Abstract
Introduction:
Exposure to elevated ambient ozone levels has been associated with exacerbation of asthma, likely mediated by oxidative stress. We have shown that supplementation with the antioxidant vitamin E isoform, gamma tocopherol, mitigates the inflammatory effects of inhaled endotoxin exposure, a key component of ambient air particulate matter.
Objective:
The objective of the current study was to assess the efficacy of gamma tocopherol for mitigating pulmonary effects of ozone, using an abbreviated dosing regimen that could be rapidly begun when high ozone days are anticipated.
Materials and Methods:
We conducted a randomized, double blind, placebo-controlled crossover study of adults with mild asthma who were pre-treated with gamma tocopherol-enriched supplement and exposed to 0.25 ppm ozone for 3 hours. Induced sputum samples were obtained before and after ozone exposure to measure airway inflammation. Mucociliary clearance was estimated using gamma scintigraphy.
Results:
With the short course of gamma tocopherol pre-treatment, we found no significant effect on ozone-induced airway inflammation. A transient slowing of clearance in the large airways was seen following ozone exposure in the placebo treatment period that was not present during gamma tocopherol treatment.
Discussion:
Short course gamma tocopherol did not protect against ozone-induced airway inflammation. Future work will focus on the efficacy of longer courses of gamma tocopherol supplementation for mitigating pollutant-induced health effects. The early transient ozone effect on airway clearance as well as the impact of gamma tocopherol on this effect will be further explored in future studies.
Keywords: Ozone, air pollution, asthma, gamma tocopherol, mucociliary clearance
Introduction:
Ozone (O3) is a commonly encountered oxidant pollutant, which is a frequent trigger of asthma exacerbations (Bromberg 2016). We and others have shown that short term O3 exposure results in lung function decrements and neutrophilic airway inflammation (Hernandez et al. 2010; Lay et al. 2007). We are assessing the vitamin E isoform, gamma tocopherol (γT), as an intervention for pollutant-induced respiratory health effects. In preclinical studies, γT inhibited O3-induced exacerbation of allergic airway inflammation and mucus production (Wagner et al. 2007). We recently reported that 14 days of supplementation with γT in asthmatics reduced baseline sputum eosinophilia and pathogenic mucin Muc5AC, as well as neutrophilic airway inflammation and slowing of mucociliary clearance (MCC) following inhaled endotoxin challenge (Burbank et al. 2018). We next set out to test a shorter course prophylactic regimen of γT that could be implemented on high pollutant exposure days that occur with little warning such as pollutant increases associated with wildfires, in which both particulate matter and O3 are increased. To determine if a shorter course of γT could have similar anti-inflammatory activities as longer courses, we simultaneously assessed the effect of a 2-day, 4 dose regimen on inflammatory cytokine secretion in lipopolysaccharide (LPS)-treated peripheral blood mononuclear cells obtained from treated volunteers. We reported a reduction of cytokine secretion similar to that observed following longer γT treatment regimens (Burbank et al. 2017). Guided by these results, we focused on the effect of this short course of γT pre-treatment on baseline airway eosinophilia and O3-induced neutrophilic airway inflammation and changes in MCC.
Materials and Methods:
In this protocol, adults with mild intermittent allergic asthma were treated with γT (or placebo) followed by exposure to O3 using a randomized, double blind, placebo-controlled crossover study design. Participants consumed two γT-enriched geltabs containing approximately 600 mg of γT each (d-gamma tocopherol 89.5%, total tocopherols 93.2%, canola oil ≤ 10%) or matching placebo (safflower oil 700 mg per capsule) every 12 hours for 4 doses, with the final dose administered the morning of O3 exposure. Participants then entered an exposure chamber where they were exposed to 0.25 ppm O3 for three hours. During exposure, participants alternated 15 minutes of rest with 15 minutes of treadmill exercise (at a level sufficient to achieve minute ventilation of 20 Liters/minute/meter2 body surface area). Induced sputum samples were obtained before and 6 hours after O3 exposure and analyzed for inflammatory cells using methods previously described (Hernandez et al. 2010). Sputum samples were analyzed with the V-PLEX Human Proinflammatory Panel II kit (MesoScale Diagnostics, Rockville, MD). MCC was measured using gamma scintigraphy techniques which have been described in detail previously (Bennett et al. 2011; Bennett et al. 2014). Briefly, participants inhaled an aerosol of technetium 99m sulfur colloid (99mTc-SC), then a gamma camera was used to obtain continuous 2-minute images at 10-minute intervals for a period of 2 hours to monitor clearance of 99mTc-SC particles from the airways. Whole lung retention was calculated as a fraction of the initial counts in the lung over the 2-hour measurement period. Images of the lung were then divided into regions of interest: central (C) and peripheral (P), where C is enriched and P is absent of the large proximal airways (Bennett et al. 2015). Whole lung and regional MCC was expressed as average clearance in percent over time. Baseline MCC measurements were established at the first study visit prior to receiving any study drug. MCC was measured again one hour after exiting the O3 chamber. After a minimum 3-week washout period, participants returned for the second period of study and were crossed over to the alternate treatment group.
O3-induced changes in outcome variables from baseline were analyzed using paired t-test or Wilcoxon signed rank test depending on normality of the data. Linear regression models were fitted that accounted for the initial regional lung deposition of radioaerosol (the central-to-peripheral, or C/P, deposition ratio) as a covariate to determine if γT treatment impacts O3-induced changes in MCC compared to placebo. The local university Institutional Review Board approved the study. Written informed consent was obtained from all participants. This study was registered on clinicaltrials.gov under identifier NCT02911688.
Results:
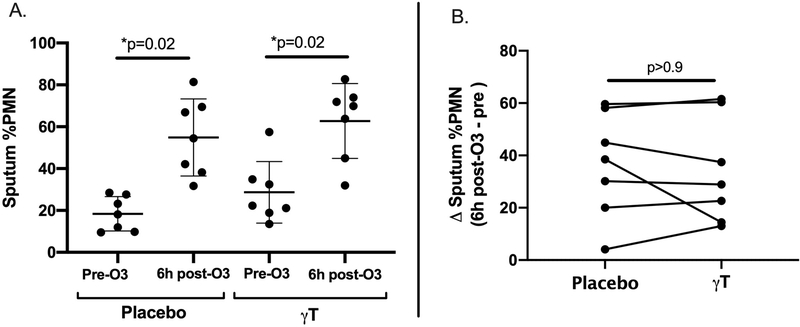
Twenty adults with mild intermittent allergic asthma were enrolled, with 15 volunteers completing all study visits, though one volunteer was unable to complete all MCC measurements for logistical reasons. Demographic information is summarized in Table 1. No participant was actively using controller therapy for asthma at any point during the study, and participants had not used short acting beta agonists for at least 12 hours prior to arriving for study visits. Due to variability in quality of induced sputum samples, paired assessments of pre and post-O3 challenge sputum endpoints were limited to 7 volunteers. We found that, in contrast to our results with 14 day dosing, the shortened treatment course of γT did not significantly reduce pre-O3 eosinophilic inflammation compared to placebo (mean of 2.2% sputum eosinophils [± 3.7 SD] following placebo treatment and 3.4% sputum eosinophils [± 4.3] following γT treatment, p=0.56). O3 exposure significantly increased sputum percentage of neutrophils during both the placebo and γT treatment periods (Figure 1A), with no significant effect on sputum percentage of eosinophils (data not shown). However, γT supplementation did not attenuate O3-induced neutrophilic airway inflammation compared to placebo (mean increase in sputum %PMN of 36.5 percentage points [± 20.2] following placebo versus 34.1 percentage points [± 20.2] following γT, p>0.9) (Figure 1B). We found no significant change in sputum inflammatory cytokines (Interleukin (IL)-1β, IL-6, IL-8 and TNFα) from baseline following O3 exposure and no difference in sputum cytokines following γT supplementation compared to placebo (data not shown).
Table 1.
Characteristics of Study Participants
| Clinical Characteristic | N = 15 |
|---|---|
| Age (years), median (range) | 23 (20–45) |
| Sex | 11 Female/ 4 Male |
| Race | 10 Caucasian 3 African-American 1 Asian 1 Other |
| Ethnicity | 2 Hispanic/Latino 13 Non-Hispanic/ Latino |
| Baseline FEV1 (Liters), Median (range) % predicted, Median (range) |
3.2 (2.3–4.8) 92 (78–109) |
| BMI (kg/m2), median (range) | 24 (17–36) |
| Baseline FeNO (ppb), median (range) | 33 (13–133) |
Forced expiratory volume in 1 second (FEV1); Body mass index (BMI); Fractional exhaled nitric oxide (FeNO); parts per billion (ppb).
Figure 1. O3-induced neutrophilic airway inflammation following γT and placebo treatment (N=7).
A) O3 exposure resulted in significantly increased sputum neutrophil burden in both treatment groups. B) No significant difference in O3-induced sputum neutrophilia was seen following γT treatment compared to placebo treatment. (Figure 1A data presented as mean and standard deviation. All p values generated from Wilcoxon signed rank tests). Neutrophils (PMN); Ozone (O3); Gamma Tocopherol (γT).
With regard to MCC measures, we found no detectable O3-related change from baseline in whole lung clearance of 99mTc-SC particles over the 120 minute measurement period in either treatment period, with mean average 120 minute clearance (Ave120Clr) of 21% (± 7% SD) at baseline, 21% (± 7%) post-O3 during placebo, and 23% (± 7%) post-O3 during γT treatment. Assessment of the central region (enriched with large proximal airways) demonstrated an early and transient decrease in clearance following O3 exposure during the placebo period, which was most pronounced during the first 30 minutes of the scan (central Ave30Clr: 12.6% [± 9.2% SD] baseline vs 9.8% [± 8.5%] post-O3, p=0.07), though the decrease did not reach statistical significance. Interestingly, this finding was not seen during γT treatment (12.6% [± 9.2%] baseline vs 12.1% [± 6.8%] post-O3, p=0.86).
Discussion:
Overall, this short course of γT did not reduce baseline eosinophilic airway inflammation nor O3-induced neutrophilic inflammation in mild asthmatics, unlike our observations of the effect of 14-day treatment on endotoxin-induced inflammation. As our preclinical studies demonstrate that γT reduces both O3 and endotoxin-induced inflammation, it seems most likely that the lack of anti-inflammatory action we observed in this study is due to shorter treatment duration, rather than a differential effect of γT on O3 vs. endotoxin-induced inflammation in humans. Future work examining asthma-specific pollutant effects will incorporate the use of longer treatment courses of gamma tocopherol.
While we did not detect an O3-mediated effect on whole lung MCC in mild asthmatics, our post-hoc analyses suggest that O3 may promote an early transient slowing of MCC within the large bronchial airways specifically, the major site of airway disease in asthma. Prior studies evaluated O3 effects on MCC in healthy non-asthmatics with mixed results, showing no effect when measured 2 hours after O3 exposure (Gerrity et al. 1993) but a temporary speeding of clearance when measured during O3 exposure (Foster and Stetkiewicz 1996). Interestingly in our study, transient slowing of clearance was not observed following short course γT treatment. This effect will be further examined in future studies to determine whether γT has a protective effect against O3-mediated slowing of MCC in the central airways. We hypothesize that asthmatics may be uniquely vulnerable to the impact of oxidative stress on airway inflammation, mucin secretion and ciliary function, factors which would impact MCC.
Acknowledgements:
We thank Heather Wells for her assistance with processing and analysis of sputum samples. We also thank Jihong Wu for her assistance with gamma scintigraphy procedures related to the study.
Funding: Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under awards R01ES023349 and P30ES010126 and Assistance Agreement No. 83578501-0 awarded by the U.S. Environmental Protection Agency to the University of North Carolina. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Environmental Protection Agency. EPA does not endorse any products or commercial services mentioned in this publication. MH is supported by R01HL135235. HZ and TW are supported by R01ES021900.
Footnotes
Disclosure of Interests: The authors declare that they have no competing interests.
Declarations
Ethics approval and consent to participate: The University of North Carolina Institutional Review Board Biomedical committee approved the study (#15–1938). Written informed consent was obtained from all participants. This study was registered on clinicaltrials.gov under identifier NCT02911688.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References:
- Bennett WD, Herbst M, Alexis NE, Zeman KL, Wu J, Hernandez ML, Peden DB. 2011. Effect of inhaled dust mite allergen on regional particle deposition and mucociliary clearance in allergic asthmatics. Clin Exp Allergy. 41(12):1719–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett WD, Alexis NE, Almond M, Herbst M, Zeman KL, Peden DB. 2014. Effect of inhaled endotoxin on mucociliary clearance and airway inflammation in mild smokers and nonsmokers. J Aerosol Med Pulm Drug Deliv. 27(6):459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett WD, Wu J, Fuller F, Balcazar JR, Zeman KL, Duckworth H, Don KH, O’Riordan TG, Boucher RC, Donaldson SH. 2015. Duration of action of hypertonic saline on mucociliary clearance in the normal lung. J Appl Physiol (1985). 118(12):1483–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg PA. Mechanisms of the acute effects of inhaled ozone in humans. 2016. Biochim Biophys Acta. 1860(12):2771–2781. [DOI] [PubMed] [Google Scholar]
- Burbank AJ, Duran CG, Almond M, Wells H, Jenkins S, Jiang Q, Yang C, Wang T, Zhou H, Hernandez ML, Peden DB. 2017. A short course of gamma-tocopherol mitigates LPS-induced inflammatory responses in humans ex vivo. J Allergy Clin Immunol. 140(4):1179–1181 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbank AJ, Duran CG, Pan Y, Burns P, Jones S, Jiang Q, Yang C, Jenkins S, Wells H, Alexis N, Kesimer M, Bennett WD, Zhou H, Peden DB, Hernandez ML. 2018. Gamma tocopherol-enriched supplement reduces sputum eosinophilia and endotoxin-induced sputum neutrophilia in volunteers with asthma. J Allergy Clin Immunol. 141(4):1231–1238 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster WM, Stetkiewicz PT. 1996. Regional clearance of solute from the respiratory epithelia: 18–20 h postexposure to ozone. J Appl Physiol (1985). 81(3):1143–1149. [DOI] [PubMed] [Google Scholar]
- Gerrity TR, Bennett WD, Kehrl H, DeWitt PJ. 1993. Mucociliary clearance of inhaled particles measured at 2 h after ozone exposure in humans. J Appl Physiol (1985). 74(6):2984–2989. [DOI] [PubMed] [Google Scholar]
- Hernandez ML, Lay JC, Harris B, Esther CR Jr., Brickey WJ, Bromberg PA, Diaz-Sanchez D, Devlin RB, Kleeberger SR, Alexis NE, Peden DB. 2010. Atopic asthmatic subjects but not atopic subjects without asthma have enhanced inflammatory response to ozone. J Allergy Clin Immunol. 126(3):537–544 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay JC, Alexis NE, Kleeberger SR, Roubey RA, Harris BD, Bromberg PA, Hazucha MJ, Devlin RB, Peden DB. 2007. Ozone enhances markers of innate immunity and antigen presentation on airway monocytes in healthy individuals. J Allergy Clin Immunol. 120(3):719–722. [DOI] [PubMed] [Google Scholar]
- Wagner JG, Jiang Q, Harkema JR, Illek B, Patel DD, Ames BN, Peden DB. 2007. Ozone enhancement of lower airway allergic inflammation is prevented by gamma-tocopherol. Free Radic Biol Med. 43(8):1176–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]