Abstract
Objectives:
To present the characteristics of neuropathic pain in individuals with chronic spinal cord injury (SCI).
Methods:
We recruited all individuals with chronic SCI referred to the Brain and Spine Injury Research center with a diagnosis of neuropathic pain from April 2013 to September 2015 into this historical cohort study.
Results:
Forty individuals with chronic SCI-induced neuropathic pain entered this study with a mean age of 43.67±13.12 years and a majority of who were male (n=30, 75%). Motor vehicle collision (n=25, 62.5%) and fall (n=7, 17.5%) were the most common causes of SCI in our participants. There were 13 (32.5%) cervical, twenty (50%) thoracic, and 7 (17.5%) lumbosacral SCI. The mean ‘maximal pain intensity’, ‘overall pain intensity during the past week’, and ‘the pain intensity at the initial consultation in pain clinic’ measured by numerical rating scale (NRS) in this cohort were 8.71±1.73, 6.32±1.60, and 6.11±2.48, respectively. Burning pain was the most frequently used description of pain reported by our participants. Pain intensity significantly decreased after six months of treatment for all three above categories.
Conclusion:
This study provides characteristics of neuropathic pain in a group of individuals with chronic SCI. Further large prospective studies are needed to determine the association between lesion level, completeness of injury, and region of pain.
Chronic pain is a frequent finding in at least 80% of individuals with chronic SCI, with one third of patients reporting it as severe pain.1 SCI-induced neuropathic pain is a debilitating syndrome frequently encountered after spinal trauma, which usually arises following diseases or injuries to the central nervous system.2 Pain can significantly affect the quality of life for individuals with chronic SCI in nearly 70% of the cases.3 Neuropathic pain is produced by an injury to the somatosensory system. It is usually described as burning, tingling, electric-shock, pins and needles, sharp, and squeezing pain. In contrast, nociceptive pain is produced by the activation of nerve endings or nociceptors in peripheral tissues, and is often described as a dull, crampy, or achy pain.4 However, distinction between neuropathic and nociceptive pain can be difficult to differentiate for patients with chronic SCI as they can experience different types of pain simultaneously.5 There are different pain classification systems of which the International SCI Pain (ISCIP) Classification is recommended for neuropathic pain according to the National Institute of Neurological Disorders and Stroke (NINDS).6 After identifying the type of pain, a numeric pain scale is used to define the intensity.6 Current pharmacological and surgical therapies can often become ineffective over time,7 and despite adequate medical treatment, two-thirds of individuals with chronic SCI do not achieve satisfactory pain relief.8 Therefore, SCI-induced neuropathic pain represents a major barrier for rehabilitation. Several studies have described pain prevalence and risk factors for individuals with chronic SCI but the underlying mechanism for neuropathic pain following SCI is poorly understood. Studying patients with chronic SCI and pain-related data could be helpful to better understand the characteristics of neuropathic pain. This study was designed to provide characteristics data for neuropathic pain in patients with chronic SCI using the International SCI Pain (ISCIP) Classification.
Methods
Ethics
This study was ethically approved by Sina Trauma and Surgery Research Center, Tehran University of Medical Sciences. The information of individuals with chronic SCI remained confidential and was used only for research purposes and the authors are obliged to the principles of Helsinki Declaration.
Study design
The inclusion criteria for this historical cohort study was all individuals with chronic SCI-induced neuropathic pain referred to the Brain and Spine Injury Research (BASIR) center, Neuroscience Institute of Tehran University of Medical Sciences (TUMS) between April 2013 and September 2015, who were followed for at least 6 months.
The SCI patients without specified neuropathic pain or those who were failed to be followed for at least 6 months were excluded from the study. The patients were examined by a fellowship-trained anesthesiologist in pain clinic and were asked to fill out a comprehensive form including demographic data (age, gender, marital status, years of education), SCI-related data (level, time, mechanism of injury), and pain-related data (severity and quality of pain, location of pain, relieving and debilitating factors, effect of pain on daily activities, frequency of accompanying symptoms, treatments). Utilizing a body diagram, participants were also asked to mark the locations where they felt the most pain. SCI were grouped according to the level of injury as upper cervical (C1-C4), lower cervical (C5-C8), upper thoracic (T1-T6), lower thoracic (T7-T12), and lumbosacral (L1-S4). We used the most current version of the International SCI Pain (ISCIP) Classification9 to present the patients responses into three tiers:
Tier 1: Pain type: neuropathic, nociceptive, other pain, unknown pain. Tier 2: Pain subtype: Nociceptive (musculoskeletal, visceral pain, etc.), Neuropathic (“at-level” vs. “below-level”, other neuropathic pain). Tier 3: Primary pain source and/or pathology: Renal calculus, nerve root compression, spinal cord trauma/ischemia, etc. The neurological level of the injury was determined based on the most caudal level with normal sensation (pinprick and light touch) or motor function. Musculoskeletal pain was defined based on the presence of the following criteria:
Change of pain intensity with movement, local tenderness, evidence of musculoskeletal pathology on imaging, and pain descriptors that support the presence of musculoskeletal pain. At-level neuropathic pain was referred to as neuropathic pain that was distributed along the dermatome of the neurological level of injury or within three dermatomes below the level of injury, unless the pain was attributed to cauda equina injury. The pain resulted from cauda equina compression was considered as at-level SCI neuropathic pain.
Below-level neuropathic pain was defined as neuropathic pain at more than three levels below the dermatome of the neurological level of injury.
Neuropathic pain was defined using the grading system introduced by Treede and colleagues.10 We evaluated the following four criteria for each participant:
1. Pain with a distinct neuroanatomically plausible distribution. 2. A history suggestive of a relevant lesion or disease affecting the peripheral or central somatosensory system 3. Demonstration of the distinct neuroanatomically plausible distribution by at least one confirmatory test 4. Demonstration of the relevant lesion or disease by at least one confirmatory test.
We considered a participant to have a definite neuropathic pain when all (1 to 4) criteria were met. Probable neuropathic pain was defined when criteria 1 and 2 were met, plus either 3 or 4; and possible neuropathic pain was considered when criteria 1 and 2 were met, without confirmatory evidence from 3 or 4.
We used the 11-point numerical rating scale (NRS) to measure the severity of pain, scoring from 0 to 10 for no pain to worst pain imaginable. Scales 1-3 refer to mild pain, 4-6 were moderate pain, and 7-10 refer to severe pain.11 We evaluated the pain intensity (measured by NRS) experienced by the participants using on 3 scales including ‘maximal pain intensity’, ‘overall pain intensity during the past week’, and ‘the pain intensity at the time of consultation’ at follow-up in the pain clinic and over the telephone.
A trained psychologist called the participants for a telephone follow-up in order to fill out a brief pain evaluation form based on the validated Persian version of the Brief Pain Inventory (BPI).12 Brief Pain Inventory asks about the ‘maximal pain intensity’, ‘overall pain intensity during the past week’, and ‘the pain intensity at the time of consultation’, in addition to questions about how pain interferes with the patient’s daily activities. Telephone follow-up at our center is performed at one, 3, 6, and 12 months and then every year thereafter. In order to report more homogenous data, we reported the 6-month post-initial consultation data for all participants.
An electronic search of PubMed literature for neuropathic pain in SCI was performed (1946 to 1 January 2017), in order to find prior related research using the following conditions: spinal cord injury (MeSH Terms) AND neuropathic pain (MeSH Terms).
Statistical analysis
All statistical analyses were performed using Statistical Package for the Social Science software version 18 (SPSS Inc, Chicago, Illinois, USA). Descriptive analysis was expressed as frequency, mean, and standard deviation (SD). Wilcoxon test was used to compare the mean difference of NRS pain scaling before and after treatment. P-values less than 0.05 were considered statistically significant.
Results
Patient-related data
Forty individuals with chronic SCI with a mean age of 43.67±13.12 years (ranging from 18 to 76 years old) were included in the study. Of the forty patients 75% (n=30) were male. The most common causes of SCI were motor vehicle collision (n=25, 62.5%) and falls (n=7, 17.5%). Detailed causes of SCI are further provided in Appendix 1.
There were 13 (32.5%) cervical (3 upper cervical, 10 lower cervical), 12 (50%) thoracic (4 upper thoracic, 16 lower thoracic), and 7 (17.5%) lumbosacral SCI. The mean time duration after injury was 5.65±7.89 years. Thirty-four participants (85%) had instrumented spinal fixation surgery after their injury (Appendix 1).
In terms of living status, 27 participants (67.5%) were married, 24 were living with their wife/husband, and 11 participants were living with their parents. Twenty-three participants used wheelchair, 4 used walker, and 9 were bedridden. With regards to other social factors, 5 participants (12.5%) were employed and the average number of years of education was 9. Seven participants had the history of admission to the hospital due to pain. Three participants were smokers with less than 1 pack/day and eight were former-smokers.
Pain-related data
The mean ‘maximal pain intensity’, ‘overall pain intensity during the past week’, and ‘the pain intensity at the initial consultation in pain clinic’ were 8.71±1.73, 6.32±1.60, and 6.11±2.48, respectively.
In terms of International SCI Pain (ISCIP) classification, 24 participants (60%) had only neuropathic pain in tier 1 while 16 participants (40%) had both neuropathic and nociceptive pains. In tier 2, 21 participants had below-level neuropathic pain (10 participants had both nociceptive musculoskeletal pain and below-level NP) and 19 participants had at-level neuropathic pain (6 participants had both nociceptive musculoskeletal pain and at-level NP). Finally, spinal cord compression (n=29), muscular pain (n=11) and cauda equina compression (n=7) were the most common types of ISCIP classification tier 3. Detailed ISCIP classification data is further provided in Appendix 1.
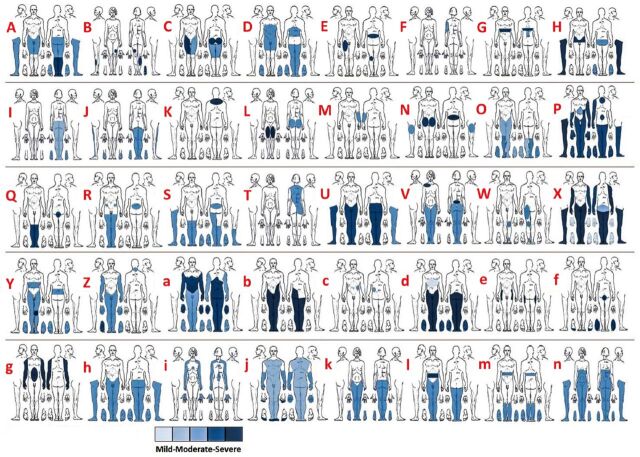
The pain diagrams demonstrating the distribution and severity of neuropathic pain for each individual are demonstrated in Figure 1. Table 1 also provides the distribution of neuropathic pain for the entire cohort. Buttocks, thighs, and legs were the most commonly reported areas of pain. Seven individuals with chronic SCI reported their pain to be most severe on the right side, 5 on the left side, 3 in the middle part, and 21 participants (52%) reported the severity of the pain to be the same on both sides of the body.
Figure 1.
Pain diagrams demonstrating the distribution and severity of neuropathic pain for each individual.
Table 1.
The anatomical distribution of pain throughout the body assessed in the initial consultation.
| Pain throughout the body | n |
|---|---|
| Head | 0 |
| Face | 0 |
| Mouth and throat | 0 |
| Shoulder | 10 |
| Arms | 9 |
| Elbows | 6 |
| Forearms | 7 |
| Wrists | 6 |
| Hands | 9 |
| Chest | 5 |
| Abdomen | 11 |
| Multiple joints | 7 |
| Groin | 10 |
| High back | 10 |
| Middle back | 10 |
| Low back | 11 |
| Buttock | 18 |
| Thighs | 15 |
| Knees | 14 |
| Legs | 15 |
| Ankles | 12 |
| Urogenital area | 5 |
| Bones | 6 |
| Different muscles | 5 |
Table 2 shows the frequency of each pain quality descriptors reported by the participants. Burning pain was the most frequently reported description. A total of 21 participants reported their pain to be continuous and nine reported it as intermittent. Cold, physical activity, and emotional stress were the most common exacerbating factors reported by participants, while warmth, resting, and massage were the most frequently reported alleviating factors (Table 3).
Table 2.
Frequency of pain quality descriptors evaluated in the first consultation.
| Pain quality descriptors | n |
|---|---|
| Burning | 30 |
| Pins & needles | 26 |
| Electric shock | 22 |
| numbness | 18 |
| Shooting | 16 |
| Stabbing | 16 |
| Pressure | 10 |
| Spontaneous pain | 16 |
| Throbbing (pulsate) | 13 |
| Allodynia | 9 |
| Sharp pain | 8 |
| Dysesthesia | 6 |
| Evoked pain | 5 |
| Hyperalgesia | 5 |
| Hyperesthesia | 5 |
| Hypoesthesia | 5 |
| Itching | 5 |
| Aching | 2 |
Table 3.
Frequency of exacerbating and alleviating factors for pain assessed in the initial consultation.
| Intensifying factors | n | Relieving factors | n |
|---|---|---|---|
| Cold | 20 | Warmth | 18 |
| Activity | 18 | Resting | 16 |
| Emotional stress | 17 | Massage | 15 |
| Coughing/sneezing | 9 | Mental relaxation | 10 |
| Bending forward | 9 | Activity | 3 |
| Bending backward | 5 | Travelling | 4 |
| Bending | 8 | Bending forward | 3 |
| Rotation the body | 5 | Cold | 1 |
| Sleeplessness | 8 | Bending backward | 0 |
| Warmth | 4 | Bending to the parties | 0 |
| Walking* | 2 | Rotation to the parties | 0 |
| Resting | 2 | Walking* | 0 |
| Oversleeping | 1 | ||
| Urination** | 2 | ||
| Defecation** | 2 | ||
| Sexual intercourse** | 4 |
Participants who could walk,
Related to pelvic pains
Twelve participants (30%) reported the worst pain occurred at nights and eleven (27.5%) reported their pain intensity had some diurnal fluctuation, but without a specific pattern.
Treatment
In terms of medical therapies, 18 individuals with chronic SCI used analgesics whenever they had pain, 12 used analgesics on a scheduled basis, and ten participants did not use analgesics at all. Table 4 shows the non-pharmacological treatments used by participants prior to their initial visit to the pain clinic. Gabapentin, amitriptyline, and capsaicin-lidocaine were the most commonly administered treatments in the pain clinic. Table 5 shows the distribution of different treatments administered for the participants. The more frequently used medications, i.e. amitriptyline, gabapentin, capsaicin and lidocaine (locally administered or IV infusion) were the most effective treatments that remained in the list of the medications during the long course of the treatment (Table 5).
Table 4.
Non-pharmacological treatments used by individuals with chronic SCI prior to their initial visit.
| Non-pharmacological treatments | n |
|---|---|
| Massage therapy | 10 |
| Exercising | 7 |
| Nerve block injections | 5 |
| TENS* | 5 |
| Acupuncture | 4 |
| Traction | 4 |
| Movement therapy | 3 |
| Thermotherapy | 2 |
| Psychotherapy | 2 |
| Hypnotism | 0 |
| Ultrasound | 0 |
| Biofeedback | 0 |
| Others | 6 |
Transcutaneous electric nerve stimulation
Table 5.
Treatments administered for the individuals with chronic SCI by the pain clinic evaluated after 6-month of treatment.
| Treatments | n |
|---|---|
| Amitriptyline | 25 |
| Gabapentin | 24 |
| Capsaicin+ Lidocaine | 23 |
| Lidocaine perfusion | 17 |
| Ketorolac | 16 |
| Baclofen | 16 |
| Venlafaxine | 11 |
| Ketamine perfusion | 8 |
| Pregabalin | 8 |
| Nitroglycerin ointment | 8 |
| Acetaminophen | 6 |
| TENS* | 5 |
| Duloxetine | 4 |
| Diclofenac sodium | 4 |
| Nortriptyline | 3 |
| All the other pharmacological treatments | 20 |
Transcutaneous electric nerve stimulation
Telephone follow-up
One patient expired and 10 were missed during the six-month follow-up period. On average, participants reported a 20.4%±20.8 relief in pain after using the treatments. The mean ‘maximal pain intensity’, ‘overall pain intensity during the past week’, and ‘the pain intensity at the time of telephone follow-up consultation’ were 7.65±1.73, 5.30±1.79 and 4.59±2.46, respectively. Pain intensity significantly decreased for ‘maximal pain intensity’, ‘overall pain intensity during the past week’, and ‘the pain intensity at the time of consultation’ after six months of treatment (Table 6).
Table 6.
Mean difference of ‘maximal pain intensity’, ‘overall pain intensity during the past week’ and ‘the pain intensity at the time of the initial consultation’ in pain clinic and after 6 months of treatment (measured by NRS) in the individuals with chronic SCI.
| Variables | Initial consultation | After 6-month of follow-up | Mean difference | P-value |
|---|---|---|---|---|
| Maximal pain intensity | 8.71±1.73 | 7.65±1.73 | -1.06 | 0.01 |
| Overall pain intensity during the past week | 6.32±1.60 | 5.30±1.79 | -1.02 | 0.01 |
| The pain intensity at the time of the consultation | 6.11±2.48 | 4.59±2.46 | -1.52 | 0.01 |
Discussion
In our study, more than half of the individuals with chronic SCI had only neuropathic pain while the rest had both neuropathic and nociceptive pains. Below-level and at-level pain were detected almost equally in our participants. Buttocks, thighs, and legs were the most commonly reported areas of pain in the body, and burning pain was the most frequently reported description of pain.
There was an improvement in all three pain scales after 6 months of treatment. Mean ‘maximal pain intensity’, ‘overall pain intensity during the past week’, and ‘the pain intensity at the time of the consultation’ were all categorized as severe at the initial visit (8.71, 6.32, and 6.11, respectively). At the six-month follow-up, their pain was severe (7.65), moderate (5.30), and moderate (4.59) for each respective category. Failed medical therapy has been reported as a common outcome for individuals with chronic SCI-induced neuropathic pain and perhaps learning to live with the pain seems to facilitate the adjustment and acceptance of the pain.13 However, several recent studies have identified promising therapies to treat chronic neuropathic pain, including botulinum toxin A and neurostimulation therapies.14,15
In our study, the lower limbs (buttock, thighs, knees, legs) were the most common areas of pain. In a descriptive study by Nakipoglu-Yuzer et al16 including 69 individuals with chronic traumatic SCI in Turkey, pain was reported in the hip and leg areas in 52.2% of their participants. Burning pain was the most common descriptor used in our study, followed by pins and needles, electric shock, and numbness. Meanwhile, pain was described as burning, aching, sharp, and stinging in the aforementioned study by Nakipoglu-Yuzer et al16 They also reported a significant association between level of injury and region of pain. However, we decided not to run a correlation analysis due to the small sample size. Nakipoglu-Yuzer et al16 did not find a significant association between demographic and SCI-related data with pain characteristics. Teixeira et al17 performed a retrospective study reported the clinical characteristics of 213 individuals with chronic SCI. However, they did not find any statistically significant association between incomplete SCI and more severe pain. In the study by Nakipoglu-Yuzer et al16 neuropathic pain localization was ‘below level’ in nearly all (97%) of the participants.In contrast to the finding of Nakipoglu-Yuzer et al,16 we showed a similar distribution for at-level and below-level SCI neuropathic pain. Werhagen et al18 retrospectively identified 402 individuals with chronic SCI in Sweden and also found no association between gender, level of SCI, or completeness of injury with the development of ‘at-level’ and ‘below-level’ neuropathic pain, except for ‘below-level’ pain which was associated with a complete injury. In a retrospective chart review and cross-sectional survey by Mann et al.19 sleep disturbance/insomnia (28.2%) was the most frequently reported comorbidities followed by depressive symptoms (25.2%) and anxiety (23.3%).
Determining the prevalence and characteristics of neuropathic pain and investigating its characteristics in individuals with chronic SCI may contribute to a better understanding to the underlying mechanism as to why some patients are refractory to certain treatments. It can also improve our understanding of the different characteristics of individuals with neuropathic pain and potentially improve the management of SCI-induced neuropathic pain.
Limitations of the study
Our center is in the public sector of the health care organizations; whereas, there is an active private sector in the country. Moreover, the center is a university and teaching hospital. Therefore, our patients do not represent the whole population and consequently our findings should be cautiously attributed to the all SCI patients. Due to serious physical disabilities, loss to follow-up is a significant limitation for any study evaluating patients with chronic SCI. Also, the small sample size limited the statistical analyses performed and a larger sample size would be required for correlation analyses.
Conclusion
This study provides demographic and pain-related data in a series of individuals with chronic SCI suffering from neuropathic pain. Our findings provide a better understanding of pain characteristics. Further studies with larger sample sizes are needed to determine the predictors of the development of the neuropathic pain in SCI patients and also the relationship between different variables such as SCI level, completeness of injury, and the region of pain.
Appendix
Appendix 1.
Characteristics of individuals with spinal cord injury referring to our clinic based on International Spinal Cord Injury Pain (ISCIP) Classification.
| No. | Age | Gender | Cause of SCI | Level of SCI | Time duration from SCI | Spine fixation surgery | ISCIP classification Tier 1 | ISCIP classification Tier 2 | ISCIP classification Tier 3 | Pain diagram |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | Male | Fall | Thoracic (T4) | 6 yrs | + | Neuropathic pain | Below level SCI neuropathic pain | Spinal cord compression | Figure1-A |
| 2 | 19 | Female | MVC | Lumbar (L1) | 3 yrs | + | Neuropathic pain | At level SCI neuropathic pain | Cauda equina compression | Figure1-B |
| 3 | 54 | Male | Fall | Thoracic (T12) | 5 yrs | + | Nociceptive pain Neuropathic pain | Musculoskeletal pain At level SCI neuropathic pain | Muscular pain Spinal cord compression | Figure1-C |
| 4 | 32 | Male | MVC | Thoracic (T6-T7) | 2 yrs | + | Neuropathic pain | Below level SCI neuropathic pain | Spinal cord compression | Figure1-D |
| 5 | 44 | Male | MVC | Cervical (C5-C6) | 3 yrs | Neuropathic pain | Below level SCI neuropathic pain | Spinal cord compression | Figure1-E | |
| 6 | 51 | Female | MVC | Cervical (C6) | 1.5 yrs | + | Neuropathic pain | At level SCI neuropathic pain | Nerve root compression | Figure1-F |
| 7 | 31 | Male | MVC | Thoracic (T4-T5) | 1 yr | + | Neuropathic pain | At level SCI neuropathic pain | Spinal cord compression | Figure1-G |
| 8 | 45 | Male | Trauma (Heavy object falling on back) | Thoracic (T12) | 3.5 yrs | + | Neuropathic pain | At level SCI neuropathic pain | Cauda equina compression | Figure1-H |
| 9 | 45 | Female | MVC | Thoracic (T8) | 2 yrs | + | Nociceptive pain Neuropathic pain | Musculoskeletal pain Below level SCI neuropathic pain | Glenohumeral arthritis, lateral epicondylitis Spinal cord compression | Figure1-I |
| 10 | 36 | Female | MVC | Thoracic (T5-T6) | 14 mo | Nociceptive pain Neuropathic pain | Musculoskeletal pain Below level SCI neuropathic pain associated with autonomic features (sweating) | Muscular pain Spinal cord compression | Figure1-J | |
| 11 | 46 | Male | MVC | Cervical (C6-C7) | 2 yrs | + | Neuropathic pain | At level SCI neuropathic pain | Spinal cord compression | Figure1-K |
| 12 | 37 | Female | MVC | Lumbar (L1-L2) | 14 yrs | + | Neuropathic pain | At level SCI neuropathic pain | Spinal cord compression | Figure1-L |
| 13 | 64 | Male | MVC | Cervical (C5-C6) | 42 yrs | Neuropathic pain | At level SCI neuropathic pain | Spinal cord compression | Figure1-M | |
| 14 | 64 | Male | Fall | Lumbar (L1-L2) | 2.5 yrs | + | Neuropathic pain | At level SCI neuropathic pain | Cauda equina compression | Figure1-N |
| 15 | 52 | Male | MVC | Thoracic (T12) | 3 yrs | + | Nociceptive pain Neuropathic pain | Other nociceptive pain Musculoskeletal pain At level SCI neuropathic pain | Surgical skin incision Muscular pain and spasm Cauda equina compression | Figure1-O |
| 16 | 31 | Male | MVC | Cervical (C5-C6-C7) | 4 yrs | + | Nociceptive pain Neuropathic pain | Musculoskeletal pain Below level SCI neuropathic pain | Muscular pain and spasm lumbar facet syndrome Spinal cord compression | Figure1-P |
| 17 | 76 | Male | Spine dissection due to sudden bending | Lumbar (L4-L5) | 8 yrs | Neuropathic pain | Below level SCI neuropathic pain | Spinal cord compression | Figure1-Q | |
| 18 | 27 | Male | MVC | Thoracic (T11-T12) | 1 yr | + | Neuropathic pain | Below level SCI neuropathic pain | Spinal cord compression | Figure1-R |
| 19 | 28 | Male | MVC | Thoracic (T10-T11) | 10 yrs | + | Nociceptive pain Neuropathic pain | Musculoskeletal pain At level SCI neuropathic pain (cauda equina syndrome) | Muscular pain Cauda equina compression | Figure1-S |
| 20 | 48 | Female | MVC | Cervical (C5-C6) | 1 yr | + | Neuropathic pain | Below level SCI neuropathic pain | Spinal cord compression | Figure1-T |
| 21 | 50 | Male | MVC | Thoracic (T11) | 4 yrs | + | Nociceptive pain Neuropathic pain | Musculoskeletal pain Below level SCI neuropathic pain | Lumbar facet syndrome Muscular pain and spasm Spinal cord compression | Figure1-U |
| 22 | 33 | Female | MVC | Cervical (C3-C4) | 2 yrs | + | Nociceptive pain Neuropathic pain | Musculoskeletal pain Below level SCI neuropathic pain | Lumbar facet syndrome Glenohumeral arthritis Spinal cord compression | Figure1-V |
| 23 | 53 | Male | MVC | Lumbar (L1) | 2 yrs | + | Nociceptive pain Neuropathic pain | Musculoskeletal pain At level SCI neuropathic pain | Ankle joint arthritis Spinal cord compression | Figure1-W |
| 24 | 54 | Male | Fall | Cervical (C4-C5) | 8 mo | + | Nociceptive pain Neuropathic pain | Musculoskeletal pain Below level SCI neuropathic pain | Muscular pain Spinal cord compression | Figure1-X |
| 25 | 51 | Male | Trauma (Heavy object falling on back) | Thoracic (T12) | 10 yrs | + | Nociceptive pain Neuropathic pain | Musculoskeletal pain At level SCI neuropathic pain | Muscular pain and spasm Spinal cord compression | Figure1-Y |
| 26 | 31 | Male | Fall | Cervical (C6) | 4 yrs | + | Neuropathic pain | Below level SCI neuropathic pain | Spinal cord compression | Figure1-Z |
| 27 | 34 | Male | Trauma (gymnastic) | Cervical (C5-C6) | 10 yrs | + | Neuropathic pain | Below level SCI neuropathic pain | Spinal cord compression | Figure1-a |
| 28 | 39 | Male | Fall | Thoracic (T10-T11) | 3 yrs | + | Nociceptive pain Neuropathic pain | Musculoskeletal pain Below level SCI neuropathic pain | Quadrates lumborum muscle spasm Spinal cord compression | Figure1-b |
| 29 | 37 | Male | T8 surgery screw malposition | Thoracic (T8) | 15 mo | + | Neuropathic pain | At level SCI neuropathic pain | Nerve root compression | Figure1-c |
| 30 | 29 | Male | MVC | Thoracic (T5-T6) | 3 yrs | + | Nociceptive pain Neuropathic pain | Musculoskeletal pain Below level SCI neuropathic pain | Muscular pain Spinal cord compression | Figure1-d |
| 31 | 31 | Male | MVC | Lumbar (L1) | 12 yrs | + | Neuropathic pain | At level SCI neuropathic pain | Cauda equina compression | Figure1-e |
| 32 | 60 | Male | Trauma (slipping) | Thoracic (T12) and L1 | 7 yrs | + | Neuropathic pain | At level SCI neuropathic pain | Cauda equina compression | Figure1-f |
| 33 | 57 | Male | MVC | Cervical (C6-C7) | 15 mo | + | Nociceptive pain Neuropathic pain | Visceral pain At level SCI neuropathic pain | Bowel impaction Spinal cord compression | Figure1-g |
| 34 | 59 | Male | MVC | Thoracic (T12) | 30 yrs | + | Neuropathic pain | Below level SCI neuropathic pain | Spinal cord compression | Figure1-h |
| 35 | 42 | Female | MVC | Cervical (C5-C6) | 6 yrs | + | Neuropathic pain | At level SCI neuropathic pain | Spinal cord compression | Figure1-i |
| 36 | 58 | Male | MVC | Cervical (C4) | 3 yrs | + | Neuropathic pain | Below level SCI neuropathic pain | Spinal cord compression | Figure1-j |
| 37 | 18 | Female | Complicated LP | Lumbar (L2) | 5 yrs | Nociceptive pain Neuropathic pain | Musculoskeletal pain At level SCI neuropathic pain (cauda equina syndrome) | Muscular spasm and pain Cauda equina injury | Figure1-k | |
| 38 | 44 | Male | Fall | Thoracic (T10-T11) | 2.5 yrs | + | Neuropathic pain | Below level SCI neuropathic pain | Spinal cord compression | Figure1-l |
| 39 | 57 | Male | Angiography of thoracic arteries | Thoracic (T7-T8) | 3 mo | Nociceptive pain Neuropathic pain | Musculoskeletal pain Below level SCI neuropathic pain | Muscular pain Spinal cord ischemia | Figure1-m | |
| 40 | 35 | Female | MVC | Thoracic (T6) | 3 yrs | + | Nociceptive pain Neuropathic pain | Musculoskeletal pain Below level SCI neuropathic pain | Thoracic facet syndrome Spinal cord compression | Figure1-n |
MVC - Motor Vehicle Crash, SCI - Spinal Cord Injury, ISCIP - International Spinal Cord Injury Pain, + - Spine fixation surgery was carried out
Footnotes
References
- 1.Cruz-Almeida Y, Martinez-Arizala A, Widerstrom-Noga EG. Chronicity of pain associated with spinal cord injury:A longitudinal analysis. J Rehabil Res Dev. 2005;42:585–594. doi: 10.1682/jrrd.2005.02.0045. [DOI] [PubMed] [Google Scholar]
- 2.Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, et al. Advances in neuropathic pain:diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524–34. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 3.Yezierski RP. Spinal cord injury:a model of central neuropathic pain. Neuro-Signals. 2005;14:182–193. doi: 10.1159/000087657. [DOI] [PubMed] [Google Scholar]
- 4.Bryce TN, Biering-Sorensen F, Finnerup NB, Cardenas DD, Defrin R, Lundeberg T, et al. International spinal cord injury pain classification:part I Background and description. Spinal cord. 2012;50:413–417. doi: 10.1038/sc.2011.156. [DOI] [PubMed] [Google Scholar]
- 5.Widerstrom-Noga E, Biering-Sorensen F, Bryce T, Cardenas DD, Finnerup NB, Jensen MP, et al. The international spinal cord injury pain basic data set. Spinal cord. 2008;46:818–823. doi: 10.1038/sc.2008.64. [DOI] [PubMed] [Google Scholar]
- 6.Biering-Sorensen F, Alai S, Anderson K, Charlifue S, Chen Y, DeVivo M, et al. Common data elements for spinal cord injury clinical research:a National Institute for Neurological Disorders and Stroke project. Spinal cord. 2015;53:265–277. doi: 10.1038/sc.2014.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hulsebosch CE. From discovery to clinical trials:treatment strategies for central neuropathic pain after spinal cord injury. Curr Pharm Des. 2005;11:1411–120. doi: 10.2174/1381612053507864. [DOI] [PubMed] [Google Scholar]
- 8.Wrigley PJ, Gustin SM, McIndoe LN, Chakiath RJ, Henderson LA, Siddall PJ. Longstanding neuropathic pain after spinal cord injury is refractory to transcranial direct current stimulation:a randomized controlled trial. Pain. 2013;154:2178–2184. doi: 10.1016/j.pain.2013.06.045. [DOI] [PubMed] [Google Scholar]
- 9.Bryce TN, Biering-Sorensen F, Finnerup NB, Cardenas DD, Defrin R, Ivan E, et al. International Spinal Cord Injury Pain (ISCIP) Classification:Part 2 Initial validation using vignettes. Spinal cord. 2012;50:404–412. doi: 10.1038/sc.2012.2. [DOI] [PubMed] [Google Scholar]
- 10.Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain:redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes AM, De Campos C, Batalha L, Perdigão A, Jacob E. Pain assessment using the adolescent pediatric pain tool:a systematic review. Pain Res Manag. 2014;19:212–218. doi: 10.1155/2014/979416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majedi H, Dehghani SS, Soleyman-Jahi S, Emami Meibodi SA, Mireskandari SM, Hajiaghababaei M, et al. Validation of the Persian Version of the Brief Pain Inventory (BPI-P) in Chronic Pain Patients. J Pain Symptom Manage. 2017;54:132–138. doi: 10.1016/j.jpainsymman.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Henwood P, Ellis JA. Chronic neuropathic pain in spinal cord injury:the patient's perspective. Pain Res Manag. 2004;9:39–45. doi: 10.1155/2004/863062. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Lv CA, Tian L, Jin LJ, Sun P, Zhao W. A randomized controlled trial of botulinum toxin A for treating neuropathic pain in patients with spinal cord injury. Medicine (Baltimore) 2017;96:6919. doi: 10.1097/MD.0000000000006919. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Nardone R, Höller Y, Leis S, Höller P, Thon N, Thomschewski A, et al. Invasive and non-invasive brain stimulation for treatment of neuropathic pain in patients with spinal cord injury:a review. J Spinal Cord Med. 2014;37:19–31. doi: 10.1179/2045772313Y.0000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakipoglu-Yuzer GF, Atci N, Ozgirgin N. Neuropathic pain in spinal cord injury. Pain physician. 2013;16:259–264. [PubMed] [Google Scholar]
- 17.Teixeira MJ, Paiva WS, Assis MS, Fonoff ET, Bor-Seng-Shu E, Cecon AD. Neuropathic pain in patients with spinal cord injury:report of 213 patients. Arq Neuropsiquiatr. 2013;71:600–603. doi: 10.1590/0004-282X20130103. [DOI] [PubMed] [Google Scholar]
- 18.Werhagen L, Budh CN, Hultling C, Molander C. Neuropathic pain after traumatic spinal cord injury--relations to gender, spinal level, completeness, and age at the time of injury. Spinal cord. 2004;42:665–673. doi: 10.1038/sj.sc.3101641. [DOI] [PubMed] [Google Scholar]
- 19.Mann R, Schaefer C, Sadosky A, Bergstrom F, Baik R, Parsons B, et al. Burden of spinal cord injury-related neuropathic pain in the United States:retrospective chart review and cross-sectional survey. Spinal cord. 2013;51:564–570. doi: 10.1038/sc.2013.34. [DOI] [PubMed] [Google Scholar]