Abstract
Aims/Introduction
The investigation of the influence of dietary fiber intake on the incidence of type 2 diabetes in a general Japanese population.
Materials and Methods
A total of 1,892 individuals aged 40–79 years without diabetes at baseline were prospectively followed up for 14 years. The glucose tolerance status of participants was defined by a 75‐g oral glucose tolerance test with the 1998 World Health Organization criteria. Dietary fiber intake was estimated by a semiquantitative food frequency questionnaire and divided to quintile levels separately by sex. A Cox proportional hazards model was applied for computing the hazard ratios and their 95% confidence intervals for the incidence of diabetes.
Results
During the follow‐up period, 280 participants had developed diabetes. The age‐adjusted cumulative diabetes incidence decreased significantly with higher total dietary fiber intake (P‐for trend = 0.01). Participants in the highest quintile of total dietary fiber intake had a 0.53‐fold (95% confidence interval 0.31–0.90) lower risk of developing diabetes than those in the lowest quintile after for the adjustment with potential confounding factors. Total dietary fiber intake showed a moderate positive correlation to the intake of soybean and soybean products, green vegetables, and other vegetables. Similar associations with diabetes and food sources were observed for both of the soluble and insoluble dietary fiber intake.
Conclusions
The present study showed that higher dietary fiber intake was associated with a lower risk of type 2 diabetes in a general Japanese population. The intake of high dietary fiber foods might be useful for diabetes prevention.
Keywords: Cohort study, Dietary fiber, Type 2 diabetes
The present study found that higher dietary fiber intake was associated with a lower risk of type 2 diabetes in a general Japanese population. The intake of foods rich in dietary fiber might be beneficial for diabetes prevention.
Introduction
Type 2 diabetes has become a worldwide epidemic 1 , especially in Asian countries as a result of rapid socioeconomic development, overnutrition and lack of physical activity 2 , 3 . In Japan, the incidence rate of type 2 diabetes increased from 8.2% in 1997 to 12.1% in 2016 4 . Type 2 diabetes has emerged recently as one of the major risk factors for geriatric diseases, such as dementia, sarcopenia, periodontal disease and cancer, in addition to large and small vessel disease 5 , 6 , 7 , 8 , 9 . Therefore, for countries with aging populations, such as Japan, reducing the load of type 2 diabetes through lifestyle modification had become a public health priority. Among the strategies for preventing type 2 diabetes, the promotion of healthy dietary habits plays an important role 10 , 11 , as nutrient intake is very closely related to diabetes etiology 11 .
Dietary fiber is the edible portion of plants that is resistant to digestion and absorption in the small intestine, and therefore dietary fiber is considered to decrease macronutrient absorption by way of controlling energy intake from the diet 12 . Several meta‐analyses, which were mainly carried out in Western countries, showed that higher dietary fiber intake has a significant association with a lower risk of the development of type 2 diabetes mellitus 13 , 14 , 15 . However, food habits are different between Asian and Western countries. Dietary fiber intake is generally lower in Japanese populations than Western populations 16 ; thus, it would be clinically helpful to clarify the association of dietary fiber intake to the development of type 2 diabetes in Asian populations.
Dietary fiber is classified by its water solubility 17 . Several prospective studies on white populations examined the associations of the risk of type 2 diabetes with soluble and insoluble dietary fiber intake separately; however, the findings were inconsistent across these studies 18 , 19 , 20 . There has been only one community‐based prospective cohort study investigating this subject in Asian populations 21 . In addition, the intake ratio of insoluble dietary fiber to that of soluble dietary fiber is higher in Asian countries than in Western countries 16 . Therefore, it is worth addressing the association of type 2 diabetes to different types of dietary fiber intake in an Asian population.
The Hisayama Study is a prospective population‐based cohort study of lifestyle‐related diseases, including cardiovascular disease and diabetes, in a Japanese community 22 . This study uses repeated 75‐g oral glucose tolerance tests (OGTTs) for the reliable determination of glucose tolerance status, which is required in order to identify effective dietary factors for preventing type 2 diabetes. The aim of the current study was to examine the influence of the total dietary fiber intake, soluble and insoluble dietary fiber intake on the incidence of type 2 diabetes in a community‐dwelling Japanese population.
Methods
Study population
The Hisayama Study has been ongoing since 1961 in Hisayama town, a suburb of Fukuoka city in the north part of Kyushu Island in Japan. The town residents have undergone a 75‐g OGTT as part of their annual health checkups since 1988. Detailed information on the health checkups have been published previously 22 . In brief, 2,587 (80.2%) of the total 3,227 residents aged 40–79 years underwent a baseline screening examination including a dietary survey in 1988. After exclusion of 82 participants who had already eaten breakfast, 10 participants who had been receiving insulin therapy and 15 participants who had general fatigue or nausea during the glucose ingestion, 2,480 participants completed the OGTT. Among them, we excluded 297 participants with diabetes at baseline, 78 participants who had a cardiovascular disease and/or cancer history, 201 participants who did not complete a semiquantitative dietary questionnaire at baseline, and 12 participants who had implausibly high or low total energy intake (more or less than mean energy intake ±3 standard deviations, <640 or >3,259 kcal/day for men, and <833 or >2,489 kcal/day for women). The remaining 1,892 participants (759 men; 1,133 women) were registered to this cohort study.
The present study was carried out under the approval of Kyushu University Institutional Review Board for Clinical Research, and all participants submitted written informed consent.
Follow‐up survey
The enrolled participants were followed up prospectively by repeated annual health examinations from December 1988 until November 2002 (median follow‐up period 14 years; range 1–14 years). For participants who had moved out of town or who did not have examinations, their health information was collected using postal mail or phone. During the follow‐up period, new development of type 2 diabetes mellitus was decided by the OGTT data or the measurements of fasting or casual plasma glucose at annual health examinations with clinical information, such as medical records and the use of antidiabetic medications (e.g., oral hypoglycemic agents or insulin). The participants underwent an average of 6.9 ± 3.9 examinations during the follow‐up period.
Definition of type 2 diabetes
The participants underwent the OGTT after an overnight fast (at least 12 h) at baseline and follow‐up examinations. A glucose oxidase method (1988–1999) or hexokinase method (2000–2002) was used to evaluate plasma glucose levels. Diabetes was defined by 1998 World Health Organization criteria as follows: (i) normal glucose tolerance (NGT) was defined as fasting plasma glucose (FPG) <6.1 mmol/L and 2‐h post‐load glucose (PG) <7.8 mmol/L; (ii) prediabetes was defined as FPG 6.1–6.9 mmol/L and 2‐h PG <7.8 mmol/L, or FPG <7.0 mmol/L and 2‐h PG 7.8–11.0 mmol/L; and (iii) diabetes was defined as FPG ≥7.0 mmol/L or 2‐h PG ≥11.1 mmol/L, or the use of antidiabetic medications.
Nutritional survey at baseline
At the 1988 baseline examination for screening, a semiquantitative food frequency questionnaire (70‐item) considering food intake 23 was used to carried out a dietary survey. This questionnaire has been validated and reported previously 24 . Each participant completed the questionnaire in advance, and trained dieticians checked that at the screening examination. The frequency of meals per week and the amount of each food portion were used to calculate the average food intake per day. Nutritional intake was calculated from the food intake base on the Standard Tables of Food Composition in Japan (Fourth Revised Edition) 25 . The density method was used to adjust all dietary nutrient for total energy 26 .
Laboratory measurements and clinical evaluation
The self‐administered questionnaire of the present study covered medical history, antidiabetic and antihypertensive treatments, alcohol intake, smoking habits, and physical activity. In the analysis, current consumption or not was used for alcohol intake and smoking habits. The regular exercise group was defined as participants engaging sports at least three times per week. Body mass index (BMI) was calculated in the usual way by height and weight measured from participants wearing light clothing with no shoes. BMI ≥25 kg/m2 was classified as obesity. Blood pressure was measured using a mercury sphygmomanometer three times in 1988 and the mean of three measurements was used in the analysis. Hypertension was classified as a systolic blood pressure ≥140 mmHg and/or a diastolic blood pressure ≥90 mmHg, or current treatment using antihypertensive agents. Serum total and high‐density lipoprotein cholesterols, and serum triglycerides were decided enzymatically. A formula (FPG [mmol/L] × fasting serum insulin [μU/mL]) / 22.5 27 was used to obtain the homeostasis model assessment of insulin resistance (HOMA‐IR). A modified version of the Behring latex‐enhanced C‐reactive protein (CRP) assay was used for deciding serum high‐sensitivity CRP (hs‐CRP) concentrations.
Statistical analysis
Dietary fiber intake was divided into five categories following the sex‐specific quintile distribution. The values of serum triglycerides, HOMA‐IR and serum hs‐CRP were natural log transformed for the analysis, because of the skew of the distributions of these variables. The trends of the mean values or the frequencies of possible risk factors among the quintiles of total dietary fiber intake were analyzed by linear regression analysis or logistic regression analysis. The person‐years method was applied for evaluating the incidence of type 2 diabetes. The adjusted hazard ratio (HR) with the 95% confidence interval (CI) of diabetes according to dietary fiber intake was evaluated using the Cox proportional hazards model. The age‐adjusted cumulative incidences of type 2 diabetes across the intake levels of each type of dietary fiber were calculated by using regression estimates from a relevant Cox model including age 28 . The trends in the risk estimates were tested using a Cox model including the intake levels of each dietary fiber with ordinal numbers (0, 1, 2, 3 and 4) and the relevant covariates. The heterogeneity in the magnitude of the association of dietary fiber intake levels with type 2 diabetes risk across subgroups (NGT and prediabetes) was tested by the relevant Cox model with the addition of a multiplicative interaction term. The Spearman’s correlation coefficients were used for estimating the correlation between dietary fiber intakes and food intakes for each food group in the Standard Tables of Food Composition in Japan 25 , where we considered correlation coefficients of 0.30–0.49 (or −0.49 to −0.30) and ≥0.50 (or ≤−0.50) as weakly positive (negative) and moderately positive (negative) correlations, respectively 29 . All statistical analysis was carried out with the SAS 9.4 software (SAS Institute, Cary, NC, USA). In all analyses, two‐sided P‐values (P < 0.05) were used for judging the statistical significance.
Results
Table 1 shows the baseline characteristics of this study population. The mean values of age, BMI, the intakes of total energy, vitamin A, vitamin C and magnesium, the ratio of polyunsaturated fatty acid : saturated fatty acid (P/S ratio), and the frequency of regular exercises all increased significantly with increasing quintile of total dietary fiber intake, whereas a higher intake of total dietary fiber was significantly associated with lower frequencies of current smoking and current alcohol drinking.
Table 1.
Baseline characteristics of potential risk factors for type 2 diabetes according to the quintile of total dietary fiber intake
| Variables | Total dietary fiber intake (g/1,000 kcal) | |||||
|---|---|---|---|---|---|---|
| Q1 (M: ≤4.13; W: ≤5.41) (n = 377) | Q2 (M: 4.14–5.01; W: 5.42–6.37) (n = 379) | Q3 (M: 5.02–5.89; W: 6.38–7.39) (n = 379) | Q4 (M: 5.90–7.06; W: 7.40–8.54) (n = 379) | Q5 (M: ≥7.07; W: ≥8.55) (n = 378) | P for trend | |
| Dietary fiber intake (median) | ||||||
| Men (g/1,000 kcal) | 3.61 | 4.61 | 5.47 | 6.45 | 7.94 | |
| Women (g/1,000 kcal) | 4.65 | 5.90 | 6.90 | 7.93 | 9.62 | |
| Clinical parameters | ||||||
| Age (years) | 55.6 (10.3) | 56.4 (10.4) | 56.2 (9.9) | 57.4 (10.2) | 59.0 (10.0) | <0.0001 |
| Men (%) | 40.1 | 40.1 | 40.1 | 40.1 | 40.2 | 0.97 |
| Family history of diabetes (%) | 8.8 | 5.0 | 7.4 | 7.4 | 9.6 | 0.36 |
| Hypertension (%) | 36.6 | 37.2 | 31.1 | 35.1 | 38.1 | 0.91 |
| Body mass index (kg/m2) | 23.0 (3.1) | 22.8 (3.1) | 22.7 (3.0) | 22.9 (3.0) | 23.4 (2.9) | 0.04 |
| Obesity, ≥25.0 kg/m2 (%) | 24.9 | 21.6 | 19.3 | 23.0 | 27.5 | 0.34 |
| Serum total cholesterol (mmol/L) | 5.27 (1.05) | 5.38 (1.08) | 5.35(1.07) | 5.38 (1.04) | 5.41 (1.06) | 0.49 |
| Serum HDL cholesterol (mmol/L) | 1.33 (0.32) | 1.31 (0.29) | 1.29 (0.29) | 1.31 (0.30) | 1.31 (0.28) | 0.38 |
| Serum triglycerides (mmol/L) | 1.07 (0.78–1.48) | 1.10 (0.76–1.56) | 1.04 (0.78–1.59) | 1.12 (0.85–1.54) | 1.07 (0.79–1.54) | 0.53 |
| HOMA‐IR | 1.32 (0.90–1.86) | 1.36 (0.96–1.86) | 1.27 (0.93–1.79) | 1.32 (0.94–1.86) | 1.40 (0.92–1.97) | 0.51 |
| Serum hs‐CRP (mg/L) | 0.41 (0.20–0.89) | 0.43 (0.22–1.03) | 0.35 (0.18–0.70) | 0.40 (0.18–0.89) | 0.44 (0.22–0.88) | 0.15 |
| Current smoking (%) | 30.0 | 24.8 | 24.0 | 19.0 | 16.9 | <0.0001 |
| Current alcohol drinking (%) | 36.3 | 30.9 | 29.0 | 28.0 | 23.8 | 0.0002 |
| Regular exercise (%) | 6.6 | 9.0 | 9.3 | 14.0 | 13.5 | 0.0002 |
| Dietary factors | ||||||
| Total energy intake (kcal/day) | 1,700 (395) | 1,660 (348) | 1,699 (383) | 1,711 (363) | 1,782 (391) | 0.003 |
| Vitamin A (IU/1,000 kcal) | 1,426 (639) | 1,572 (574) | 1,771 (594) | 1,881 (671) | 2,167 (718) | <0.0001 |
| Vitamin C (mg/1,000 kcal) | 32.8 (13.2) | 40.7 (16.1) | 48.0 (18.6) | 52.8 (19.1) | 62.0 (23.0) | <0.0001 |
| Magnesium (mg/1,000 kcal) | 86.1 (20.4) | 95.2 (16.0) | 103.4 (16.7) | 108.6 (15.2) | 126.7 (23.2) | <0.0001 |
| P/S ratio | 1.10 (0.40) | 1.21 (0.42) | 1.28 (0.42) | 1.40 (0.42) | 1.62 (0.49) | <0.0001 |
The values of serum triglycerides, homeostasis model assessment of insulin resistance (HOMA‐IR) and serum high‐sensitivity C‐reactive protein (hs‐CRP) are shown as the median (interquartile range). All other values are given as the mean (standard deviations) or percentage. HDL, high density lipoprotein; M, men; P/S ratio, polyunsaturated fatty acid : saturated fatty acid ratio; Q, quintile; W, women.
During the follow‐up period, type 2 diabetes was developed by 280 participants (139 men and 141 women). The age‐adjusted cumulative incidence of diabetes decreased significantly with increasing quintile of the amount of total dietary fiber intake (P for trend = 0.01; Figure 1). The age‐adjusted HR of the development of type 2 diabetes decreased significantly with increasing amount of total dietary fiber intake (P for trend = 0.01) and insoluble dietary fiber (P for trend = 0.002), and also decreased, but not significantly with an increasing amount of intake of soluble dietary fiber (P for trend = 0.08; model 1), as shown in Table 2. The risk of incident type 2 diabetes significantly decreased with higher intakes of total dietary fiber, soluble dietary fiber and insoluble dietary fiber after adjustment for age, family history of diabetes mellitus, hypertension, BMI, serum total cholesterol, serum high‐density lipoprotein cholesterol, serum triglycerides, current smoking, current alcohol drinking, regular exercise and dietary factors, such as total energy intake, vitamin A, vitamin C, magnesium and polyunsaturated fatty acid/saturated fatty acid ratio (model 2, all P for trends≤0.02). For type 2 diabetes, the multivariate‐adjusted HR was significantly lower for participants at the highest quintile compared with those at the lowest quintiles of dietary fiber (with total dietary fiber HR 0.52, 95% CI 0.31–0.89; with soluble dietary fiber HR 0.50, 95% CI 0.30–0.83; with insoluble dietary fiber HR 0.54, 95% CI 0.32–0.90). These associations were unchanged even after the additional adjustment with HOMA‐IR and serum hs‐CRP (model 3, all P for trends ≤0.02). The ratio of insoluble dietary fiber to soluble dietary fiber showed no clear association with the risk of the development of type 2 diabetes (Table S1).
Figure 1.
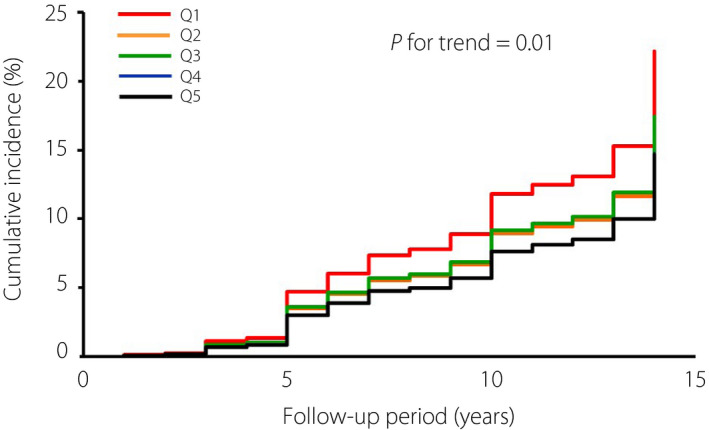
Age‐adjusted cumulative incidence of type 2 diabetes according to the quintile of total dietary fiber intake.
Table 2.
Incidence rates and hazard ratios for the development of type 2 diabetes according to total dietary fiber, soluble dietary fiber and insoluble dietary fiber intake
| Dietary fiber intake levels (g/1,000 kcal) | No. events/ PYs | Age‐adjusted incidence rate (per 103 PYs) | Hazard ratio (95% confidence interval) | ||
|---|---|---|---|---|---|
| Model 1 (Age‐adjusted) | Model 2 † (Multivariable‐adjusted) | Model 3 ‡ (Model 2 + HOMA‐IR and serum hs‐CRP) | |||
| Total dietary fiber intake | |||||
| Q1 (M: ≤4.13; W: ≤5.41) | 70/4,345 | 16.5 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 (M: 4.14–5.01; W: 5.42–6.37) | 55/4,497 | 12.2 | 0.75 (0.52–1.06) | 0.73 (0.51–1.06) | 0.72 (0.50–1.03) |
| Q3 (M: 5.02–5.89; W: 6.38–7.39) | 59/4,651 | 12.4 | 0.76 (0.54–1.08) | 0.77 (0.53–1.13) | 0.76 (0.52–1.12) |
| Q4 (M: 5.90–7.06; W: 7.40–8.54) | 49/4,599 | 10.6 | 0.64 (0.44–0.92) | 0.63 (0.41–0.97) | 0.63 (0.41–0.96) |
| Q5 (M: ≥7.07; W: ≥8.55) | 47/4,456 | 9.9 | 0.63 (0.44–0.92) | 0.52 (0.31–0.89) | 0.53 (0.31–0.90) |
| P for trend | 0.01 | 0.02 | 0.02 | ||
| Soluble dietary fiber intake | |||||
| Q1 (M: ≤0.56; W: ≤0.77) | 65/4,304 | 15.4 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 (M: 0.57–0.79; W: 0.78–1.08) | 49/4,544 | 10.6 | 0.70 (0.49–1.02) | 0.73 (0.50–1.06) | 0.70 (0.48–1.03) |
| Q3 (M: 0.80–1.06; W: 1.09–1.39) | 63/4,560 | 13.7 | 0.90 (0.64–1.27) | 0.82 (0.57–1.19) | 0.78 (0.54–1.14) |
| Q4 (M: 1.07–1.40; W: 1.40–1.76) | 58/4,648 | 12.1 | 0.80 (0.56–1.14) | 0.70 (0.47–1.05) | 0.65 (0.44–0.97) |
| Q5 (M: ≥1.41; W: ≥1.77) | 45/4,492 | 9.7 | 0.64 (0.44–0.94) | 0.50 (0.30–0.83) | 0.50 (0.30–0.82) |
| P for trend | 0.08 | 0.02 | 0.01 | ||
| Insoluble dietary fiber intake | |||||
| Q1 (M: ≤3.54; W: ≤4.53) | 76/4,299 | 20.5 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 (M: 3.55–4.18; W: 4.54–5.28) | 57/4,564 | 16.3 | 0.69 (0.49–0.97) | 0.70 (0.49–1.00) | 0.69 (0.48–0.99) |
| Q3 (M: 4.19–4.87; W: 5.29–6.04) | 50/4,630 | 9.3 | 0.59 (0.41–0.84) | 0.64 (0.43–0.95) | 0.64 (0.43–0.95) |
| Q4 (M: 4.88–5.66; W: 6.05–6.91) | 47/4,579 | 8.7 | 0.56 (0.39–0.80) | 0.55 (0.36–0.84) | 0.56 (0.37–0.86) |
| Q5 (M: ≥5.67; W: ≥6.92) | 50/4,476 | 9.2 | 0.61 (0.42–0.87) | 0.54 (0.32–0.90) | 0.56 (0.34–0.94) |
| P for trend | 0.002 | 0.008 | 0.001 | ||
Adjusted for age, family history of diabetes mellitus, hypertension, body mass index, serum total cholesterol, serum high density lipoprotein cholesterol, log‐transformed serum triglycerides, current smoking, current alcohol drinking, regular exercise, intakes of total energy, vitamin A, vitamin C, magnesium and polyunsaturated fatty acid : saturated fatty acid ratio.
Adjusted for age, family history of diabetes mellitus, hypertension, body mass index, serum total cholesterol, serum high density lipoprotein cholesterol, log‐transformed serum triglycerides, current smoking, current alcohol drinking, regular exercise, intakes of total energy, vitamin A, vitamin C, magnesium, polyunsaturated fatty acid : saturated fatty acid ratio, log‐transformed homeostasis model assessment of insulin resistance and log‐transformed high‐sensitivity C‐reactive protein (hs‐CRP). M, men; PYs, person‐years; Q, quintile; W, women.
Next, we carried out a subgroup analysis of glucose tolerance status (NGT and prediabetes) to compare the magnitude of the association of total dietary fiber intake with the development risk of type 2 diabetes between subgroups (Table 3). The results showed a significant inverse association of the intake of total dietary fiber with type 2 diabetes risk in participants with prediabetes (P for trend = 0.04) after adjustment with confounders including HOMA‐IR and serum hs‐CRP, but there was no evidence of significant heterogeneity for participants with NGT and those with prediabetes (P for heterogeneity = 0.99).
Table 3.
Incidence rates and hazard ratios for the development of diabetes according to total dietary fiber intake in participants with normal glucose tolerance and prediabetes
| Total dietary fiber intake levels (g/1,000 kcal) | No. events/PYs | Age‐adjusted incidence rate (per 103 PYs) | Hazard ratio (95% confidence interval) | ||
|---|---|---|---|---|---|
| Model 1 (age‐ and sex‐adjusted) | Model 2 † (multivariable‐adjusted) | Model 3 ‡ (model 2 + HOMA‐IR and serum hs‐CRP) | |||
| Normal glucose tolerance (n = 1,366) | |||||
| Q1 (M: ≤4.21; W: ≤5.45) | 30/3,274 | 9.6 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 (M: 4.22–5.06; W: 5.46–6.35) | 20/3,365 | 5.7 | 0.64 (0.37–1.13) | 0.69 (0.38–1.26) | 0.69 (0.38–1.25) |
| Q3 (M: 5.07–5.93; W: 6.36–7.31) | 21/3,484 | 5.8 | 0.64 (0.37–1.12) | 0.73 (0.39–1.36) | 0.72 (0.38–1.35) |
| Q4 (M: 5.94–7.03; W: 7.32–8.46) | 19/3,435 | 5.4 | 0.59 (0.33–1.06) | 0.62 (0.31–1.23) | 0.63 (0.31–1.25) |
| Q5 (M: ≥7.04; W: ≥8.47) | 16/3,388 | 4.4 | 0.52 (0.28–0.96) | 0.42 (0.17–1.06) | 0.42 (0.17–1.07) |
| P for trend | 0.04 | 0.09 | 0.11 | ||
| Prediabetes § (n = 526) | |||||
| Q1 (M: ≤4.00; W: ≤5.28) | 40/1,080 | 38.0 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 (M: 4.01–4.93; W: 5.29–6.41) | 35/1,120 | 32.0 | 0.85 (0.54–1.34) | 0.77 (0.49–1.23) | 0.76 (0.47–1.21) |
| Q3 (M: 4.94–5.80; W: 6.42–7.69) | 41/1,149 | 35.1 | 0.95 (0.61–1.47) | 0.83 (0.53–1.30) | 0.77 (0.48–1.25) |
| Q4 (M: 5.81–7.28; W: 7.70–8.70) | 32/1,149 | 28.8 | 0.75 (0.47–1.19) | 0.66 (0.41–1.06) | 0.62 (0.36–1.10) |
| Q5 (M: ≥7.29; W: ≥8.71) | 27/1,109 | 23.5 | 0.66 (0.40–1.08) | 0.53 (0.32–0.89) | 0.48 (0.24–0.94) |
| P for trend | 0.09 | 0.01 | 0.04 | ||
| P for heterogeneity between subjects with normal glucose tolerance and prediabetes | 0.73 | 0.96 | 0.99 | ||
Adjusted for age, sex, family history of diabetes mellitus, hypertension, body mass index, serum total cholesterol, serum high density lipoprotein cholesterol, log‐transformed serum triglycerides, current smoking, current alcohol drinking, regular exercise, intakes of total energy, vitamin A, vitamin C, magnesium and polyunsaturated fatty acid/saturated fatty acid ratio.
Adjusted for age, sex, family history of diabetes mellitus, hypertension, body mass index, serum total cholesterol, serum high density lipoprotein cholesterol, log‐transformed serum triglycerides, current smoking, current alcohol drinking, regular exercise, intakes of total energy, vitamin A, vitamin C, magnesium, polyunsaturated fatty acid : saturated fatty acid ratio, log‐transformed homeostasis model assessment of insulin resistance (HOMA‐IR) and log‐transformed high‐sensitivity C‐reactive protein (hs‐CRP).
Prediabetes was defined as fasting plasma glucose 6.1–6.9 mmol/L and 2‐h post‐load glucose <7.8 mmol/L, or fasting plasma glucose <7.0 mmol/L and 2‐h post‐load glucose 7.8–11.0 mmol/L. M, men; PYs, person‐years; Q, quintile; W, women.
Finally, the correlation of the intake of dietary fiber to that of food groups was estimated (Table 4). Total dietary fiber intake was correlated positively to intakes of soybean and soybean products, green and other vegetables, fruits, fruits juices, and algae. Similar correlations were observed on soluble and insoluble fiber intakes, except for algae intake, with soluble dietary fiber.
Table 4.
Spearman’s correlation coefficients between food groups and total dietary fiber, soluble dietary fiber and insoluble dietary fiber intake
| Food group | Total dietary fiber | Soluble dietary fiber | Insoluble dietary fiber |
|---|---|---|---|
| Rice | 0.02 | −0.11 | 0.07 |
| Breads | 0.14 | 0.15 | 0.14 |
| Noodles and other cereals | 0.16 | 0.21 | 0.14 |
| Potatoes | 0.27 | 0.24 | 0.27 |
| Soybean and soybean products | 0.60 ‡ | 0.64 ‡ | 0.57 ‡ |
| Miso | 0.18 | 0.11 | 0.20 |
| Pickles | 0.06 | 0.02 | 0.06 |
| Green vegetables | 0.60 ‡ | 0.42 † | 0.65 ‡ |
| Other vegetables | 0.60 ‡ | 0.39 † | 0.66 ‡ |
| Fruits and fruit juices | 0.31 † | 0.31 † | 0.30 † |
| Algae | 0.33 † | 0.29 | 0.34 † |
| Fish | 0.15 | 0.14 | 0.15 |
| Meat | 0.04 | 0.02 | 0.05 |
| Eggs | 0.17 | 0.19 | 0.20 |
| Milk and dairy products | 0.18 | 0.19 | 0.22 |
| Fats and oils | 0.13 | 0.12 | 0.13 |
| Sugar and confectioneries | −0.07 | −0.07 | −0.07 |
| Alcoholic beverages | −0.0009 | 0.01 | −0.005 |
| Salt | −0.01 | −0.03 | −0.01 |
Correlation coefficients of ≥0.30 or ≤−0.30.
Correlation coefficients of ≥0.50 or ≤−0.50.
Discussion
The present study showed that higher intake of total dietary fiber had an association with a lower risk of type 2 diabetes in a general Japanese population. Furthermore, individuals with both higher intakes of soluble and insoluble dietary fiber had a lower risk of type 2 diabetes. This inverse association was found for both participants with prediabetes and those with NGT. In addition, dietary fiber had a positive correlation with the intakes of soybean and soybean products, green and other vegetables, fruits, fruit juices, and algae, which were considered the main sources of dietary fiber in the present study. These findings show that higher dietary fiber intake might be effective to lighten the burden of type 2 diabetes among the Japanese population.
Several prospective studies have explored the association of total dietary fiber intake with the risk of type 2 diabetes in Western populations, but the findings are inconsistent 30 , 31 , 32 , 33 , 34 , 35 . The Nurses’ Health Study showed that women in the highest quintile of total dietary fiber intake showed the effect of lowering the risk of developing type 2 diabetes by approximately 20% compared with those in the lowest quintile 30 , 31 . A Finnish study showed a similar significant inverse association of intake of total dietary fiber with diabetes risk 32 . The present findings are consistent with these previous results. In contrast, several studies did not show clear associations (e.g., the Health Professionals Follow‐up Study, the Atherosclerosis Risk in Communities study and the Nurses’ Health Study II) 33 , 34 , 35 . A meta‐analysis of 19 cohort studies clarified that the multivariable‐adjusted risk of type 2 diabetes lowered significantly by 9% for every 10‐g/day increase in intake of total dietary fiber with low between‐study heterogeneity (I 2 = 29.4%) 13 . Two other meta‐analyses of prospective studies showed that individuals with a higher intake level of total dietary fiber (above the median, the mean or comparing the highest and lowest categories for each study) had an approximately 20% reduced risk of diabetes compared with those with a lower intake level, but the association was heterogeneous across the studies (I 2 = 53.6% 14 and I 2 = 44.1% 15 , respectively). These inconsistent results might be by over‐ or underestimation of the intake of total dietary fiber due to differences in the dietary assessment methods, and/or the possibility of misclassification of diagnostic criteria for diabetes. Furthermore, ethnicity is an important determinant of the etiology of type 2 diabetes 36 . A major difference between Asians and white people in regulating the postprandial glucose metabolism is the β‐cell response to glucose 37 . It is widely recognized that type 2 diabetes in East Asians is characterized primarily by β‐cell dysfunction rather than insulin resistance 36 . In the present study, an inverse association between dietary fiber intake and diabetes risk was observed, because dietary fiber is resistant to carbohydrate absorption in the small intestine 12 , and its ability to suppress a rapid rise of blood glucose levels contributes to the prevention of type 2 diabetes in East Asians with limited insulin secretion capacity 38 . The present findings make a valuable contribution by showing that high total dietary fiber intake could play some role in the prevention of type 2 diabetes for Asian populations, as well as for Western populations. However, the effect of dietary fiber intake on the prevention of type 2 diabetes has not yet been fully addressed in Asian populations. Further epidemiological studies are required to determine whether a high‐fiber diet can lessen the future risk of type 2 diabetes in Asian populations.
The present study showed that higher intake of soluble and insoluble dietary fiber clearly reduced the risk of type 2 diabetes. Previous epidemiological studies, such as the Iowa Women’s Health Study and the Finnish Mobile Clinic Health Examination Survey, showed that the type 2 diabetes risk decreased significantly with higher intake of insoluble dietary fiber, but did not decrease with higher intake of soluble dietary fiber 18 , 19 . In contrast, neither soluble dietary fiber nor insoluble dietary fiber intake showed an association with diabetes risk in the European Prospective Investigation into Cancer and Nutrition‐Potsdam Study 20 . The inconsistency in these findings might be caused by differences in the source of dietary fiber among the populations. For example, the main food sources of dietary fiber in the Japanese population are legumes, vegetables, cereals and fruits 39 , and the majority (97%) of the legume intake is soybean and soybean products 40 . Also in the present study, soybean and soybean products and vegetables were the major sources of dietary fiber. In contrast, Western studies showed that cereals were the main source of dietary fiber, and small amounts of dietary fiber were derived from legumes 18 , 19 , 20 . Soybean and soybean products and vegetables have low carbohydrate content 25 and low glycemic indexes 41 , which has the effect of suppressing an increase in postprandial blood glucose concentrations 42 . These differences in food habits among countries might influence the differential findings on soluble and insoluble dietary fiber.
Intriguingly, a significant inverse association was found between total dietary fiber intake and type 2 diabetes risk in the present participants with prediabetes. Considering the high incidence of type 2 diabetes among participants with prediabetes in the present study, it would be assumed that the absolute benefit of intake of dietary fiber for the prevention of type 2 diabetes would be greater in those with prediabetes. Nevertheless, there was no significant heterogeneity in the preventive effects of dietary fiber on our participants with prediabetes and those with NGT. Therefore, an optimal amount of dietary fiber intake might be one of the important protective factors preventing the incidence of type 2 diabetes not only for individuals with prediabetes, but also for those with NGT.
The exact mechanism underlying the significant association between intake of dietary fiber and the risk of type 2 diabetes remains unclear; however, several possible explanations would be shown. Dietary fiber delays the absorption of carbohydrates and adequately secretes insulin, which leads to reduced postprandial blood glucose and insulin levels 12 . Furthermore, dietary fiber is fermented and generates short‐chain fatty acids inside the large intestine 12 , which might improve gut microbiota balance, and short‐chain fatty acids might increase the secretion of incretins 43 , which stimulate insulin secretion from β‐cells 44 . Higher intake of total and insoluble dietary fiber could lower markers of inflammation (e.g., plasminogen activator inhibitor‐1, resistin, CRP and interleukin‐6) 45 , 46 , thereby leading to a reduced risk of type 2 diabetes 46 . Finally, soluble dietary fiber has a favorable effect on the glucose and insulin responses by delaying gastric emptying and the absorption of nutrients 12 .
The strengths of the present study are its prospective population‐based study design, long follow‐up period, high rates of participation and the use of an OGTT for precisely determining the glucose tolerance status. Although, some potential limitations need to be addressed. At first, the dietary assessment was carried out at baseline only once using a semiquantitative food frequency questionnaire, and dietary fiber intake was evaluated only on the limited food list in the questionnaire. Additionally, the estimated dietary fiber intake based on self‐reported information does not necessarily reflect actual dietary fiber intake based on a direct biological analysis 47 . These limitations are likely to have caused some degree of misclassification of dietary fiber intake. Such residual misclassification would lower the association shown in the current study and lead the results to the null hypothesis. To minimize the measurement errors, we added in‐person interviews carried out by dieticians who were trained on the dietary questionnaire. Second, it was hard to fully exclude the effects of residual confounders (e.g., health awareness, the quality of the whole meal, eating habits, the effects of other nutrients and socioeconomic status) 48 on the association between dietary fiber intake and the risk of type 2 diabetes. It is known that individuals with good lifestyle habits and high health consciousness have a low risk of type 2 diabetes 49 , 50 . Dietary fiber is considered to be a key component of a healthy diet, as recommended in several nutritional guidelines 51 , 52 . Therefore, high dietary fiber intake might serve as an index for the high quality of the whole meal, favorable eating habits for diabetes prevention or health awareness. Finally, the generalizability of the findings might be limited, because the present study was carried out only in a community in Japan.
In conclusion, the results of the present study suggest that individuals with higher total dietary fiber intake had a lower risk of type 2 diabetes in a general Japanese population. Furthermore, both the soluble and insoluble dietary fiber intakes were inversely associated with the risk of type 2 diabetes. Participants with higher total dietary fiber intake consumed soybean and soybean products, vegetables, fruits, fruits juices, and algae, and the food sources of soluble and insoluble dietary fiber were generally similar. Therefore, the intake of high dietary fiber foods might be beneficial for diabetes prevention.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 | Incidence rates and hazard ratios for the development of diabetes according to the insoluble : soluble dietary fiber ratio.
Acknowledgments
This study was supported in part by Grants‐in‐Aid for Scientific Research (A) (JP16H02692) and (B) (JP16H05850, JP17H04126 and JP18H02737) and (C) (JP17K09114, JP17K09113, JP17K01853, JP18K07565, JP18K09412 and JP19K07890) and Early‐Career Scientists (JP18K17925 and JP18K17382) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by Health and Labor Sciences Research Grants of the Ministry of Health, Labor and Welfare of Japan (H29‐Junkankitou‐Ippan‐003 and H30‐Shokuhin‐[Sitei]‐005); and by the Japan Agency for Medical Research and Development (JP19dk0207025, JP19ek0210082, JP19ek0210083, JP19km0405202, JP19ek0210080, JP19fk0108075). There was no study sponsor that had any role in the study design, data collection, study data interpretation or preparation of the manuscript.
J Diabetes Investig. 2021
References
- 1. Unnikrishnan R, Pradeepa R, Joshi SR, et al. Type 2 diabetes: demystifying the global epidemic. Diabetes 2017; 66: 1432–1442. [DOI] [PubMed] [Google Scholar]
- 2. Nanditha A, Ma RC, Ramachandran A, et al. Diabetes in Asia and the Pacific: implications for the global epidemic. Diabetes Care 2016; 39: 472–485. [DOI] [PubMed] [Google Scholar]
- 3. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009; 301: 2129–2140. [DOI] [PubMed] [Google Scholar]
- 4. Ministry of Health Labour and Walfare, Japan . The National Health and Nutrition Survey in Japan, 2016. Tokyo: Dai‐ichi Publishing, 2017. (Japanese). [Google Scholar]
- 5. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet 2017; 389: 2239–2251. [DOI] [PubMed] [Google Scholar]
- 6. Li W, Huang E. An update on type 2 diabetes mellitus as a risk factor for dementia. J Alzheimers Dis 2016; 53: 393–402. [DOI] [PubMed] [Google Scholar]
- 7. Fung FY, Koh YLE, Malhotra R, et al. Prevalence of and factors associated with sarcopenia among multi‐ethnic ambulatory older Asians with type 2 diabetes mellitus in a primary care setting. BMC Geriatr 2019; 19: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu YY, Xiao E, Graves DT. Diabetes mellitus related bone metabolism and periodontal disease. Int J Oral Sci 2015; 7: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsilidis KK, Kasimis JC, Lopez DS, et al. Type 2 diabetes and cancer: umbrella review of meta‐analyses of observational studies. BMJ 2015; 350: g7607. [DOI] [PubMed] [Google Scholar]
- 10. Wu Y, Ding Y, Tanaka Y, et al. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci 2014; 11: 1185–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ley SH, Hamdy O, Mohan V, et al. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 2014; 383: 1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients 2010; 2: 1266–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The Inter Act Consortium . Dietary fiber and incidence of type 2 diabetes in eight European countries: the EPIC‐InterAct Study and a meta‐analysis of prospective studies. Diabetologia 2015; 58: 1394–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yao B, Fang H, Xu W, et al. Dietary fiber intake and risk of type 2 diabetes: a dose‐response analysis of prospective studies. Eur J Epidemiol 2014; 29: 79–88. [DOI] [PubMed] [Google Scholar]
- 15. Ye EQ, Chacko SA, Chou EL, et al. Greater whole‐grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J Nutr 2012; 142: 1304–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bingham S. Patterns of dietary fiber consumption in humans. In: Spiller GA (ed). CRC Handbook of Dietary Fiber in Human Nutrition, 2nd edn. Boca Raton, FL: CRC Press, 1993; 509–523. [Google Scholar]
- 17. Weickert MO, Pfeiffer AFH. Impact of dietary fiber consumption on insulin resistance and the prevention of type 2 diabetes. J Nutr 2018; 148: 7–12. [DOI] [PubMed] [Google Scholar]
- 18. Meyer KA, Kushi LH, Jacobs DR, et al. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 2000; 71: 921–930. [DOI] [PubMed] [Google Scholar]
- 19. Montonen J, Knekt P, Jarvinen R, et al. Whole‐grain and fiber intake and the incidence of type 2 diabetes. Am J Clin Nutr 2003; 77: 622–629. [DOI] [PubMed] [Google Scholar]
- 20. Schulze MB, Schulz M, Heidemann C, et al. Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta‐analysis. Arch Intern Med 2007; 167: 956–965. [DOI] [PubMed] [Google Scholar]
- 21. Weng LC, Lee NJ, Yeh WT, et al. Lower intake of magnesium and dietary fiber increases the incidence of type 2 diabetes in Taiwanese. J Formos Med Assoc 2012; 111: 651–659. [DOI] [PubMed] [Google Scholar]
- 22. Ninomiya T. Japanese legacy cohort studies: the Hisayama Study. J Epidemiol 2018; 28: 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kiyohara Y, Shinohara A, Kato I, et al. Dietary factors and development of impaired glucose tolerance and diabetes in a general Japanese population: the Hisayama Study. J Epidemiol 2003; 13: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shirota T, Yoshizumi E. A study on convenient dietary assessment. Jpn Public Health 1990; 37: 100–108 (Japanease). [PubMed] [Google Scholar]
- 25. Resources Council of Science and Technology Agency . Standard Tables of Food Composition in Japan, 4th edn. Tokyo, 1982. (Japanese). [Google Scholar]
- 26. Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986; 124: 17–27. [DOI] [PubMed] [Google Scholar]
- 27. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 28. SAS Institute Inc . SAS/STAT 15.1 User’s Guide. The PHREG Procedure. Example 89.8 Survival Curves, 2019. Available from: http://documentation.sas.com/?docsetId=statug&docsetTarget=statug_phreg_examples08.htm&docsetVersion=15.1&locale=ja. Accessed August 17, 2019.
- 29. Hinkle DE, Jurs SG. Applied Statistics for the Behavioral Sciences. 5th edn. Boston, MA: Houghton Mifflin, 2002. (English). [Google Scholar]
- 30. Salmeron J, Manson JE, Stampfer MJ, et al. Dietary fiber, glycemic load, and risk of non‐insulin‐dependent diabetes mellitus in women. JAMA 1997; 277: 472–477. [DOI] [PubMed] [Google Scholar]
- 31. AlEssa HB, Bhupathiraju SN, Malik VS, et al. Carbohydrate quality and quantity and risk of type 2 diabetes in US women. Am J Clin Nutr 2015; 102: 1543–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lindstrom J, Peltonen M, Eriksson JG, et al. High‐fiber, low‐fat diet predicts long‐term weight loss and decreased type 2 diabetes risk: the Finnish Diabetes Prevention Study. Diabetologia 2006; 49: 912–920. [DOI] [PubMed] [Google Scholar]
- 33. Salmeron J, Ascherio A, Rimm EB, et al. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 1997; 20: 545–550. [DOI] [PubMed] [Google Scholar]
- 34. Stevens J, Ahn K, Juhaeri, et al. Dietary fiber intake and glycemic index and incidence of diabetes in African‐American and white adults: the ARIC Study. Diabetes Care 2002; 25: 1715–1721. [DOI] [PubMed] [Google Scholar]
- 35. Schulze MB, Liu S, Rimm EB, et al. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle‐aged women. Am J Clin Nutr 2004; 80: 348–356. [DOI] [PubMed] [Google Scholar]
- 36. Yabe D, Seino Y, Fukushima M, et al. β cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep 2015; 15: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cho YM. Incretin physiology and pathophysiology from an Asian perspective. J Diabetes Investig 2015; 6: 495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fujimoto WY. Overview of non‐insulin‐dependent diabetes mellitus (NIDDM) in different population groups. Diabet Med 1996; 13: S7–S10. [PubMed] [Google Scholar]
- 39. Ikegami S. Changes in dietary fiber intake in Japan. J Jpn Assoc Dietary Fiber Res 1997; 1: 3–12 (Japanese). [Google Scholar]
- 40. Ministry of Health Labour and Walfare, Japan . The National Health and Nutrition Survey in Japan, 1988. Available from http://www.nibiohn.go.jp/eiken/chosa/kokumin_eiyou/1988.html. Accessed September 26, 2019 (Japanese).
- 41. Foster‐Powell K, Holt SH, Brand‐Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 2002; 76: 5–56. [DOI] [PubMed] [Google Scholar]
- 42. Barclay AW, Petocz P, McMillan‐Price J, et al. Glycemic index, glycemic load, and chronic disease risk‐a meta‐analysis of observational studies. Am J Clin Nutr 2008; 87: 627–637. [DOI] [PubMed] [Google Scholar]
- 43. Tolhurst G, Heffron H, Lam YS, et al. Short‐chain fatty acids stimulate glucagon‐like peptide‐1 secretion via the G‐protein‐coupled receptor FFAR2. Diabetes 2012; 61: 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chia CW, Egan JM. Incretins in obesity and diabetes. Ann N Y Acad Sci 2019; 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miller SJ, Batra AK, Shearrer GE, et al. Dietary fiber linked to decreased inflammation in overweight minority youth. Pediatr Obes 2016; 11: 33–39. [DOI] [PubMed] [Google Scholar]
- 46. Wannamethee SG, Whincup PH, Thomas MC, et al. Associations between dietary fiber and inflammation, hepatic function, and risk of type 2 diabetes in older men: potential mechanisms for the benefits of fiber on diabetes risk. Diabetes Care 2009; 32: 1823–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shim JS, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health 2014; 36: e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Le C, Jun D, Zhankun S, et al. Socioeconomic differences in diabetes prevalence, awareness, and treatment in rural southwest China. Trop Med Int Health 2011; 16: 1070–1076. [DOI] [PubMed] [Google Scholar]
- 49. Uusitupa M, Khan TA, Viguiliouk E, et al. Prevention of type 2 diabetes by lifestyle changes: a systematic review and meta‐analysis. Nutrients 2019; 11: 2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sami W, Ansari T, Butt NS, et al. Effect of diet on type 2 diabetes mellitus: a review. Int J Health Sci 2017; 11: 65–71. [PMC free article] [PubMed] [Google Scholar]
- 51. Lichtenstein AH, Appel LJ, Brands M, et al. Summary of American Heart Association diet and lifestyle recommendations revision 2006. Arterioscler Thromb Vasc Biol 2006; 26: 2186–2191. [DOI] [PubMed] [Google Scholar]
- 52. Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006; 114: 82–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Incidence rates and hazard ratios for the development of diabetes according to the insoluble : soluble dietary fiber ratio.


