Abstract
Antibodies against two G-protein coupled receptors (GPCRs), angiotensin II type 1 receptor (AT1R) and endothelin A receptor (ETAR) are among a growing number of autoantibodies that are found to be associated with allograft dysfunction. AT1R antibodies (AT1Rabs) and ETAR antibodies (ETARabs) have been shown to activate their target receptors and affect signaling pathways. Multiple single center reports have shown an association between presence of these antibodies and acute or chronic rejection and graft loss in kidney, heart, liver, lung and composite tissue transplantations. However, the characteristics of patients that are most likely to develop adverse outcomes, the phenotypes associated with graft damage solely due to these antibodies, and the antibody titer required to cause dysfunction are areas that remain controversial. This review compiles existing knowledge on the effect of antibodies against GPCRs in other diseases in order to bridge the gap in knowledge within transplantation biology. Future areas for research are highlighted and include the need for functional assays and treatment protocols for transplant patients who present with AT1Rabs and ETARabs. Understanding how antibodies that activate GPCRs influence transplantation outcome will have direct clinical implications for pre-emptive evaluation of transplant candidates as well as the post-transplant care of organ recipients.
Keywords: Non-HLA antibodies, Angiotensin II type 1 receptor antibody, Endothelin A receptor antibody, Allograft dysfunction
1. Introduction
Antibody mediated injury following organ transplantation results most often from development of an alloimmune response against non-self-antigens [1]. This response is primarily due to reactivity against HLA antigen mismatch between donor and recipient, but may also involve reactivity against other alloantigens expressed on the allograft [2]. Antibody mediated injury may also result from an autoimmune response developed against self-antigens [3]. Although a role for antigenic targets other than HLA in transplantation outcome had been proposed [2,4], progress in characterizing these targets, understanding the mechanism of antibody damage, and developing clinically useful detection tools is lagging. This slow progress may reflect skepticism about the importance of autoantibodies and a lack of mechanistic studies to support the involvement of these autoantibodies in transplantation outcome. Nevertheless, single center reports of acute and chronic antibody mediated injuries noted on transplant biopsies as well as cases of allograft loss [5] in the absence of donor specific HLA antibodies cannot be ignored if the goal is to extend the lifetime of a transplanted organ [6].
Antibodies against two G protein coupled receptors (GPCRs), angiotensin II type 1 receptor (AT1R) and endothelin A receptor (ETAR), have been detected in the sera of transplant recipients who experience allograft dysfunction [7]. Importantly, AT1R antibodies (AT1Rabs) and ETAR antibodies (ETARabs) were shown to activate their target receptors and are therefore considered not only biomarkers but potential contributors to allograft injury [8,9]. Yet, the pathophysiologic consequences associated with presence of AT1Rabs and ETARabs in the sera of transplant recipients are not clearly understood. Not all transplant recipients with positive AT1Rab and ETARab experience allograft dysfunction [10,11]; and antibody titers reported in studies that utilize commercially available enzyme linked assays vary widely within the literature [12–14]. Additionally, the close correlation between the levels of AT1Rabs and ETARabs in some patients [5] makes it difficult to determine which of these antibodies contribute to abnormal phenotypes. Finally, there are limited clinical data on long-term allograft outcomes that compare no treatment versus use of plasmapheresis, receptor antagonists, or other therapeutics, in cases of antibody mediated injury and detectable AT1Rabs or ETARabs.
In addition to allograft dysfunction, autoantibodies that activate GPCRs have been linked to cardiovascular disease, preeclampsia, hypertension, aging, autoimmune diseases, and cancer [9,15–21]. In this review, we bring together existing knowledge on GPCR activation and the development and function of autoantibodies in other diseases, to better understand how antibodies that activate GPCRs could influence transplantation outcome. We also offer suggestions for future mechanistic and clinical studies that focus on understanding the functional properties of these antibodies, the effect of environmental stimuli, patient characteristics, and treatment interactions.
2. Angiotensin II type 1 and endothelin A receptors: structure, function and expression
While the structure of ETAR has been determined through modeling of the other GPCRs, the crystal structure of the human AT1R has been recently elucidated, providing new details on the extracellular domains of AT1R and molecular targets for new potential therapeutics [22,23]. Both AT1R and ETAR share most features of GPCRs [24], which consist of an extracellular glycosylated N-terminal domain followed by transmembrane regions containing seven hydrophobic α-helices. The transmembrane domains are linked via three shorter extracellular and intracellular loops. The structure ends with an intracellular C-terminal domain containing serine and threonine residues that are targets for phosphorylation. Subgroups of GPCRs are distinguished based on the length of the N- and C-terminal domains and a few residue differences within the transmembrane domains. Binding of molecules, such as hormones or antibodies, to GPCRs causes a rotational movement of these complex structures within the membrane of cells and promotes interaction with G-protein subunits [25]. This resulting tripartite connection between ligand (or autoantibody), receptor, and G-protein permits rapid signal transduction.
Both AT1R and ETAR are widely expressed in the human body [26], including the vascular endothelium, smooth muscle cells, immune cells, kidney, lung, heart, and placental tissues [27–29]. AT1R is activated through binding of its natural ligand, angiotensin II, to residues within transmembrane domains and the second extracellular loop [30]. Fig. 1 summarizes the effects of a transient activation of AT1R as well as consequences of overstimulation. When transiently stimulated by angiotensin II, AT1R regulates important processes such as blood flow [31], sodium retention, and aldosterone secretion [32]. In addition, AT1R stimulation plays a role in wound healing and tissue repair [33] (Fig. 1: The Good). ETAR activation by its natural ligand, the potent vasoconstrictor endothelin 1 [34], also mediates similar physiologic functions, but most importantly plays a role in the regulation of normal blood flow through alternate vasoconstriction and vasodilation, as endothelin 1 binds to ETAR and other endothelin receptors [22].
Fig. 1.
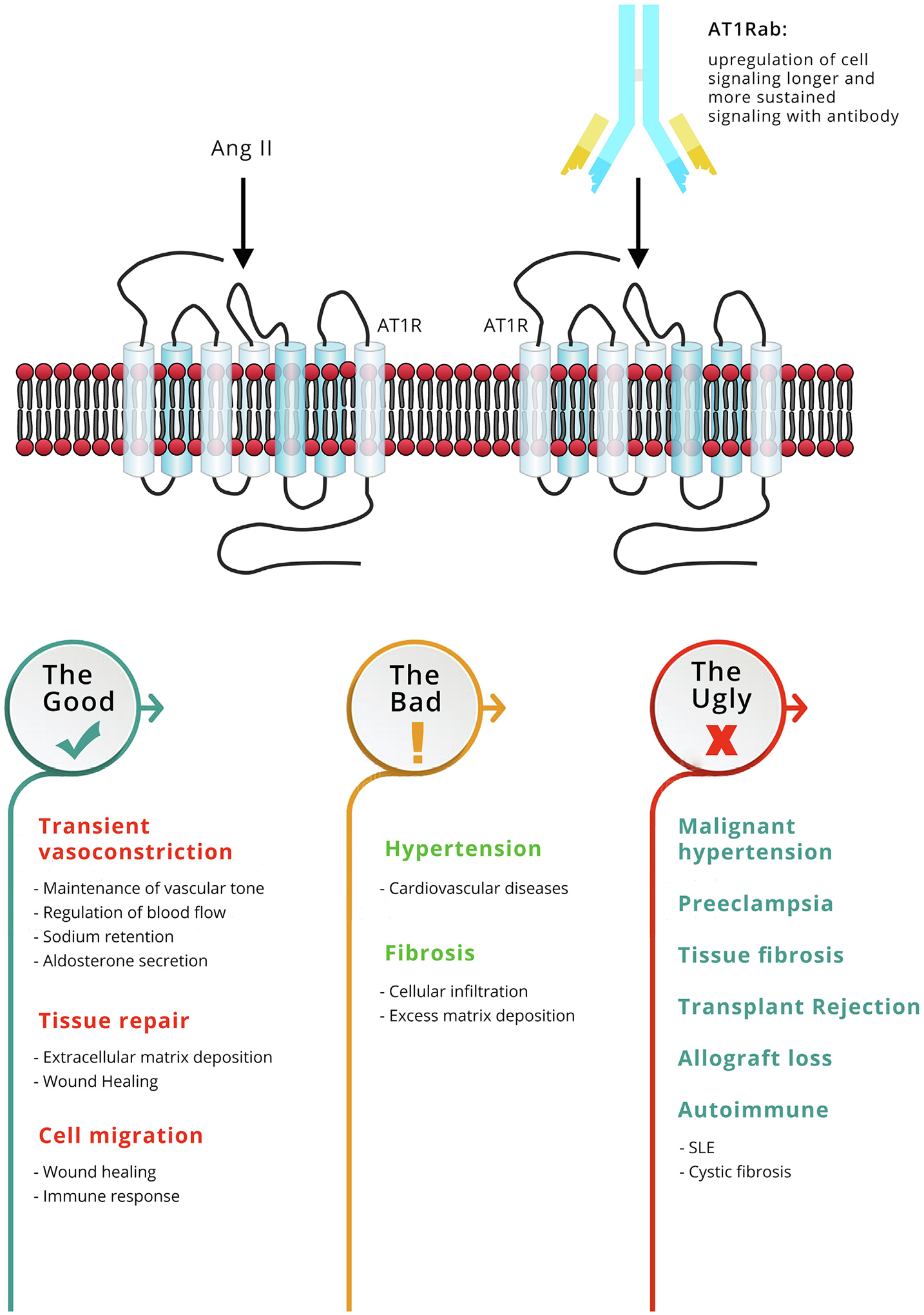
Angiotensin II type 1 receptor function in homeostasis and dysregulation: AT1R is widely distributed among many tissues and cells and mediates biological effects that maintain normal cellular homeostasis. The Good - AT1R activation by angiotensin II (Ang II) leads to contraction of vascular smooth muscles, accelerates the release of aldosterone and causes sodium retention, which all contribute to blood pressure regulation. Furthermore, AT1R activation leads to increased expression of collagen, proliferation and thickening of cells, and regulates cellular migration. These processes are important in tissue repair. The Bad - when over-activated, either due to increase presence of the natural ligand, Ang II or development of the autoantibody, AT1Rab, the receptor is associated with hypertension and development of fibrosis. AT1Rabs were shown to bind to the receptor and cause phosphorylation of ERK and activation of downstream signaling cascades. Activation of ERK pathways by AT1Rab binding to AT1R leads to an increase in vasoconstriction of arteries and increase in cell migration. The Ugly - AT1Rabs cause a more sustained activation of AT1R as compared to Ang II. These antibodies have been detected in patients with autoimmune diseases, preeclampsia and older individuals; conditions associated with progression of fibrosis, vascular constriction, and increased cellular migration. These same mechanisms may contribute to injury, rejection and graft loss in transplantation.
Levels of AT1R and ETAR are influenced by genetic and environmental factors. Increases or decreases in receptor expression can result in changes in receptor activation patterns that can disrupt normal cellular homeostasis (Fig. 1: The Bad). This has been demonstrated with in vitro studies where silencing of AT1R expression leads to a decrease in the deposition of extracellular matrix proteins by hepatic stellate cells [35]. Additionally, several gene variants of AT1R have been identified, none, to date, affecting primary protein structure or binding affinity, but instead altering levels of expression. The most studied AT1R allele variant, which has been associated with hypertension, consists of a substitution of adenine to cytosine at position 1166 within the 3’ untranslated region of the gene (A1166C) [36]. This cytosine substitution results in increased AT1R protein levels as it alters a binding site for miR-155, a microRNA that controls AT1R expression [36]. Single nucleotide polymorphisms have also been identified for ETAR [37], some of which may result in an increase in endothelin 1 plasma concentration [38]. Polymorphic variants of AT1R and ETAR genes have been linked to a number of diseases and abnormalities [36,38].
In vivo environmental factors, such as inflammation, and other chronic changes resulting from infection or ischemia reperfusion injury, also affect receptor expression. An inflammatory milieu, simulated by increased concentrations of the pro-inflammatory cytokine, IL-6, is associated with increased endothelial cell AT1R expression and oxidative stress [39], both of which may cause endothelial dysfunction. Recurrent acute rejection also upregulates AT1R mRNA and protein expression, which has been noted in biopsies of heart transplant recipients [40]. Increased ETAR expression results in the breakdown of endothelial cell membrane integrity in patients with capillary leak syndrome [41]. There is also evidence of crosstalk between these two receptors and their ligands. In vitro treatment of vascular smooth muscle cells with angiotensin II results in upregulation of AT1R, activation of extracellular signal-regulated kinases (ERKs), and upregulation of ETAR [42]. The increased expression of AT1R and ETAR due to genetic differences or resulting from environmental stimuli may generate more targets to which autoantibodies can bind. These factors may therefore contribute to the development of an autoimmune pathology observed when antibody mediated rejection is reported in patients who present with circulating AT1Rabs and ETARabs.
3. Pathogenic features associated with presence of antibodies against angiotensin II type 1 and endothelin A receptors
AT1Rabs and ETARabs are “functional” and “agonistic” because they activate their target receptors in a similar fashion as the endogenous ligands, angiotensin II and endothelin 1 [9]. These autoantibodies were found to induce the intracellular signaling pathway, ERK [8]. Activation of signaling by these autoantibodies resulted in similar downstream responses as activation with the natural ligands, including vasoconstriction, extracellular matrix remodeling, and proinflammatory cascades [43,44]. Both the endogenous ligands and the autoantibodies have been shown, in structural and functional studies, to require contact with amino acid residues in the second extracellular loop, for receptor activation [9]. Interestingly, this region includes an evolutionarily conserved disulfide bond that links a pair of cysteine residues, present in over 90% of GPCRs [25]. Disulfide bonds are known to play a role in maintaining the stability of a protein as well as affecting the folding process. Point mutations in sequences AFHYESQ and ENTNIT, contained within the second extracellular loop of AT1R, resulted in loss of AT1Rab reactivity [8]. Similar experiments have identified sequences “EQHKTCMLNATSK” within the second extracellular loop of ETAR to which ETARab bind [9]. Importantly, only antibodies targeting the second extracellular loop have been demonstrated to activate these receptors [8,9].
While receptor activation by the natural ligand is transient, AT1Rabs and ETARabs binding to their respective receptor result in a more sustained and prolonged activation. As an example, AT1R mediated vasoconstriction induced by AT1Rabs was maintained more than 10 times longer compared with Ang II stimulation [45]. This prolonged response may be due to the skewing of receptor activation. The natural ligand, Ang II activates both AT1R and a second receptor, angiotensin II type 2 (AT2R) [46,47]. AT2R signaling is important because it leads to vasodilation and affects anti-inflammatory and anti-fibrotic mechanisms, modulating the vasoconstrictive and pro-inflammatory effects of AT1R [47]. In contrast, AT1Rabs activate AT1R but do not activate AT2R, resulting in enhanced signaling via AT1R. Similarly, ETARabs activate ETAR but not endothelin B receptor (ETBR) resulting is similar skewing of the activation [43,44].
3.1. Vasoconstriction
Importantly, the enhanced signaling driven by these autoantibodies may directly contribute to poor transplant outcome through increased blood pressure, enhanced fibrosis and immune cell recruitment (Fig. 1: The Ugly). This has been demonstrated using both in vitro and in vivo models. AT1Rabs caused increased contractility in a rat cremaster arteriole assay that could be blocked with losartan, which is an angiotensin-receptor blocker (ARB) [48]. Further, the passive transfer of AT1Rab into normal animals generates phenotypic changes resembling vascular diseases [44]. In vivo, AT1Rabs are linked to pre-eclampsia, a condition associated with new-onset hypertension, endothelial dysfunction, proteinuria, end-organ damage, and placental insufficiency [49]. In these patients, AT1Rabs may stimulate vasoconstriction, endothelin secretion, and the generation of reactive oxygen species via the NADH/NADPH-oxidase pathways [50]. In renal transplant patients with refractory vascular rejection, increased levels of AT1RAbs were associated with accelerated hypertension [8].
3.2. Fibrosis
Given the role of AT1R and ETAR activation in tissue repair and fibrosis (Fig. 1), AT1Rabs and ETARabs are also associated with fibrotic diseases, such as cystic fibrosis [17] and systemic sclerosis [18]. Several studies have found a positive correlation between AT1Rabs and ETARabs levels in these diseases [51,52]. This suggests that both antibodies may work synergistically to activate their respective receptors in patients with fibrosis and support earlier observations showing crosstalk between the two receptors [42]. Of note, studies have reported that hepatic stellate cells have different levels of ETAR and AT1R expression at different stages of fibrosis progression. AT1R has enhanced expression during early fibrosis and expression decreases in later fibrosis [29]. ETAR shows the opposite expression and is more prevalent in late fibrosis [53]. It is possible that AT1Rabs drive pathogenic processes in early stages of fibrosis and ETARabs in later fibrosis. AT1Rabs and ETARabs have also been implicated in fibrosis progression in transplanted liver allografts and are associated with an increased risk of rejection in these liver transplant recipients [54]. This crosstalk can also be illustrated in patients who received kidney transplants. Using data [55] extracted from the customizable gene annotation portal, BioGPS (biogps.org) [56], we compared AT1R and ETAR expression in a cohort of kidney transplant recipients at one-year post-transplantation. Recipients with only interstitial fibrosis had higher AT1R mRNA expression, while development of interstitial inflammation along with fibrosis resulted in a decrease in AT1R mRNA expression and a corresponding increase in ETAR mRNA expression (Fig. 2). For both liver and kidney transplants, development of fibrosis in the transplanted organ affects long-term graft survival [57]. This dynamic interplay between the expression patterns of these receptors may result in an enhanced, continuous and prolonged effect leading to fibrosis, cirrhosis or allograft failure over time.
Fig. 2.
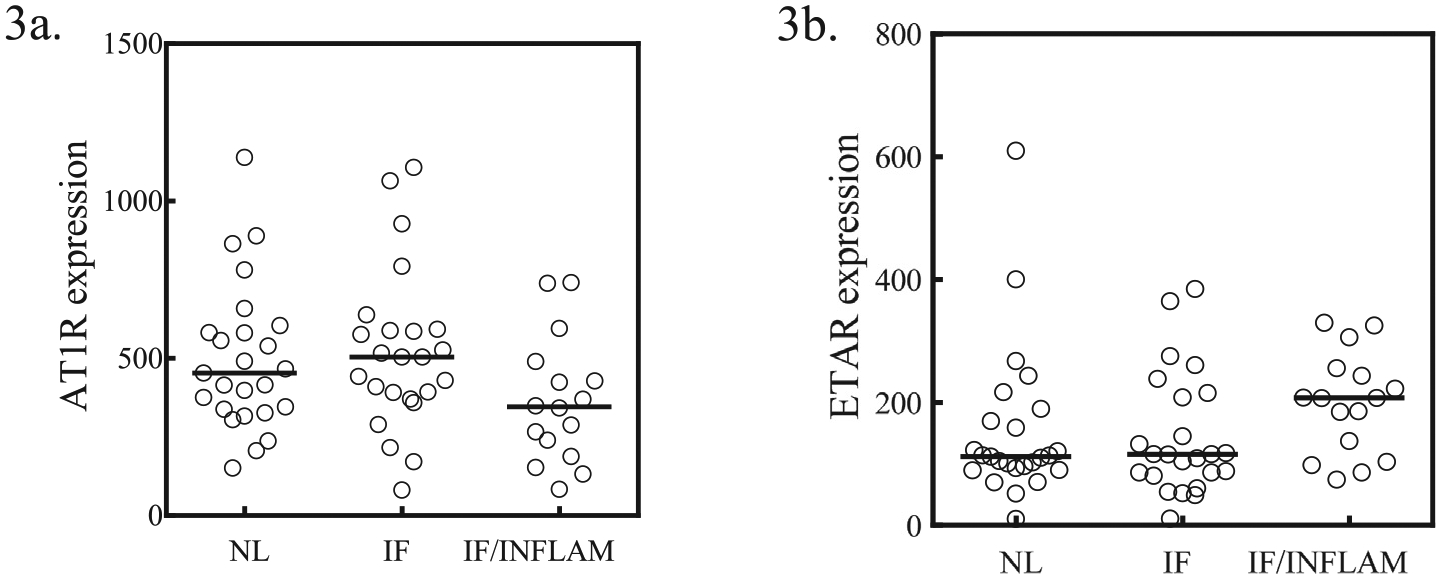
AT1R and ETAR expression from a group of 65 renal transplant recipients. AT1R and ETAR transcripts from patients that received renal transplants were measured by quantitative polymerase chain reaction (qPCR). The patients included in this cohort received a living donor transplant and immunosuppression that consisted of Tacrolimus, Mycophenolate Mofetil and Prednisone. Patients had no delayed graft function, BK viremia nor acute rejection. At one year post-transplant, biopsies were examined and scores for inflammation (i), interstitial fibrosis (ci) and transplant glomerulopathy (cg) were compared to AT1R mRNA and ETAR mRNA expression. Patients were classified based on histopathological examination as normal (n = 25; i/cg/ct = 0); interstitial fibrosis only (n = 24; i/cg = 0; ci > 1) and interstitial fibrosis and inflammation (n = 16; i/cg/ct > 1). (A) AT1R mRNA was higher in patients with interstitial fibrosis but levels decreased in those with both interstitial fibrosis and inflammation. (B) Conversely, ETAR mRNA expression is lower in patients with interstitial fibrosis and increased while AT1R mRNA decreases in patients with interstitial fibrosis and inflammation (3B).
3.3. Cell migration
AT1Rabs and ETARabs may also cause damage by promoting migration of immune regulatory cells to target tissues. Patients with systemic sclerosis typically have elevated levels of AT1Rabs and ETARabs in their serum [58]. Mononuclear cell infiltrates are found in the early disease stage in these patients [59]. AT1RAb and ETARab from patients with systemic sclerosis, were found, in in vitro studies, to induce activation of human microvascular endothelial cells, and resulted in increased secretion of the proinflammatory and profibrotic chemokine, IL-8. Induction of IL-8 secretion was followed by neutrophil migration, fibroblast type 1 collagen production and generation of reactive oxygen species all in a dose dependent manner [18,60]. In transplant patients, AT1Rabs was associated with increased migration of immune cells in the kidney resulting in increased peritubular capillaritis and glomerulitis [12]. Further, AT1Rabs isolated from the sera of transplant recipients, via induction of ERK signaling in endothelial and vascular smooth muscle cells, activated the transcription factor, nuclear factor κB, which plays a role in initiation of immune responses [8]. T cells also express AT1R and activation of this receptor induces migration and recruitment of T cells to areas of injury and infection [61] as well as modulate function of antigen-specific T cells [62]. ETARabs were also shown to play a role in neutrophil migration [63]. Collectively these findings suggest that autoantibodies against GPCRs may directly contribute to immune cell recruitment to the transplanted organ and per-petuate further immune activation and inflammation.
3.4. Complement activation
AT1Rabs were characterized as IgG1 and IgG3 subtypes [8] and ETARabs as IgG1 [15], which are immunoglobulin subclasses that bind complement with highest affinity. While these antibodies are often associated with C4d-negative biopsies [64], there are reported cases correlating high antibody levels with C4d-positive biopsies [13]. In liver transplant patients, de-novo development of AT1Rabs and ETARab at 1-year post transplantation was associated with a pattern of sinusoidal C4d staining on liver biopsies [54]. It is possible that autoantibody density, and proximity, may elicit complement activation and subsequent binding of complement components and C4d deposition in tissue in a similar fashion as with HLA antibodies. The presence of AT1Rabs and ETARabs may also precede development of antibodies against HLA antigens [14,65] and therefore increasing the risk of antibody mediated graft injury due to de-novo donor specific HLA antibodies which in turn result in complement activation. There may be a relationship with these autoantibodies and complement activation in other patient populations as well, especially in women with preeclampsia, who can develop C4d deposits in kidney and placental tissue [66,67].
4. Mechanism of antibody development
In the context of organ transplantation, AT1Rabs and ETARabs are proposed to develop when there is damage to the endothelium of the host or transplanted organ, and subsequent shedding of the extracellular portions of the receptors [68]. In patients with end-stage organ failure or recent organ transplants, this endothelial damage occurs in a pro-inflammatory environment, leading to increased phagocytosis and skewed processing of the shed receptors. The self-antigens can then undergo posttranslational modifications, resulting in the production of a neoantigen that are recognized as foreign, thus eliciting an immune response [69]. Autoantibodies are also more likely to develop in conditions associated with oxidative stress and increased apoptosis [70].
5. Patient characteristics and incidence
AT1Rabs and ETARabs are present in healthy individuals and strength and prevalence varies based on age, and gender [63]. In adults over the age of 70 years old, AT1Rabs were associated with frailty, worse blood pressure control, and increased inflammation [71]. Bjerre et al. [72] show that children between the ages 5 and 8 years old, born from mothers who had complicated pregnancies also expressed high levels of AT1Rab, although the antibody concentrations were lower in this group compared to antibody concentrations in pediatric kidney transplant patients with stable graft function. In this study, healthy adults had the lowest AT1Rab concentrations compared to both pediatric groups. More recently, Pearl et al. reported a strong association between presence of AT1Rab and poor allograft survival in pediatric transplant patients [73]. The study further reported elevated serum cytokine levels in this cohort and is in line with previous evidence for a role of AT1Rab in vascular inflammation.
The incidence of AT1Rab prior to transplantation ranged between 15 and 40% depending on the organ type, and concentration used to report a positive test [68]. The incidence of ETARabs detected prior to transplantation in kidney transplant recipients was 40% [74]. AT1Rab was more prevalent in serum collected prior to transplantation among kidney recipients who were Caucasian (70% versus 30.1% for all other races), younger (mean age 44 years old versus 50 years old; p = 0.04), men (63% men versus 37% women; p = 0.008) and patients who were retransplanted (p < 0.0001) [75]. AT1Rab and/or ETARab with antibodies against HLA antigens had higher incidence of allograft loss [14]. Heart transplant candidates who were placed on mechanical circulatory devices as a bridge to transplantation developed high AT1Rab concentrations post-implantation of the devices and this was associated with higher incidence of death post-implantation [76]. Therefore, an algorithm for pre-transplant screening for GPCR antibodies in transplant candidates could focus on those more likely to have endothelial cell damage resulting from repeated transplantation, use of mechanical circulatory devices or presence of HLA antibodies.
6. Clinical implications, therapeutics and future studies
The preponderance of studies on the function of AT1R and ETAR, coupled with mechanistic studies that show the ability of autoantibodies that bind to epitopes on the second extracellular loop of these GPCRs to cause sustained receptor activation, make the case for the functional role of AT1Rabs and ETARabs [8,9,44,45]. Additionally, evidence of vascular abnormalities such as increase in blood pressure, inflammation, or fibrosis in the presence of AT1Rabs and ETARabs provide additional evidence for their role in disease pathogenesis. In the context of organ transplantation, detection of AT1Rabs and ETARabs with evidence of antibody-mediated injury on biopsies in the absence of HLA antibody, the primary offender, further support their contribution in an injurious process [68]. Nevertheless, autoantibodies are often present without adverse clinical events. Variations in clinical outcomes can be explained in part, by absence of pro-inflammatory environmental conditions that triggers upregulation of receptors thus generating antigenic targets for antibodies, genotypic differences that modify receptor expression and adverse patient profiles.
Variations in autoantibody agonistic function may also explain differences in outcome in patients with positive antibody. These differences may not be readily detectable in commonly used detection assays. Initially, the functional properties of AT1Rabs and ETARabs were determined by exposing cultured neonatal-rat cardiomyocytes to an IgG fraction of antibody-positive serum, which elicited spontaneous heartbeats [8]. More recently, less labor-intensive enzyme-linked im-munosorbent assays (ELISAs) have been developed, although these assays do not characterize autoantibody functionality. Some of the ELISAs detect immunoreactivity against specific epitopes within loop 2 of AT1R, as in experiments showing binding of human serum to ELISA wells coated with the AFHYES sequence within loop 2 of AT1R [71]. For most transplantation studies, a commercially available ELISA is used to detect autoantibodies. This assay consists of microtiter plates coated with human full length AT1R or ETAR protein derived from Chinese hamster ovary cell extracts and treated to allow the receptors to maintain their conformational structure as they remain bound to cell membranes. This ELISA was validated according to the Food and Drug Administration’s Guidance for Industry: Bioanalytical Method Validation [58]. Additional functional assays are needed to further characterize epitope types and functional property of the autoantibodies detected in these ELISA. It is possible that ETARabs or AT1Rabs that recognize GPCR domains other than loop 2 are functional, whether by activating or inhibiting these receptors. Differences in autoantibody epitopes may distinguish between patients with pathogenic or non-pathogenic antibodies. Of note, when autoantibodies are present in high concentration, they may be able to activate complement regardless of epitope specificity.
Because AT1Rabs and ETARabs are associated with adverse clinical effects, therapeutics targeting these autoantibodies or their receptors may be useful. In older adults with detectable AT1Rabs, use of an angiotensin receptor blocker such as losartan, correlated with improvement in frailty and blood pressure control [71]. In patients who have had organ transplants, angiotensin receptor blockers are of particular interest because these medications are relatively common, in-expensive, and well tolerated. Structural studies that evaluate the interaction between the AT1R and a few angiotensin receptor blockers, have found differences in the ability of an ARB to effectively inactivate AT1R [77]. For example, candesartan is reported to be more effective compared to losartan, because of higher receptor avidity and half-life [77]. Furthermore, mutant models of AT1R that mimic the active conformational state of this receptor, showed that epitopes that are targeted by losartan become inaccessible in a constitutively active form of AT1R compared to a receptor that alternates between resting and active state [77]. These are important observations to consider in the context of AT1R stimulation in the presence of autoantibody compared to the transient activation with Ang II. Therefore, studies that compare the efficacy of various ARBs, particularly in the context of AT1R activation by an autoantibody compared to Ang II would inform on best therapies for autoimmune diseases and transplantation. Endothelin antagonists, such as ambrisentan, bosentan, and macitentan, have been used in patients with pre-eclampsia and pulmonary hypertension and could be considered for treatment of antibody mediated rejection [6,22].
Treatment with plasmapheresis can remove circulating autoantibodies and has been used clinically in patients with antibody-mediated injury [78]. A perioperative regimen consisting of a combination of angiotensin receptor blocker and plasmapheresis treatment was found to reduce the risk of acute rejection in kidney transplant recipients [79]. Both therapies are used in cases of severe antibody mediated rejection, to decrease the circulating antibody levels while inhibiting receptor activation. Following plasmapheresis treatments, AT1Rabs often rebound back to pretreatment levels even with resolution of the antibody-mediated rejection [80]. In addition to reducing the antibody levels in serum, plasmapheresis treatments may also remove pro-inflammatory cytokines and thus reduce the effect of an inflammatory milieu. Immediate resurgence of antibody may not be detrimental in the absence of an inflammatory environment. However, in the absence of acute rejection, the continued presence of these autoantibodies may drive a slower and more chronic injurious process. Presence of AT1Rabs prior to kidney transplantation correlated with increases in serum creatinine within three months post-transplantation [75], and an increased incidence of graft loss was reported to occur up to three years after detection of AT1Rabs [81]. Finally, the presence of these antibodies prior to transplantation and their persistence post-transplantation were associated with development of antibody mediated rejection and graft loss [14]. Despite observations of worse outcome in the presence of AT1Rabs and/or ETARabs and HLA antibodies [14,54,82], very few transplant centers routinely measure AT1Rabs and ETARabs levels in patients with graft dysfunction, once HLA antibodies are detected in patient sera. Therefore, data correlating the effect of these antibodies on chronic rejection are lacking but may be important for the design of treatment protocols for chronic antibody mediated rejection.
Because of the limitations for available therapies, new treatments may need to aim at other parts of the AT1R and ETAR signaling pathways. Signaling through AT1R and ETAR is complex and varies depending on cell type [22,83]. Both AT1R and ETAR signal via G protein coupling and kinase activation. AT1R and ETAR function can also be modulated via pathways that lead to receptor desensitization, internalization or recycling, all contributing to silence receptor effect. It is unclear if the autoantibodies that target epitopes on loop 2 can induce signals through all of these pathways. One potential target is the AT1R associated protein (ATRAP), which downregulates AT1R induced signal transduction and leads to receptor internalization [84]. The interaction between the two receptors and the effect of treatment based on this interaction remain an open question and may affect treatment protocol. Treatment that target both receptors may be a more useful approach. Finally, it is worth investigating the effect of standard immunosuppression regimen that target plasma cells producing antibodies, or activated and memory B cells.
7. Conclusion
AT1Rabs and ETARabs have been shown to have detrimental effects in several diseases and cause acute or chronic rejection and graft loss. It is possible that routine clinical monitoring, when coupled with potential therapeutic strategies, can prevent long-term detrimental impacts and lead to improvements in long-term allograft survival. For these reasons, there is an urgent need for more research, which will require collaboration between a variety of specialists to determine and address these complex biological interactions, develop functional assays, and create effective treatment protocols.
Acknowledgments
We thank Sabrina Yuchi Liao, Digital media specialist, iCloud9 Media, for designing the graphics.
Abbreviations:
- Ang II
angiotensin II
- ARB
angiotensin receptor blocker
- AT1R
angiotensin II type 1 receptor
- AT2R
angiotensin II type 2 receptor
- AT1Rab
angiotensin type 1-receptor antibody
- ATRAP
angiotensin II type 1 receptor associated protein
- BioGPS
customizable gene annotation portal at biogps.org
- ERK
extracellular signal-regulated kinases
- ETAR
endothelin A receptor
- ETARab
endothelin A receptor antibody
- ETBR
endothelin B receptor
- GPCR
G-protein coupled receptor
References
- [1].Terasaki PI, A personal perspective: 100-year history of the humoral theory of transplantation, Transplantation 93 (8) (2012) 751–756. [DOI] [PubMed] [Google Scholar]
- [2].Terasaki PI, Deduction of the fraction of immunologic and non-immunologic failure in cadaver donor transplants, Clin. Transpl (2003) 449–452. [PubMed] [Google Scholar]
- [3].Angaswamy N, Tiriveedhi V, Sarma NJ, et al. , Interplay between immune responses to HLA and non-HLA self-antigens in allograft rejection, Hum. Immunol 74 (11) (2013) 1478–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Opelz G, Collaborative Transplant Study. Non-HLA transplantation immunity revealed by lymphocytotoxic antibodies, Lancet 365 (9470) (2005) 1570–1576. [DOI] [PubMed] [Google Scholar]
- [5].Meyer C, Heidecke H, Antibodies against GPCR, Front. Biosci. (Landmark Ed.) 23 (2018) 2177–2194. [DOI] [PubMed] [Google Scholar]
- [6].Dragun D, Catar R, Philippe A, Non-HLA antibodies against endothelial targets bridging allo- and autoimmunity, Kidney Int. 90 (2) (2016) 280–288. [DOI] [PubMed] [Google Scholar]
- [7].Banasik M, Boratynska M, Koscielska-Kasprzak K, et al. , Non-HLA antibodies: angiotensin II type 1 receptor (anti-AT1R) and endothelin-1 type A receptor (anti-ETAR) are associated with renal allograft injury and graft loss, Transpl. Proc 46 (8) (2014) 2618–2621. [DOI] [PubMed] [Google Scholar]
- [8].Dragun D, Muller DN, Brasen JH, et al. , Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection, N. Engl. J. Med 352 (6) (2005) 558–569. [DOI] [PubMed] [Google Scholar]
- [9].Wallukat G, Pruss H, Muller J, Schimke I, Functional autoantibodies in patients with different forms of dementia, PLoS One 13 (3) (2018) e0192778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pinelli DF, Friedewald JJ, Haarberg KMK, et al. , Assessing the potential of angiotensin II type 1 receptor and donor specific anti-endothelial cell antibodies to predict long-term kidney graft outcome, Hum. Immunol 78 (5–6) (2017) 421–427. [DOI] [PubMed] [Google Scholar]
- [11].Deltombe C, Gillaizeau F, Anglicheau D, et al. , Is pre-transplant sensitization against angiotensin II type 1 receptor still a risk factor of graft and patient outcome in kidney transplantation in the anti-HLA luminex era? A retrospective study, Transpl. Int 30 (11) (2017) 1150–1160. [DOI] [PubMed] [Google Scholar]
- [12].Philogene MC, Bagnasco S, Kraus ES, et al. , Anti-angiotensin II type 1 receptor and anti-endothelial cell antibodies: a cross-sectional analysis of pathological findings in allograft biopsies, Transplantation (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Reinsmoen NL, Lai CH, Heidecke H, et al. , Anti-angiotensin type 1 receptor antibodies associated with antibody mediated rejection in donor HLA antibody negative patients, Transplantation 90 (12) (2010) 1473–1477. [DOI] [PubMed] [Google Scholar]
- [14].Taniguchi M, Rebellato LM, Cai J, et al. , Higher risk of kidney graft failure in the presence of anti-angiotensin II type-1 receptor antibodies, Am. J. Transplant 13 (10) (2013) 2577–2589. [DOI] [PubMed] [Google Scholar]
- [15].Wallukat G, Jandrig B, Kunze R, et al. , Autoantibodies directed against the endothelin A receptor in patients with benign prostatic hyperplasia, Prostate 77 (5) (2017) 458–465. [DOI] [PubMed] [Google Scholar]
- [16].Becker M, Kill A, Undeutsch R, et al. , Pathogenic effects of autoantibodies against vascular receptors in patients with SSc, Rheumatology (UK) 51 (2012) ii42–ii43. [Google Scholar]
- [17].Budding K, van de Graaf EA, Hoefnagel T, et al. , Anti-ETAR and anti-AT1R autoantibodies are elevated in patients with endstage cystic fibrosis, J. Cyst. Fibros 14 (1) (2015) 42–45. [DOI] [PubMed] [Google Scholar]
- [18].Günther J, Rademacher J, van Laar JM, Siegert E, Riemekasten G, Functional autoantibodies in systemic sclerosis, Semin. Immunopathol 37 (5) (2015) 529–542. [DOI] [PubMed] [Google Scholar]
- [19].Aggarwal S, Sunderland N, Thornton C, Xu B, Hennessy A, Makris A, A longitudinal analysis of angiotensin II type 1 receptor antibody and angiogenic markers in pregnancy, Am. J. Obstet. Gynecol 216 (2) (2017) 170.e1–170.e8. [DOI] [PubMed] [Google Scholar]
- [20].Landsberger M, Autoantibodies in type 2 diabetes patients with left ventricular dilatation: biomarkers and/or risk markers? Cardiology 129 (3) (2014) 189–190. [DOI] [PubMed] [Google Scholar]
- [21].Li Y, Tian J, Ma XR, et al. , Increase in G protein-coupled receptor autoantibodies with decline of cardiac function in hypercholesterolemic rats, Eur. Rev. Med. Pharmacol. Sci 21 (5) (2017) 1065–1073. [PubMed] [Google Scholar]
- [22].Horinouchi T, Terada K, Higashi T, Miwa S, Endothelin receptor signaling: New insight into its regulatory mechanisms, J. Pharmacol. Sci 123 (2) (2013) 85–101. [DOI] [PubMed] [Google Scholar]
- [23].Zhang H, Unal H, Gati C, et al. , Structure of the angiotensin receptor revealed by serial femtosecond crystallography, Cell 161 (4) (2015) 833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Edward Zhou X, Melcher K, Eric Xu H, Structural biology of G protein-coupled receptor signaling complexes, Protein Sci. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Unal H, Jagannathan R, Bhat MB, Karnik SS, Ligand-specific conformation of extracellular loop-2 in the angiotensin II type 1 receptor, J. Biol. Chem 285 (21) (2010) 16341–16350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Abadir PM, Walston JD, Carey RM, Subcellular characteristics of functional intracellular renin-angiotensin systems, Peptides 38 (2) (2012) 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sas A, Banasik M, Dionizy P, et al. , The expression of angiotensin II type 1 receptors (AT1R) in microcirculation of renal biopsy for cause might be significant, Transplant Int. 30 (2017) 245. [Google Scholar]
- [28].Dzau VJ, Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis, Hypertension 37 (2001) 1047. [DOI] [PubMed] [Google Scholar]
- [29].Schulte S, Oidtmann A, Kociok N, et al. , Hepatocyte expression of angiotensin II type 1 receptor is downregulated in advanced human liver fibrosis, Liver Int. 29 (3) (2009) 384–391. [DOI] [PubMed] [Google Scholar]
- [30].Boucard AA, Wilkes BC, Laporte SA, Escher E, Guillemette G, Leduc R, Photolabeling identifies position 172 of the human AT(1) receptor as a ligand contact point: receptor-bound angiotensin II adopts an extended structure, Biochemistry 39 (32) (2000) 9662–9670. [DOI] [PubMed] [Google Scholar]
- [31].Madsen K, Marcussen N, Pedersen M, et al. , Angiotensin II promotes development of the renal microcirculation through AT1 receptors, J. Am. Soc. Nephrol 21 (3) (2010) 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Qian C, Schoemaker RG, van Gilst WH, Roks AJ, The role of the renin-angiotensin-aldosterone system in cardiovascular progenitor cell function, Clin. Sci. (Lond.) 116 (4) (2009) 301–314. [DOI] [PubMed] [Google Scholar]
- [33].Kurosaka M, Suzuki T, Hosono K, et al. , Reduced angiogenesis and delay in wound healing in angiotensin II type 1a receptor-deficient mice, Biomed. Pharmacother 63 (9) (2009) 627–634. [DOI] [PubMed] [Google Scholar]
- [34].McPherson A, Larson SB, The X-ray crystal structure of human endothelin 1, a polypeptide hormone regulator of blood pressure, Acta Crystallogr. Sect: F, Struct. Biol. Commun 75 (Pt 1) (2019) 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dong P, Yu F, Fan X, Lin Z, Chen Y, Li J, Inhibition of ATIR by shRNA prevents collagen synthesis in hepatic stellate cells, Mol. Cell. Biochem 344 (1–2) (2010) 195–202. [DOI] [PubMed] [Google Scholar]
- [36].Ceolotto G, Papparella I, Bortoluzzi A, et al. , Interplay between miR-155, AT1R A1166C polymorphism, and AT1R expression in young untreated hypertensives, Am. J. Hypertens 24 (2) (2011) 241–246. [DOI] [PubMed] [Google Scholar]
- [37].Zhang L, Sui R, Effect of SNP polymorphisms of EDN1, EDNRA, and EDNRB gene on ischemic stroke, Cell Biochem. Biophys 70 (1) (2014) 233–239. [DOI] [PubMed] [Google Scholar]
- [38].Kosior-Jarecka E, Wrobel-Dudzinska D, Lukasik U, et al. , Plasma endothelin-1 and single nucleotide polymorphisms of endothelin-1 and endothelin type A receptor genes as risk factors for normal tension glaucoma, Mol. Vis 22 (2016) 1256–1266. [PMC free article] [PubMed] [Google Scholar]
- [39].Wassmann S, Stumpf M, Strehlow K, et al. , Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor, Circ. Res 94 (4) (2004) 534–541. [DOI] [PubMed] [Google Scholar]
- [40].Yamani MH, Cook DJ, Rodriguez ER, et al. , Increased expression of angiotensin II type 1 receptor (AGTR1) in heart transplant recipients with recurrent rejection, J. Heart Lung Transplant 25 (11) (2006) 1283–1289. [DOI] [PubMed] [Google Scholar]
- [41].Sek AC, Xie Z, Terai K, et al. , Endothelial expression of endothelin receptor A in the systemic capillary leak syndrome, PLoS One 10 (7) (2015) e0133266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lin YJ, Kwok CF, Juan CC, et al. , Angiotensin II enhances endothelin-1-induced vasoconstriction through upregulating endothelin type A receptor, Biochem. Biophys. Res. Commun 451 (2) (2014) 263–269. [DOI] [PubMed] [Google Scholar]
- [43].Dechend R, Dragun D, Herse F, et al. , Activating autoantibodies against the AT1-receptor in vascular disease, Transplantationsmedizin 24 (1) (2012) 20–26. [Google Scholar]
- [44].Lukitsch I, Kehr J, Chaykovska L, et al. , Renal ischemia and transplantation predispose to vascular constriction mediated by angiotensin II type 1 receptor-activating antibodies, Transplantation 94 (1) (2012) 8–13. [DOI] [PubMed] [Google Scholar]
- [45].Zhang S, Zheng R, Yang L, et al. , Angiotensin type 1 receptor autoantibody from preeclamptic patients induces human fetoplacental vasoconstriction, J. Cell. Physiol 228 (1) (2013) 142–148. [DOI] [PubMed] [Google Scholar]
- [46].Pandey A, Gaikwad AB, AT2 receptor agonist compound 21: a silver lining for diabetic nephropathy, Eur. J. Pharmacol 815 (2017) 251–257. [DOI] [PubMed] [Google Scholar]
- [47].Wang Y, Del Borgo M, Lee HW, et al. , Anti-fibrotic potential of AT2 receptor agonists, Front. Pharmacol 8 (2017) 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kem DC, Li H, Velarde-Miranda C, et al. , Autoimmune mechanisms activating the angiotensin AT1 receptor in ‘primary’ aldosteronism, J. Clin. Endocrinol. Metab 99 (5) (2014) 1790–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Adamczyk M, Brashear RJ, Hsu SC, Mattingly PG, Autoantibodies to the angiotensin II type 1 receptor in preeclampsia, J. Clin. Hypertens 13 (4) (2011) A160. [Google Scholar]
- [50].Dechend R, Homuth V, Wallukat G, et al. , AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor, Circulation 101 (20) (2000) 2382–2387. [DOI] [PubMed] [Google Scholar]
- [51].Hiemann NE, Meyer R, Wellnhofer E, et al. , Non-HLA antibodies targeting vascular receptors enhance alloimmune response and microvasculopathy after heart transplantation, Transplantation 94 (9) (2012) 919–924. [DOI] [PubMed] [Google Scholar]
- [52].Delville M, Lamarthee B, Pagie S, et al. , Early acute microvascular kidney transplant rejection in the absence of anti-HLA antibodies is associated with pre-formed IgG antibodies against diverse glomerular endothelial cell antigens, J. Am. Soc. Nephrol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Cho JJ, Hocher B, Herbst H, et al. , An oral endothelin-A receptor antagonist blocks collagen synthesis and deposition in advanced rat liver fibrosis, Gastroenterology 118 (6) (2000) 1169–1178. [DOI] [PubMed] [Google Scholar]
- [54].O’Leary JG, Demetris AJ, Philippe A, et al. , Non-HLA antibodies impact on C4d staining, stellate cell activation and fibrosis in liver allografts, Transplantation 101 (10) (2017) 2399–2409. [DOI] [PubMed] [Google Scholar]
- [55].Park WD, Griffin MD, Cornell LD, Cosio FG, Stegall MD, Fibrosis with inflammation at one year predicts transplant functional decline, J. Am. Soc. Nephrol 21 (11) (2010) 1987–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wu C, Jin X, Tsueng G, Afrasiabi C, Su AI, BioGPS: building your own mash-up of gene annotations and expression profiles, Nucleic Acids Res. 44 (D1) (2016) D313–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ohe H, Uchida Y, Yoshizawa A, et al. , Association of anti-human leukocyte antigen and anti-angiotensin II type 1 receptor antibodies with liver allograft fibrosis after immunosuppression withdrawal, Transplantation 98 (10) (2014) 1105–1111. [DOI] [PubMed] [Google Scholar]
- [58].Riemekasten G, Philippe A, Nather M, et al. , Involvement of functional autoantibodies against vascular receptors in systemic sclerosis, Ann. Rheum. Dis 70 (3) (2011) 530–536. [DOI] [PubMed] [Google Scholar]
- [59].Gunther J, Kill A, Becker MO, et al. , Angiotensin receptor type 1 and endothelin receptor type A on immune cells mediate migration and the expression of IL-8 and CCL18 when stimulated by autoantibodies from systemic sclerosis patients, Arthritis Res. Ther 16 (2) (2014) R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kill A, Becker MO, Guenther J, Heidecke H, Dragun D, Riemekaste G, Anti-AT(1)R and anti-ET(A)R autoantibodies in systemic sclerosis: clues for possible involvement in disease pathology, Ann. Rheum. Dis 71 (2012) A39. [Google Scholar]
- [61].Silva-Filho JL, Caruso-Neves C, Pinheiro AA, Angiotensin II type-1 receptor (AT1R) regulates expansion, differentiation, and functional capacity of antigen-specific CD8(+) T cells, Sci. Rep 6 (2016) 35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Silva-Filho JL, Souza MC, Ferreira-Dasilva CT, et al. , Angiotensin II is a new component involved in splenic T lymphocyte responses during plasmodium berghei ANKA infection, PLoS One 8 (4) (2013) e62999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cabral-Marques O, Marques A, Giil LM, et al. , GPCR-specific autoantibody signatures are associated with physiological and pathological immune homeostasis, Nat. Commun 9 (1) (2018) 5224-018-07598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Scornik JC, Guerra G, Schold JD, Srinivas TR, Dragun D, Meier-Kriesche HU, Value of posttransplant antibody tests in the evaluation of patients with renal graft dysfunction, Am. J. Transplant 7 (7) (2007) 1808–1814. [DOI] [PubMed] [Google Scholar]
- [65].Cuevas E, Arreola-Guerra JM, Hernandez-Mendez EA, et al. , Pretransplant angiotensin II type 1-receptor antibodies are a risk factor for earlier detection of de novo HLA donor-specific antibodies, Nephrol. Dial. Transplant 31 (10) (2016) 1738–1745. [DOI] [PubMed] [Google Scholar]
- [66].Buurma A, Cohen D, Veraar K, et al. , Preeclampsia is characterized by placental complement dysregulation, Hypertension 60 (5) (2012) 1332–1337. [DOI] [PubMed] [Google Scholar]
- [67].Penning M, Chua JS, van Kooten C, et al. , Classical complement pathway activation in the kidneys of women with preeclampsia, Hypertension 66 (1) (2015) 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhang Q, Reed EF, The importance of non-HLA antibodies in transplantation, Nat. Rev. Nephrol 12 (8) (2016) 484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Darrah E, Kim A, Zhang X, et al. , Proteolysis by granzyme B enhances presentation of autoantigenic peptidylarginine deiminase 4 epitopes in rheumatoid arthritis, J. Proteome Res 16 (1) (2017) 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Liu C, Wang W, Parchim N, et al. , Tissue transglutaminase stabilizes placental AT1 receptor and contributes to pathophysiology in preeclampsia, Hypertension (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Abadir PM, Jain A, Powell LJ, et al. , Discovery and validation of agonistic angiotensin receptor autoantibodies as biomarkers of adverse outcomes, Circulation 135 (5) (2017) 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bjerre A, Tangeraas T, Heidecke H, Dragun D, Dechend R, Staff AC, Angiotensin II type 1 receptor antibodies in childhood kidney transplantation, Pediatr. Transplant 20 (5) (2016) 627–632. [DOI] [PubMed] [Google Scholar]
- [73].Pearl MH, Zhang Q, Palma Diaz MF, et al. , Angiotensin II type 1 receptor antibodies are associated with inflammatory cytokines and poor clinical outcomes in pediatric kidney transplantation, Kidney Int. 93 (1) (2018) 260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Banasik M, Boratynska M, Koscielska-Kasprzak K, et al. , The impact of non-HLA antibodies directed against endothelin-1 type A receptors (ETAR) on early renal transplant outcomes, Transpl. Immunol 30 (1) (2014) 24–29. [DOI] [PubMed] [Google Scholar]
- [75].Philogene MC, Zhou S, Lonze BE, et al. , Pre-transplant screening for non-HLA antibodies: who should be tested? Hum. Immunol (2018). [DOI] [PubMed] [Google Scholar]
- [76].Zhang X, Mirocha J, Aintablian T, et al. , Revealing a new mode of sensitization induced by mechanical circulatory support devices (MCS): impact of anti-AT1R antibodies, J. Heart Lung Transplant 36 (4) (2017) S32–S33. [DOI] [PubMed] [Google Scholar]
- [77].Takezako T, Unal H, Karnik SS, Node K, Current topics in angiotensin II type 1 receptor research: Focus on inverse agonism, receptor dimerization and biased agonism, Pharmacol. Res 123 (2017) 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Yamada C, Huang Y, Norman S, et al. , Efficacy of therapeutic plasma exchange on angiotensin II type-1 receptor antibodies on two kidney transplant recipients, J Clin Apher. 33 (6) (2018) 673–677. [DOI] [PubMed] [Google Scholar]
- [79].Carroll RP, Riceman M, Hope CM, et al. , Angiotensin II type-1 receptor antibody (AT1Rab) associated humoral rejection and the effect of peri operative plasma exchange and candesartan, Hum. Immunol 77 (12) (2016) 1154–1158. [DOI] [PubMed] [Google Scholar]
- [80].Philogene MC, Jackson AM, Non-HLA antibodies in transplantation: when do they matter? Curr. Opin. Organ. Transplant 21 (4) (2016) 427–432. [DOI] [PubMed] [Google Scholar]
- [81].Giral M, Foucher Y, Dufay A, et al. , Pretransplant sensitization against angiotensin II type 1 receptor is a risk factor for acute rejection and graft loss, Am. J. Transplant 13 (10) (2013) 2567–2576. [DOI] [PubMed] [Google Scholar]
- [82].Gareau AJ, Wiebe C, Pochinco D, et al. , Pre-transplant AT1R antibodies correlate with early allograft rejection, Transpl. Immunol 46 (2018) 29–35. [DOI] [PubMed] [Google Scholar]
- [83].Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S, Angiotensin II signal transduction through the AT1 receptor: Novel insights into mechanisms and pathophysiology, Clin. Sci. (Lond.) 112 (8) (2007) 417–428. [DOI] [PubMed] [Google Scholar]
- [84].Tamura K, Wakui H, Azushima K, et al. , Angiotensin II type 1 receptor binding molecule ATRAP as a possible modulator of renal sodium handling and blood pressure in pathophysiology, Curr. Med. Chem 22 (28) (2015) 3210–3216. [DOI] [PubMed] [Google Scholar]


