Abstract
The American Thoracic Society Core Curriculum updates clinicians annually in adult and pediatric pulmonary disease, medical critical care, and sleep medicine in a 3- to 4-year recurring cycle of topics. The topics of the 2020 Pulmonary Core Curriculum include pulmonary vascular disease (submassive pulmonary embolism, chronic thromboembolic pulmonary hypertension, and pulmonary hypertension) and pulmonary infections (community-acquired pneumonia, pulmonary nontuberculous mycobacteria, opportunistic infections in immunocompromised hosts, and coronavirus disease [COVID-19]).
Keywords: submassive pulmonary embolism, nontuberculous mycobacteria, COVID-19, community-acquired pneumonia, pulmonary hypertension
Key Points
Risk stratification and treatment (particularly reperfusion therapies) for submassive pulmonary embolism involve a complex decision-making process that can be best accomplished with involvement of a multidisciplinary pulmonary embolism response team.
Echocardiography is the recommended screening test for pulmonary hypertension. To confirm a diagnosis of pulmonary hypertension, right heart catheterization is required, with guidelines now recommending a mean pulmonary arterial pressure cutoff of >20 mm Hg to make a diagnosis of pulmonary hypertension.
The treatments available for chronic thromboembolic pulmonary hypertension have expanded to include medical, surgical, and percutaneous options. Thus, selection of the most appropriate treatment strategy requires multidisciplinary assessment by a center with chronic thromboembolic pulmonary hypertension expertise.
Recent updates in the guidelines for diagnosis and treatment of community-acquired pneumonia have downplayed the role of sputum cultures and procalcitonin in nonsevere community-acquired pneumonia.
The nodular–bronchiectatic phenotype of nontuberculous mycobacterial pulmonary disease is commonly seen in tall, thin postmenopausal women without significant smoking history, and the fibrocavitary phenotype predominantly affects elderly male smokers with underlying tobacco-related lung disease. Treatment of Mycobacterium avium complex pulmonary disease should be continued for 12 months beyond negative culture conversion.
Opportunistic infections in the immunocompromised host carry high morbidity and mortality. Culture confirmation and histopathologic confirmation are often the gold-standard diagnostics tools but lack sensitivity. Laboratory tests (polymerase chain reaction [PCR] assays, antigen testing, and serology) in conjunction with compatible clinical and radiologic findings are alternative tools that can rapidly and accurately aid in the diagnosis.
The test currently recommended for the diagnosis of coronavirus disease (COVID-19) is the nasopharyngeal swab for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse transcription PCR. Treatment focuses on supportive care, with close monitoring for rapidly increasing oxygen demands and impending respiratory failure. Although there are no randomized controlled trials of ventilator management of severe COVID-19, use of strategies beneficial in other causes of acute respiratory distress syndrome is recommended. COVID-19–specific therapies are under intense investigation with preliminary data emerging.
Submassive Pulmonary Embolism
Rami Alashram and Parth Rali
Pulmonary embolism is the third most common cause of death in hospitalized patients, with a presentation that ranges from no symptoms to sudden death (1). In the absence of hypotension, pulmonary embolism with objective evidence of right ventricular dysfunction noted on computed tomographic (CT) pulmonary angiography (CTPA) or transthoracic echocardiography and/or because of cardiac biomarker elevation (BNP [brain natriuretic peptide], NT-proBNP [N-terminal pro-BNP], or troponin) is termed submassive with right ventricular strain by the American Heart Association or intermediate risk by the European Society of Cardiology (2, 3). In addition, the European Society of Cardiology incorporates the Pulmonary Embolism Severity Index (PESI) or the simplified PESI, validated prognostic scores, into pulmonary embolism classification and further subclassifies intermediate-risk pulmonary embolism into two categories: intermediate–low risk and intermediate–high risk (2) (Table 1).
Table 1.
Definitions of submassive (intermediate-risk) pulmonary embolism according to the American Heart Association and the European Society of Cardiology guidelines
| Shock | PESI Class III–V or sPESI Score ≥ 1 | RV Dysfunction on CTPA or TTE | Elevated Troponin | Elevated NT-proBNP or BNP | EKG (RV Strain Pattern) | Available Treatment Options | |
|---|---|---|---|---|---|---|---|
| AHA 2011 | |||||||
| Submassive with RV strain (any one positive) | No | N/A | + | + | + | + | |
| ESC 2019 | |||||||
| Intermediate–low risk (either one or none positive for RV dysfunction and elevated troponin) | No | + | ± | ± | N/A | N/A | |
| Intermediate–high risk (both positive for RV dysfunction and elevated troponin) | No | + | + | + | N/A† | N/A |
Definition of abbreviations: AHA = American Heart Association; BNP = brain natriuretic peptide; CTPA = computed tomographic pulmonary angiography; EKG = electrocardiogram; ESC = European Society of Cardiology; N/A = not applicable; NT-proBNP = N-terminal pro-BNP; PESI = Pulmonary Embolism Severity Index; RV = right ventricular; sPESI = simplified PESI; tPA = tissue plasminogen activator; TTE = transthoracic echocardiography.
Reperfusion therapies should be considered on a case-by-case basis, and in a multidisciplinary pulmonary embolism response team setting, given very limited evidence.
Elevation of NT-proBNP or BNP may provide additional prognostic information but is not used to classify pulmonary embolism as per ESC guidelines.
Use only when therapeutic anticoagulation is absolutely contraindicated.
The clinical course of submassive pulmonary embolism is highly variable. A subset of patients will progress to high-risk (massive) pulmonary embolism (pulmonary embolism with hypotension) (2, 3). An elevated PESI or simplified PESI score, severe pulmonary embolism–related functional impairment, and signs of reduced end-organ perfusion (e.g., increased lactate or renal impairment) are associated with an increased risk of hemodynamic deterioration (1).
The primary focus of submassive pulmonary embolism management is prompt and effective anticoagulation, unless contraindicated (1, 4). Advanced therapies, including systemic thrombolysis and catheter-based treatments (catheter-directed thrombolysis and catheter-based embolectomy), are available to treat selected patients with submassive pulmonary embolism (1, 4). These therapies have been shown to reduce clot burden, improve hemodynamics, and improve right ventricular function in the short term. However, their ability to impact short- and long-term mortality, long-term symptoms, and functional status has not been clearly established (1). Advanced therapies are not without risk, as bleeding and procedure-related complications can be fatal (1, 4). Quantifying these risks is critical during decision-making and can be achieved by using available bleeding-risk scores such as the El Registro Informatizado de Pacientes con Enfermedad TrombóEmbolica score, which assesses the risk of major bleeding during anticoagulant therapy, and the Pulmonary Embolism–Intracranial Hemorrhage score, which assesses the risk of intracranial hemorrhage (1, 4).
Advanced therapies are most appropriate for those patients with the highest mortality risk (i.e., clinical deterioration) and are avoided in patients with an unacceptably high risk of bleeding (particularly intracranial hemorrhage). The location of the thrombus, operator expertise, and individual patient preference should also be weighed when considering advanced therapies. The extent of the thrombus by itself does not necessarily inform the need for intervention (1, 4). Surgical embolectomy can be considered when there is an absolute contraindication to thrombolysis, failure of thrombolysis, or clot traversing the foramen ovale (4). Given uncertainty in the optimal management of some patients with life-threatening pulmonary embolism, formal multidisciplinary teams such as the pulmonary embolism response team can leverage the expertise of local clinical specialists who can provide treatment recommendations in a time-sensitive manner (1, 4).
References
- 1.Giri J, Sista AK, Weinberg I, Kearon C, Kumbhani DJ, Desai ND, et al. Interventional therapies for acute pulmonary embolism: current status and principles for the development of novel evidence. A scientific statement from the American Heart Association. Circulation. 2019;140:e774–e801. doi: 10.1161/CIR.0000000000000707. [DOI] [PubMed] [Google Scholar]
- 2.Konstantinides SV, Meyer G. The 2019 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2019;40:3453–3455. doi: 10.1093/eurheartj/ehz726. [DOI] [PubMed] [Google Scholar]
- 3.Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, et al. American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 4.Rali PM, Criner GJ. Submassive pulmonary embolism. Am J Respir Crit Care Med. 2018;198:588–598. doi: 10.1164/rccm.201711-2302CI. [DOI] [PubMed] [Google Scholar]
Chronic Thromboembolic Pulmonary Hypertension
Tyler Peck and Alison Witkin
Chronic thromboembolic pulmonary hypertension (CTEPH) is the most feared long-term complication of acute pulmonary embolism. The incidence of CTEPH among survivors of pulmonary embolism is estimated to be as high as 4% (1). This syndrome is defined by the accumulation of occlusive fibrotic tissue within the pulmonary vasculature, resulting in elevations in the resting mean pulmonary artery pressure (>20 mm Hg) and an increased pulmonary vascular resistance (PVR; ≥3 Wood units [WU]) (2). More recently, there has been increased recognition that CTEPH represents the most severe end of the spectrum of chronic pulmonary embolism. Patients with pulmonary embolism can also develop chronic thromboembolic disease (CTED), involving similar pathophysiology and symptoms without resting pulmonary hypertension. These patients may report exercise limitation owing to either exercise-induced pulmonary hypertension or increased dead-space ventilation.
Clinical Features
Dyspnea and exercise intolerance are common in both CTEPH and CTED, although symptoms are nonspecific and may be mistaken for those of other diseases (3). As many patients with diagnosed CTEPH have not previously received a diagnosis of acute pulmonary embolism or deep vein thrombosis (DVT), it is important to maintain a high degree of clinical suspicion. Risk factors for the development of CTEPH include antiphospholipid antibody syndrome, chronic inflammatory states, splenectomy, and prior pulmonary embolism (4).
Diagnostic Testing
All patients with diagnosed pulmonary hypertension should receive screening for CTEPH, as it is the only form of pulmonary hypertension that is potentially curable without lung transplantation. The ventilation–perfusion (/) lung scan is the preferred screening test. Although CTPA often shows signs of CTEPH, it is less sensitive than a / scan in detecting the vascular filling defects associated with CTEPH (Video 1) (5). Right-heart catheterization (RHC) is required to quantify the presence and severity of pulmonary hypertension. Pulmonary angiography is performed to define the location and severity of pulmonary vascular obstruction, an important step in assessing treatment options. For patients with CTED, exercise testing may be useful to help quantify the degree of disease and to evaluate for a pulmonary vascular cause of exercise limitation.
Video 1.
This video shows the complementary use of ventilation–perfusion (/) scan and pulmonary angiography in the diagnosis and treatment of chronic thromboembolic pulmonary hypertension. The / scan shows absent perfusion to the bilateral lower lobes. A right-heart catheterization and pulmonary angiogram is then performed (only the right side is shown), confirming complete occlusion of the descending pulmonary artery with pulmonary hypertension. The patient underwent successful pulmonary thromboendarterectomy, and the surgical specimen is shown.
Treatment Options
Pulmonary thromboendarterectomy (PTE) is first-line therapy for patients with surgically accessible disease. Although invasive, PTE offers an opportunity for cure. Survival and functional status are markedly improved in appropriately selected patients (6). For patients who are not surgical candidates, either owing to comorbidities or disease location, or for those who have residual disease despite PTE, balloon pulmonary angioplasty and pharmacologic therapy are available alternatives. Balloon pulmonary angioplasty is a staged procedure and has been associated with improvements in both hemodynamics and symptoms as measured by functional class (7). Riociguat, a soluble guanylate cyclase stimulator, is currently the only U.S. Food and Drug Administration (FDA)-approved medical therapy for residual or inoperable CTEPH. All patients should be treated with indefinite anticoagulation.
Role of Multidisciplinary Care in CTEPH
As CTEPH is a rare disease with complex and invasive treatment options, it is essential that patients be evaluated at a specialized CTEPH center that can provide multidisciplinary care (8). In such a setting, pulmonologists with expertise in pulmonary hypertension, interventional specialists (typically cardiologists or radiologists), and surgeons can review individual cases to determine the best course of treatment.
References
- 1.Ende-Verhaar YM, Cannegieter SC, Vonk Noordegraaf A, Delcroix M, Pruszczyk P, Mairuhu AT, et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J. 2017;49:1601792. doi: 10.1183/13993003.01792-2016. [DOI] [PubMed] [Google Scholar]
- 2.Moser KM, Bloor CM. Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest. 1993;103:685–692. doi: 10.1378/chest.103.3.685. [DOI] [PubMed] [Google Scholar]
- 3.Kim NH, Delcroix M, Jenkins DP, Channick R, Dartevelle P, Jansa P, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013;62:D92–D99. doi: 10.1016/j.jacc.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Lang IM, Pesavento R, Bonderman D, Yuan JX. Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: a current understanding. Eur Respir J. 2013;41:462–468. doi: 10.1183/09031936.00049312. [DOI] [PubMed] [Google Scholar]
- 5.Tunariu N, Gibbs SJ, Win Z, Gin-Sing W, Graham A, Gishen P, et al. Ventilation–perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med. 2007;48:680–684. doi: 10.2967/jnumed.106.039438. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins D, Madani M, Fadel E, D’Armini AM, Mayer E. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26:160111. doi: 10.1183/16000617.0111-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kataoka M, Inami T, Hayashida K, Shimura N, Ishiguro H, Abe T, et al. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2012;5:756–762. doi: 10.1161/CIRCINTERVENTIONS.112.971390. [DOI] [PubMed] [Google Scholar]
- 8.Mahmud E, Madani MM, Kim NH, Poch D, Ang L, Behnamfar O, et al. Chronic thromboembolic pulmonary hypertension: evolving therapeutic approaches for operable and inoperable disease. J Am Coll Cardiol. 2018;71:2468–2486. doi: 10.1016/j.jacc.2018.04.009. [DOI] [PubMed] [Google Scholar]
Updates in Pulmonary Hypertension
Amita Krishnan and Matthew R. Lammi
Definition
At the 2018 sixth World Symposium on Pulmonary Hypertension, major revisions were made to the definition of pulmonary hypertension from a mean pulmonary arterial pressure (mPAP) ≥ 25 mm Hg to a new definition of >20 mm Hg. A PVR cutoff of 3 WU was also recommended to discriminate elevation of mPAP owing to precapillary pulmonary vascular disease from other causes, such as high cardiac output or elevations in pulmonary artery wedge pressure (PAWP) (1, 2).
Pulmonary hypertension is grouped into five clinical classifications on the basis of pathophysiologic mechanisms, clinical presentation, hemodynamic characteristics, and treatment. The two main modifications to group 1 pulmonary arterial hypertension (PAH) were the inclusion of the subgroups “PAH with overt features of venous/capillary involvement” and “PAH long-term responders to calcium channel blockers (CCBs).” In addition, methamphetamines and dasatinib were added as drugs that definitely cause drug-induced PAH (2).
Diagnosis
Echocardiography is the most important screening tool in patients suspected of having pulmonary hypertension on the basis of history, symptoms, physical examination findings, and biomarkers (Video 2). RHC remains the gold standard for establishing the diagnosis of pulmonary hypertension. / scanning should be performed in all patients to screen for CTEPH. Patients with scleroderma, the human immunodeficiency virus (HIV), and portal hypertension have elevated risk for developing pulmonary hypertension and require more aggressive screening (3).
Patients with idiopathic, heritable, and drug- or toxin-associated PAH should undergo acute vasoreactivity testing during RHC to predict responsiveness to CCBs. If used, adequate response should be confirmed by sustained hemodynamic improvement after 1 year of treatment.
Video 2.
Echocardiographic findings in pulmonary hypertension. (Clip 1) Right ventricular enlargement. (Clip 2) Straightening of the interventricular septum. (Clip 3) Tricuspid regurgitant jet velocity elevation. (Clip 4) Inferior vena cava diameter without respiratory variability. (Clip 5) Right ventricular pressure volume overload.
Management of Pulmonary Hypertension
A multiparametric risk-stratification approach is recommended to assess the severity and prognosis of PAH patients and to guide treatment, both at time of diagnosis and on follow-up. With rare exception, patients who are at low/intermediate risk should initially be treated with oral combination therapy. In high-risk patients, initial combination therapy including intravenous prostacyclin analogs is recommended. If the treatment approach results in a low-risk status within 3–6 months, the regimen should be continued. If the patient is still in intermediate- or high-risk status, escalation of therapy is recommended (4).
Pulmonary hypertension is a common complication of left-heart disease (LHD) owing to an increase in left-sided filling pressures. The hemodynamic definition of pulmonary hypertension–LHD is as follows: 1) isolated postcapillary pulmonary hypertension (PAWP > 15 mm Hg; mPAP > 20 mm Hg; PVR < 3 WU) and 2) combined pre- and postcapillary pulmonary hypertension (PAWP > 15 mm Hg; mPAP > 20 mm Hg; PVR ≥ 3 WU]). Distinguishing between PAH and pulmonary hypertension–LHD may be difficult; therefore, determining pretest probability and evaluating hemodynamics is vital for the confirmation of any PAH diagnosis. Evidence-based guidelines recommend against using PAH-specific drugs in the treatment of pulmonary hypertension–LHD (1, 5).
Chronic lung disease is frequently complicated by pulmonary hypertension, resulting in reduced functional status and worse outcomes (6). RHC should be performed only when significant pulmonary hypertension is suspected and management would be influenced by the RHC results (transplant referral, inclusion in clinical trials, treatment of LHD, and compassionate use of PAH therapy). Overall, PAH-targeted therapy has not shown benefit in this group (2, 4).
References
- 1.Galiè N, McLaughlin VV, Rubin LJ, Simonneau G. An overview of the 6th world symposium on pulmonary hypertension. Eur Respir J. 2019;53:1802148. doi: 10.1183/13993003.02148-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frost A, Badesch D, Gibbs JSR, Gopalan D, Khanna D, Manes A, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53:1801904. doi: 10.1183/13993003.01904-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galiè N, Channick RN, Frantz RP, Grünig E, Jing ZC, Moiseeva O, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53:1801889. doi: 10.1183/13993003.01889-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vachiéry JL, Tedford RJ, Rosenkranz S, Palazzini M, Lang I, Guazzi M, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi: 10.1183/13993003.01897-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan SD, Barbera JA, Gaine SP, Harari S, Martinez FJ, Olschewski H, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. 2019;53:1801914. doi: 10.1183/13993003.01914-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Community-acquired Pneumonia
Bronwyn L. Small and Kristina L. Bailey
Community-acquired pneumonia (CAP) remains a major cause of morbidity and mortality. Recently, new American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) guidelines highlighted existing evidence and recommendations for the diagnosis and treatment of CAP in adults (1). Several important updates are summarized in Table 2.
Table 2.
Comparison between 2019 and 2007 ATS/IDSA CAP guidelines
| 2007 Guidelines | 2019 Guidelines | |
|---|---|---|
| Diagnostic recommendations | ||
| Use of the HCAP category | Endorsed, previously included in 2005 guidelines | Recommend abandoning this category |
| Sputum culture | Recommended with severe CAP | Recommended in severe CAP and for inpatients empirically treated for MRSA or P. aeruginosa |
| Blood culture | Recommended with severe CAP | Recommended in severe CAP and for inpatients empirically treated for MRSA or P. aeruginosa |
| Use of procalcitonin | Not addressed | Not recommended to determine need for antibacterial therapy |
| Initial treatment recommendations | ||
| Outpatient | Macrolide as strong recommendation | No comorbidities: |
| Amoxicillin, doxycycline, or macrolide (only if local pneumococcal resistance is <25%) | ||
| With comorbidities: | ||
| 1) Amoxicillin/clavulanate or cephalosporin, and macrolide or doxycycline | ||
| or 2) Monotherapy with a respiratory quinolone | ||
| Inpatient | β-lactam/macrolide and β-lactam/fluoroquinolone combinations given equal weighting for severe CAP | Both accepted, but stronger evidence in favor of β-lactam/macrolide combination |
Definition of abbreviations: ATS = American Thoracic Society; CAP = community-acquired pneumonia; HCAP = health care–associated pneumonia; IDSA = Infectious Diseases Society of America; MRSA = methicillin-resistant Staphylococcus aureus; P. = Pseudomonas.
Diagnostic Considerations
The diagnosis of CAP is based on patient history, physical examination, and chest imaging. Previously, a distinction was made between pneumonia acquired as an outpatient and pneumonia acquired as an inpatient (health care–associated pneumonia) to guide evaluation and therapy. The 2019 guidelines recommend abandoning the category of health care–associated pneumonia and using local epidemiology and specific risk factors for infection with methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa to guide the diagnostic evaluation and initial treatment.
Adjunctive tests such as sputum and blood cultures, Legionella testing, and procalcitonin are often used clinically. Sputum and blood cultures from patients with CAP do not improve patient outcomes and often have a poor yield (1–4). Therefore, the ATS/IDSA 2019 guidelines recommend obtaining sputum and blood cultures only in hospitalized patients with severe CAP and in those empirically treated for MRSA and P. aeruginosa. The results of blood and sputum cultures should be used to de-escalate therapy if possible. Likewise, routine testing for Legionella and pneumococcal pneumonia are not recommended and should be limited to patients with severe CAP or risk factors for the infection, as randomized clinical trials have failed to show benefit to this testing (5). Procalcitonin testing is occasionally used to determine whether CAP is bacterial or viral in origin. However, an appropriate threshold for procalcitonin in bacterial pneumonia has not been determined. Therefore, use of procalcitonin as the sole metric for withholding antibiotic treatment is not recommended, and adults with clinical and radiologic features of CAP should receive appropriate initial antibiotic treatment even when procalcitonin is low (1).
Treatment Considerations
The recommendations for initial antibiotic coverage have shifted slightly in the new guidelines (1). Healthy outpatients can be treated with amoxicillin, doxycycline, or a macrolide when pneumococcal resistance is low (<25%). Outpatients with comorbidities, including heart, lung, or renal disease, should receive amoxicillin/clavulanate or a cephalosporin and a macrolide or monotherapy with a respiratory fluoroquinolone.
Initial inpatient management depends on CAP severity and the presence of risk factors for MRSA and P. aeruginosa. Initial therapy for inpatients with nonsevere CAP is the same as for outpatients with comorbidities unless there is a history of previous infection with MRSA or P. aeruginosa, in which case coverage for that organism should be added. Inpatients with nonsevere CAP and risk factors for MRSA or P. aeruginosa should be cultured, and coverage should be added if cultures are positive. For inpatients with severe pneumonia, a β-lactam plus a macrolide or a β-lactam plus a respiratory fluoroquinolone may be initiated with additional coverage for MRSA or P. aeruginosa if there is previous infection with or risk factors for these organisms.
In the 2019 ATS/IDSA CAP guideline, the routine use of corticosteroids is not recommended; however, it may be considered in patients with refractory septic shock (1).
New guidelines for CAP recommend shifting our focus toward which pathogens to target in therapy, and the guidelines delineate treatment and investigational recommendations primarily on the basis of severe and nonsevere CAP.
References
- 1.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lidman C, Burman LG, Lagergren A, Ortqvist A. Limited value of routine microbiological diagnostics in patients hospitalized for community-acquired pneumonia. Scand J Infect Dis. 2002;34:873–879. doi: 10.1080/0036554021000026967. [DOI] [PubMed] [Google Scholar]
- 3.Sanyal S, Smith PR, Saha AC, Gupta S, Berkowitz L, Homel P. Initial microbiologic studies did not affect outcome in adults hospitalized with community-acquired pneumonia. Am J Respir Crit Care Med. 1999;160:346–348. doi: 10.1164/ajrccm.160.1.9806048. [DOI] [PubMed] [Google Scholar]
- 4.Campbell SG, Marrie TJ, Anstey R, Dickinson G, Ackroyd-Stolarz S. The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community-acquired pneumonia: a prospective observational study. Chest. 2003;123:1142–1150. doi: 10.1378/chest.123.4.1142. [DOI] [PubMed] [Google Scholar]
- 5.Carratalà J, Fernández-Sabé N, Ortega L, Castellsagué X, Rosón B, Dorca J, et al. Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low-risk patients. Ann Intern Med. 2005;142:165–172. doi: 10.7326/0003-4819-142-3-200502010-00006. [DOI] [PubMed] [Google Scholar]
Pulmonary Nontuberculous Mycobacteria
Rebecca Kapolka and Colin Swenson
Nontuberculous mycobacteria (NTM) are a group of ubiquitous environmental organisms found mainly in water and soil. Of the over 200 known species of NTM, only a fraction are considered human pathogens (1). Human exposure may occur through the municipal water supply, recreational water activities, and gardening (1). NTM are opportunistic pathogens with variable mechanisms of pathogenicity. Common host risk factors include underlying structural lung disease, thoracic skeletal abnormalities, low body weight, gastroesophageal reflux disease, and chronic aspiration (2).
The prevalence of NTM pulmonary disease (NTM-PD) is increasing worldwide because of improved microbiologic culture techniques, increased clinician awareness, increased use of radiographic imaging, and more specific diagnostic criteria (3).
NTM clinical isolates are differentiated into two main groups by their rates of growth: slow growers, such as Mycobacterium avium complex (MAC), appear on growth media after 7 days; and rapid growers, such as M. abscessus complex, are isolated within 7 days of culture (3). The most common clinically encountered slow-growing species are avium, intracellulare, and chimaera, and M. kansasii. The most common rapidly growing clinical isolates include M. abscessus complex (which includes abscessus, bolletii, and massiliense), M. chelonae, and M. fortuitum (3).
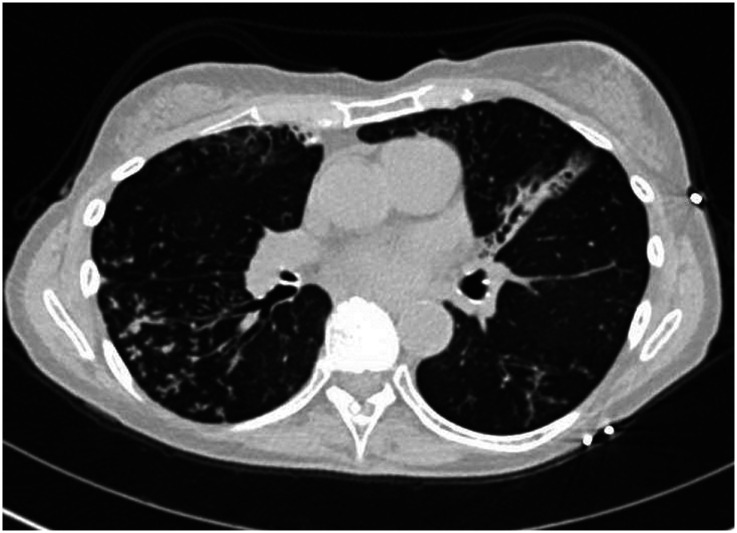
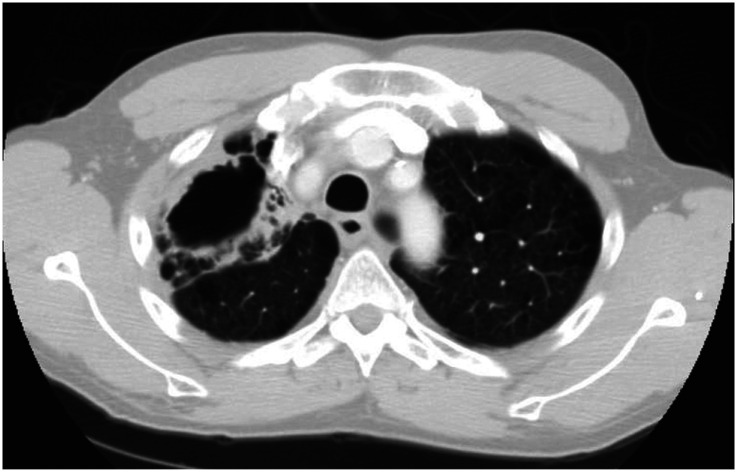
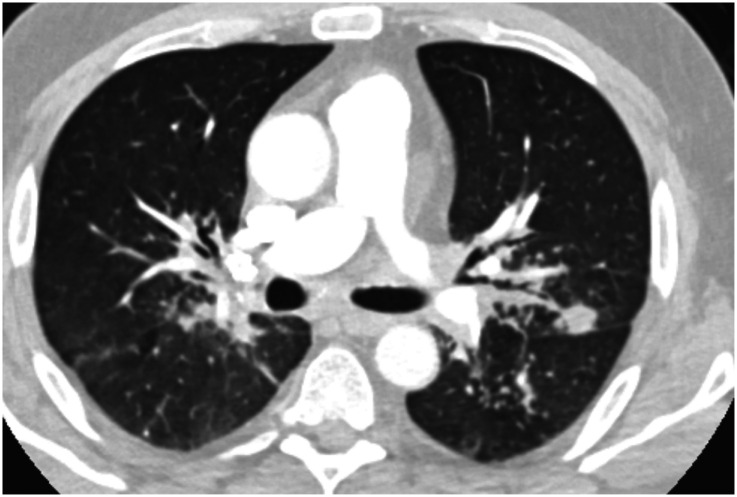
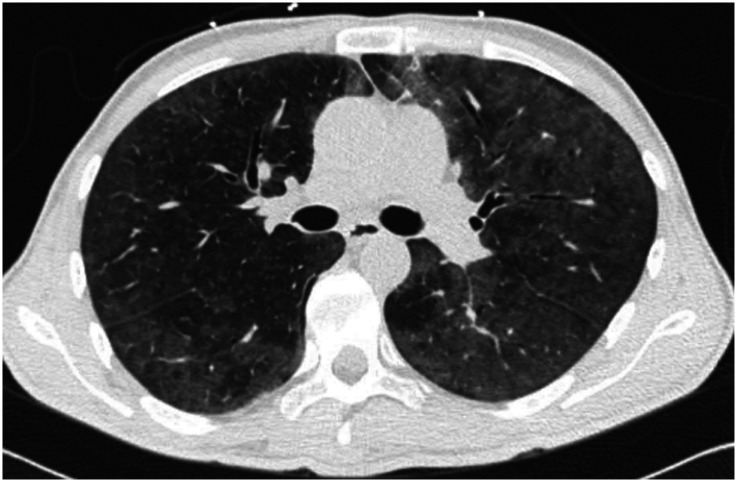
The most common clinical manifestations of NTM-PD are cough, fatigue, malaise, dyspnea, weight loss, subjective fever, and sputum production that may be accompanied by hemoptysis (4). There are two primary disease phenotypes in NTM-PD. The nodular–bronchiectatic phenotype is commonly seen in tall, thin, postmenopausal women without significant smoking history, although nonsmoking males increasingly compose part of this disease cohort. Radiographically, the phenotype is characterized by centrilobular pulmonary nodules, tree-in-bud opacities, and/or cylindrical bronchiectasis, often within the same lobe (4) (Figure 1). The fibrocavitary disease phenotype is more commonly seen in older male smokers with underlying chronic lung disease, most commonly emphysema. Chest imaging typically shows an upper-lobe thick-walled cavitary lesion, which may have an appearance similar to that of active pulmonary tuberculosis (Figure 2). Although most cases with these patterns are caused by MAC, both slow and rapid growers may have similar presentations.
Figure 1.
Chest computed tomographic image of the nodular–bronchiectatic phenotype of nontuberculous mycobacterial pulmonary disease showing centrilobular pulmonary nodules, tree-in-bud opacities, and cylindrical bronchiectasis.
Figure 2.
Chest computed tomographic image of the fibrocavitary disease phenotype of nontuberculous mycobacterial pulmonary disease with an upper-lobe thick-walled cavitary lesion.
Not all NTM pulmonary infections are symptomatic, and not all require antibiotic treatment. The 2007 ATS and IDSA guidelines have outlined three main criteria for the diagnosis of NTM-PD: clinical symptoms of pulmonary disease, radiographic findings suggestive of NTM infection, and microbiologic data from either two positive sputum cultures, one positive bronchoscopic culture, or lung tissue with a positive culture for NTM and granulomatous inflammation (5). All three criteria are required to establish a diagnosis of NTM-PD and help distinguish clinically active disease from indolent infection or asymptomatic colonization (5).
Before initiating treatment for NTM-PD, it is important to optimize therapy of any underlying pulmonary comorbidities (e.g., airway clearance for bronchiectasis or bronchodilators for obstructive diseases). For patients with nodular–bronchiectatic MAC-PD, the current guidelines recommend a thrice-weekly regimen of a macrolide (typically azithromycin), ethambutol, and a rifamycin (typically rifampin). For patients with fibrocavitary disease or more severe nodular–bronchiectatic disease, a daily regimen including a macrolide, ethambutol, and a rifamycin, with consideration of a thrice-weekly intravenous aminoglycoside, is recommended. It is important to avoid macrolide monotherapy, as this therapy may promote macrolide resistance (6). Sputum acid-fast bacillus cultures should be monitored every 1–3 months while on treatment, and therapy is continued for 12 months after sputum culture conversion (7).
References
- 1.Falkinham JO., III Nontuberculous mycobacteria in the environment. Clin Chest Med. 2002;23:529–551. doi: 10.1016/s0272-5231(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 2.Falkinham JO., III Current epidemiologic trends of the nontuberculous mycobacteria (NTM) Curr Environ Health Rep. 2016;3:161–167. doi: 10.1007/s40572-016-0086-z. [DOI] [PubMed] [Google Scholar]
- 3.Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med. 2015;36:13–34. doi: 10.1016/j.ccm.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winthrop KL, McNelley E, Kendall B, Marshall-Olson A, Morris C, Cassidy M, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182:977–982. doi: 10.1164/rccm.201003-0503OC. [DOI] [PubMed] [Google Scholar]
- 5.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [Published erratum appears in Am J Respir Crit Care Med 175:744–745.] [DOI] [PubMed] [Google Scholar]
- 6.McShane PJ, Glassroth J. Pulmonary disease due to nontuberculous mycobacteria: current state and new insights. Chest. 2015;148:1517–1527. doi: 10.1378/chest.15-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abubakar I, Gupta RK, Rangaka MX, Lipman M. Update in tuberculosis and nontuberculous mycobacteria 2017. Am J Respir Crit Care Med. 2018;197:1248–1253. doi: 10.1164/rccm.201801-0106UP. [DOI] [PubMed] [Google Scholar]
Approach to the Diagnosis of Opportunistic Fungal Infections in Immunocompromised Hosts
Kelly M. Pennington and Eva M. Carmona
Immunocompromised hosts are susceptible to life-threating pathogens that typically cause minimal or no disease in normal hosts (1). Infections owing to these pathogens are termed “opportunistic infections”.
Patients at Risk for Opportunistic Infections
Risk of opportunistic infections is determined by host risk factors, including environmental exposure (occupation, home environment, travel, and hobbies) and type and degree of immunodeficiency (1). Defects can affect humoral immunity (B cell–mediated), cell-mediated immunity (T cell–mediated), or neutrophil function (2). Non-HIV cases of acquired immunodeficiency may result from medications, solid-organ transplant or bone marrow transplant, malignancy, autoimmune disease, or structural lung disease (2).
Clinical Syndrome and Diagnostic Approach of Common Opportunistic Infections
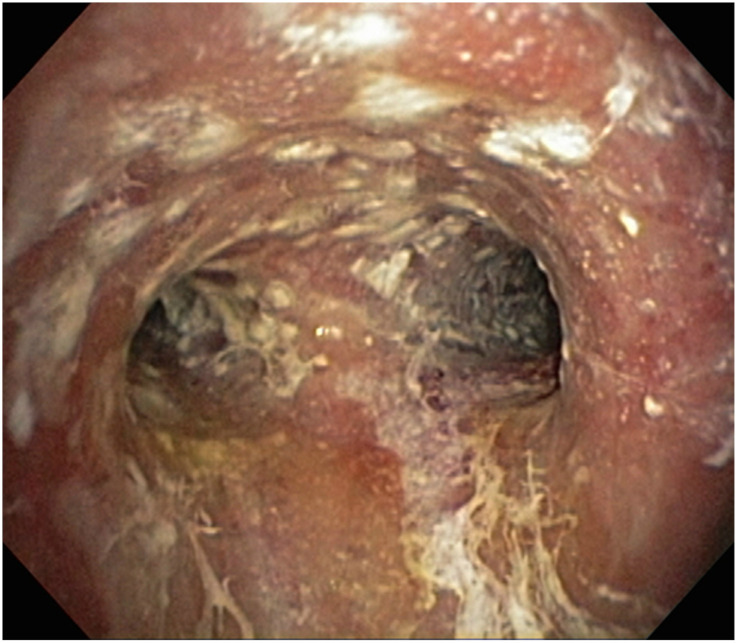
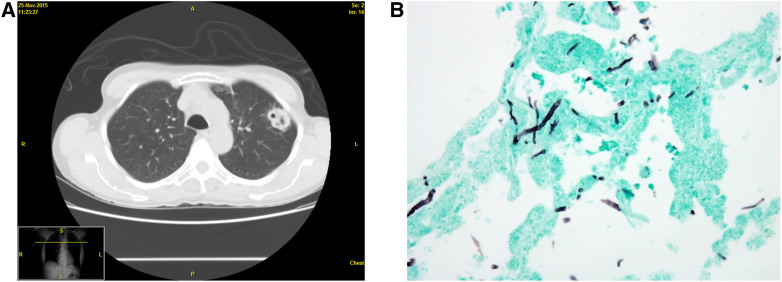
Bacteria, viruses, and fungi can cause opportunistic infections. Herein, we focus on fungal pathogens, including Aspergillus species, Cryptococcus species, and Pneumocystis jirovecii (Table 3). Aspergillus species cause disease in hosts with impaired cellular immunity, neutrophil dysfunction, or structural lung disease. Invasive pulmonary aspergillosis is the most severe manifestation and presents as fever, cough, pleuritic chest pain, hemoptysis, and nonspecific nodular or ground-glass infiltrates on chest imaging (Figures 3 and 4). Hosts with prolonged neutropenia, those receiving prolonged high-dose systemic corticosteroids, and those after solid-organ transplant or bone marrow transplant are at greatest risk (3). Diagnosis requires direct visualization of Aspergillus species invading tissue or blood vessels on histopathologic examination. As biopsies can be difficult to obtain because of illness severity, antigen testing (galactomannan cell-wall component) and Aspergillus polymerase chain reaction (PCR) measured on either serum or bronchoalveolar lavage (BAL) may be a less invasive approach to aid diagnosis (4).
Table 3.
Common opportunistic fungal infections: clinical presentation and diagnostic tests
| Pathogen | Host Risk Factors | Clinical Presentation | Common Diagnostic Tests |
|---|---|---|---|
| Aspergillus species | Invasive pulmonary aspergillosis | Invasive pulmonary aspergillosis | • Culture from sterile site: gold standard/low sensitivity |
| • Impaired cellular immunity | • Fever, cough, pleuritic chest pain, hemoptysis, and nodular or ground-glass infiltrate on chest imaging | • Histology: gold standard/invasive | |
| • Neutropenia | • Serum or BAL galactomannan: less invasive and rapid turnaround; antifungal therapy may reduce sensitivity, false positives in mucositis, cross-react with Histoplasma and other fungi | ||
| • Bone marrow transplant | • Serum or BAL Aspergillus PCR: less invasive and rapid turnaround; lack of standard technique, contamination, and inability to distinguish colonization from infection | ||
| • Solid-organ transplant | |||
| • Chronic high-dose corticosteroids | |||
| Tracheobronchitis | Tracheobronchitis | ||
| • Lung transplant | • Ulceration/eschar at airway anastomosis | ||
| C. neoformans | • Impaired cellular immunity | • Cough, fever, headache ± meningismus, pulmonary nodules, and lymphadenopathy | • Culture/India-ink stain from BAL or CSF: gold standard |
| • Chronic high-dose corticosteroids | • Lumbar puncture should be completed to evaluate for CNS disease | • Antigen testing on serum, BAL, or CSF | |
| • Sarcoidosis | • β-1,3-d-glucan (Fungitell): characteristically negative | ||
| • Exposure to pigeons | |||
| P. jirovecii | • Impaired cellular immunity ± lung disease | • Nonproductive cough, fever, severe hypoxemic respiratory failure, and diffuse ground-glass or cystic lesions on chest imaging | • Direct visualization: gold standard/requires trained personnel |
| • High-dose corticosteroids | • PCR on BAL or sputum: rapid turnaround/inability to distinguish colonization from infection | ||
| • Rituximab | • β-1,3-d-glucan: typically elevated (nonspecific) |
Definition of abbreviations: BAL = bronchoalveolar lavage; C. = Cryptococcus; CNS = central nervous system; CSF = cerebrospinal fluid; P. = Pneumocystis; PCR = polymerase chain reaction.
Figure 3.
Aspergillus tracheobronchitis. A 78-year-old man receiving chemotherapy for diffuse large B-cell lymphoma was found to have diffuse mucosal plaques and ulcerations on bronchoscopy. Bronchial washings grew A. fumigatus.
Figure 4.
Invasive pulmonary aspergillosis. (A) A 58-year-old woman on high-dose inhaled corticosteroids presented with an incidentally discovered left upper-lobe cavitary nodule on chest computed tomography (CT). (B) CT-guided biopsy of the nodule revealed acute branching and septate hyphae consistent with Aspergillus species.
C. neoformans causes disease in hosts with severe defects in cellular immunity. Hosts with exposure to pigeons or other bird droppings may be at higher risk. C. neoformans enters the host via inhalation and has a predilection for the central nervous system. Patients present with fever, cough, and headache or meningeal signs. Chest imaging may show pulmonary nodules, lymphadenopathy, and/or pleural effusions (Figure 5). Culture or direct visualization is the gold standard for diagnosis, but antigen testing of serum or cerebrospinal fluid is often used, and thus lumbar puncture is recommended during evaluation (5).
Figure 5.
Cryptococcus neoformans pulmonary infection. A 66-year-old man with pulmonary sarcoidosis on high-dose prednisone presented with fever, altered mental status, and progressive cough. A computed tomographic scan of the chest revealed bilateral peri-hilar and nodular opacities consistent with sarcoidosis. Serum and cerebrospinal-fluid cryptococcal antigens were positive.
P. jirovecii pneumonia (PJP) typically occurs in patients with defects in cell-mediated immunity, but reports of PJP in patients receiving rituximab exist (6). Presentation ranges from mild nonproductive cough to severe hypoxemic respiratory failure with diffuse infiltrates on chest imaging (Figure 6). Because P. jirovecii cannot be cultured, direct visualization of the organism on histopathologic specimens is the gold standard for diagnosis. The diagnosis is often made using PCR testing on BAL specimens (7). Serum β-1,3-d-glucan may also be significantly elevated in PJP but is a nonspecific marker of disease.
Figure 6.
Pneumocystis jirovecii pneumonia. A 41-year-old man with a history of kidney and pancreas transplant 3 years prior presented with progressive hypoxemic respiratory failure and diffuse bilateral ground-glass opacities on chest computed tomographic images. Serum β-1,3-d-glucan was greater than 10 times the upper limit of normal. Sputum P. jirovecii pneumonia polymerase chain reaction was positive.
Limitations of Common Diagnostic Tests
The gold standard for diagnosing most opportunistic infections is direct visualization and/or culture, but this is usually not feasible secondary to illness severity, bleeding risk, and time to culture positivity. Limitations exist with antigen and PCR testing modalities. For example, galactomannan may be falsely positive in patients with mucositis (4, 8) and falsely negative in patients on antifungal prophylaxis (3). Serum β-1,3-d-glucan is nonspecific and can be falsely positive in patients receiving hemodialysis, intravenous immunoglobulin, cardiopulmonary bypass, or blood transfusion (9). PCR testing is limited by contamination and inability to distinguish colonization from infection. Serum Aspergillus PCR has high sensitivity and low specificity with single-sample testing, but sensitivity decreases and specificity increases with repeated samples (3).
References
- 1.Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis. 2002;34:1098–1107. doi: 10.1086/339548. [DOI] [PubMed] [Google Scholar]
- 2.de Marie S. Diseases and drug-related interventions affecting host defence. Eur J Clin Microbiol Infect Dis. 1993;12:S36–S41. doi: 10.1007/BF02389876. [DOI] [PubMed] [Google Scholar]
- 3.Hage CA, Carmona EM, Epelbaum O, Evans SE, Gabe LM, Haydour Q, et al. Microbiological laboratory testing in the diagnosis of fungal infections in pulmonary and critical care practice: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2019;200:535–550. doi: 10.1164/rccm.201906-1185ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urabe N, Sakamoto S, Sano G, Suzuki J, Hebisawa A, Nakamura Y, et al. Usefulness of two Aspergillus PCR assays and Aspergillus galactomannan and β-d-glucan testing of bronchoalveolar lavage fluid for diagnosis of chronic pulmonary aspergillosis. J Clin Microbiol. 2017;55:1738–1746. doi: 10.1128/JCM.02497-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon-Chung KJ, Fraser JA, Doering TL, Wang Z, Janbon G, Idnurm A, et al. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb Perspect Med. 2014;4:a019760. doi: 10.1101/cshperspect.a019760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin-Garrido I, Carmona EM, Specks U, Limper AH. Pneumocystis pneumonia in patients treated with rituximab. Chest. 2013;144:258–265. doi: 10.1378/chest.12-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujisawa T, Suda T, Matsuda H, Inui N, Nakamura Y, Sato J, et al. Real-time PCR is more specific than conventional PCR for induced sputum diagnosis of Pneumocystis pneumonia in immunocompromised patients without HIV infection. Respirology. 2009;14:203–209. doi: 10.1111/j.1440-1843.2008.01457.x. [DOI] [PubMed] [Google Scholar]
- 8.Busca A, Locatelli F, Barbui A, Limerutti G, Serra R, Libertucci D, et al. Usefulness of sequential Aspergillus galactomannan antigen detection combined with early radiologic evaluation for diagnosis of invasive pulmonary aspergillosis in patients undergoing allogeneic stem cell transplantation. Transplant Proc. 2006;38:1610–1613. doi: 10.1016/j.transproceed.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 9.Tran T, Beal SG. Application of the 1,3-β-d-glucan (Fungitell) assay in the diagnosis of invasive fungal infections. Arch Pathol Lab Med. 2016;140:181–185. doi: 10.5858/arpa.2014-0230-RS. [DOI] [PubMed] [Google Scholar]
COVID-19
Naomi Habib and Raed Alalawi
On December 31, 2019, an outbreak of pneumonia of unknown cause was first announced by the Chinese Center for Disease Control and Prevention (1). Less than 3 months later, the World Health Organization declared the global outbreak of this infection a pandemic (2). The genomic sequence of the virus was identified as a severe acute respiratory syndrome (SARS)-like coronavirus and named SARS coronavirus 2 (SARS-CoV-2), with the associated disease termed coronavirus disease (COVID-19) (3).
SARS-CoV-2, a novel, enveloped, single-stranded RNA β-coronavirus, is believed to have a zoonotic source, with bats as a possible reservoir. Person-to-person transmission via respiratory droplets and direct contact are believed to be the primary mechanisms of spread, although other routes of transmission are under investigation (4, 5).
The clinical manifestations of COVID-19 range from asymptomatic to fulminant respiratory failure. A median incubation period of 4 days (interquartile range, 2–7) has been reported (5). Commonly reported symptoms include fevers, cough, dyspnea, fatigue, myalgias, and headache, with gastrointestinal symptoms being less common (5–7).
SARS-CoV-2 PCR is the primary method used to diagnose COVID-19. Test characteristics are unclear because of the lack of a reference standard; however, specificity is likely high. Sensitivity depends in part on adequate sample collection (8). Nasopharyngeal swab samples are the preferred specimen source because of higher yields than oropharyngeal samples. Methods that result in aerosol generation, such as induced sputum, are relatively contraindicated because of concern for nosocomial spread. Bronchoscopy should only be considered if upper respiratory samples are negative and other diagnoses under consideration would significantly change clinical management (9). Serologic tests are commercially available, but they have variable sensitivity, particularly early in the course of disease. Antibody production is reported to start 1 week after the onset of infection (8).
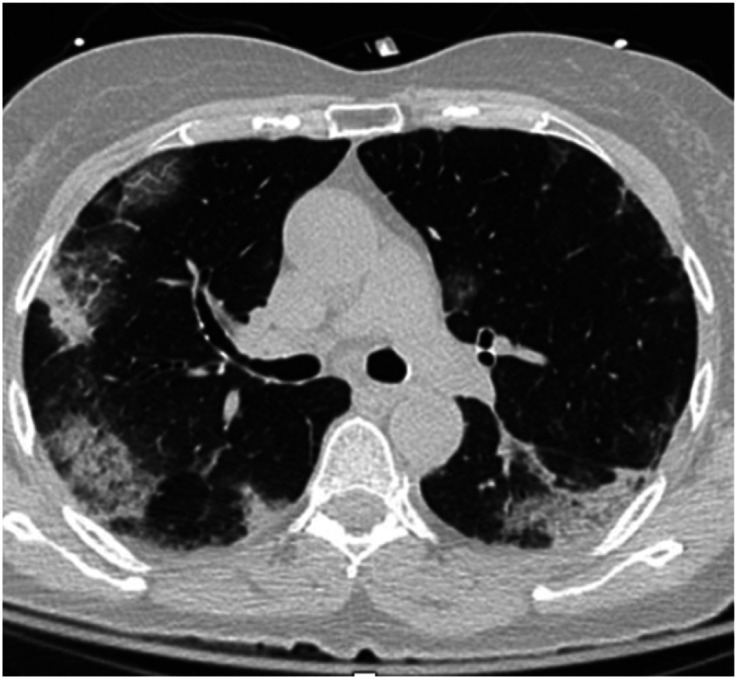
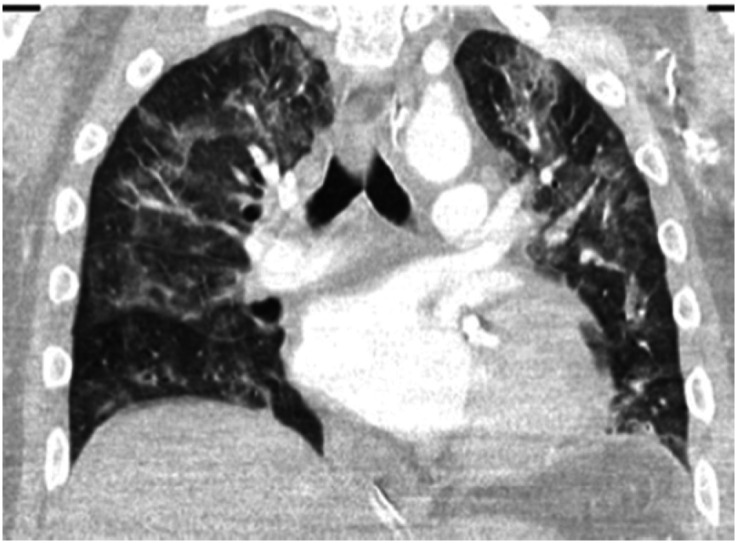
Characteristic imaging findings at chest radiography include consolidation or ground-glass opacities that are usually bilateral, peripheral, and lower-lobe predominant (10). Chest CT images often show peripheral, bilateral ground-glass opacities in early or mild disease, whereas cases with severe disease demonstrate more diffuse ground-glass opacities and consolidation; often with reticular abnormality and traction bronchiectasis reflecting more severe lung injury (11) (Figures 7 and 8). Lung ultrasound shows thickening and irregularity of the pleural line, B-lines, and consolidation, with pleural effusions being uncommon (12).
Figure 7.
Chest computed tomographic image showing pattern of bilateral patchy peripheral infiltrates in patient with coronavirus disease (COVID-19) infection.
Figure 8.
Chest computed tomographic image showing pattern of bilateral ground-glass opacities in patient with coronavirus disease (COVID-19) infection.
Laboratory findings are nonspecific and often show an elevation in inflammatory markers (C-reactive protein, erythrocyte sedimentation rate, ferritin), leukopenia, and elevated D-dimer, lactate dehydrogenase, and interleukin-6 levels (13).
Risk factors for severe COVID-19 infection include advanced age; chronic medical conditions such as advanced cardiopulmonary disease, hypertension, diabetes, and obesity; cancer; and immunosuppression (14). African American, Native American, and Hispanic persons are much more likely to be hospitalized than non-Hispanic white persons (14).
Mortality rates have varied (1.4–3.2%), with a larger series of 44,672 confirmed cases reporting a case fatality rate of 2.3% (1, 5). Reports of mortality among critically ill patients have ranged from 16.7% to >40% (15–17). Renal failure requiring renal replacement therapy and mechanical ventilation are risk factors associated with a higher mortality (16, 17). Scarcity of resources to provide advanced respiratory and renal support may have contributed to increased mortality rates in locations where the caseload has exceeded capacity.
Treatment focuses on supportive care, with close monitoring for rapidly increasing oxygen demands and impending respiratory failure. Intubated patients should receive best practices for acute respiratory distress syndrome, including lung-protective ventilation strategies, prone positioning, and conservative fluid management (18–20). There are currently no FDA-approved therapies for COVID-19, but multiple agents are under investigation. Initial enthusiasm for hydroxychloroquine based on in vitro benefit has waned, as trials have failed to demonstrate a clear benefit for hospitalized patients while highlighting potential, harms including QT interval prolongation and arrhythmias (18, 21, 22). The antiviral drug remdesivir was shown in a preliminary report to reduce time to recovery in patients who need supplemental oxygen or mechanical ventilation (23). Treatment with convalescent plasma appears safe, but rigorous studies have not yet demonstrated a benefit (24, 25). These studies evaluated the clinical response as well as the anti–SARS-CoV-2 antibody levels.
A number of antiinflammatory approaches are under investigation, including the interleukin-6 inhibitors tocilizumab and sarilumab. A retrospective study suggested a reduction in the need for mechanical ventilation or death with tocilizumab use (26). Prospective studies are underway. Preliminary data from the RECOVERY (Randomised Evaluation of COVID-19 Therapy) trial (a large multicenter, randomized, open-label study) suggest a 17% reduction in 28-day mortality for hospitalized patients with low-dose dexamethasone (29.0% vs. 40.7%) (27). The benefit appeared to be greatest in patients with severe disease, whereas no benefit was observed in patients not requiring supplemental oxygen. These data have not yet been published in a peer-reviewed journal; however, the observed benefit led the National Institutes of Health COVID-19 Treatment Guidelines Panel to recommend dexamethasone 6 mg daily for up to 10 days for patients receiving mechanical ventilation (18).
Patients with COVID-19 appear to have an increased incidence of thromboembolic events (28). Although hospitalized patients frequently have coagulation parameters measured, it is unclear how results should influence therapy (18). Hospitalized patients should receive DVT prophylaxis on the basis of risk stratification independent of COVID-19 status. Those with confirmed or suspected thromboembolic events should receive full-dose anticoagulation (18). Multiple trials evaluating anticoagulation regimens and thrombolytic use are underway. Multiple COVID-19 vaccines are in development.
It is important to recall that data before the pandemic demonstrate that viruses result in 25–44% of all cases of CAP and that 16–49% of pneumonias lead to severe respiratory illness (29, 30). Coinfections with COVID-19 and other respiratory viruses appear relatively uncommon; however, a case series demonstrated that 20% of samples from patients with COVID-19 infection had other respiratory pathogens identified (6, 31). A case series describes four patients who presented with COVID-19 and influenza (32). Diagnostic testing should assess for influenza depending on community activity, as antiviral therapy is indicated for hospitalized patients with influenza (33). A positive test result for other respiratory viruses does not exclude a diagnosis of COVID-19.
Public health efforts aimed at controlling the spread of COVID-19 include social distancing with an emphasis on hand washing and wearing masks in public where social distancing is not possible (particularly indoors). Testing programs and contact tracing are additional public health measures employed to control the spread of disease. Although these results are preliminary, the global race continues as we seek to curb the effects of this highly contagious and virulent organism.
Supplementary Material
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. doi: 10.1001/jama.2020.2648. [online ahead of print] 24 Feb 2020. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Geneva, Switzerland: World Health Organization; 2020. WHO Director-General’s opening remarks at the media briefing on COVID-19: 11 March 2020. [accessed 2020 Mar 25]. Available from: http://who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. [Google Scholar]
- 3.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. China Medical Treatment Expert Group for COVID-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caruana G, Croxatto A, Coste AT, Opota O, Lamoth F, Jaton K, et al. Diagnostic strategies for SARS-CoV-2 infection and interpretation of microbiological results. Clin Microbiol Infect. doi: 10.1016/j.cmi.2020.06.019. [online ahead of print] 25 Jun 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wahidi MM, Shojaee S, Lamb CR, Ost D, Maldonado F, Eapen G, et al. The use of bronchoscopy during the coronavirus disease 2019 pandemic: CHEST/AABIP guideline and expert panel report. Chest. doi: 10.1016/j.chest.2020.04.036. [online ahead of print] 1 May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong HYF, Lam HYS, Fong AH, Leung ST, Chin TW, Lo CSY, et al. Frequency and distribution of chest radiographic findings in patients positive for COVID-19. Radiology. 2020;296:E72–E78. doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 12.Peng QY, Wang XT, Zhang LN Chinese Critical Care Ultrasound Study Group (CCUSG) Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020;46:849–850. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Latin American Network of Coronavirus Disease 2019-COVID-19 Research (LANCOVID-19). Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Centers for Disease Control and Prevention. Atlanta, GA: U.S. Centers for Disease Control and Prevention; 2020. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19) [accessed 2020 Jul 7]. Available from: http://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. [Google Scholar]
- 15.Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201:1560–1564. doi: 10.1164/rccm.202004-1163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suleyman G, Fadel RA, Malette KM, Hammond C, Abdulla H, Entz A, et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Netw Open. 2020;3:e2012270. doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.COVID-19 Treatment Guidelines Panel. Bethesda, MD: National Institutes of Health; 2020. Coronavirus disease 2019 (COVID-19) treatment guidelines. [accessed 2020 Jul 7]. Available from: https://www.covid19treatmentguidelines.nih.gov/ [PubMed] [Google Scholar]
- 19.Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng L, Qiu H, Wan L, Ai Y, Xue Z, Guo Q, et al. Intubation and ventilation amid the COVID-19 outbreak: Wuhan’s experience. Anesthesiology. 2020;132:1317–1332. doi: 10.1097/ALN.0000000000003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. Food and Drug Administration. Silver Spring, MD: U.S. Food and Drug Administration; 2020. FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. [accessed 2020 Jul 7]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or. [Google Scholar]
- 23.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19 - preliminary report. N Engl J Med. doi: 10.1056/NEJMc2022236. [online ahead of print] 22 May 2020. [DOI] [PubMed] [Google Scholar]
- 24.Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. doi: 10.1002/jmv.25882. [online ahead of print] 15 Apr 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Randomised Evaluation of COVID-19 Therapy (RECOVERY) Oxford, England: University of Oxford; 2020. Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19. [accessed 2020 Jul 7]. Available from: https://www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19. [Google Scholar]
- 28.Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18:1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Britto CJ, Brady V, Lee S, Dela Cruz CS. Respiratory viral infections in chronic lung diseases. Clin Chest Med. 2017;38:87–96. doi: 10.1016/j.ccm.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burk M, El-Kersh K, Saad M, Wiemken T, Ramirez J, Cavallazzi R. Viral infection in community-acquired pneumonia: a systematic review and meta-analysis. Eur Respir Rev. 2016;25:178–188. doi: 10.1183/16000617.0076-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323:2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuadrado-Payán E, Montagud-Marrahi E, Torres-Elorza M, Bodro M, Blasco M, Poch E, et al. SARS-CoV-2 and influenza virus co-infection. Lancet. 2020;395:e84. doi: 10.1016/S0140-6736(20)31052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uyeki TM, Bernstein HH, Bradley JS, Englund JA, File TM, Fry AM, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis. 2019;68:e1–e47. doi: 10.1093/cid/ciy866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.