Abstract
Myeloablative (MAC) as compared to reduced-intensity conditioning (RIC) is generally associated with lower relapse risk after allogeneic hematopoietic cell transplantation (HCT) for acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS). However, disease specific risk factors in AML/MDS can further inform when MAC vs. RIC may yield differential outcomes. We analyzed HCT outcomes stratified by the disease risk index (DRI) in 4387 adults (age 40–65 years) to identify the impact of conditioning intensity. In the low/intermediate risk DRI cohort, RIC was associated with lower non-relapse mortality (NRM) (HR=0.74, 95% CI 0.62–0.88; p<0.001), but significantly higher relapse risk (HR=1.54, 95% CI 1.35–1.76; p<0.001) and thus inferior disease-free survival (DFS) (HR=1.19, 95% CI 1.07–1.33; p=0.001). In the high/very high risk DRI cohort, RIC resulted in marginally lower NRM (HR=0.83, 95% CI 0.68–1.00; p=0.051), and significantly higher relapse risk (HR=1.23, 95% CI 1.08–1.41; p=0.002) leading to similar DFS using either RIC or MAC.
These data support MAC over RIC as the preferred conditioning intensity for AML/MDS with low/intermediate risk DRI, but similar benefit to RIC in high/very high risk DRI. Novel MAC regimens with less toxicity could benefit all, but more potent anti-neoplastic approaches are needed for the high/very high risk DRI group.
INTRODUCTION:
Allogeneic hematopoietic cell transplantation (HCT) is the only curative therapy for most adults with acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS). Since HCT using conventional myeloablative conditioning (MAC) can be associated with higher toxicity and mortality rates, reduced intensity conditioning (RIC) regimens have been increasingly used in the past two decades for HCT in older and less fit patients with AML or MDS.1,2 A recent prospective randomized Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0901 trial demonstrated significantly improved disease-free survival (DFS) benefit with use of MAC compared with RIC for HCT in patients (age 18–65 years) with AML and MDS.3 Long term follow-up of this trial showed also significantly longer overall survival (OS) for MAC recipients.4 In addition, retrospective analysis of AML patients in this study demonstrated that OS benefit of MAC is observed in patients with molecular MRD, but not in those who are MRD negative at transplant.5 Although BMT CTN 0901 study defined MAC as current standard of care for younger and fit patients with AML, this trial eligibility was restricted only to patients with fewer comorbidities (HCT-CI ≤4) and with <5% marrow myeloblasts at HCT. While some studies reported advantages of using MAC over RIC in patients with AML and MDS,6–8 others observed similar outcomes across cohorts of various age and comorbidities at HCT.9–16 Further confounding the choice of conditioning intensity in this setting, several studies showed no advantage in using MAC over RIC in patients receiving HCT for AML and MDS with high-risk cytogenetic abnormalities.9,10
Disease Risk Index (DRI) that considers the cytogenetic risk and the disease status at HCT for AML and MDS has been identified as a strong independent predictor of OS in patients receiving HCT for hematological malignancies.17 Since high-risk cytogenetic abnormalities and persistent leukemia before allograft are well-recognized independent prognostic factors for relapse and treatment failure after HCT in AML and MDS,6,18–23 we hypothesized that the choice of conditioning intensity for HCT could be better informed by DRI applied at HCT. Thus, we conducted this large Center for International Blood and Marrow Transplant Research (CIBMTR) registry study to identify a preferred conditioning intensity choice for low/intermediate risk vs. high/very high risk DRI groups in adults with AML and MDS receiving HCT.
METHODS:
Data Source
The CIBMTR collects consecutive detailed HCT data from a volunteer network of >450 transplant centers worldwide. These patient data, including the information from yearly longitudinal observation, are reported to a centralized statistical center of the CIBMTR research headquarters located at the Medical College of Wisconsin and the National Marrow Donor Program. The observational studies conducted by the CIBMTR meet the compliance requirements of all applicable Federal regulations in order to protect all human research subjects. The Medical College of Wisconsin Institutional Board and the Privacy Officer granted a waiver of informed consent for this study that follows Health Insurance Portability and Accountability Act regulations.
Patient Selection
Adult patients with AML or MDS who were 40–65 years old at their first HCT (2009–2015) and thereby potentially eligible to receive either MAC or RIC were included in this analysis. Peripheral blood, bone marrow and umbilical cord blood (UCB) graft sources and all related (except identical twin) and unrelated donor (URD) types24 were included. We excluded data from embargoed centers and those patients who were missing conditioning intensity, cytogenetic or pre-HCT disease status information, or their comprehensive research data or informed consent forms. CIBMTR consensus criteria were used to define the conditioning intensity.25 Cytogenetic risks of AML18 and MDS26 were classified as previously reported and considered for the revised DRI classification.17 For the purpose of this analysis, DRI was stratified as low/intermediate risk and high/very high risk since the number of patients receiving HCT for low risk DRI (n=157 with AML in CR with favorable cytogenetics) and very high risk DRI (n=90 with advanced AML with adverse cytogenetics) were small. More specifically, based on the revised DRI classification, low/intermediate risk DRI group included the AML in CR with favorable or intermediate cytogenetics, low risk MDS defined as ≤5% blasts (refractory anemia with or without ringed sideroblasts and refractory cytopenia with multilineage dysplasia), or high-risk (refractory anemia with excess blasts 1 or 2) MDS with intermediate cytogenetics at early stage prior to HCT.17 High/very high risk DRI group included the AML in CR with adverse cytogenetics, advanced stage (induction failure or active relapse) AML regardless of cytogenetic risk, early stage high-risk MDS with adverse cytogenetics, or advanced stage high-risk MDS with either intermediate or adverse cytogenetics.17
Study Endpoints
Clinical outcomes included non-relapse mortality (NRM), incidence of relapse, DFS and OS. NRM was defined as the time from HCT to death of any cause without evidence of AML or MDS relapse considering relapse as a competing event. Relapse was defined as recurrence of AML or MDS after HCT, and death in remission was considered as a competing event. DFS was defined as the time to AML or MDS relapse or death from any cause, while OS was defined as the time from HCT to death from any cause. Surviving patients were censored at time of last follow-up.
Statistical Analysis
In this observational retrospective study, Chi-square test for categorical variables and the Wilcoxon two sample tests for continuous variables were used to compare patient, disease and transplant related characteristics between conditioning intensity groups (MAC vs. RIC) within low/intermediate risk DRI and high/very high risk DRI risk cohorts separately. Cumulative incidence estimator was used to calculate probabilities of NRM and relapse adjusting for competing risks. The Kaplan-Meier method was used to estimate DFS and OS probabilities.27 Cox proportional hazards regression model was used to examine the association between treatment groups and DFS and OS outcomes within low/intermediate and high/very high risk DRI groups.28 We used the forward stepwise selection method to build the regression model for the NRM, relapse, DFS and OS outcomes. Regardless of level of significance, the conditioning intensity type (reference group; myeloablative) as the main interest of this study was included in all steps of model building. The effect of the conditioning intensity was assessed across low/intermediate and high/very high DRI categories. The risk factors with a significance level of p<0.05 were retained in the model. Any potential interaction between conditioning intensity and other significant covariates were examined and further adjustment applied if the interactions were significant. The Cox regression model was used to estimate adjusted DFS and OS probabilities, stratified by treatment groups, and weighted by the pooled sample proportion value for all significant risk factors. These adjusted probabilities estimate likelihood of outcomes in populations that have similar prognostic factors. All study analyses were performed by using SAS 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS:
Patient Characteristics
We identified 4387 adult patients who received their first allogeneic HCT for AML (68%) or MDS (32%) between 2009 and 2015 reported to CIBMTR. Patient and treatment characteristics are summarized in Table 1. DRI was stratified as low/intermediate risk (1539 patients received MAC and 999 RIC) and high/very high risk (1121 MAC and 728 RIC). Median age for the entire cohort was 56 years (range, 40–65) and the median follow-up of survivors was 46 months (range, 2–102). Half of the study patients (49.9%) had HCT-CI ≥3 and 40.8% had Karnofsky performance score of <90%. The majority (64.2%) were CMV seropositive. Disease status at HCT was first complete remission (CR1) in 58.5% and active leukemia in 9.2% of patients with AML, and advanced MDS in 65.5% of patients. Well-matched URD (40.0%) and HLA-identical sibling donor (MSD, 29.8%) were the predominant donor types used for HCT. Graft source was most often filgrastim-mobilized peripheral blood (75.2%), followed by UCB (13.6%) and bone marrow (11.2%). In vivo T-cell depletion with anti-thymocyte globulin (ATG) or Alemtuzumab was used in 26.7% of patients as part of conditioning. GVHD prophylaxis was most often tacrolimus-based with either methotrexate or mycophenolate mofetil (MMF, 76.3%).
Table 1.
Patient and HCT Characteristics
| Variable | MAC and Low/Intermediate risk | RIC and Low/Intermediate risk | MAC and High/Very High risk | RIC and High/Very High Risk |
|---|---|---|---|---|
| Number of patients | 1539 | 999 | 1121 | 728 |
| Number of centers | 100 | 105 | 100 | 95 |
| Age at HCT, years | ||||
| Median (range) | 53 (40–65) | 59 (40–65) | 55 (40–65) | 60 (40–65) |
| 40–50 | 620 (40) | 167 (17) | 347 (31) | 80 (11) |
| 51–60 | 768 (50) | 470 (47) | 583 (52) | 355 (49) |
| 61–65 | 151 (10) | 362 (36) | 191 (17) | 293 (40) |
| Recipient Sex | ||||
| Male | 781 (51) | 553 (55) | 639 (57) | 426 (59) |
| Female | 758 (49) | 446 (45) | 482 (43) | 302 (41) |
| Karnofsky score | ||||
| <90 | 525 (34) | 398 (40) | 498 (44) | 367 (50) |
| ≥90 | 991 (64) | 588 (59) | 594 (53) | 357 (49) |
| Missing | 23 (1) | 13 (1) | 29 (3) | 4 (<1) |
| HCT-CI | ||||
| 0 | 398 (26) | 198 (20) | 227 (20) | 107 (15) |
| 1 | 229 (15) | 153 (15) | 150 (13) | 96 (13) |
| 2 | 240 (16) | 129 (13) | 146 (13) | 89 (12) |
| 3 | 308 (20) | 187 (19) | 221 (20) | 120 (16) |
| 4 | 167 (11) | 122 (12) | 148 (13) | 91 (13) |
| ≥5 | 192 (12) | 201 (20) | 219 (20) | 215 (30) |
| Missing | 5 (0) | 9 (1) | 10 (1) | 10 (1) |
| Disease | ||||
| AML | 1294 (84) | 800 (80) | 606 (54) | 285 (39) |
| MDS | 245 (16) | 199 (20) | 515 (46) | 443 (61) |
| Disease status prior to HCT for AML | ||||
| Primary induction failure | 0 | 0 | 277 (25) | 113 (16) |
| CR1 | 940 (61) | 601 (60) | 118 (11) | 86 (12) |
| CR2 | 335 (22) | 188 (19) | 13 (1) | 7 (<1) |
| ≥CR3 | 19 (1) | 11 (1) | 0 | 3 (<1) |
| Relapse | 0 | 0 | 198 (18) | 76 (10) |
| Disease status prior to HCT for MDS | ||||
| MDS early | 174 (11) | 152 (15) | 68 (6) | 89 (12) |
| MDS advanced | 71 (5) | 47 (5) | 447 (40) | 354 (49) |
| WBC at diagnosis, (x 10^9) AML only* | ||||
| Median (range) | 10 (<1–450) | 7 (<1–428) | 6 (<1–399) | 5 (<1–375) |
| ≤ 10 | 602 (47) | 403 (50) | 347 (57) | 172 (60) |
| 11 – 100 | 437 (34) | 240 (30) | 163 (27) | 69 (24) |
| > 100 | 127 (10) | 62 (8) | 40 (7) | 18 (6) |
| Missing | 128 (10) | 95 (12) | 56 (9) | 26 (9) |
| Donor type | ||||
| HLA-identical sibling | 534 (35) | 228 (23) | 356 (32) | 191 (26) |
| Other relative | 71 (5) | 113 (12) | 62 (6) | 68 (9) |
| Well-matched (8/8) unrelated | 633 (41) | 340 (34) | 497 (44) | 284 (39) |
| Unrelated (≤7/8) and matching unknown | 133 (9) | 87 (9) | 114 (10) | 78 (11) |
| Umbilical cord blood | 168 (11) | 231 (23) | 92 (8) | 107 (15) |
| Donor/recipient sex match | ||||
| Male-Male | 501 (33) | 355 (36) | 407 (36) | 266 (37) |
| Male-Female | 436 (28) | 251 (25) | 310 (28) | 170 (23) |
| Female-Male | 276 (18) | 194 (19) | 232 (21) | 155 (21) |
| Female-Female | 320 (21) | 186 (19) | 171 (15) | 130 (18) |
| Missing | 6 (<1) | 13 (1) | 1 (<1) | 7 (<1) |
| Donor/Recipient CMV serostatus | ||||
| Recipient + | 986 (64) | 642 (64) | 716 (64) | 472 (65) |
| Recipient −/Donor − | 354 (23) | 210 (21) | 277 (25) | 173 (24) |
| Other | 199 (13) | 147 (15) | 128 (11) | 83 (11) |
| Conditioning regimen | ||||
| TBI/Cy ± Flu | 382 (25) | 259 (26) | 211 (19) | 125 (17) |
| TBI/Other | 68 (4) | 71 (7) | 59 (4) | 82 (11) |
| Bu/Cy | 476 (31) | - | 345 (31) | - |
| Bu/Flu ± TT | 531 (34) | 358 (36) | 422 (37) | 252 (35) |
| Flu/Mel ± TT | 24 (2) | 271 (27) | 21 (2) | 233 (32) |
| Other | 58 (3) | 40 (4) | 63 (5) | 36 (3) |
| ATG/Alemtuzumab | ||||
| Yes | 344 (22) | 322 (32) | 259 (23) | 247 (34) |
| No | 1191 (77) | 673 (67) | 862 (77) | 481 (66) |
| Missing | 4 (<1) | 4 (<1) | 0 | 0 |
| GVHD prophylaxis | ||||
| TCD/CD34 selected | 41 (3) | 35 (4) | 20 (2) | 26 (4) |
| Tac + MMF/MTX +/− others | 1250 (81) | 653 (65) | 940 (84) | 505 (69) |
| CSA + MMF/MTX +/− others | 187 (12) | 194 (19) | 105 (9) | 116 (16) |
| PT-Cy + others | 37 (2) | 75 (8) | 46 (4) | 48 (7) |
| Others/Missing | 24 (2) | 42 (4) | 10 (<1) | 33 (5) |
| Graft source | ||||
| Bone marrow | 181 (12) | 105 (11) | 132 (12) | 73 (10) |
| Peripheral blood | 1190 (77) | 663 (66) | 897 (80) | 548 (75) |
| Umbilical cord blood | 168 (11) | 231 (23) | 92 (8) | 107 (15) |
| Year of transplant | ||||
| 2009–2012 | 893(58) | 415 (42) | 610 (54) | 290 (39) |
| 2013–2015 | 646 (42) | 584 (59) | 511 (46) | 438 (61) |
| Median follow-up of survivors (range), months | 50 (4–98) | 37 (3–103) | 47 (3–98) | 38 (1–97) |
HR, hazard ratio. RIC, reduced-intensity conditioning. MAC, myeloablative conditioning. L/I, low/intermediate risk disease risk index (DRI). High-risk, high/very high risk DRI. HCT-CI, hematopoietic cell transplant comorbidity index.
Relapse and Non-Relapse Mortality
Adjusted 3-year probabilities of relapse were: 28% (95% CI 26–31) for MAC + low/intermediate risk DRI; 40% (95% CI 37–43) for RIC + low/intermediate risk DRI; 47% (95% CI 44–50) for MAC + high/very high risk DRI; and 52% (95% CI 48–56) for RIC + high/very high risk DRI groups (total p <0.001) (Figure 1). Corresponding adjusted 3-year probabilities of NRM were 25% (95% CI 22–27) for MAC + low/intermediate risk DRI; 19% (95% CI 16–21) for RIC + low/intermediate risk DRI; 26% (95% CI 23–28) for MAC + high/very high risk DRI and 22% (95% CI 19–25) for RIC + high/very high risk DRI groups (total p <0.001). In multiple regression analysis, in the low/intermediate risk DRI cohort, RIC compared to MAC was associated with significantly higher risk of relapse (HR=1.54, 95% CI 1.35–1.76; p<0.001), but lower risk of NRM (HR=0.74, 95% CI 0.62–0.88; p<0.001) (Table 2). In high/very high risk DRI cohort, RIC also led to a significantly higher risk of relapse (HR=1.23, 95% CI 1.08–1.41; p=0.002). However, in this cohort RIC resulted only in marginally lower NRM (HR=0.83, 95% CI 0.68–1.00; p=0.051) compared to MAC. We also examined the RIC and MAC cohorts separately and found that high/very high risk DRI is associated with significantly higher risk of relapse in both RIC (HR=1.51, 95% CI1.31–1.74; p<0.001) and MAC (HR=1.89, 95% CI 1.67–2.13; p<0.001) cohorts compared to low/intermediate risk DRI. However, NRM was not significantly influenced by the DRI risk in either conditioning intensity group.
Figure 1.
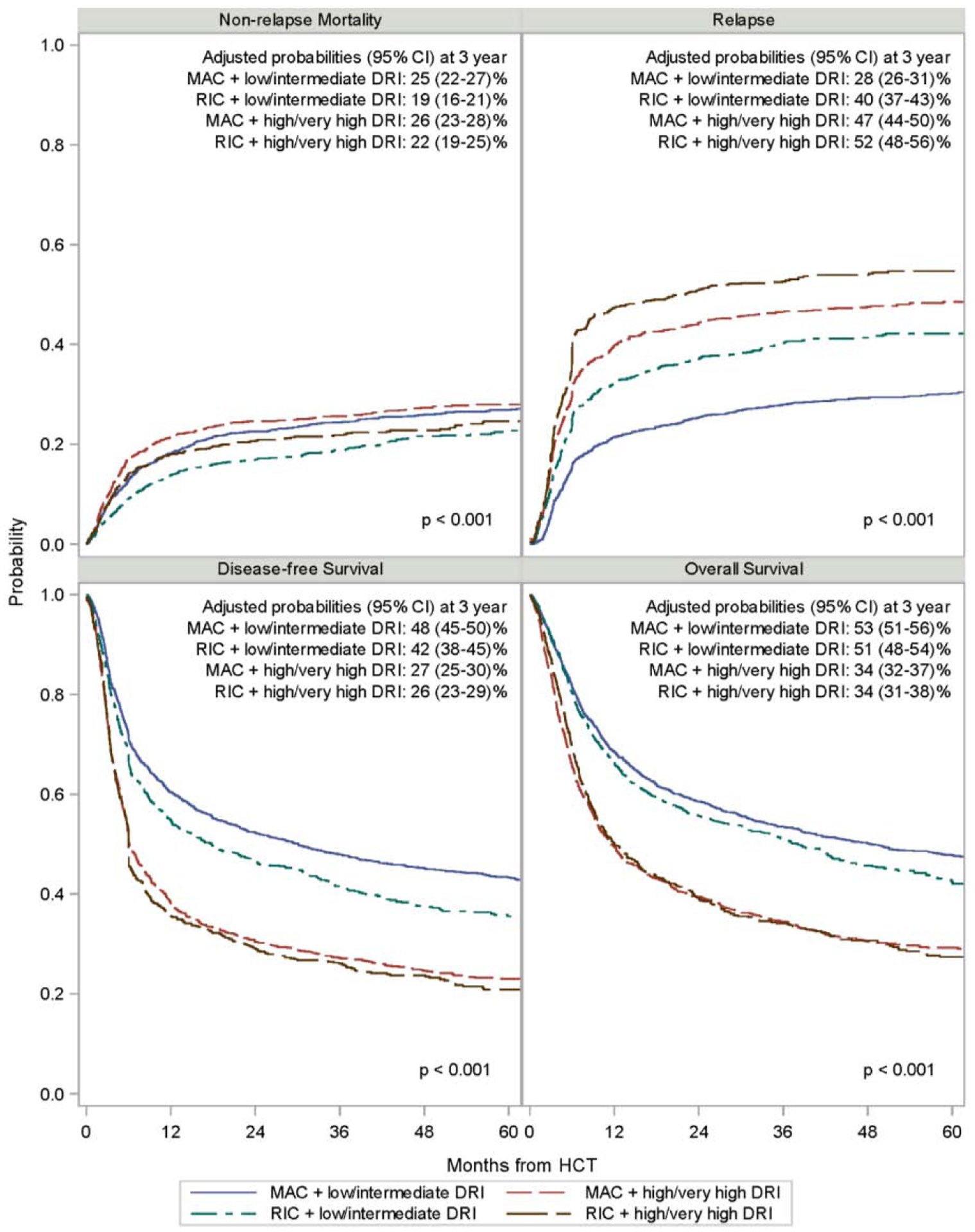
Adjusted clinical outcomes of AML and MDS HCT by DRI and conditioning intensity
Table 2:
Multivariable Analysis of Relapse and Non-Relapse Mortality
| Variables | Number | Relapse | Non-Relapse Mortality | ||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Main effect | <0.001 | <0.001 | |||
| RIC-L/I-Risk vs MAC-L/I-Risk: | 999/1539 | 1.54 (1.35–1.76) | <0.001 | 0.74 (0.62–0.88) | <0.001 |
| RIC-High-Risk vs MAC-High-Risk: | 728/1121 | 1.23 (1.08–1.41) | 0.0019 | 0.83(0.68–1.00) | 0.0510 |
| MAC-High-Risk vs MAC-L/I-Risk: | 1121/1539 | 1.89 (1.67–2.13) | <0.001 | 1.06 (0.91–1.24) | 0.453 |
| RIC-High-Risk vs RIC-L/I-Risk: | 728/999 | 1.51 (1.31–1.74) | <0.001 | 1.18(0.97–1.44) | 0.0966 |
| Age, years | 0.0002 | ||||
| 40–50 | 1214 | - | - | 1.00 | |
| 51–60 | 2176 | - | - | 1.24 (1.07–1.44) | 0.0040 |
| 61–65 | 997 | - | - | 1.45 (1.21–1.75) | <0.001 |
| Donor | <0.001 | <.0001 | |||
| HLA identical Sibling | 1309 | 1.00 | 1.00 | ||
| Mismatched relative (≥7/8) / Other relatives (missing HLA) | 314 | 0.81 (0.67–0.98) | 0.027 | 1.24 (0.93–1.65) | 0.14 |
| Matched unrelated donor (8/8) | 1754 | 0.79 (0.70–0.88) | <0.001 | 1.41 (1.21–1.65) | <0.001 |
| Unrelated (≤7/8) and matching unknown | 412 | 0.74 (0.62–0.88) | <0.001 | 2.02 (1.64–2.49) | <0.001 |
| Umbilical cord blood | 598 | 0.76 (0.65–0.89) | <0.001 | 2.18 (1.79–2.64) | <0.001 |
| HCT-CI | <.0001 | ||||
| 0 | 930 | - | - | 1.00 | |
| 1 | 628 | - | - | 1.02 (0.82–1.26) | 0.87 |
| 2 | 604 | - | - | 1.06 (0.86–1.31) | 0.60 |
| ≥3 | 2191 | - | - | 1.34 (1.15–1.57) | <0.001 |
| Missing | 34 | - | - | 0.66 (0.29–1.48) | 0.31 |
| Year of Transplant | <.0001 | ||||
| 2009–2012 | 2208 | - | - | 1.35 (1.20–1.53) | <0.001 |
| 2013–2015 | 2179 | - | - | 1.00 | |
HR, hazard ratio. RIC, reduced-intensity conditioning. MAC, myeloablative conditioning. L/I, low/intermediate risk disease risk index (DRI). High-risk, high/very high risk DRI. HCT-CI, hematopoietic cell transplant comorbidity index.
Donor type was the only additional factor associated with risk of relapse. All donor types except MSD led to lower relapse (p<0.001). Significantly higher NRM was observed with increasing patient age (p<0.001), HCT-CI ≥3 (p<0.001), HCT performed 2009–2012 (p<0.001) and HCT using either matched or mismatched URD or UCB donors (p<0.001).
Disease-Free and Overall Survival
Adjusted 3-year probabilities of DFS were: 48% (95% CI 45–50) for MAC + low/intermediate risk DRI; 42% (95% CI 38–45) for RIC + low/intermediate risk DRI; 27% (95% CI 25–30) for MAC + high/very high risk DRI; and 26% (95% CI 23–29) for RIC + high/very high risk DRI groups (total p <0.001). Corresponding adjusted 3-year probabilities of OS were 53% (95% CI 51–56) for MAC + low/intermediate risk DRI; 51% (95% CI 48–54) for RIC + low/intermediate risk DRI; 34% (95% CI 32–37) for MAC + high/very high risk DRI and 34% (95% CI 31–38) for RIC + high/very high risk DRI groups (total p <0.001). In multiple regression analysis, in the low/intermediate risk DRI cohort, RIC resulted in statistically significantly worse DFS (HR=1.19, 95% CI 1.07–1.33; p=0.001), but similar OS (HR=1.11, 95% CI 0.99–1.25; p=0.06) compared with MAC (Table 3). In high/very high risk DRI cohort, however, RIC and MAC had similar DFS (HR=1.07, 95% CI 0.96–1.19; p=0.24) and OS (HR=1.0, 95% CI 0.90–1.12; p=0.98). Importantly, high/very high risk DRI compared to low/intermediate risk DRI was associated with significantly worse DFS in both RIC (HR=1.63, 95% CI 1.45–1.83; p<0.001) and MAC (HR=1.82, 95% CI 1.65–2.00; p<0.001) cohorts. Similarly, OS in RIC (HR=1.60, 95% CI 1.42–1.80; p<0.001) and MAC (HR=1.77, 95% CI 1.61–1.96; p<0.001) cohorts was also significantly worse with high/very high risk DRI. Karnofsky score <90% (p<0.001), HCT-CI ≥3 (p<0.001), HCT performed 2009–2012 (p≤0.02), the use of in vivo T-cell depletion (p≤0.005) and UCB donor for HCT (p≤0.004) were additional factors associated with significantly worse DFS and OS. The use of ≤7/8 HLA-matched URD led to worse OS (HR=1.26, 95% CI 1.09 – 1.45; p=0.001), but not DFS (HR=1.11, 95% CI 0.97–1.27; p=0.14).
Table 3:
Multivariable Analysis of Disease-Free and Overall Survival
| Variables | Number | Disease-Free Survival | Overall Survival | ||
|---|---|---|---|---|---|
| HR 95% CI) | P value | HR (95%CI) | P value | ||
| Main effect | <0.001 | <0.001 | |||
| RIC-L/I-Risk vs MAC-L/I-Risk: | 999/1539 | 1.19 (1.07–1.33) | 0.0012 | 1.11 (0.99–1.25) | 0.0611 |
| RIC-High-Risk vs MAC-High-Risk: | 728/1121 | 1.07 (0.96–1.19) | 0.240 | 1.00 (0.90–1.12) | 0.978 |
| MAC-High-Risk vs MAC-L/I-Risk: | 1121/1539 | 1.82 (1.65–2.00) | <0.001 | 1.77 (1.61–1.96) | <0.001 |
| RIC-High-Risk vs RIC-L/I-Risk: | 728/999 | 1.63 (1.45–1.83) | <0.001 | 1.60 (1.42–1.80) | <0.001 |
| Donor | 0.0041 | <.0001 | |||
| HLA identical Sibling | 1309 | 1.00 | 1.00 | ||
| Mismatched relative (≥7/8) / Other relatives (missing HLA) | 314 | 0.96 (0.80–1.17) | 0.70 | 0.98 (0.83–1.17) | 0.85 |
| Matched unrelated donor (8/8) | 1754 | 0.96 (0.88–1.06) | 0.44 | 0.99 (0.90–1.10) | 0.91 |
| Unrelated (≤7/8) and matching unknown | 412 | 1.11 (0.97–1.27) | 0.14 | 1.26 (1.09–1.45) | 0.0014 |
| Umbilical cord blood | 598 | 1.20 (1.06–1.37) | 0.0041 | 1.49 (1.32–1.69) | <0.001 |
| Karnofsky Score | <.0001 | <.0001 | |||
| <90 | 1788 | 1.00 | 1.00 | ||
| ≥90 | 2530 | 0.79 (0.74–0.86) | <0.001 | 0.76 (0.70–0.82) | <0.001 |
| Missing | 69 | 0.97 (0.73–1.29) | 0.83 | 1.08 (0.81–1.45) | 0.59 |
| HCT-CI | <.0001 | <.0001 | |||
| 0 | 930 | 1.00 | 1.00 | ||
| 1 | 628 | 1.08 (0.95–1.23) | 0.25 | 1.09 (0.95–1.25) | 0.20 |
| 2 | 604 | 1.07 (0.94–1.22) | 0.28 | 1.14 (0.99–1.30) | 0.065 |
| ≥3 | 2191 | 1.24 (1.13–1.37) | <0.001 | 1.35 (1.22–1.50) | <0.001 |
| Missing | 34 | 1.40 (0.87–2.23) | 0.17 | 1.21 (0.80–1.83) | 0.36 |
| GVHD prophylaxis | 0.0157 | ||||
| TCD/CD34 | 122 | 1.00 | - | - | |
| Tac + MMF/MTX +/− others | 3348 | 0.95 (0.76–1.20) | 0.67 | - | - |
| CSA + MMF/MTX +/− others | 602 | 1.15 (0.90–1.46) | 0.26 | - | - |
| Post-cy + others | 206 | 1.02 (0.76–1.38) | 0.88 | - | - |
| Others/Missing | 109 | 1.15 (0.84–1.57) | 0.39 | - | - |
| ATG/Alemtuzumab | 0.0011 | 0.0053 | |||
| Yes | 1172 | 1.00 | 1.00 | ||
| No | 3207 | 0.86 (0.79–0.94) | <0.001 | 0.88 (0.80–0.96) | 0.0049 |
| Missing | 8 | 0.36 (0.11–1.15) | 0.085 | 0.30 (0.07–1.19) | 0.086 |
| Year of transplant | 0.0204 | 0.0003 | |||
| 2013–2015 | 2179 | 1.00 | 1.00 | ||
| 2009–2012 | 2208 | 1.10 (1.01–1.18) | 0.020 | 1.16 (1.07–1.26) | <0.001 |
GVHD, graft-versus-host disease. IPS, idiopathic pulmonary syndrome. ARDS, acute respiratory distress syndrome.
Relapse of the primary disease (AML or MDS) was the most common cause of death in all groups, ranging from 22% after MAC in low/intermediate risk DRI cohort to 40% after RIC in high/very high risk DRI cohort (Supplemental figure). GVHD followed by infection and organ failure were the other common causes of death and were of similar frequency between the 4 groups.
DISCUSSION:
In this large observational study of HCT outcomes stratified by DRI, we observed that in adults with AML or MDS, relapse is significantly influenced by both DRI and conditioning intensity. DRI risk modified the impact of the association between conditioning intensity and DFS as MAC significantly improved DFS in low/intermediate DRI group, but not in the high/very high risk group. While all AML patients with low/intermediate DRI were in CR prior to HCT, 75% of AML patients with high/very high risk DRI had morphologically persistent leukemia and therefore were less likely to benefit from additional chemotherapy intensification. Consistent with previous CIBMTR report by Armand et al.,17 we observed a significant influence of DRI on OS in our study. However, the impact of conditioning intensity on OS was not significant in either DRI risk cohorts.
Various prior studies report inconsistent findings about conditioning intensity, which fail to clarify the decision-making for alloHCT in AML and MDS.3,7,9,10,13,14,29 This inconsistency is likely due to the different methods used across these studies for pre-transplant disease risk assessment. Thus, DRI, as the only validated prognostic tool available for pre-HCT disease-risk assessment, can further inform the decision making in regards to the conditioning intensity choice. While the BMT CTN 0901 randomized trial showed a benefit of MAC over RIC in patients with AML or MDS, we observed similar results only in low/intermediate risk DRI but not in high/very high risk DRI cohort.3 The difference in these findings can be explained by expected higher proportion of patients with low/intermediate risk DRI (75% of AML patients) in BMT CTN 0901 study since trial participation was limited to patients in morphological complete remission. In addition, while BMT CTN 0901 trial only included patients with HCT-CI ≤4, our study had a high proportion of patients with many comorbidities across all conditioning intensity and DRI groups. Similarly, a DFS advantage with MAC over RIC was also reported in recent European Group for Blood and Marrow Transplantation (EBMT) study in <50 years old patients with AML in CR1 who had detectable MRD at transplant.29 In contrast, a large EBMT analysis comparing MAC vs. RIC in 1555 patients with AML that included advanced disease (29%) cases at HCT found conditioning intensity not affecting the DFS or OS, despite RIC resulting in higher relapse risk in patients younger than 50 years of age (72% of the study population).14 Similarly, no clinical outcome differences have been reported between MAC and RIC in several prior multicenter or single institution retrospective studies that included patients with AML and MDS who had either adverse risk cytogenetics or advanced disease at HCT.9,10,13 These reported data in patients with high-risk AML and MDS are consistent with our observation of MAC and RIC resulting in similar DFS and OS in our high/very high risk DRI cohort.
Although we observed higher risk of primary disease relapse after RIC HCT which was the leading cause of death, net DFS and OS outcomes were similar in high/very high risk DRI cohort due to slightly lower relapse risk being offset by slightly higher NRM with use of MAC. In contrast to our observation, one registry study reported relapse being not significantly affected by conditioning intensity in patients receiving HCT for high-risk AML with monosomal karyotype.10 However, that study also showed similar DFS and OS outcomes between MAC and RIC despite higher observed NRM with MAC HCT. Another large registry study by EBMT examining the effect of conditioning intensity in 40–60 years old adults with AML reported similar DFS and OS in a setting of lower relapse and higher NRM rates with MAC in both high and intermediate cytogenetic risk groups.9 While this EBMT report focused mainly on the cytogenetic risk, our study considered both the disease status and cytogenetic risk as part of DRI in each individual patient, which can explain some of the relapse and survival outcome differences seen between the studies. We thereby conclude that MAC in general leads to modestly higher NRM, but often lower relapse risk compared to RIC, particularly in patients with low/intermediate risk DRI or with detectable MRD at transplant. However, in patients with high/very high risk DRI, where reduction in relapse incidence with MAC is less prominent, the higher NRM does not lead to overall improvement in survival.
An inherent limitation in all retrospective studies, our analysis could not adjust for the clinical decision-making factors (specific comorbidities, performance score, disease status, genetic risk etc.) which prompted treating physicians to choose MAC or RIC for each individual study patient. We were unable to adjust for factors such as molecular abnormalities or MRD status that can also affect the risk of relapse and subsequent survival after HCT.5,6,29–36 However, since no widely used standardized methods for the high-sensitivity quantification of residual disease burden prior to HCT are yet widely available for AML(5), future studies could prospectively reexamine the role of conditioning intensity on HCT outcomes where MRD status and molecular genetic risk are both considered. Novel MAC or augmented anti-neoplastic regimens with a better safety profile could benefit patients, particularly those with high/very high risk DRI at highest risk of relapse.
Supplementary Material
Highlights:
MAC results in lower relapse and better DFS after HCT for AML/MDS with low/intermediate risk DRI
MAC and RIC yield similar DFS and OS for AML/MDS with high/very high risk DRI
Acknowledgements
The CIBMTR is supported primarily by Public Health Service grant/cooperative agreement U24CA076518 with the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); grant/cooperative agreement U24HL138660 with NHLBI and NCI; grant U24CA233032 from the NCI; grants OT3HL147741, R21HL140314 and U01HL128568 from the NHLBI; contract HHSH250201700006C with Health Resources and Services Administration (HRSA); grants N00014-18-1-2888 and N00014-17-1-2850 from the Office of Naval Research; subaward from prime contract award SC1MC31881-01-00 with HRSA; subawards from prime grant awards R01HL131731 and R01HL126589 from NHLBI; subawards from prime grant awards 5P01CA111412, 5R01HL129472, R01CA152108, 1R01HL131731, 1U01AI126612 and 1R01CA231141 from the NIH; and commercial funds from Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies; Allovir, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Anthem, Inc.; Astellas Pharma US; Atara Biotherapeutics, Inc.; BARDA; Be the Match Foundation; bluebird bio, Inc.; Boston Children’s Hospital; Bristol Myers Squibb Co.; Celgene Corp.; Children’s Hospital of Los Angeles; Chimerix, Inc.; City of Hope Medical Center; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Dana Farber Cancer Institute; Enterprise Science and Computing, Inc.; Fred Hutchinson Cancer Research Center; Gamida-Cell, Ltd.; Genzyme; Gilead Sciences, Inc.; GlaxoSmithKline (GSK); HistoGenetics, Inc.; Immucor; Incyte Corporation; Janssen Biotech, Inc.; Janssen Pharmaceuticals, Inc.; Janssen Research & Development, LLC; Janssen Scientific Affairs, LLC; Japan Hematopoietic Cell Transplantation Data Center; Jazz Pharmaceuticals, Inc.; Karius, Inc.; Karyopharm Therapeutics, Inc.; Kite, a Gilead Company; Kyowa Kirin; Magenta Therapeutics; Mayo Clinic and Foundation Rochester; Medac GmbH; Mediware; Memorial Sloan Kettering Cancer Center; Merck & Company, Inc.; Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; Mundipharma EDO; National Marrow Donor Program; Novartis Oncology; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncoimmune, Inc.; OptumHealth; Orca Biosystems, Inc.; PCORI; Pfizer, Inc.; Phamacyclics, LLC; PIRCHE AG; Regeneron Pharmaceuticals, Inc.; REGiMMUNE Corp.; Sanofi Genzyme; Seattle Genetics; Shire; Sobi, Inc.; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; The Medical College of Wisconsin; University of Minnesota; University of Pittsburgh; University of Texas-MD Anderson; University of Wisconsin - Madison; Viracor Eurofins and Xenikos BV. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Alyea EP, Kim HT, Ho V, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2006;12:1047–55. [DOI] [PubMed] [Google Scholar]
- 2.Craddock CF. Full-intensity and reduced-intensity allogeneic stem cell transplantation in AML. Bone marrow transplantation 2008;41:415–23. [DOI] [PubMed] [Google Scholar]
- 3.Scott BL, Pasquini MC, Logan BR, et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017;35:1154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott BL. Long-Term Follow up of BMT CTN 0901, a Randomized Phase III Trial Comparing Myeloablative (MAC) to Reduced Intensity Conditioning (RIC) Prior to Hematopoietic Cell Transplantation (HCT) for Acute Myeloid Leukemia (AML) or Myelodysplasia (MDS) (MAvRIC Trial). Biology of Blood and Marrow Transplantation 2020; 26:S11. [Google Scholar]
- 5.Hourigan CS, Dillon LW, Gui G, et al. Impact of Conditioning Intensity of Allogeneic Transplantation for Acute Myeloid Leukemia With Genomic Evidence of Residual Disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019:JCO1903011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ustun C, Courville E, DeFor T, et al. Myeloablative, but not Reduced-Intensity, Conditioning Overcomes the Negative Effect of Flow-Cytometric Evidence of Leukemia in AML. Biol Blood Marrow Transplant. 2016. April; 22(4): 669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martino R, de Wreede L, Fiocco M, et al. Comparison of conditioning regimens of various intensities for allogeneic hematopoietic SCT using HLA-identical sibling donors in AML and MDS with <10% BM blasts: a report from EBMT. Bone marrow transplantation 2013;48:761–70. [DOI] [PubMed] [Google Scholar]
- 8.Solh MM, Solomon SR, Morris LE, Zhang X, Holland HK, Bashey A. The Dilemma of Conditioning Intensity: When Does Myeloablative Conditioning Improve Outcomes for Allogeneic Hematopoietic Cell Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2019;25:606–12. [DOI] [PubMed] [Google Scholar]
- 9.Passweg JR, Labopin M, Cornelissen J, et al. Conditioning intensity in middle-aged patients with AML in first CR: no advantage for myeloablative regimens irrespective of the risk group-an observational analysis by the Acute Leukemia Working Party of the EBMT. Bone marrow transplantation 2015;50:1063–8. [DOI] [PubMed] [Google Scholar]
- 10.Poire X, Labopin M, Cornelissen JJ, et al. Outcome of conditioning intensity in acute myeloid leukemia with monosomal karyotype in patients over 45 year-old: A study from the acute leukemia working party (ALWP) of the European group of blood and marrow transplantation (EBMT). American journal of hematology 2015;90:719–24. [DOI] [PubMed] [Google Scholar]
- 11.Sebert M, Porcher R, Robin M, et al. Equivalent outcomes using reduced intensity or conventional myeloablative conditioning transplantation for patients aged 35 years and over with AML. Bone marrow transplantation 2015;50:74–81. [DOI] [PubMed] [Google Scholar]
- 12.Luger SM, Ringden O, Zhang MJ, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone marrow transplantation 2012;47:203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khabori MA, El-Emary M, Xu W, et al. Impact of intensity of conditioning therapy in patients aged 40–60 years with AML/myelodysplastic syndrome undergoing allogeneic transplantation. Bone marrow transplantation 2011;46:516–22. [DOI] [PubMed] [Google Scholar]
- 14.Ringden O, Labopin M, Ehninger G, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009;27:4570–7. [DOI] [PubMed] [Google Scholar]
- 15.Weisdorf DJ. Reduced-intensity versus myeloablative allogeneic transplantation. Hematol Oncol Stem Cell Ther 2017;10:321–6. [DOI] [PubMed] [Google Scholar]
- 16.Savani BN, Labopin M, Kroger N, et al. Expanding transplant options to patients over 50 years. Improved outcome after reduced intensity conditioning mismatched-unrelated donor transplantation for patients with acute myeloid leukemia: a report from the Acute Leukemia Working Party of the EBMT. Haematologica 2016;101:773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 2014;123:3664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armand P, Kim HT, Zhang MJ, et al. Classifying cytogenetics in patients with acute myelogenous leukemia in complete remission undergoing allogeneic transplantation: a Center for International Blood and Marrow Transplant Research study. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2012;18:280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oran B, Popat U, Rondon G, et al. Significance of persistent cytogenetic abnormalities on myeloablative allogeneic stem cell transplantation in first complete remission. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2013;19:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemmati PG, Terwey TH, Na IK, et al. Allogeneic stem cell transplantation for refractory acute myeloid leukemia: a single center analysis of long-term outcome. European journal of haematology 2015. [DOI] [PubMed] [Google Scholar]
- 21.Michallet M, Thomas X, Vernant JP, et al. Long-term outcome after allogeneic hematopoietic stem cell transplantation for advanced stage acute myeloblastic leukemia: a retrospective study of 379 patients reported to the Societe Francaise de Greffe de Moelle (SFGM). Bone marrow transplantation 2000;26:1157–63. [DOI] [PubMed] [Google Scholar]
- 22.Fung HC, Stein A, Slovak M, et al. A long-term follow-up report on allogeneic stem cell transplantation for patients with primary refractory acute myelogenous leukemia: impact of cytogenetic characteristics on transplantation outcome. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2003;9:766–71. [DOI] [PubMed] [Google Scholar]
- 23.Oyekunle AA, Kroger N, Zabelina T, et al. Allogeneic stem-cell transplantation in patients with refractory acute leukemia: a long-term follow-up. Bone marrow transplantation 2006;37:45–50. [DOI] [PubMed] [Google Scholar]
- 24.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2008;14:748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2009;15:1628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armand P, Deeg HJ, Kim HT, et al. Multicenter validation study of a transplantation-specific cytogenetics grouping scheme for patients with myelodysplastic syndromes. Bone marrow transplantation 2010;45:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan ELM P Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81. [Google Scholar]
- 28.Cox DR. Regression models and life tables. Journal of the Royal Stastistical Society 1972:187–220. [Google Scholar]
- 29.Gilleece MH, Labopin M, Yakoub-Agha I, et al. Measurable residual disease, conditioning regimen intensity, and age predict outcome of allogeneic hematopoietic cell transplantation for acute myeloid leukemia in first remission: A registry analysis of 2292 patients by the Acute Leukemia Working Party European Society of Blood and Marrow Transplantation. American journal of hematology 2018;93:1142–52. [DOI] [PubMed] [Google Scholar]
- 30.Araki D, Wood BL, Othus M, et al. Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia: Time to Move Toward a Minimal Residual Disease-Based Definition of Complete Remission? Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016;34:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buckley SA, Wood BL, Othus M, et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: a meta-analysis. Haematologica 2017;102:865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129:424–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Getta BM, Devlin SM, Levine RL, et al. Multicolor Flow Cytometry and Multigene Next-Generation Sequencing Are Complementary and Highly Predictive for Relapse in Acute Myeloid Leukemia after Allogeneic Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2017;23:1064–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kharfan-Dabaja MA, Komrokji RS, Zhang Q, et al. TP53 and IDH2 Somatic Mutations Are Associated With Inferior Overall Survival After Allogeneic Hematopoietic Cell Transplantation for Myelodysplastic Syndrome. Clin Lymphoma Myeloma Leuk 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thol F, Gabdoulline R, Liebich A, et al. Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood 2018;132:1703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindsley RC, Saber W, Mar BG, et al. Prognostic Mutations in Myelodysplastic Syndrome after Stem-Cell Transplantation. The New England journal of medicine 2017;376:536–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


