Abstract
Angiocentric glioma is a rare brain tumor commonly found in frontal or temporal lobes. It has a benign course, and surgical resection can be curative. Brainstem location is extremely rare, with only six cases reported so far in the literature. In the present study, the seventh case of brainstem angiocentric glioma has been reported, and its course in comparison with supratentorial location and the role of molecular diagnosis has been discussed.
Angiocentric glioma is a rare brain tumor, which was initially reported in 2005.1 It is classified as a grade I distinct clinicopathological entity by WHO CNS-2016 classification.2 Less than 100 cases have been reported in literature, with common locations being in the cerebral cortex of frontal or temporal/ lobe or hippocampus.1 In these series, patients are typically presented with seizures at 14-17 years of age. Brainstem location is extremely rare, with only six cases ever reported in the literature studies.3–6 Here, we are reporting a rare case of brainstem angiocentric glioma with myxoid features with 7 years follow up.
Case Report
Patient information
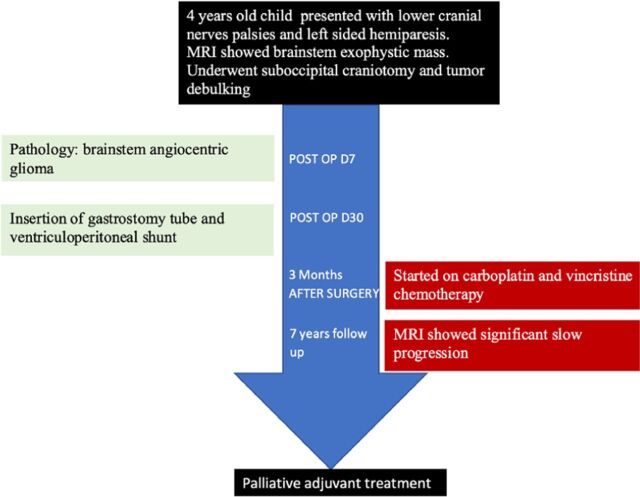
A 4-year-old female came to our institute with complains of headache and vomiting, which started 4 months prior to her presentation (Figure 1). She reported repeated episodes of coughing while drinking water along with change of voice and progressive difficulty in walking.
Figure 1.
Timeline showing the course of the patient during follow up and outcome.
Clinical findings
Physical Examination showed GCS of 15/15. Cranial nerves examination showed left 6th and 7th cranial nerves palsies, weak cough and absent gag reflex on left side. Her power was 4/5 on left side and 5/5 on the right. Other general and neurological examination were unremarkable.
Diagnostic assessment
Magnetic resonance imaging (MRI) of the brain showed left dorsal large exophytic brainstem and left middle cerebellar peduncle lesion causing mild degree of obstructing hydrocephalus. The lesion was heterogeneously hyperintense on T2, and none enhancing with IV gadolinium contrast injection on T1 images (Figure 2 a&b). It also showed asymmetric diffusion restriction on diffusion weighted images (DWI).
Figure 2.
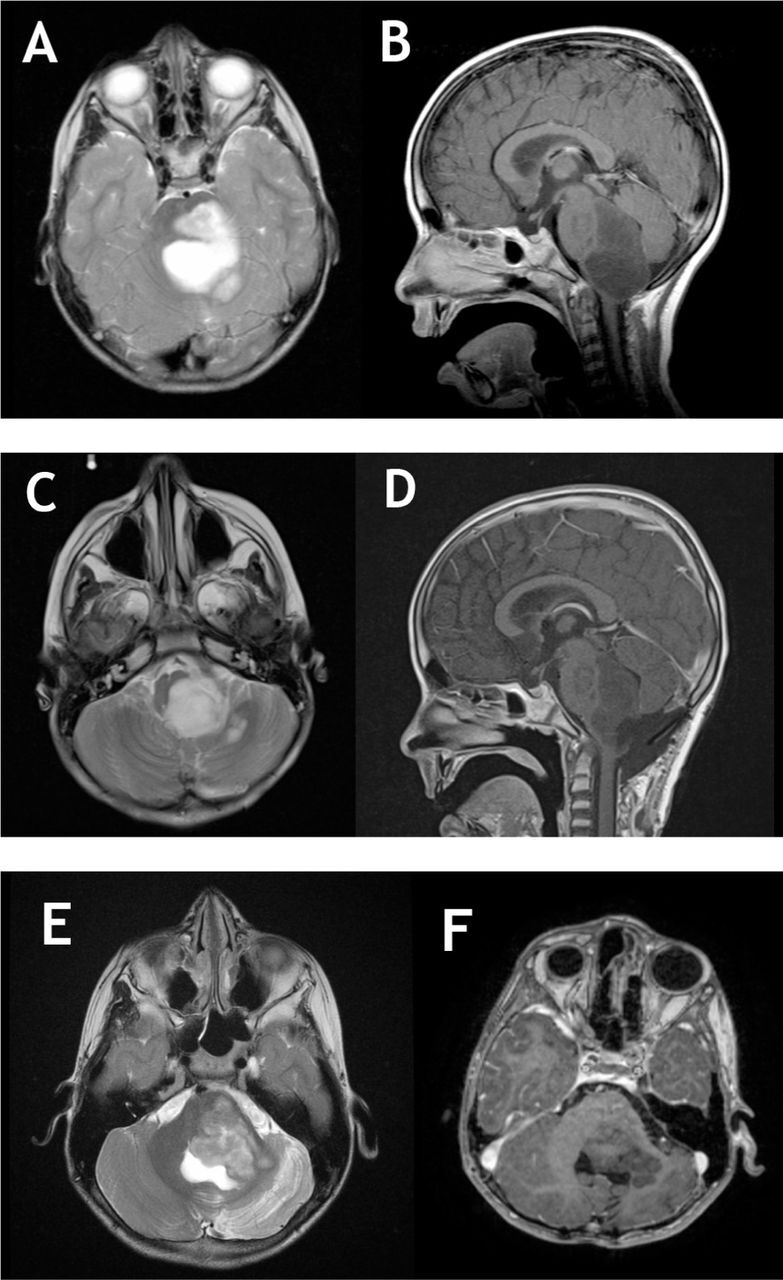
Pre and post-operative MRI images of brainstem angiocentric glioma. a,b) T2 and T1 with gadolinium MRI showing a large brainstem none enhancing lesion with dorsal exophytic component compression the cerebellum, notice the significant compression on medulla. c,d) Postoperative images showing a large residual left intentionally on T2 and T1C+ images. e,f) After 7 years of follow up. Notice the progression of the lesion with further compression on cerebellum. No enhancement noticed or signs of transformation into a higher grade. The tumor is filling the entrapped CSF area on T2 weighted images.
Therapeutic intervention
Suboccipital craniotomy using telovelar approach for extended biopsy of the exophytic portion under neurophysiology monitoring was performed. Debulking of the lesion was continued until change in motor evoked potentials (MEPs) signals was seen bilaterally, which halted the resection. There was a clear plane of cleavage between the tumor and neural tissue.
Pathological examination showed a glial neoplasm composed of bipolar spindled cells with alternating relatively compact and myxoid patterns. Intraoperative smears represented the myxoid component and showed cytological morphology identical to pilocytic astrocytoma (Figure 3a). Histologically, the cells were diffusely infiltrative of CNS parenchyma with prominent parallel arrangement around the blood vessels (Figure 3b). Despite the cellular resemblance to pilocytic astrocytoma, there were no Rosenthal fibers or eosinophilic granular bodies. In some areas, these arrangements resembled perivascular ependymal pseudorosettes. In the myxoid component, the cells resembled pilomyxoid astrocytoma (Figure 3c). The cells strongly and diffusely expressed glial fibrillary acidic protein (GFAP) and D2-40 (Figures 3d & e). Epithelial membrane antigen (EMA) moderately stained the tumor cells with strong paranuclear staining in some of the angiocentric cells (Figure 3f). Blood whole genome sequencing showed no pathogenic or variants in genes related to the disease.
Figure 3.
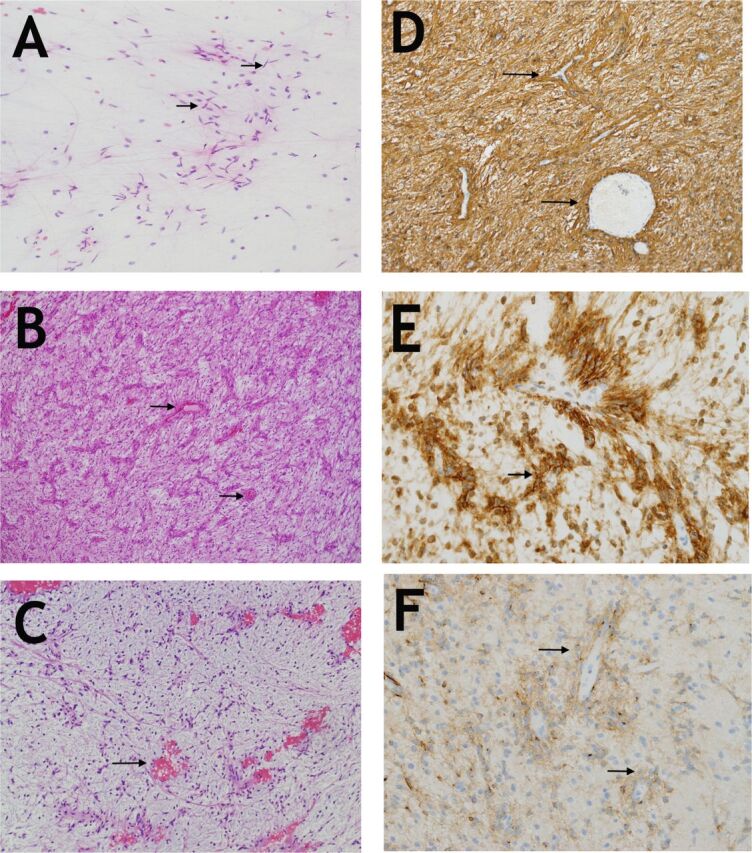
Histopathological examination of the lesion a) Smear preparation showing bipolar cells (arrows) with long processes reminiscent of pilocytic astrocytoma (Hematoxylin and Eosin, x200). b) A low magnification image showing the neoplastic bipolar cells with their distinct angiocentric arrangement which is mostly parallel in this tumor (arrows). (Hematoxylin and Eosin, x100). c) The myxoid component is very hypocellular but retains the angiocentric pattern(arrows). (Hematoxylin and Eosin, x100). d) GFAP is diffusely expressed in tumor cells (arrows) and particularly highlights the angiocentric pattern (GFAP, polyclonal, Ventana, x200). e) D2-40 is strongly expressed (arrows), (clone D2-4, DakoCytomation, x400). f) EMA showing moderate staining of cells and their processes. Note the perinuclear accentuation of expression in some cells (arrows), (EMA, clone E29, Dako x400).
Follow-up and outcomes
Postoperative MRI showed huge residual cells (Figure 2c & d). The patient needed gastrostomy tube due to lower cranial nerves palsy. She also needed ventriculoperitoneal shunt due to postoperative hydrocephalus. She received adjuvant chemotherapy consisting of carboplatin and vincristine due to presence of a residual. The patient continued follow up in clinic with annual brain imaging with stable residual. After 7 years of follow up, MRI showed significant progression of the mass (Figure 2 e& f). The patient eventually was referred for palliative treatment.
Discussion
Angiocentric glioma has been considered under sub classification “other glioma” along with choroid glioma of third ventricle and remained unchanged in the recent 2016 WHO classification.2 The most common presentation is seizures in young adults, with mean onset at the age of 17 years. Due to it’s epileptogencity, it has been considered under “long term epilepsy associated tumors” (LEASTs) group, along with other lesions.7 The AG is commonly located in the cortex of frontotemporal region or the hippocampus.1 The tumor has excellent prognosis and indolent course when located supratentorially. Gross total resection was found to be curative that resulted in seizure-free status.1,8
The AG typically appears as a low signal on MRI T1 weighted images and high signal on T2 weighted images/FLAIR sequences which can extend to the ventricle. It typically does not enhance with gadolinium on T1WI. It may have a high signal cortical rim on T1WI, which is pathogonomic for angiocentric glioma.8 Unlike our case, the lesion typically does not restrict on DWI/ADC. Morphologically, brainstem AG is composed of bipolar cells with overlapping feature with pilocytic astrocytoma and pilomyxoid astrocytoma. It is distinguished by its angiocentricity and expression of EMA and D2-40.
The genetic alteration in AG was not studied extensively as with other low grade gliomas. Qaddoumi examined 15 cases of AG; 13 of them harbored MYB-QKI fusion (87%).9 This was also mentioned by Bandopadhayay et al in which MYB-QKI fusion was tightly associated with AG histology and it was found in 6/7 of AGs, but was absent in other gliomas in same series, proposing that this genetic alteration can be diagnostic for AG.10
The AG is extremely rare in the brainstem, with only six cases reported in literature,3–6 as summarized in Table 1. Average age was five years. All patients were presented with cranial nerve palsies. Hydrocephalus was present in 4/7 cases, managed by endoscopic third ventriculostomies (ETV) or ventriculoperitoneal shunts. All cases had exophtic component, which was the main indication for craniotomy and debulking in only 3 cases. Progression was noted in the selected cases who underwent sterotactic biopsy. Our case also showed progression after 7 years of follow up, however, debulking was minimal and there was huge residual left due to drop in MEP signals intraoperatively. In this review, it has been shown that the extent of resection correlates with the prognosis of the patient. Due its eloquent location, safe gross total or near total resection is not possible, and chemotherapy was used in 4 out of 6 cases who had incomplete resection or biopsy, which failed to halt its progression. Angiocentric glioma of brainstem have worse prognosis than cortical location, and similar course to pilocytic astrocytoma of brainstem.11
Table 1.
Summary of published cases of brainstem angiocentric gliomas. M: male, F:female, CN: cranial nerve, ETV: endoscopic third ventriculostomy, NTR: near total resection, VPS: ventriculoperitoneal shunt.
| No. | Author (year) | Age (gender) | Location | Presentation/exam | Surgery | Chemotherapy | Follow up | Outcome | MYB-QKI fusion |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Covington et al (2009) | 5 years (F) | Right exophytic tectal lesion | Unsteady gait, 4th and 7th CN palsies, hydrocephalus | ETV, craniotomy and debulking | No | 2 years | Stable lesion | Yes |
| 2 | Weaver et al (2017) | 5 years (F) | Midline midbrain tegmentam | Diplopia, 6th CN nerve palsy, hydrocephalus | ETV, craniotomy and NTR | NO | 6 years | Stable after resection | No data |
| 3 | Weaver et al (2017) | 6 years (M) | Right pontine exophytic lesion | Hemiparesis, facial palsy | Stereotactic biopsy and ETV | No | 1.5 years | Stable lesion | No data |
| 4 | D’Aronco et al (2017) | 7 years (M) | Pontomedullary exophytic lesion | respiratory failure, repated pneumonia | Stereotactic biopsy | Yes (carboplatin/vincristine) | 10 months | Progression | Yes |
| 5 | D’Aronco et al (2017) | 3 years (F) | Pontomedullary exophytic lesion | Facial palsy | Stereotactic biopsy | Yes (vinblastine and bevacizumab) | 4 years | Progression | Yes |
| 6 | Chan et al (2017) | 7 Years (M) | Pons | 6th CN palsy | Stereotactic biopsy | No data | No data | No data | Yes |
| 7 | Current case (2019) | 4 years (F) | Left brainstem dorsal exophytic lesion | Hydrocephalus, unsteady gait, hoarseness, choking with food, 6th,7th, and lower CN palsies | Craniotomy and debulking, VPS | Yes (carboplatin/vincristine) | 8 years | Progression | Not done |
In conclusion, angiocentric glioma of brainstem has worse outcome, different clinical presentation and variable course in comparison to supranational location. The role of extent of resection and adjuvant treatment is not yet established in this region, which requires further research.
Acknowledgement
We would like to show our gratitude to Dr. Emad Alrasheed from Radiology Department for reviewing the images provided in this report.
Case Reports
Case reports will only be considered for unusual topics that add something new to the literature. All Case Reports should include at least one figure. Written informed consent for publication must accompany any photograph in which the subject can be identified. Figures should be submitted with a 300 dpi resolution when submitting electronically. The abstract should be unstructured, and the introductory section should always include the objective and reason why the author is presenting this particular case. References should be up to date, preferably not exceeding 15.
References
- 1.Wang M, Tihan T, Rojiani AM, Bodhireddy SR, Prayson RA, Iacuone JJ, et al. Monomorphous angiocentric glioma: a distinctive epileptogenic neoplasm with features of infiltrating astrocytoma and ependymoma. J Neuropathol Exp Neurol. 2005;64:875–881. doi: 10.1097/01.jnen.0000182981.02355.10. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 3.Covington DB, Rosenblum MK, Brathwaite CD, Sandberg DI. Angiocentric glioma-like tumor of the midbrain. Pediatric neurosurgery. 2009;45:429–433. doi: 10.1159/000277616. [DOI] [PubMed] [Google Scholar]
- 4.Chan E, Bollen AW, Sirohi D, Van Ziffle J, Grenert JP, Kline CN, et al. Angiocentric glioma with MYB-QKI fusion located in the brainstem, rather than cerebral cortex. Acta Neuropathol. 2017;134:671–673. doi: 10.1007/s00401-017-1759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Aronco L, Rouleau C, Gayden T, Crevier L, Decarie JC, Perreault S, et al. Brainstem angiocentric gliomas with MYB-QKI rearrangements. Acta Neuropathol. 2017;134:667–669. doi: 10.1007/s00401-017-1763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weaver KJ, Crawford LM, Bennett JA, Rivera-Zengotita ML, Pincus DW. Brainstem angiocentric glioma: report of 2 cases. J Neurosurg Pediatr. 2017;20:347–351. doi: 10.3171/2017.5.PEDS16402. [DOI] [PubMed] [Google Scholar]
- 7.Giulioni M, Marucci G, Martinoni M, Marliani AF, Toni F, Bartiromo F, et al. Epilepsy associated tumors: Review article. World J Clin Cases. 2014;2:623–641. doi: 10.12998/wjcc.v2.i11.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lellouch-Tubiana A, Boddaert N, Bourgeois M, Fohlen M, Jouvet A, Delalande O, et al. Angiocentric neuroepithelial tumor (ANET): a new epilepsy-related clinicopathological entity with distinctive MRI. Brain Pathol. 2005;15:281–286. doi: 10.1111/j.1750-3639.2005.tb00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qaddoumi I, Orisme W, Wen J, Santiago T, Gupta K, Dalton JD, et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 2016;131:833–845. doi: 10.1007/s00401-016-1539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandopadhayay P, Ramkissoon LA, Jain P, Bergthold G, Wala J, Zeid R, et al. MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat Genet. 2016;48:273–282. doi: 10.1038/ng.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kestle J, Townsend JJ, Brockmeyer DL, Walker ML. Juvenile pilocytic astrocytoma of the brainstem in children. J Neurosurg. 2004;101:1–6. doi: 10.3171/ped.2004.101.2.0001. [DOI] [PubMed] [Google Scholar]