Abstract
The American Thoracic Society Core Curriculum updates clinicians annually in adult and pediatric pulmonary disease, medical critical care, and sleep medicine, in a 3–4-year recurring cycle of topics. These topics will be presented at the 2020 Virtual Conference. Below is the adult sleep medicine core that includes topics pertinent to sleep-disordered breathing and insomnia.
Keywords: sleep apnea, insomnia, positive airway pressure adherence, cognitive behavioral therapy, hypnotics
Key Points
• Untreated obstructive sleep apnea (OSA) increases the risk of cardiovascular morbidity and mortality and is associated with neurocognitive dysfunction.
• An individualized approach incorporating technological advancements can improve positive airway pressure adherence and successful OSA treatment.
• Oral appliances remain the most widely accepted alternative to continuous positive airway pressure (CPAP). For select patients, hypoglossal nerve stimulation or surgical approaches may also be considered. Weight loss should be part of OSA management for all overweight patients but generally does not replace OSA-specific therapy. For patients with OSA with residual sleepiness, several wake-promoting medications have now been approved as an adjunct to (not a replacement for) OSA therapies.
• CPAP therapy is first line for treatment of central sleep apnea associated with heart failure after the treatment of heart failure has been optimized. Supplemental oxygen is recommended for those patients who are unable to tolerate or fail CPAP therapy.
• Cognitive behavioral therapy for insomnia is recommended as first-line treatment of insomnia.
• The decision to treat chronic insomnia disorder with long-term hypnotic medications should be individualized and balance the risks of long-term hypnotic use against the risks of untreated chronic insomnia and associated functional limitations.
• Despite accumulating evidence suggesting improved sleep quality with cannabinoids, more information is needed to understand optimal prescription, dosing, timing, and duration. Also, adverse effects of chronic use and dependency need to be addressed.
Cardiovascular and Neurocognitive Consequences of Untreated Obstructive Sleep Apnea
Robert M. Marron and Maria Elena Vega Sanchez
Patients with obstructive sleep apnea (OSA) experience apneic and hypopneic events that, when untreated, have detrimental cardiovascular and neurocognitive consequences. Under normal conditions, blood pressure and heart rate decrease during non–rapid eye movement (REM) sleep and increase commensurately upon waking. This is attributed to a decrease in sympathetic nervous system activation and a subsequent increase in cardiac vagal tone during sleep (1). The transient episodes of hypoxemia and hypercapnia caused by apneas or hypopneas, as well as arousals, result in an increase in cardiac output and heart rate that leads to sympathetically induced peripheral vasoconstriction that causes a marked increase in blood pressure. The result of this chronic sympathetic excitation and inflammation does not resolve upon waking, and over time, together with the loss of the normal nocturnal blood pressure dip, it can lead to pathophysiologic changes such as impaired vascular function and stiffness (1–3). This impairment in the untreated patient with moderate to severe OSA has been found to increase the risk of both acute coronary syndrome and sudden cardiac death (4, 5). The increased sympathetic nervous activity, inflammation, and oxidative stress seen in OSA can lead to hypertension. The prevalence of hypertension in moderate to severe OSA ranges between 13% and 60%, and OSA is considered the most common cause of secondary hypertension (6).
Arrhythmias can be common in patients with OSA, and the prevalence of atrial fibrillation is higher in these patients than in patients without OSA. In fact, severe sleep-disordered breathing is associated with twofold to fourfold higher odds of having complex arrhythmias. In addition, untreated OSA has been associated with higher rates of failure to maintain sinus rhythm after cardioversion or ablation therapy (7).
Inflammation, atrial fibrillation, and atherosclerosis are all associated with OSA and overlap with risk factors for cerebrovascular disease. OSA may be frequently diagnosed after stroke, and it can be difficult to determine whether the condition is causal or resultant. Evidence suggests that OSA is associated with an increased risk of stroke in elderly patients, and untreated OSA after stroke increases mortality risk during 10-year follow-up (8–10).
Another disease state affected by sleep apnea is heart failure. Both OSA and central sleep apnea are common in patients with acute and chronic systolic and diastolic heart failure. Untreated OSA in this patient population has been associated with an increased risk of death. However, screening for sleep-disordered breathing can be difficult because patients with OSA and heart failure often do not report excessive daytime sleepiness. This absent symptom raises challenges in diagnosis and treatment adherence for OSA (11, 12).
Untreated OSA can affect many cognitive domains, including learning, memory, attention, and executive functioning. Data suggest that OSA is linked with cognitive impairment and may advance cognitive decline or dementia (13). In addition, intermittent hypoxemia and sleep fragmentation have been linked to structural changes in the brain that may be responsible for cognitive impairment (14).
Given the increased prevalence of obesity and the common nature of diagnoses such as hypertension, coronary artery disease, atrial fibrillation, heart failure, and neurocognitive impairment, healthcare providers should be cognizant of the hazards of untreated OSA (summarized in Table 1).
Table 1.
Association between common cardiac and neurological disorders and untreated OSA
| Disease Process | Association with Untreated OSA |
|---|---|
| Hypertension | OSA is the most common cause of secondary hypertension |
| OSA causes activation of sympathetic nervous system, leading to increased diastolic as well as systolic blood pressure | |
| ACS | Increased risk of:
|
| May be due to increased plaque vulnerability | |
| Atrial fibrillation | Strong association between moderate to severe OSA and atrial fibrillation |
| Recurrence of atrial fibrillation after cardioversion or ablation therapy | |
| Stroke | Increased incidence of stroke, especially in elderly patients |
| OSA is often diagnosed after stroke | |
| Untreated OSA after stroke increases 10-yr mortality risk | |
| Heart failure | Both OSA and CSA (frequently with Cheyne-Stokes respiration) are associated with heart failure |
| OSA > CSA in women with heart failure | |
| CSA is more common in older men (>65 yr old) with atrial fibrillation and hypocapnia (PaCO2 < 38 mm Hg) (1) | |
| Patients often do not report excessive daytime sleepiness | |
| Neurocognitive impairment | Can affect learning, memory, attention, and executive function |
| Structural changes to the brain are believed to be from intermittent hypoxemia and sleep fragmentation |
Definition of abbreviations: ACS = acute coronary syndrome; CSA = central sleep apnea; OSA = obstructive sleep apnea; PaCO2 = partial pressure of arterial carbon dioxide.
References
- 1.Floras JS. Sleep apnea and cardiovascular disease: an enigmatic risk factor. Circ Res. 2018;122:1741–1764. doi: 10.1161/CIRCRESAHA.118.310783. [DOI] [PubMed] [Google Scholar]
- 2.Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307:2169–2176. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 4.Nakashima H, Kurobe M, Minami K, Furudono S, Uchida Y, Amenomori K, et al. Effects of moderate-to-severe obstructive sleep apnea on the clinical manifestations of plaque vulnerability and the progression of coronary atherosclerosis in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2015;4:75–84. doi: 10.1177/2048872614530865. [DOI] [PubMed] [Google Scholar]
- 5.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–1214. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 6.Haas DC, Foster GL, Nieto FJ, Redline S, Resnick HE, Robbins JA, et al. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health study. Circulation. 2005;111:614–621. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- 7.Shukla A, Aizer A, Holmes D, Fowler S, Park DS, Bernstein S, et al. Effect of obstructive sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC Clin Electrophysiol. 2015;1:41–51. doi: 10.1016/j.jacep.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Catalan-Serra P, Campos-Rodriguez F, Reyes-Nuñez N, Selma-Ferrer MJ, Navarro-Soriano C, Ballester-Canelles M, et al. Increased incidence of stroke, but not coronary heart disease, in elderly patients with sleep apnea. Stroke. 2019;50:491–494. doi: 10.1161/STROKEAHA.118.023353. [DOI] [PubMed] [Google Scholar]
- 9.Sahlin C, Sandberg O, Gustafson Y, Bucht G, Carlberg B, Stenlund H, et al. Obstructive sleep apnea is a risk factor for death in patients with stroke: a 10-year follow-up. Arch Intern Med. 2008;168:297–301. doi: 10.1001/archinternmed.2007.70. [DOI] [PubMed] [Google Scholar]
- 10.Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49:2028–2034. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 11.Pak VM, Strouss L, Yaggi HK, Redeker NS, Mohsenin V, Riegel B. Mechanisms of reduced sleepiness symptoms in heart failure and obstructive sleep apnea. J Sleep Res. 2019;28:e12778. doi: 10.1111/jsr.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Randerath W, Verbraecken J, Andreas S, Arzt M, Bloch KE, Brack T, et al. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J. 2017;49:1600959. doi: 10.1183/13993003.00959-2016. [DOI] [PubMed] [Google Scholar]
- 13.Leng Y, McEvoy CT, Allen IE, Yaffe K. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: a systematic review and meta-analysis. JAMA Neurol. 2017;74:1237–1245. doi: 10.1001/jamaneurol.2017.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baril AA, Gagnon K, Brayet P, Montplaisir J, De Beaumont L, Carrier J, et al. Gray matter hypertrophy and thickening with obstructive sleep apnea in middle-aged and older adults. Am J Respir Crit Care Med. 2017;195:1509–1518. doi: 10.1164/rccm.201606-1271OC. [DOI] [PubMed] [Google Scholar]
OSA: New Advances in Positive Airway Pressure Therapy to Improve Management and Adherence
Michael T. Lam and Bernie Y. Sunwoo
Positive airway pressure (PAP) therapy is highly efficacious in treating OSA, but its effectiveness relies on adherence. There is a dose–response relationship between continuous PAP (CPAP) usage and clinical outcomes in OSA, although the optimal adherence threshold may vary depending on the clinical outcome of interest (1, 2). Consequently, recognition of barriers to use and interventions to augment adherence are pivotal to the successful management of patients with OSA.
Studies have explored potential modifiable and nonmodifiable predictors of PAP adherence with inconsistent results (3). Most adherent patients have higher baseline daytime somnolence, possibly worse OSA severity based on the apnea–hypopnea index (AHI), higher self-efficacy (i.e., believing the device is going to improve quality of life), and confidence for troubleshooting as well as greater social support, including bed partner engagement. Patients who have challenges with PAP adherence tend to have lower socioeconomic status, type D (distressed) personality, high expectations in treatment outcome, claustrophobia, and small nasal passages. Patient age, sex, marital status, and amount of anxiety and depression have not been shown to consistently predict PAP adherence (4).
Therefore, an individualized patient-centered approach is recommended to optimize PAP adherence in OSA. Interrogation of PAP tracking systems can reveal patterns and the duration of PAP use and may help identify potential modifiable targets to improve adherence, such as mask leak (5). High residual AHI can point toward suboptimally treated obstructive events or the emergence of central events. Prompt and early troubleshooting of any side effects is important, as the pattern of PAP usage is established early and has been shown to predict long-term use. Attention to mask fitting is essential, with otolaryngologic evaluation helpful in patients with narrow nasal passages or nasal congestion that may be amenable to surgery (6). Given the psychological influences on PAP adherence, educational and behavioral interventions aim to address patient perceptions and promote self-efficacy. Motivational counseling by psychologists during appointments with follow-up phone calls has been shown to increase adherence by 99 min/night compared with control subjects (7). In a meta-analysis, behavioral therapy improved average PAP usage by 1.44 h/night and increased the number of nights with >4 hours usage from 28% to 47%, although the quality of evidence was low (8). In addition, studies exploring educational and behavioral strategies are limited by heterogeneity, often combining various modalities and thus making generalizations difficult.
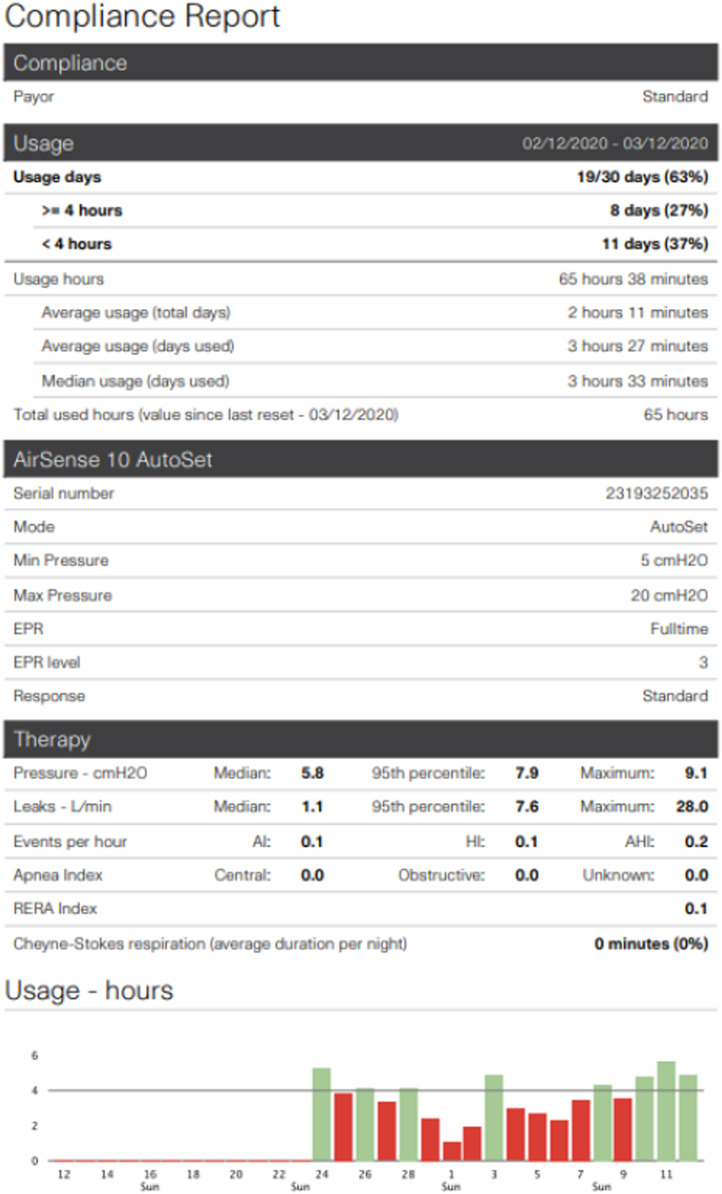
Increasingly, technological innovations are being used to improve PAP management and adherence. Cloud-based platforms and wireless capabilities offer real-time monitoring and active patient engagement. In a retrospective analysis of two cloud-based databases, patients who actively engaged in real-time feedback through a website connected to their PAP devices (n = 42,679) had 87% compliance compared with 70% compliance in the usual-care group (n = 85,358), as defined by the U.S. Medicare criteria for compliance (>4 h/night of PAP usage on at least 70% of nights in a consecutive 30-day period) (9). These technologies are also being incorporated in telemedicine. In a recent trial on telemedicine education and telemonitoring on CPAP adherence, patients randomized to receive web-based education and automated message feedback through telemonitoring had a Medicare adherence rate of 73% compared with 55% in the usual-care group (10). Modern PAP devices are including features in an attempt to improve comfort and adherence, including ramp, automatically adjusting pressures (e.g., automatic PAP), expiratory pressure relief technologies, lighter interfaces such as nasal pillows, and heated humidification (see Figure 1). Although none of these have been shown to consistently improve adherence, these technological advancements reflect ongoing efforts to personalize OSA management.
Figure 1.
Example of a standard positive airway pressure compliance report depicting 30 nights’ objective downloaded data from automatic positive airway pressure device. AHI = apnea–hypopnea index; AI = apnea index; EPR = expiratory pressure relief; HI = hypopnea index; RERA = respiratory effort–related arousal.
References
- 1.Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanchina ML, Welicky LM, Donat W, Lee D, Corrao W, Malhotra A. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J Clin Sleep Med. 2013;9:767–772. doi: 10.5664/jcsm.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sunwoo BY, Light M, Malhotra A. Strategies to augment adherence in the management of sleep-disordered breathing. Respirology. 2020;25:363–371. doi: 10.1111/resp.13589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15:343–356. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwab RJ, Badr SM, Epstein LJ, Gay PC, Gozal D, Kohler M, et al. ATS Subcommittee on CPAP Adherence Tracking Systems. An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems. The optimal monitoring strategies and outcome measures in adults. Am J Respir Crit Care Med. 2013;188:613–620. doi: 10.1164/rccm.201307-1282ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakata S, Noda A, Yagi H, Yanagi E, Mimura T, Okada T, et al. Nasal resistance for determinant factor of nasal surgery in CPAP failure patients with obstructive sleep apnea syndrome. Rhinology. 2005;43:296–299. [PubMed] [Google Scholar]
- 7.Bakker JP, Wang R, Weng J, Aloia MS, Toth C, Morrical MG, et al. Motivational enhancement for increasing adherence to CPAP: a randomized controlled trial. Chest. 2016;150:337–345. doi: 10.1016/j.chest.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wozniak DR, Lasserson TJ, Smith I. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2014:CD007736. doi: 10.1002/14651858.CD007736.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Malhotra A, Crocker ME, Willes L, Kelly C, Lynch S, Benjafield AV. Patient engagement using new technology to improve adherence to positive airway pressure therapy: a retrospective analysis. Chest. 2018;153:843–850. doi: 10.1016/j.chest.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang D, Chang JW, Benjafield AV, Crocker ME, Kelly C, Becker KA, et al. Effect of telemedicine education and telemonitoring on continuous positive airway pressure adherence: the tele-OSA randomized trial. Am J Respir Crit Care Med. 2018;197:117–126. doi: 10.1164/rccm.201703-0582OC. [DOI] [PubMed] [Google Scholar]
OSA: Update on Current Non-PAP Therapies
Christopher Schmickl and Jeremy E. Orr
OSA affects up to 1 billion people globally and has a number of associated adverse health effects (1). CPAP is recommended as first-line treatment of OSA, but several alternative therapies may be considered for patients reluctant to use PAP or for those who struggle with adherence.
Oral Appliances
Oral appliances (i.e., custom titratable mandibular advancement devices) have been shown to improve sleepiness (2) as well as blood pressure (3). However, results may depend on the particular device selected, experience of the dentist, and appropriate follow-up. Approximately 50% of patients will have a therapeutic response as assessed by reduction of the AHI, with responses predicted modestly by clinical characteristics (lower body mass index [BMI], younger age, female sex, and lower pretreatment AHI). More recently identified predictors of oral appliance success include a lower therapeutic CPAP pressure (4) and OSA physiological traits such as less upper airway collapsibility (5).
Hypoglossal Nerve Simulation
Implantable hypoglossal nerve stimulation has been U.S. Food and Drug Administration (FDA) approved since 2014 as an alternative therapy for OSA, with data showing sustained AHI improvement at 5-year follow-up (6). However, only a subset of patients are eligible based on the criteria of BMI <32–33 kg/m2 and findings during drug-induced sleep endoscopy (palatal concentric collapse is unfavorable). In this select population, AHI reduction averages 50%. Recent findings from a registry suggest that female sex, older age, and lower BMI may be predictors of good response to hypoglossal nerve stimulation treatment (7).
Airway Surgeries
A number of upper airway surgeries are available, including uvulopalatopharyngoplasty, tongue reduction or advancement, and maxillomandibular advancement, with observational data suggesting highly variable polysomnographic response rates. Robust data in this area are limited by variable criteria used for patient and procedure selection and evolving surgical techniques. For uvulopalatopharyngoplasty, reported polysomnographic response rates are variable, but meta-analysis indicates an average of 49.5% reduction in AHI. Furthermore, randomized (nonsham) trials suggest improvements in sleepiness and quality of life (8). Maxillomandibular advancement has been shown to be beneficial in those with mandibular retrognathia, although high-quality data for patient selection are lacking.
Weight Loss
Weight loss is recommended for adult patients with OSA who are overweight or obese on the basis of improvements in AHI as well as improvements in associated symptoms and comorbidities (9). Programs that include comprehensive lifestyle interventions, including a reduced calorie diet, physical activity, and behavioral counseling, are recommended. For those with a BMI ≥35 kg/m2, referral to bariatric surgery may be considered. Although weight loss commonly reduces OSA severity, resolution is uncommon, and patients often regain weight over time. Therefore, weight loss is regarded as an adjunctive therapy and should not be considered a substitute for primary OSA therapies (10).
Pharmacotherapy
No pharmacotherapies are currently FDA approved as primary treatments for OSA. Medications currently under study include acetazolamide, dronabinol, and atomoxetine plus oxybutynin. Solriamfetol and pitolisant have recently been FDA approved for the treatment of sleepiness in patients with OSA, adding to other alerting agents such as modafanil. It is important to realize that these are symptomatic therapies only that do not address the underlying OSA and thus should not be recommended for use in the absence of optimal OSA treatment. An overview of alternative non-PAP therapies has been summarized in Table 2.
Table 2.
Overview of alternative non-PAP therapies
| Intervention | When to Consider | Key Predictors of Good Response | Key Predictors of Poor Response |
|---|---|---|---|
| Oral appliance | Acceptable dentition | Low AHI | High loop gain |
| Mild to moderate OSA | Low BMI | ||
| Availability of experienced sleep dentist | Younger age | ||
| Female sex | |||
| Hypoglossal nerve stimulation | FDA approval criteria include:
|
Low BMI | CCC on DISE (contraindication) |
| Older age | |||
| Female sex | |||
| Upper airway surgery (e.g., uvulopalatopharyngoplasty) | Surgical problem (e.g., tonsillar hypertrophy) | Low BMI | High loop gain |
| Patient is interested in surgery and has acceptable surgical risk | High Friedman/Mallampati class | ||
| Tonsillar hypertrophy | |||
| Low AHI | |||
| Weight loss (complementary to intervention targeting the underlying obstruction) | Patient is overweight or has obesity | N/A | N/A |
| Specific intervention (lifestyle, pharmacological, or surgical) depends on degree of excess weight and other patient factors | |||
| Wake-promoting medications (solriamfetol, modafinil, and armodafinil) | Residual sleepiness despite control of underlying obstruction (e.g., CPAP) | N/A | N/A |
| Avoid modafinil/armodafinil in women on hormonal contraceptives |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; CCC = complete concentric collapse; CPAP = continuous PAP; DISE = drug-induced sleep endoscopy; FDA = U.S. Food and Drug Administration; N/A = not applicable; OSA = obstructive sleep apnea; PAP = positive airway pressure.
References
- 1.Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7:687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bratton DJ, Gaisl T, Schlatzer C, Kohler M. Comparison of the effects of continuous positive airway pressure and mandibular advancement devices on sleepiness in patients with obstructive sleep apnoea: a network meta-analysis. Lancet Respir Med. 2015;3:869–878. doi: 10.1016/S2213-2600(15)00416-6. [DOI] [PubMed] [Google Scholar]
- 3.Gotsopoulos H, Kelly JJ, Cistulli PA. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: a randomized, controlled trial. Sleep. 2004;27:934–941. doi: 10.1093/sleep/27.5.934. [DOI] [PubMed] [Google Scholar]
- 4.Sutherland K, Phillips CL, Davies A, Srinivasan VK, Dalci O, Yee BJ, et al. CPAP pressure for prediction of oral appliance treatment response in obstructive sleep apnea. J Clin Sleep Med. 2014;10:943–949. doi: 10.5664/jcsm.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards BA, Andara C, Landry S, Sands SA, Joosten SA, Owens RL, et al. Upper-airway collapsibility and loop gain predict the response to oral appliance therapy in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2016;194:1413–1422. doi: 10.1164/rccm.201601-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodson BT, Strohl KP, Soose RJ, Gillespie MB, Maurer JT, de Vries N, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg. 2018;159:194–202. doi: 10.1177/0194599818762383. [DOI] [PubMed] [Google Scholar]
- 7.Heiser C, Steffen A, Boon M, Hofauer B, Doghramji K, Maurer JT, et al. ADHERE registry investigators. Post-approval upper airway stimulation predictors of treatment effectiveness in the ADHERE registry. Eur Respir J. 2019;53:1801405. doi: 10.1183/13993003.01405-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stuck BA, Ravesloot MJL, Eschenhagen T, de Vet HCW, Sommer JU. Uvulopalatopharyngoplasty with or without tonsillectomy in the treatment of adult obstructive sleep apnea - a systematic review. Sleep Med. 2018;50:152–165. doi: 10.1016/j.sleep.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Hudgel DW, Patel SR, Ahasic AM, Bartlett SJ, Bessesen DH, Coaker MA, et al. American Thoracic Society Assembly on Sleep and Respiratory Neurobiology. The role of weight management in the treatment of adult obstructive sleep apnea: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e70–e87. doi: 10.1164/rccm.201807-1326ST. [DOI] [PubMed] [Google Scholar]
- 10.Bakker JP, Tavakkoli A, Rueschman M, Wang W, Andrews R, Malhotra A, et al. Gastric banding surgery versus continuous positive airway pressure for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2018;197:1080–1083. doi: 10.1164/rccm.201708-1637LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
Controversies and Consensus: Strategies in the Management of Central Sleep Apnea
Snigdha Sharma and Abdulghani Sankari
Central sleep apnea (CSA) is caused by a temporary failure of the pontomedullary pacemaker in generating a breathing rhythm, which results in the loss of ventilatory effort. If this lasts for ≥10 seconds, it is defined as a CSA event. There is no brainstem inspiratory neural output, and the nerves innervating the inspiratory thoracic pump muscles are silent. Therefore, on polysomnography (PSG), it is characterized by the absence of both naso-oral airflow and thoracoabdominal excursions (i.e., muscle effort).
CSA can be seen in the settings of high altitude, medication use, substance abuse (most commonly opioids), or severe heart failure, manifesting in a Cheyne-Stokes respiration pattern. When CSA is not due to a primary medical disorder, it is termed primary CSA. Treatment-emergent CSA is another type of CSA characterized by the emergence of CSA during the treatment of OSA with PAP therapy (1, 2).
Clinically, patients with CSA may be asymptomatic or present with excessive daytime sleepiness, poor sleep quality, insomnia, paroxysmal nocturnal dyspnea, morning headaches, or nocturnal angina. In addition, apneic episodes may be reported by bed partners.
Diagnosis
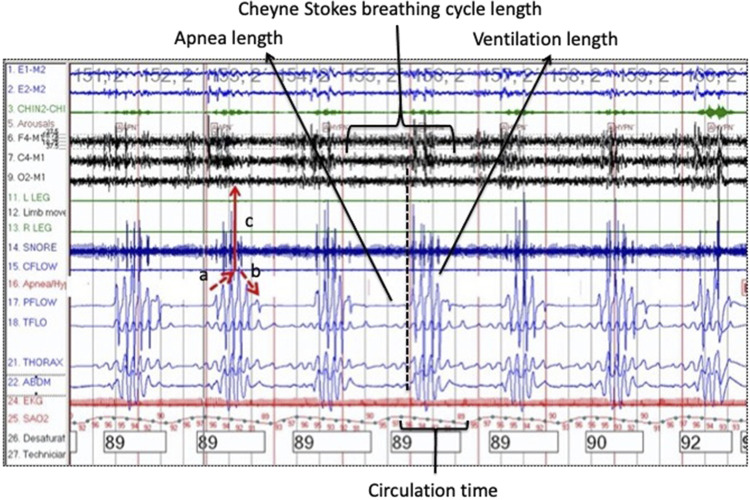
In-laboratory PSG is the gold standard for establishing a diagnosis. However, more recently, ambulatory testing has been validated in CSA using the peripheral arterial tonometry–based WatchPAT devices (3). The diagnosis of CSA with Cheyne-Stokes breathing requires three or more consecutive central apneas and/or central hypopneas separated by a crescendo–decrescendo respiratory pattern with a cycle length of ≥40 seconds (see Figure 2). In primary CSA, PSG reveals five or more central apneas and/or central hypopneas per hour of sleep, and the number of central apneas and central hypopneas is >50% of the total number of apneas and hypopneas without any other associated disorder (4).
Figure 2.
A polygraph illustrating a case of consecutive central sleep apnea (CSA) events with Cheyne-Stokes respiration. Red arrows’ abbreviations: (a) crescendo phase; (b) decrescendo phase; (c) electroencephalographic arousal at peak ventilation phase. The dotted vertical line indicates the end of a CSA event. Different CSA-related timing definitions are highlighted. Apnea length = duration of apnea; Circulation time = the time between the end of CSA event and the nadir corresponding O2 saturation (SaO2); Ventilation length = duration of respiration following apnea. Adapted by permission from Reference 12.
Treatments
When confronted with opioid-associated CSA, discontinuation of the offending agent or at least lowering the dose is recommended whenever possible. Although most cases of treatment-emergent CSA resolve spontaneously over weeks to months, some cases may persist (4). CSA can also be seen at sleep onset in patients without sleep disorders and is typically not treated because it is physiological and reflects differences in respiratory drive between wake and sleep. In patients with heart failure, efforts should be made to optimize treatment of the underlying heart failure using medical therapy, cardiac resynchronization, or other modalities before considering PAP therapy. CPAP is first-line therapy for symptomatic patients with hyperventilation-related CSA associated with heart failure (5). Treatment options for those who are unable to tolerate or fail CPAP include supplemental oxygen therapy (especially useful for those with nighttime hypoxemia), and adaptive servo ventilation. However, on the basis of the results of the SERVE-HF (Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure) trial, the American Academy of Sleep Medicine now recommends against the use of adaptive servo ventilation to treat heart failure–associated CSA in patients with an ejection fraction ≤45% and moderate to severe CSA because of increased all-cause and cardiovascular mortality (6, 7). Bilevel PAP therapy in spontaneous-timed mode may be considered if there is no response to other interventions (8). In small clinical trials, acetazolamide and theophylline have demonstrated an improvement in heart failure–associated CSA (9, 10). The FDA also recently approved the use of transvenous neurostimulation of the phrenic nerve for the treatment of CSA on the basis of the results of a randomized trial demonstrating a significant reduction in the severity of CSA (11).
References
- 1.Liu D, Armitstead J, Benjafield A, Shao S, Malhotra A, Cistulli PA, et al. Trajectories of emergent central sleep apnea during CPAP therapy. Chest. 2017;152:751–760. doi: 10.1016/j.chest.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nigam G, Riaz M, Chang ET, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13:86–91. doi: 10.4103/atm.ATM_321_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pillar G, Berall M, Berry R, Etzioni T, Shrater N, Hwang D, et al. Detecting central sleep apnea in adult patients using WatchPAT-a multicenter validation study. Sleep Breath. 2020;24:387–398. doi: 10.1007/s11325-019-01904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. American Academy of Sleep Medicine; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arzt M, Floras JS, Logan AG, Kimoff RJ, Series F, Morrison D, et al. CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP) Circulation. 2007;115:3173–3180. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 6.Aurora RN, Bista SR, Casey KR, Chowdhuri S, Kristo DA, Mallea JM, et al. Updated adaptive servo-ventilation recommendations for the 2012 AASM guideline: “the treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses”. J Clin Sleep Med. 2016;12:757–761. doi: 10.5664/jcsm.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowie MR, Woehrle H, Wegscheider K, Angermann C, d’Ortho MP, Erdmann E, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373:1095–1105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aurora RN, Chowdhuri S, Ramar K, Bista SR, Casey KR, Lamm CI, et al. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep (Basel) 2012;35:17–40. doi: 10.5665/sleep.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Javaheri S. Acetazolamide improves central sleep apnea in heart failure: a double-blind, prospective study. Am J Respir Crit Care Med. 2006;173:234–237. doi: 10.1164/rccm.200507-1035OC. [DOI] [PubMed] [Google Scholar]
- 10.Javaheri S, Parker TJ, Wexler L, Liming JD, Lindower P, Roselle GA. Effect of theophylline on sleep-disordered breathing in heart failure. N Engl J Med. 1996;335:562–567. doi: 10.1056/NEJM199608223350805. [DOI] [PubMed] [Google Scholar]
- 11.Costanzo MR, Ponikowski P, Javaheri S, Augostini R, Goldberg L, Holcomb R, et al. remedé System Pivotal Trial Study Group. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388:974–982. doi: 10.1016/S0140-6736(16)30961-8. [DOI] [PubMed] [Google Scholar]
- 12.Sankari A. clinical sleep medicine self-assessment, 1st ed. 2017. p. 40. [Google Scholar]
Practical Approaches to Cognitive Behavioral Therapy for Insomnia for Clinicians
Sogol Javaheri and Suzanne Bertisch
Chronic insomnia, characterized by difficulties falling asleep, staying asleep, or early morning awakenings, is the most prevalent sleep disorder in the United States, affecting an estimated 10–15% of Americans. Symptoms occur despite adequate opportunity to sleep and are associated with daytime impairment, including impaired attention and cognition, increased risk of industrial and motor vehicle accidents, reduced work productivity, and increased healthcare costs (1). Insomnia is also a risk factor for multiple chronic health conditions, including cardiovascular disease (2), mood disorders, and pain conditions (1).
Despite the widespread public health impact, insomnia remains both underrecognized and undertreated. Nearly two-thirds of patients with insomnia are unaware of available treatment options, and ∼40% self-medicate with alcohol and unproven over-the-counter sleep aids (3). Evaluation of insomnia should include a comprehensive sleep history, including sleep/wake routines (time to bed, time to fall asleep, number and duration of awakenings, wake time, time out of bed, and daytime naps), daily behaviors that impact sleep (e.g., use of electronic devices, caffeine, alcohol, and nicotine), and screening for comorbid sleep disorders (e.g., sleep-disordered breathing, restless legs syndrome, periodic limb movement disorder, and circadian disorders) (1, 4). Given the high prevalence of concurrent mood disorders and other comorbidities, a thorough medical and psychiatric history is also warranted. Prior treatments for insomnia should also be reviewed. Use of a sleep diary to capture the patient’s habitual sleep patterns, including differences between weekday and weekend sleep routines, is very helpful. A sleep study is not routinely recommended unless OSA or periodic limb movement disorder are suspected. Comorbid depression and anxiety as well as pain syndromes are distinct but overlapping entities and should be treated concurrently (1).
Current guidelines recommend multicomponent cognitive behavioral therapy for insomnia (CBT-I) as first-line treatment for chronic insomnia in adults (see Table 3). Data suggest that compared with pharmacotherapy, the effects of CBT-I are similar (5) but more durable and have a better safety profile (5–7). CBT-I is also effective for insomnia in adults with comorbid mood and medical conditions, including conditions in which sleep medication may be contraindicated. The key components of CBT-I include sleep hygiene, stimulus control, sleep restriction, cognitive restructuring, and relaxation techniques (Table 3) (8). It is important to note that sleep hygiene alone is not efficacious for chronic insomnia. CBT-I should be implemented by a trained provider or web-based program supported by clinical data. In-person CBT-I options are often limited by an insufficient number of trained clinicians, cost/insurance barriers, and time intensity (8). Recent studies support the use of validated web-based CBT-I programs (9), group formats, and condensed versions, such as brief behavioral treatment for insomnia (9, 10). Behavioral treatments can also be implemented in patients who are concurrently receiving sleep aids (11).
Table 3.
Cognitive behavioral therapy for insomnia components
| Technique | Aim |
|---|---|
| Stimulus control | Extinguish the association between wakefulness and the bedroom environment and establish a consistent wake time |
| Sleep restriction | Increase sleep drive and consolidate sleep by limiting time in bed |
| Relaxation training | Reduce physical and cognitive arousals |
| Cognitive restructuring | Restructure maladaptive thinking regarding insomnia, such as unhelpful beliefs about sleep or performance anxiety |
| Sleep hygiene | Reduce behaviors and environmental factors that interfere with sleep or increase arousals |
References
- 1.Buysse DJ. Insomnia. JAMA. 2013;309:706–716. doi: 10.1001/jama.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Javaheri S, Redline S. Insomnia and risk of cardiovascular disease. Chest. 2017;152:435–444. doi: 10.1016/j.chest.2017.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. II. Sleep. 1999;22:S354–S358. [PubMed] [Google Scholar]
- 4.Richardson C, Micic G, Cain N, Bartel K, Maddock B, Gradisar M. Cognitive “insomnia” processes in delayed sleep-wake phase disorder: do they exist and are they responsive to chronobiological treatment? J Consult Clin Psychol. 2019;87:16–32. doi: 10.1037/ccp0000357. [DOI] [PubMed] [Google Scholar]
- 5.Rossman J. Cognitive-behavioral therapy for insomnia: an effective and underutilized treatment for insomnia. Am J Lifestyle Med. 2019;13:544–547. doi: 10.1177/1559827619867677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Zweerde T, Bisdounis L, Kyle SD, Lancee J, van Straten A. Cognitive behavioral therapy for insomnia: a meta-analysis of long-term effects in controlled studies. Sleep Med Rev. 2019;48:101208. doi: 10.1016/j.smrv.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Wilt TJ, MacDonald R, Brasure M, Olson CM, Carlyle M, Fuchs E, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2016;165:103–112. doi: 10.7326/M15-1781. [DOI] [PubMed] [Google Scholar]
- 8.Zhou ES, Gardiner P, Bertisch SM. Integrative medicine for insomnia. Med Clin North Am. 2017;101:865–879. doi: 10.1016/j.mcna.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Vedaa Ø, Hagatun S, Kallestad H, Pallesen S, Smith ORF, Thorndike FP, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15:101–110. doi: 10.5664/jcsm.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunn HE, Tutek J, Buysse DJ. Brief behavioral treatment of insomnia. Sleep Med Clin. 2019;14:235–243. doi: 10.1016/j.jsmc.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Winkelman JW.Insomnia disorder N Eng J Medicine 20153731437–1444.. [DOI] [PubMed] [Google Scholar]
Risks and Management of Long-Term Use of Sleep-Inducing Medications
Kara Dupuy-McCauley and Bhanu Kolla
Chronic insomnia disorder is characterized by perceived difficulty initiating or maintaining sleep or waking up earlier than desired with difficulty returning to sleep, coupled with daytime impairment. It is a common condition in the United States, with an approximate prevalence of 10–24% (1). In addition to being a source of emotional distress, chronic insomnia can create problems with mood and cognitive function and can lead to increased risk of automobile accidents, increased healthcare costs, perceived poor health, and loss of productivity or errors in the workplace (1).
Insomnia is frequently persistent if left untreated. Chronic insomnia, by definition, lasts for more than 3 months, but 56–74% of patients will continue to have insomnia at 1 year and 46% of patients have insomnia for at least 3 years (2). The mainstay of treatment for chronic insomnia is CBT-I when available. There is moderate existing evidence to suggest that CBT-I improves sleep outcomes and has limited potential for harm (1).
Little is known about the effects of using hypnotic medication on a long-term basis (1), yet many patients remain on pharmacologic treatments for insomnia beyond what can be thought of as an acute period; ∼2.5% of the U.S. population receives hypnotics for insomnia, and 25% of that group has been on nightly treatment for 4 months or longer (3). The initial guidelines for pharmacotherapy in chronic insomnia were based on short-term hypnotic use, with the mean duration of clinical trials being 1 week. Two more recent trials have examined the use of hypnotic medications over a 12-month period, and as a result, eszopiclone and zolpidem extended release are now FDA approved for long-term use (3, 4). Many providers and patients remain hesitant to use hypnotics on a long-term basis because of fear of potential health-related consequences. One matter of particular concern to patients is the association of long-term hypnotic use with neurodegenerative diseases, mainly Alzheimer’s dementia. Multiple prospective and retrospective studies have linked long-term benzodiazepine and nonbenzodiazepine hypnotic use with dementia (5). Long-term hypnotic use has also been linked to increased mortality, cardiovascular disease, psychiatric disorders, and falls (6–8). Although alarming, support for these concerns comes mainly from association studies, and furthermore, these possible risks have not formally been studied against the risks of untreated insomnia. Insomnia with short sleep, defined as a duration of less than 6 hours, has been shown to increase risk of hypertension, acute coronary syndrome, and mortality (9). There is also evidence that insomnia and sleep fragmentation may be risk factors for neurodegenerative diseases, including Alzheimer’s disease (10).
Recently, several hypnotic agents, including doxepin (a tricyclic antidepressant that acts exclusively as a hypnotic at low doses), orexin antagonists, suvorexant, and lemborexant have been approved by the FDA for the treatment of insomnia. Current data examining their safety and efficacy in the long term are sparse.
In conclusion, for the treatment of chronic insomnia, CBT-I should be first line when available, but long-term pharmacotherapy may be appropriate in a select group of patients. We need more rigorous and dedicated research to fully elucidate the safety of long-term hypnotic use, but one must also consider the consequences of untreated insomnia with respect to quality of life and risk of morbidity and mortality. A careful risk–benefit analysis should be assessed for each patient, and the provider must engage in shared decision-making when considering the long-term use of hypnotics for chronic insomnia (see Table 4).
Table 4.
Risks of long-term hypnotic use versus risks of untreated chronic insomnia disorder
| Potential Risks of Long-Term Hypnotic Use | Potential Risks of Untreated Chronic Insomnia |
|---|---|
| All-cause mortality | Hypertension |
| Cancer | Cardiovascular disease |
| Abuse | All-cause mortality |
| Dependence | Psychiatric disorders, including depression |
| Infections | Diabetes |
| Falls | Neurocognitive deficits |
| Dementia | |
| Cardiovascular disease | |
| Alcohol use disorder | |
| Major depressive disorder |
References
- 1.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125–133. doi: 10.7326/M15-2175. [DOI] [PubMed] [Google Scholar]
- 2.Morin CM, Bélanger L, LeBlanc M, Ivers H, Savard J, Espie CA, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169:447–453. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 3.Roth T, Walsh JK, Krystal A, Wessel T, Roehrs TA. An evaluation of the efficacy and safety of eszopiclone over 12 months in patients with chronic primary insomnia. Sleep Med. 2005;6:487–495. doi: 10.1016/j.sleep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Roehrs TA, Randall S, Harris E, Maan R, Roth T. Twelve months of nightly zolpidem does not lead to rebound insomnia or withdrawal symptoms: a prospective placebo-controlled study. J Psychopharmacol. 2012;26:1088–1095. doi: 10.1177/0269881111424455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ettcheto M, Olloquequi J, Sánchez-López E, Busquets O, Cano A, Manzine PR, et al. Benzodiazepines and related drugs as a risk factor in Alzheimer’s disease dementia. Front Aging Neurosci. 2020;11:344. doi: 10.3389/fnagi.2019.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kripke DF, Langer RD, Kline LE. Hypnotics’ association with mortality or cancer: a matched cohort study. BMJ Open. 2012;2:e000850. doi: 10.1136/bmjopen-2012-000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weich S, Pearce HL, Croft P, Singh S, Crome I, Bashford J, et al. Effect of anxiolytic and hypnotic drug prescriptions on mortality hazards: retrospective cohort study. BMJ. 2014;348:g1996. doi: 10.1136/bmj.g1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandt J, Leong C. Benzodiazepines and Z-drugs: an updated review of major adverse outcomes reported on in epidemiologic research. Drugs R D. 2017;17:493–507. doi: 10.1007/s40268-017-0207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17:241–254. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shamim SA, Warriach ZI, Tariq MA, Rana KF, Malik BH. Insomnia: risk factor for neurodegenerative diseases. Cureus. 2019;11:e6004. doi: 10.7759/cureus.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tetrahydrocannabinol, Cannabidiol, Edibles, Oils, and All That: Cannabis and Sleep
Ikuyo Imayama and Bharati Prasad
The legalization of cannabinoids in the United States has opened avenues for using cannabinoids to treat medical conditions. Cannabinoids are active constituents of cannabis. Tetrahydrocannabinol, cannabidiol, edibles, and oils are different forms of cannabinoids. Cannabinoid products are approved for appetite stimulation, nausea and vomiting, specific types of seizure disorders, and pain. Prior studies have reported their therapeutic potential in sleep disorders, including insomnia, REM behavior disorder, nightmare disorder, sleep disturbances in pain conditions, daytime sleepiness, and sleep apnea (1–4).
Endogenous cannabinoids are a brain lipid involved in the regulation of the sleep–wake cycle (5). Effects of cannabinoids are mediated by cannabinoid 1 and 2 receptors, and by modifying effects of other receptors (6). Animal studies have shown that endogenous cannabinoids play a significant role in regulating wakefulness and sleep architecture (6). Alteration of endogenous cannabinoid concentrations associated with sleep deprivation, diurnal variation of endogenous cannabinoids in the brain, and synthetic cannabinoids induced sleep in multiple animal models, suggesting its pivotal role in sleep–wake homeostasis (5).
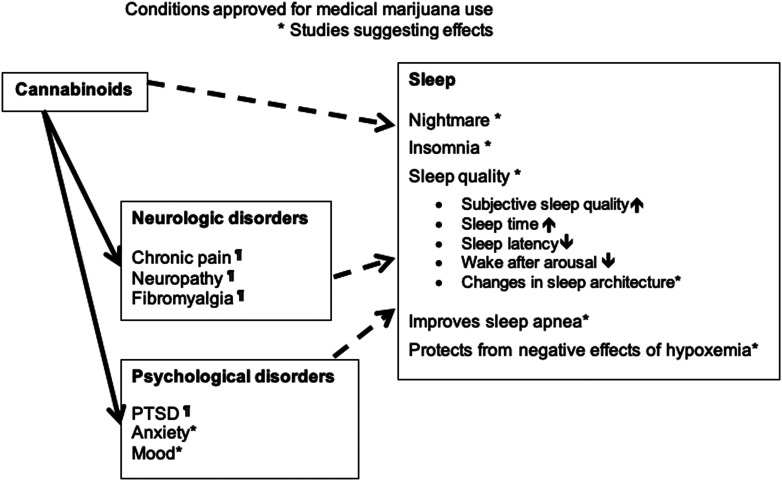
Short-term studies have shown that cannabinoids improve subjective sleep quality (i.e., decreased sleep fragmentation, nightmares, and daytime sleepiness) and objective sleep parameters (i.e., shortened sleep onset latency, reduced wake after sleep onset, and normalized REM sleep onset) (1, 4, 7). One study reported a reduction in OSA severity as measured by the AHI (3). Effects of cannabinoids on sleep is detailed in Figure 3. Cannabis withdrawal symptoms in chronic users include sleep disturbance and vivid dreams (2). Abrupt cessation of cannabinoids was associated with decreased total sleep time, sleep efficiency, and percentage of REM and slow-wave sleep and increased wake after sleep onset, sleep onset latency, and periodic limb movement (8, 9). These studies suggest that cannabinoids interact with the sleep–wake cycle and in the short term, may improve subjective and objective sleep quality in human subjects.
Figure 3.
How cannabinoids may improve sleep (potential pathways). *Studies suggesting effects. ¶Conditions approved for medical marijuana use. PTSD = post-traumatic stress disorder.
Despite animal and human studies suggesting positive effects of cannabinoids on sleep, limitations of current evidence in examining therapeutic effects of cannabinoids for sleep disorders include 1) small sample size, 2) comorbid conditions such as pain and substance use disorders that may confound the true effects of cannabinoids on sleep, 3) examination of sleep as a secondary outcome, 4) short duration of the trials (<6 mo), 5) mixed findings among reported studies, and 6) unclear optimal dosing and side effect profile of different products, especially in the long term (1, 7). Cannabinoids cannot be recommended as a treatment for any sleep disorder at this time (10).
Supplementary Material
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kuhathasan N, Dufort A, MacKillop J, Gottschalk R, Minuzzi L, Frey BN. The use of cannabinoids for sleep: a critical review on clinical trials. Exp Clin Psychopharmacol. 2019;27:383–401. doi: 10.1037/pha0000285. [DOI] [PubMed] [Google Scholar]
- 2.Babson KA, Sottile J, Morabito D. Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. 2017;19:23. doi: 10.1007/s11920-017-0775-9. [DOI] [PubMed] [Google Scholar]
- 3.Carley DW, Prasad B, Reid KJ, Malkani R, Attarian H, Abbott SM, et al. Pharmacotherapy of apnea by cannabimimetic enhancement, the PACE clinical trial: effects of dronabinol in obstructive sleep apnea. Sleep (Basel) 2018;41:zsx184. doi: 10.1093/sleep/zsx184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456–2473. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Bazan NG. Lipid signaling: sleep, synaptic plasticity, and neuroprotection. Prostaglandins Other Lipid Mediat. 2005;77:65–76. doi: 10.1016/j.prostaglandins.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Prospéro-García O, Amancio-Belmont O, Becerril Meléndez AL, Ruiz-Contreras AE, Méndez-Díaz M. Endocannabinoids and sleep. Neurosci Biobehav Rev. 2016;71:671–679. doi: 10.1016/j.neubiorev.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Gates PJ, Albertella L, Copeland J. The effects of cannabinoid administration on sleep: a systematic review of human studies. Sleep Med Rev. 2014;18:477–487. doi: 10.1016/j.smrv.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Bolla KI, Lesage SR, Gamaldo CE, Neubauer DN, Wang NY, Funderburk FR, et al. Polysomnogram changes in marijuana users who report sleep disturbances during prior abstinence. Sleep Med. 2010;11:882–889. doi: 10.1016/j.sleep.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen-Zion M, Drummond SP, Padula CB, Winward J, Kanady J, Medina KL, et al. Sleep architecture in adolescent marijuana and alcohol users during acute and extended abstinence. Addict Behav. 2009;34:976–979. doi: 10.1016/j.addbeh.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramar K, Rosen IM, Kirsch DB, Chervin RD, Carden KA, Aurora RN, et al. American Academy of Sleep Medicine Board of Directors. Medical cannabis and the treatment of obstructive sleep apnea: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2018;14:679–681. doi: 10.5664/jcsm.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.