 | sec |
| Hb | 13.4 | g/dL | Cre | 1.14 | mg/dL | FDP | 3.0 | μg/ml |
| Ht | 40.1 | % | Na | 137 | mEq/L | D-dimer | 0.7 | μg/ml |
| Platelets | 17.6 × 104 | /μL | K | 3.9 | mEq/L | Fibrinogen | 644 | ng/dl |
| Cl | 104 | mEq/L | ||||||
Abbreviations: WBC, white blood cell; RBC, red blood cell; Hb, hemoglobin; Ht, hematocrit; TP, total protein; Alb, albumin; T-bil, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; BUN, blood urea nitrogen; Cre, creatinine; CRP, c-reactive protein; PT, prothrombin time; PT-INR, prothrombin time-international normalized ratio; APTT, activated partial thromboplastin time; FDP, fibrin/fibrinogen degradation products.
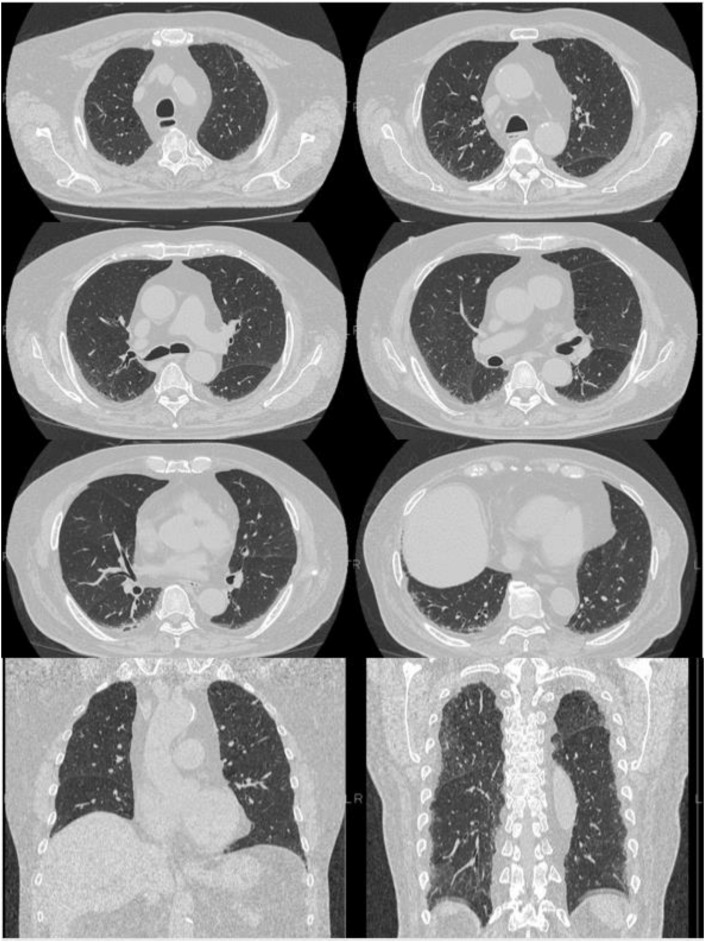
Fig. 1.
Chest CT on admission. Chest CT showing slight fibrosis bilaterally in the lung bases; there is no evidence of viral pneumonia, such as ground-glass attenuation. CT: computed tomography.
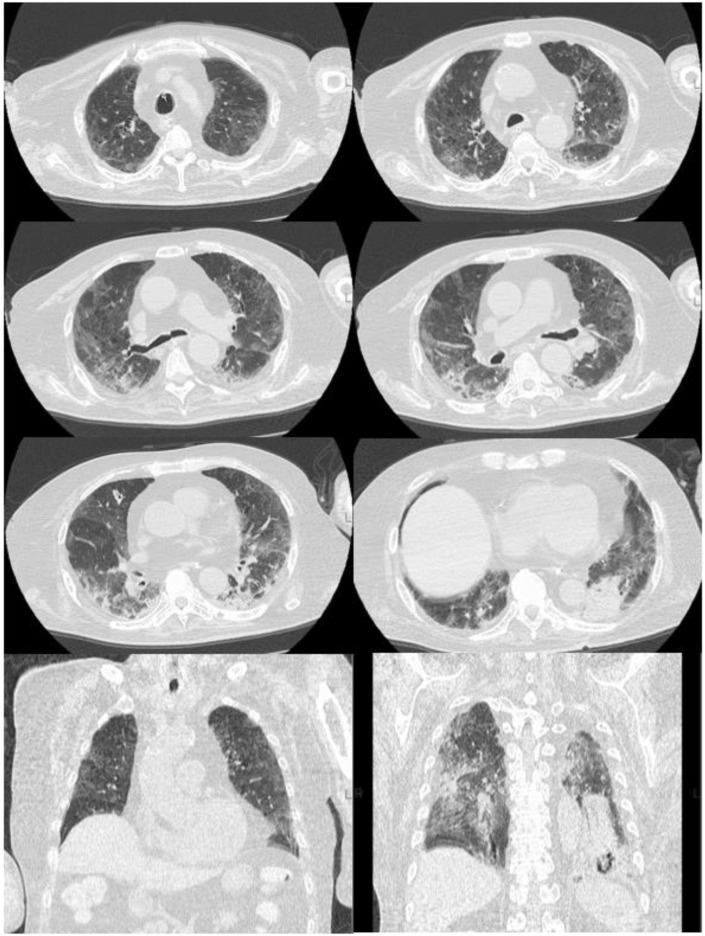
Fig. 2.
Chest CT on the 23rd day after admission. Chest CT showing bilateral ground-glass attenuations and an approximately 3-cm nodule in the left lower lung. CT: computed tomography.
Fig. 3.
Papanicolaou stain of the tracheal aspirate. A fungus similar to Aspergillus fumigatus was observed on the Papanicolaou stain of the tracheal aspirate ( × 100).
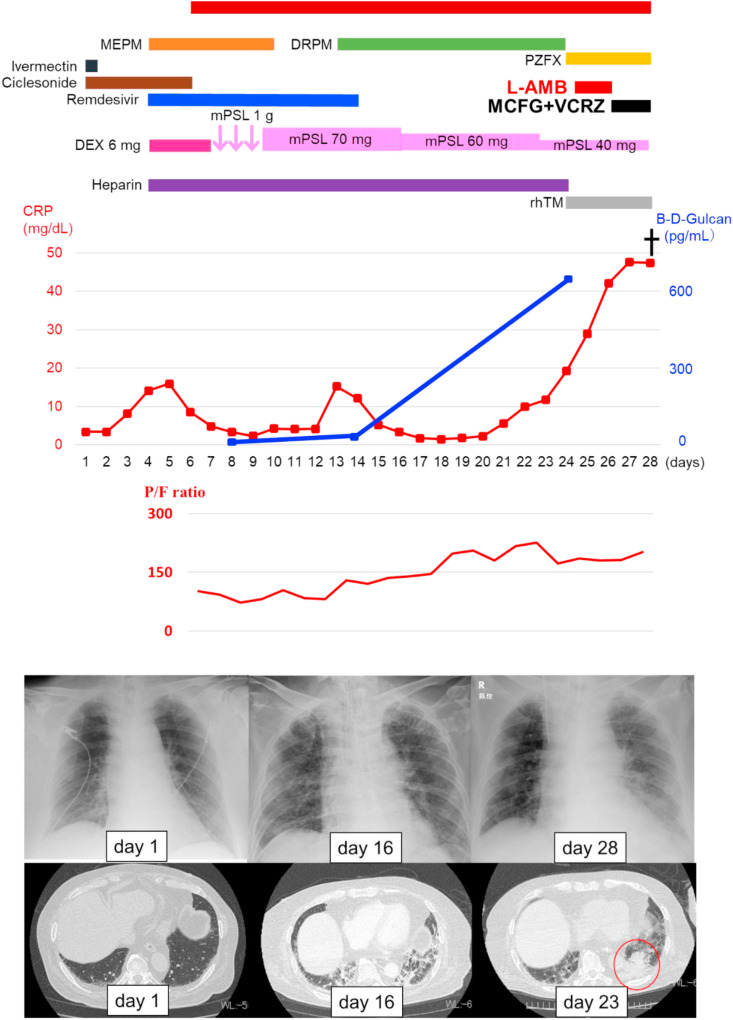
According to the proposed screening and diagnostic algorithm for CAPA [4], our patient was “CAPA highly likely” based on the following features: 1) positive Aspergillus spp. in tracheal aspirate culture, 2) deteriorating or persistent poor respiratory function with no other explanation, or progressive radiology, and 3) positive serum biomarker, BDG. Liposomal amphotericin B and recombinant human soluble thrombomodulin were initiated on the 24th day. Liposomal amphotericin B was administered for treating not only Aspergillosis but also other fungi such as Mucor spp. according to the Guidelines for Management of Deep-seated Mycoses 2014 (Japanese). However, rapid progression of bilateral pulmonary infiltrations; a nodular shadow in the lower left lobe on chest CT, which was suspected to be Aspergillus (Fig. 4 , red circle); and hypoxemia were observed, and the patient died of multiple organ failure, including respiratory and renal failure, on the 28th day.
Fig. 4.
Clinical course of the patient. MEPM: meropenem; DRPM: doripenem; PZFX: pazufloxacin; L-AMB: liposomal amphotericin B; DEX: dexamethasone; MCFG micafungin; VRC: Voriconazole; mPSL: methylprednisolone; rhTM: recombinant human soluble thrombomodulin.
3. Discussion
The patient’s initial clinical and radiological findings were not indicative of pulmonary Aspergillus infection. Limited diagnostic procedures, such as bronchoscopic examinations and measurements of galactomannan antigen and BDG, in Japan contribute to the difficulties in diagnosing CAPA, and CAPA may be underdiagnosed in Japan. In-hospital measurement of serum BDG was practicable in our hospital, and the concurrent identification of a large amount of Aspergillus-like fungus observed in tracheal aspirate cytology and increased level of serum BDG led to a diagnosis of CAPA in our patient. Transient improvement was observed with treatment for COVID-19 using mechanical ventilation in the first two weeks, but even though antifungal agents and comprehensive therapy were administered, he died owing to rapid worsening of his respiratory condition, most likely due to CAPA [4].
The European Organization for Research and Treatment of Cancer/Mycoses Study Group recently revised the definition of invasive pulmonary aspergillosis to apply to immunocompromised patients [5]; this is based on typical radiographic findings such as nodular lesions with or without halo signs and cavitation. Applying these criteria to patients with COVID-19 with ARDS and bilateral ground-glass opacity may be difficult, and there are no standard diagnostic algorithms and definitions of CAPA, which makes it even more challenging. The reported cases were diagnosed based on cultured Aspergillus species from bronchoalveolar lavage fluid (BALF) or tracheal aspirate and/or a positive galactomannan test or Aspergillus-specific lateral-flow device test of the BALF or tracheal aspirate [6]. Although galactomannan antigen detection and fungal culture using BALF are considered to be the most sensitive, these tests were rarely performed in patients with COVID-19 due to the infectious risk of aerosols [6]. To date, several dozens of patients with CAPA have been reported, and the incidence of CAPA was about 27.7% among critically ill patients with COVID-19 after a median of 4 days from intensive care unit admission, with 30-day mortality rate being 44% [7]. Most patients with CAPA did not have classical host risk factors of angioinvasive aspergillosis, such as organ transplant immunosuppression and neutrophilia, but a significant proportion (46%) received corticosteroids. On comparing the 46 patients who died of CAPA with the 39 who survived, it was reported that older age (70.6 vs. 63.2 years, p = 0.0098), presence of pulmonary comorbidities (28% vs. 10%, p = 0.056), and treatment without voriconazole (74% vs. 57%, p = 0.11) were associated with higher mortality [8].
According to the recently proposed screening and diagnostic algorithm for CAPA [4], CAPA should be suspected in patients with COVID-19 having the following two features: 1) microbiological: positive sputum/tracheal aspirate aspergillus culture and 2) clinical: deteriorating or persistent poor respiratory function with no other explanation, or progressive radiology. Patients should then be classified into four categories based on BALF findings (detection of Aspergillus spp. by antigen testing, culture, and PCR) and serum biomarkers (detection of Aspergillus by antigen, PCR, and BDG tests) as follows: “CAPA highly likely,” “CAPA likely,” “CAPA not excluded,” and “CAPA unlikely.” Chest CT of our patient showed rapid progression of bilateral pulmonary infiltrations without typical findings such as cavitary lesions, which was typical for non-neutropenic patients such as those with COVID-19 [9]. A. fumigatus was cultured from the BALF sample and serum BDG was positive; therefore, he was diagnosed with “CAPA highly likely” and treated with an anti-fungal agent.
Our patient was on betamethasone and prednisolone for polymyalgia rheumatica for two years, and he was treated with high-dose corticosteroids and broad-range antibiotics after admission, suggesting he was at high risk for developing invasive pulmonary aspergillosis, such as CAPA. Previous studies demonstrated that 29% of CAPA patients received systemic corticosteroids [6], and long-term steroid administration was frequently used to treat CAPA [7]. Our patient was treated with dexamethasone (6 mg/day), methylprednisolone (1000 mg/day for 3 days, followed by 70 mg/day for 1 week and subsequent dose of 60 mg/day for 1 week) after admission, these high doses of corticosteroid treatment may increase the risk of developing CAPA. In addition, disturbing gut microbiome and impaired host immunity and increased susceptibility to fungal infections caused by the use of antibiotics [6].
To date, there is no standard treatment for CAPA, and it is unknown whether antifungal treatments and their use at appropriate time intervals would improve prognosis. Our patient died even though we promptly initiated antifungal therapy after diagnosing CAPA. Perhaps an earlier diagnosis and treatment of CAPA might improve the prognosis. An increasing number of cases using corticosteroids for the treatment of COVID-19 is being seen, and clinicians should be aware of CAPA, especially while treating severe cases of COVID-19. Although our patient died despite receiving antifungal treatment, early diagnosis and proper treatment introduction were necessary for treating CAPA. Repetitive BDG measurement and fungal smear and culture of tracheal aspirate/sputum samples are required in patients with COVID-19 who are treated with moderate-to high-dose corticosteroids. When increased BDG levels and/or microscopic findings suggestive of Aspergillus spp. are observed, immediate administration of antifungal drugs may contribute to prognostic improvement in patients with CAPA.
In summary, COVID-19 is associated with several well-known complications including anosmia, venous thromboembolism, and ARDS. Clinicians should also be aware of the risk of CAPA, especially in patients with severe COVID-19 admitted to the intensive care unit, and aggressive and continuous testing for the presence of Aspergillus spp. through tracheal aspirate cultures and serum BDG testing should be considered.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of interest
None.
Authorship statement
All authors meet the ICMJE authorship criteria. Yuto Iwanaga wrote the draft manuscript. Kei Yamasaki supervised the writing of the manuscript and was responsible for the clinical data. Toshinori Kawanami, Hideki Sakakibara, Issei Ikusima, Hiroaki Ikegami, Masahiro Tahara, Kentaro Akata, and Hiroshi Mukae contributed to the critical review of the manuscript. Kazuhiro Yatera organized and contributed to the management of this case report. All authors contributed to the writing of the final manuscript.
Acknowledgements
None.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schauwvlieghe A., Rijnders B.J.A., Philips N., Verwijs R., Vanderbeke L., Van Tienen C., et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6(10):782–792. doi: 10.1016/s2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 3.Mohamed A., Rogers T.R., Talento A.F. COVID-19 associated invasive pulmonary aspergillosis: diagnostic and therapeutic challenges. J Fungi (Basel) 2020;6(3) doi: 10.3390/jof6030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong-James D., Youngs J., Bicanic T., Abdolrasouli A., Denning D.W., Johnson E., et al. Confronting and mitigating the risk of COVID-19 associated pulmonary aspergillosis. Eur Respir J. 2020;56(4) doi: 10.1183/13993003.02554-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnelly J.P., Chen S.C., Kauffman C.A., Steinbach W.J., Baddley J.W., Verweij P.E., et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the Mycoses study Group education and research consortium. Clin Infect Dis. 2020;71(6):1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arastehfar A., Carvalho A., van de Veerdonk F.L., Jenks J.D., Koehler P., Krause R., et al. COVID-19 associated pulmonary aspergillosis (CAPA)-From immunology to treatment. J Fungi (Basel). 2020;6(2) doi: 10.3390/jof6020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartoletti M., Pascale R., Cricca M., Rinaldi M., Maccaro A., Bussini L., et al. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apostolopoulou A., Esquer Garrigos Z., Vijayvargiya P., Lerner A.H., Farmakiotis D. Invasive pulmonary aspergillosis in patients with SARS-CoV-2 infection: a systematic review of the literature. Diagnostics. 2020;10(10) doi: 10.3390/diagnostics10100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenks J.D., Mehta S.R., Taplitz R., Aslam S., Reed S.L., Hoenigl M. Point-of-care diagnosis of invasive aspergillosis in non-neutropenic patients: Aspergillus Galactomannan Lateral Flow Assay versus Aspergillus-specific Lateral Flow Device test in bronchoalveolar lavage. Mycoses. 2019;62(3):230–236. doi: 10.1111/myc.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]