Observational studies show that COPD is associated with increased coronavirus disease 2019 (COVID-19) severity and mortality [1]. Inhaled corticosteroids (ICS), which are commonly used to treat COPD, have been associated with increased risk of bacterial pneumonia in COPD and impaired immune response to viruses. Whether this class of medication affects the airway expression of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptors and cofactors (changes which may modify COVID-19 susceptibility and outcomes) is currently unclear. Therefore, we examined the effects of ICS treatment on SARS-CoV-2-related gene expression in lower airway bronchial epithelial cells (BECs) in a randomised controlled trial of COPD patients.
Short abstract
In a RCT of 63 patients with COPD, 12 weeks of ICS/LABA therapy downregulated bronchial epithelial expression of the SARS-CoV-2-related genes ACE2 and ADAM17 compared to LABA alone. This may have implications for COVID-19 susceptibility/severity in COPD. https://bit.ly/3vZnBVO
To the Editor:
Observational studies show that COPD is associated with increased coronavirus disease 2019 (COVID-19) severity and mortality [1]. Inhaled corticosteroids (ICS), which are commonly used to treat COPD, have been associated with increased risk of bacterial pneumonia in COPD and impaired immune response to viruses. Whether this class of medication affects the airway expression of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptors and cofactors (changes which may modify COVID-19 susceptibility and outcomes) is currently unclear. Therefore, we examined the effects of ICS treatment on SARS-CoV-2-related gene expression in lower airway bronchial epithelial cells (BECs) in a randomised controlled trial of COPD patients.
We conducted the DISARM trial (recruitment October 2015–June 2019; clinicaltrials.gov identifier NCT02833480; University of British Columbia/Providence Health Care ethics approval H14-02277) to examine the effects of two long-acting β2-agonist (LABA)/ICS combinations on the airway microbiome in people with COPD. After a 4-week formoterol (FOR) run-in, we randomised participants to receive ongoing FOR 12 μg, formoterol/budesonide (FOR/BUD) 12/400 μg, or salmeterol/fluticasone propionate (SAL/FLU) 25/250 μg, twice daily for 12 weeks. We collected bronchial brush specimens according to a standard protocol (sixth- to eighth-generation airways, right upper lobe) before and after treatment, and measured BEC gene expression by RNA-sequencing as described previously [2]. The co-primary outcomes (change in total bacterial population and diversity at 12 weeks) are yet to be reported. However, in response to the urgency of the COVID-19 pandemic, we performed an ad hoc analysis of genes encoding SARS-CoV-2 entry receptors (ACE2, BSG) and host co-factors (TMPRSS2, ADAM17, FURIN).
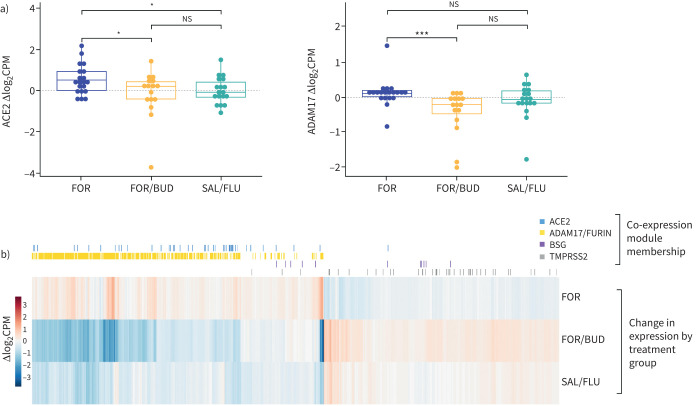
63 participants (median age 64 years, 83% male, 46% current smokers, mean forced expiratory volume in 1 s 61.36% predicted) were randomised. There were no differences between the treatment groups with regard to demographics, lung function, comorbidities or recent ICS use (Kruskal–Wallis and Fisher's exact tests p>0.05). Principal component analysis of overall pre-treatment gene expression showed no differences between the treatment groups, or between pre-enrolment ICS users and non-users. 54 participants had both pre- and post-treatment gene expression data available (seven participants failed to attend the post-treatment bronchoscopy, and two had insufficient pre-treatment RNA). After 12 weeks of treatment, the FOR/BUD and SAL/FLU arms both showed significantly lower changes in ACE2 expression relative to FOR monotherapy (Wilcoxon rank sum test p=0.049 and p=0.041, respectively), and the FOR/BUD arm showed significantly lower changes in ADAM17 expression relative to FOR monotherapy (Wilcoxon rank sum test p=1.36×10−4) (figure 1a). This suggests that ICS have a suppressive effect on the transcription of these genes. There was no effect of ICS treatment on BEC expression of BSG, TMPRSS2 or FURIN. When stratified by baseline smoking status, the results were similar for ADAM17 (FOR/BUD versus FOR p=0.01 in former smokers and p=0.002 in current smokers), but were not significant for ACE2.
FIGURE 1.
Bronchial epithelial cell (BEC) gene expression in the DISARM study. Gene expression in BECs collected during bronchoscopy before and after treatment was determined by RNA-sequencing (Illumina NextSeq 500; Illumina, San Diego, CA, USA) with paired-end 42×42 bp reads). Sequencing data were aligned to GENCODE genome reference assembly GRCh37 release 31 using Salmon. Low-abundance genes (log2 counts per million (log2CPM) <1 or transcripts per million (TPM) <2 in >80% of the samples) were filtered out, leaving a total of 15 667 genes. a) Box plots showing pre- to post-treatment change in expression (Δlog2CPM) of ACE2 (encodes the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptor) and ADAM17 (encodes a metalloproteinase that cleaves the angiotensin-converting enzyme (ACE)2 protein and facilitates endocytosis of the ACE2–SARS-CoV-2 complex). Only participants with both pre- and post-treatment gene expression data available are shown (54 out of 63 randomised participants). Between-group comparisons were by Wilcoxon rank-sum test. *: p<0.05, ***: p<0.001. b) Heat map of pre- to post-treatment change in gene expression (median Δlog2CPM) of genes with at least one significant between-group Wilcoxon rank-sum test at false discovery rate (FDR) <0.1 (977 out of the 15 667 total genes). Columns represent single genes, and are arranged using hierarchical clustering (“aheatmap” function in the NMF packing in R). Treatment with formoterol (FOR)/budesonide (BUD) and salmeterol (SAL)/fluticasone (FLU) tended to have the opposite direction of effect on gene expression compared to treatment with FOR, suggesting a class effect of inhaled corticosteroids (ICS) on the expression of these genes. Additionally, each plotted gene was annotated according to its membership of a SARS-CoV-2-related gene co-expression module determined by weighted gene correlation network analysis (WGCNA) of pre-treatment expression (as log2(TPM+1), soft threshold power β=6, minimum module size 50 genes). ICS treatment tended to decrease the expression of genes that are co-expressed with ACE2 and ADAM17/FURIN, whereas genes co-expressed with TMPRSS2 and BSG tended to be upregulated by ICS treatment. ns: nonsignificant.
In addition, we qualitatively explored how ICS treatment affects transcriptome-wide BEC gene expression in COPD using a clustered heat map (figure 1b). LABA-only and LABA/ICS treatment had modest but opposite effects on gene expression: upregulation in the FOR arm was met with downregulation in the FOR/BUD and SAL/FLU arms, and vice versa, suggesting an ICS class effect. Overall, FOR/BUD appeared to have a greater effect on gene expression than SAL/FLU despite similar doses (in beclomethasone-equivalents). The reason for this is unknown, but may be due to the greater relative retention of BUD in airway epithelial cells [3].
Next, we determined how genes that were co-expressed with each of the key SARS-CoV-2-related genes (determined by a weighted gene correlation network analysis [4]) changed with ICS treatment. ACE2 was contained in a module of 444 genes; using Gene Ontology and Kyoto Encyclopedia of Genes and Genomes annotations, this module was highly enriched for genes related to type I interferons (IFN-I) and viral infections. ADAM17 and FURIN were in the same module of 1900 genes, which was enriched for genes related to the regulation of innate immunity, cytokine production and infectious and autoimmune diseases. BSG and TMPRSS2 were each contained in modules of 788 and 985 genes, respectively, both of which were enriched for genes related to intracellular processes. We then annotated the clustered heat map according to each gene's co-expression module membership. ACE2 and ADAM17/FURIN module genes were clustered in areas of the heat map where genes were downregulated by FOR/BUD and SAL/FLU treatment, suggesting that ICS downregulate genes that are highly connected to these key SARS-CoV-2-related genes.
Our analysis extends our previous findings that ACE2 gene and protein expression was increased in BECs [2] and lung tissue [5] of people with COPD, by showing that ACE2 was downregulated by ICS treatment. Our data, which are from a randomised controlled trial of ICS/LABA therapy, confirm a recent study that indicated a downregulatory effect of ICS on ACE2 expression in the sputum of COPD patients (BUD and FLU) and in mouse lungs (BUD, FLU and beclomethasone) [6]. In this study, in concordance with our results, ICS did not affect the expression of BSG or TMPRSS2 [6]. Another group has recently conducted a post hoc analysis [7] of the Groningen and Leiden Universities study of Corticosteroids in Obstructive Lung Disease (GLUCOLD) trial, showing that ACE2 expression in airway biopsy specimens was downregulated following treatment with FLU in patients with COPD. Our data complement these results by showing that ACE2 downregulation is probably the result of an ICS class effect, and can occur more acutely (after only 12 weeks of treatment, compared to 26 weeks in the GLUCOLD study).
The relative importance of differences in ACE2 expression for COVID-19 is debated. Increased availability of ACE2 protein in the airways may increase COVID-19 susceptibility and severity; in theory, the ICS-mediated downregulation of ACE2 reported here and by others [6, 7] could therefore be protective. Conversely, since ACE2 is a critical negative regulator of the renin–angiotensin system, its downregulation could predispose to lung injury [8]. Our gene network analysis showed that ACE2 was co-expressed with genes related to the innate immune response to viruses, particularly IFN-I, and that genes in this module tended to be suppressed by ICS therapy. Animal models suggest that a delayed IFN-I response to SARS-CoV infection may lead to excessive inflammation and death [9]. Indeed, COPD patients already have impaired IFN-I responses following viral infection [10]. Therefore, ACE2 expression in COVID-19 may represent a double-edged sword, but any interaction between COPD and ICS treatment in COVID-19 is likely to be more complex than can be explained by alterations in ACE2 expression alone.
A novel, truncated isoform of ACE2 that is transcriptionally independent and highly expressed in lung epithelium has been reported recently [11]. This isoform does not have an extracellular domain and does not bind SARS-CoV-2 spike protein, meaning that changes in its expression may have no effect on COVID-19 risk. This important finding challenges the notion that functional ACE2 is an IFN-stimulated gene, since it appears to be only the truncated isoform that is induced by IFN-I. We attempted exon-level analysis to quantify this isoform, but our sequencing depth was insufficient to produce reliable results. Future investigation of this novel isoform will be critical to understand the implications of our current findings.
To our knowledge, our finding that ICS therapy downregulates ADAM17 expression in human BECs is novel. The SARS-CoV-2 spike protein induces ADAM17-dependent shedding of the ACE2 ectodomain, creating the soluble form of ACE2 and facilitating fusion of the viral and cell membranes [12]. Inhibition of ADAM17 at least partially blocks SARS-CoV entry in cultured epithelial cells [12, 13]. In addition, ADAM17 plays a crucial role in interleukin (IL)-6 signalling, which is activated in severe COVID-19 [14]; it has been described as a “master switch” between the pro-inflammatory trans- and anti-inflammatory classical- (i.e. via membrane-bound IL-6 receptor) IL-6 signalling pathways [15]. However, any impact the ICS-mediated downregulation of ADAM17 may have on COVID-19 susceptibility or outcomes would be speculative at this stage.
Despite the limitations (including relatively small sample size, short follow-up period and lack of accompanying protein expression or functional data), our results show that ICS modifies lower-airway BEC expression of genes relevant to SARS-CoV-2 and COVID-19 biology. The relative importance of upper (nasal) versus lower (bronchial) expression of these genes for SARS-CoV-2 transmission needs further study. However, our results provide potential mechanistic support for the results of the Steroids in COVID-19 (STOIC) trial (clinicaltrials.gov identifier NCT04416399), which showed a reduction in urgent care/hospitalisation in early-stage COVID-19 with inhaled BUD [16]. The trial results require confirmation in a COPD population, which has increased BEC ACE2 expression, before the clinical relevance of our findings can be determined. In the absence of any epidemiological evidence that ICS therapy increases COVID-19 severity or mortality [17], we agree with the international consensus that ICS treatment in COPD patients should be continued if clinically indicated until further evidence is available.
Shareable PDF
Acknowledgements
The authors would like to thank the generous fundraising and donation efforts by Irving Ding and Chun Hong Tao to St. Paul's Foundation COVID-19 Response Fund. We are grateful to Ryan Vander Werff and Tara Stach at BRC-Seq (University of British Columbia (UBC), Vancouver, BC, Canada) for conducting the RNA sequencing; and Chung Cheung and Julia Yang (UBC Centre for Heart Lung Innovation) for their assistance with laboratory specimens.
Footnotes
Data sharing statement: Individual participant gene expression data are publicly available via Gene Expression Omnibus (GEO) at www.ncbi.nlm.nih.gov/geo/ (accession number GSE162120). The DISARM study protocol is available at https://clinicaltrials.gov (identifier NCT02833480). Additional individual participant data that underlie the results reported in this article, after de-identification, will be made available immediately following publication to any investigators and for any purpose, upon reasonable request. Proposals should be made directly to the principal investigator, Don D. Sin (don.sin@hli.ubc.ca).
Author contributions: Data acquisition: S. Milne, F.S. Leitao Filho, T. Shaipanich, S.F. van Eeden, J.M. Leung, S. Lam and D.D. Sin; data analysis and interpretation: S. Milne, X. Li, A.I. Hernández Cordero, C.X. Yang, F.S. Leitao Filho, C.W.T. Yang, J.M. Leung and D.D. Sin; drafting of manuscript: S. Milne and X. Li; critical revision of draft manuscript: S. Milne, X. Li, A.I. Hernández Cordero, C.X. Yang, F.S. Leitao Filho, C.W.T. Yang, S.F. van Eeden, J.M. Leung, S. Lam and D.D. Sin; all authors reviewed and approved the final version of the manuscript; all authors agree to be accountable for all aspects of the work including data integrity.
Conflict of interest: S. Milne reports personal fees from Novartis and Boehringer Ingelheim, outside the submitted work.
Conflict of interest: X. Li has nothing to disclose.
Conflict of interest: C.X. Yang has nothing to disclose.
Conflict of interest: F.S. Leitao Filho has nothing to disclose.
Conflict of interest: A.I. Hernández Cordero has nothing to disclose.
Conflict of interest: C.W.T. Yang has nothing to disclose.
Conflict of interest: T. Shaipanich has nothing to disclose.
Conflict of interest: S.F. van Eeden has nothing to disclose.
Conflict of interest: J.M. Leung has nothing to disclose.
Conflict of interest: S. Lam has nothing to disclose.
Conflict of interest: D.D. Sin reports grants from AstraZeneca, during the conduct of the study; personal fees for lectures from Novartis and Boehringer Ingelheim, grants and personal fees for lectures from AstraZeneca, outside the submitted work.
Support statement: The DISARM study was funded by an investigator-initiated grant from AstraZeneca. RNA sequencing work was funded by the St. Paul's Foundation-Rapid COVID-19 Response Fund. S. Milne and A.I. Hernández Cordero are supported by the MITACS Accelerate Program. J.M. Leung is supported by Michael Smith Foundation for Health Research-Health Professional-Investigator Award and the Providence Health Care Research Institute. D.D. Sin is supported by a Tier 1 Canada Research Chair in COPD Award and the de Lazzari Family Chair at HLI. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 2020; 55: 2000547. doi: 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung JM, Yang CX, Tam A, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J 2020; 55: 2000688. doi: 10.1183/13993003.00688-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borchard G, Cassará ML, Roemelé PEH, et al. Transport and local metabolism of budesonide and fluticasone propionate in a human bronchial epithelial cell line (Calu-3). J Pharm Sci 2002; 91: 1561–1567. doi: 10.1002/jps.10151 [DOI] [PubMed] [Google Scholar]
- 4.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 2008; 9: 559. doi: 10.1186/1471-2105-9-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milne S, Yang CX, Timens W, et al. SARS-CoV-2 receptor ACE2 gene expression and RAAS inhibitors. Lancet Respir Med 2020; 8: e50–e51. doi: 10.1016/S2213-2600(20)30224-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finney LJ, Glanville N, Farne H, et al. Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon. J Allergy Clin Immunol 2021; 147: 510–519. doi: 10.1016/j.jaci.2020.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aliee H, Massip F, Qi C, et al. Determinants of SARS-CoV-2 receptor gene expression in upper and lower airways. medRxiv 2020; preprint [ 10.1101/2020.08.31.20169946]. [DOI] [Google Scholar]
- 8.Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005; 11: 875–879. doi: 10.1038/nm1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 2016; 19: 181–193. doi: 10.1016/j.chom.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mallia P, Message SD, Gielen V, et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med 2011; 183: 734–742. doi: 10.1164/rccm.201006-0833OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onabajo OO, Banday AR, Stanifer ML, et al. Interferons and viruses induce a novel truncated ACE2 isoform and not the full-length SARS-CoV-2 receptor. Nat Genet 2020; 52: 1283–1293. doi: 10.1038/s41588-020-00731-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haga S, Yamamoto N, Nakai-Murakami C, et al. Modulation of TNF-α-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-α production and facilitates viral entry. Proc Natl Acad Sci USA 2008; 105: 7809–7814. doi: 10.1073/pnas.0711241105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heurich A, Hofmann-Winkler H, Gierer S, et al. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol 2014; 88: 1293–1307. doi: 10.1128/JVI.02202-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tay MZ, Poh CM, Rénia L, et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020; 20: 363–374. doi: 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahmud-Al-Rafat A, Majumder A, Taufiqur Rahman KM, et al. Decoding the enigma of antiviral crisis: does one target molecule regulate all? Cytokine 2019; 115: 13–23. doi: 10.1016/j.cyto.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramakrishnan S, Nicolau DV, Langford B, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med 2021; 9: 763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schultze A, Walker AJ, MacKenna B, et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med 2020; 8: 1106–1120. doi: 10.1016/S2213-2600(20)30415-X [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00130-2021.Shareable (431.7KB, pdf)