Abstract
Aims/Introduction
In this meta‐analysis, we aimed to explore the association between bodyweight cycling (weight fluctuation) and the risk of developing diabetes.
Materials and Methods
We analyzed data from eligible cohort studies that assessed the association between weight cycling in adults and the risk of developing diabetes from online databases PubMed, Cochrane Library and EMBASE databases (1966 to April 2020). We pooled data using relative risks (RRs) with a random effects model.
Results
A total of 14 studies involving 253,766 participants, including 8,904 diabetes events, were included. One study included eight independent reports, resulting in 21 reports in 14 studies. Summary analysis showed that individuals who suffered weight cycling had a higher risk of diabetes (RR 1.23. 95% confidence interval 1.07–1.41; P = 0.003). However, the association between weight cycling and the risk of developing diabetes was not observed in obese participants (body mass index ≥30 kg/m2; P = 0.08).
Conclusions
The present meta‐analysis showed that weight cycling was a strong independent predictor of new‐onset diabetes. Future studies are required to detect the causal links between weight cycling and the risk of developing diabetes.
Keywords: Body‐weight fluctuation, Diabetes, Weight cycling
Flowchart of the study selection. aExact reasons for exclusion were not documented.

Introduction
Obesity is increasing worldwide 1 . In 2015, a total of 711.4 million people were obese, and overweight or obesity contributed to 4.0 million deaths in 1 year alone 2 . Obesity is an independent risk factor for diabetes 3 . Therefore, weight loss is widely advised by physicians for obese patients, and it has been estimated that >30 million people in the USA are overweight and in a battle to lose weight 4 . However, the success rate is not impressive on account of multiple factors, such as genetic predisposition, emotions and barriers in a person’s social or cultural environment 5 , 6 . According to the Nurses’ Health Study II (NHSII), just <10% of the women who lost weight successfully were able to maintain their loss of weight 7 . Repeated weight loss followed by weight gain caused weight fluctuation or weight cycling. The National Task Force on the Prevention and Treatment of Obesity summarized in reports weight cycling from 1966 to 1994 carried out in normal weight, overweight and obese individuals. They concluded that the available evidence regarding increased morbidity as a result of weight cycling is not sufficiently compelling to override the potential benefits of moderate weight loss 8 . Since 1994, numerous studies investigating weight cycling have been carried out and have yielded controversial results 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 . Therefore, we undertook the present meta‐analysis based on available evidence from published cohort studies to establish whether participants with weight cycling had a higher risk of diabetes.
Methods
The present meta‐analysis was prospectively registered with PROSPERO International Prospective Register of Systematic Reviews (PROSPERO identifier CRD42018110985). We carried out this meta‐analysis of studies assessing the association between weight cycling and the risk of developing diabetes according to the Conducting Systematic Reviews and Meta‐Analyses of Observational Studies of Etiology (COSMOS‐E) Guidelines 18 .
Search strategy and selection criteria
PubMed, EMBASE databases and the Cochrane library were searched from 1966 to 24 September 2018. Then, hand‐searching was undertaken according to references from these relevant papers. The search was subsequently updated to 7 April 2020. One newly identified study was included in the analyses 19 . The search strategies are shown in Appendix S1.
Our analyses included cohort studies if they had published relative risk (RR) estimates with 95% confidence intervals (CI) of the association between weight cycling and the risk of developing diabetes in populations free of diabetes at baseline aged ≥18 years. As no single definition and measurement are currently endorsed, we defined weight cycling as weight gain or loss in a specific period, and change in the opposite direction (loss or gain) in the next period. Detailed descriptions for definitions and measurements of weight cycling in different studies are shown in Table S1. The primary outcome was new‐onset diabetes. If multiple publications from the same cohort study were reviewed, we included the publication with the longest follow‐up period.
Statistical analysis
We assessed pooled data using RRs with a random effects model, and any results in studies that were stratified by sex were treated as two separate reports 20 . Weight cycling was measured in two different ways; that is, weight cycles as categorical variables (such as weight loss and gain or gain and loss ≥2.27 kg 9 , 4.54 kg 10 or ≥1.125 kg/year 11 ) and deviation degree of weight as continuous variables (such as root mean square error 21 , coefficient of variation 22 , body mass index [BMI] variability or average successive variability of weight 17 ). All RRs for outcomes were transformed as categorical variables (details are shown in Appendix S2).
The modified Newcastle–Ottawa scale (NOS) was used for grading the quality of cohort studies (Appendix S2) 23 . Publication bias was assessed using funnel plots, and Egger’s and Begg’s tests 24 , 25 . The sensitivity analyses were carried out at the levels of both report and study to assess the effect of each report or each study on the overall findings. For the report level of analysis, we omitted one report at a time from the analyses and summarized the RR of the remaining reports. For the study level of analysis, we omitted one study, which might include several reports, at each time and summarized the RR of the remaining studies. We estimated heterogeneity among studies with the I 2 statistic 26 , and used meta‐regression to assess the contribution to heterogeneity of sex, age, location, number of participates, percentage of events, follow‐up duration, study quality, original measurement of weight cycling, method for weight ascertainment, follow‐up rate and duration of assessing weight change. In the meta‐regression, variables in the univariable analyses with P‐values <0.1 were considered statistically significant and were then included in the multivariable models, and an overall P‐value <0.05 was considered statistically significant in the multivariable regression analysis 27 , 28 . We undertook subgroup analyses based on sex (male, female or both), age (≤60 or >60 years), location (North America, Europe or Asia), follow‐up duration (>10 or ≤10 years), study quality (NOS score >7 or ≤7), original measurement of weight cycling (deviation degree or weight cycle), method of weight ascertainment (self‐reported or measured at each visit), intentional or unintentional weight cycling, BMI (BMI <25 kg/m2, 25 kg/m2 ≤ BMI < 30 kg/m2 or BMI ≥30 kg/m2) and follow‐up rate (>80% or ≤80%).
Data were analyzed with Stata statistical software version 12.0 (StataCorp, College Station, TX, USA), and all statistical tests were two‐sided with a significance level of 0.05.
Results
Study selection and study characteristics
After excluding 4,992 ineligible studies identified in the initial search, 14 cohort studies were eligible for the present study (Figure 1) 9 , 10 , 11 , 12 , 13 , 14 , 16 , 17 , 19 , 29 , 30 , 31 , 32 , 33 . Notably, two studies 34 , 35 were excluded because RRs of 1 kg average successive variability of weight or root mean square error increase were reported, and could not be transformed to categorical variables for further analysis. In addition, one publication 36 was excluded from our analysis because the participants came from the same study, and we included the article with a longer duration 30 .
Figure 1.
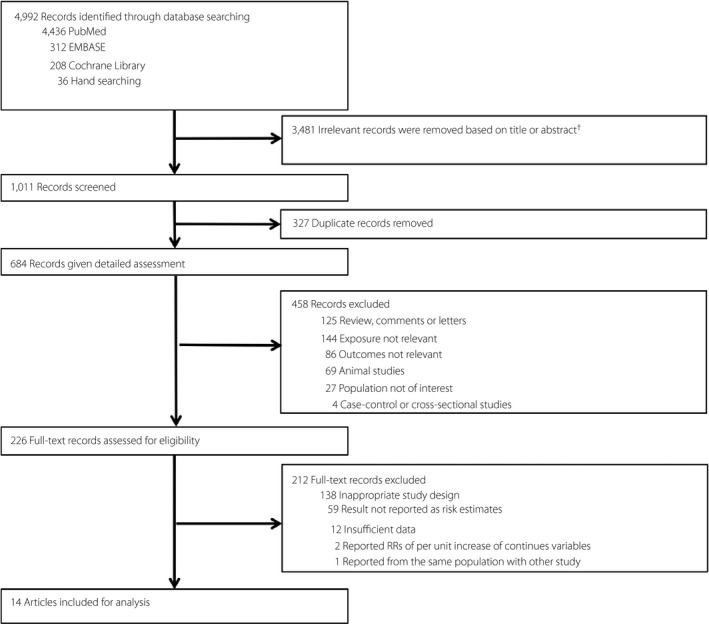
Flowchart of the study selection. †Exact reasons for exclusion were not documented. RRs, relative risks.
In total, 14 studies with 253,766 individuals (8,904 new‐onset diabetes) were included. The age of participants ranged from 20 to 75 years, and the majority of the participants were aged >50 years. The participants in six studies 9 , 10 , 13 , 16 , 19 , 31 had an average BMI ≥25 kg/m2 (n = 119,517, 47.1%), participants in three studies 12 , 17 , 29 had an average BMI <25 kg/m2 (n = 36,112, 14.2%) and the remaining studies 11 , 14 , 30 , 32 , 33 did not provide a BMI. Three studies involved weight cycling as a result of intentional weight loss, and the remaining studies could not be distinguished based on the available information. The median follow‐up time of these cohort studies ranged from 2.5 to 32 years. Of the 14 publications, 12 were rated high quality (≥6.5) based on the Newcastle–Ottawa scale score (Table S2). Yokomichi et al. 12 divided participants into groups of rural men, rural women, urban men and urban women. They then reported results as weight cycling end at weight loss and end at weight gain, respectively. Therefore, eight reports were generated and added to the other 13 studies, resulting in 21 reports in 14 studies.
Weight cycling and risk of developing diabetes
The available evidence shows that weight cycling was significantly associated with the risk of developing diabetes (RR 1.23, 95% CI 1.07–1.41, P = 0.003; Figure 2). The heterogeneity was evident among the studies (I 2 = 73.9%, P < 0.001 for heterogeneity).
Figure 2.
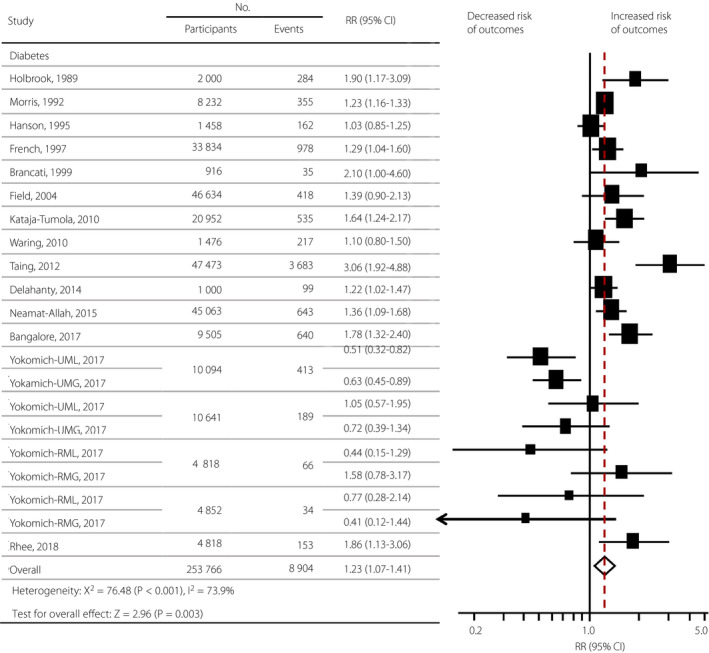
Summary relative risks (RRs) of the association between weight cycling and diabetes. The size of data markers is proportional to the weight of each report. RRs and 95% confidence intervals (CI) were calculated using a random effects model to pool the data. Error bars indicate the 95% CIs. RR data are rounded to two decimal places; error bars reflect unrounded values. RMG, weight cycle in rural men ending at gain; RML, weight cycle in rural men ending at loss; RWG, weight cycle in rural women ending at gain; RWL, weight cycle in rural women ending at loss; UMG, weight cycle in urban men ending at gain; UML, weight cycle in urban men ending at loss; UWG, weight cycle in urban women at gain; UWL, weight cycle in urban women ending at loss.
Meta‐regression
Significant heterogeneity (I 2 > 50%) was detected among these studies. Therefore, we undertook meta‐regression analyses to assess the contribution to heterogeneity. The results suggested that the age of participants (P = 0.04 in the univariable models), location (P = 0.02 in the univariable models), follow‐up duration (P = 0.01 in the univariable models), study quality (P = 0.007 in the univariable models), method of weight ascertainment (P = 0.01 in the univariable models) and duration of the assessment of weight change (P = 0.01 in the univariable models) were the major sources of heterogeneity of the studies (the overall P = 0.001 in the multivariable models; Table S3).
Sensitivity and subgroup analyses
The results of the sensitivity analyses showed that the observed association between weight cycling and the risk of developing diabetes was not altered after excluding any report (Figure S1) or study (Table S4). Furthermore, three of the 21 reports of diabetes involved intentional weight loss. The subgroup analyses of three reports showed that individuals with intentional weight cycling had a higher risk of diabetes (P < 0.001; Table 1); however, the small number of studies limits the conclusion drawn based on these studies. Furthermore, the association was not detected in several subgroup analyses (Table 1), such as in participants aged ≤60 years (P = 0.16), in studies using weight cycle as the original measurement for cycling (P = 0.55), participants with normal weight (P = 0.20) or obesity (P = 0.55). This might be due to the fact that null association for weight cycling and risk of developing diabetes was observed in eight reports from the Yokomichi et al. 12 study among 21 reports. Therefore, we also summarized the overall RR and carried out the subgroup analyses after omission of the Yokomichi et al. study. The overall result after omitting the Yokomichi et al. study was consistent with the original findings of increased risk of diabetes with weight cycling (RR 1.41, 95% CI 1.25–1.59, P < 0.001). The significance of the association between weight cycling and the risk of diabetes was detected in most subgroups after omitting the Yokomichi et al. study (P < 0.05; Table S5), such as in participants aged ≤60 years (P < 0.001), in studies using weight cycle as the original measurement for cycling (P < 0.001) and participants with normal weight (P = 0.002). However, null association was still detected in obese individuals (BMI ≥30 kg/m2), even after removing the Yokomichi et al. study (P = 0.08; Table S5).
Table 1.
Subgroup analyses for the association of weight cycling and the risk of diabetes
| Reports (n) | RR (95% CI) | P 1 | I 2 (%) | P 2 | |
|---|---|---|---|---|---|
| All studies | 21 | 1.23 (1.07–1.41) | 0.003 | 73.9 | <0.001 |
| Sex | |||||
| Male | 6 | 0.97 (0.57–1.66) | 0.92 | 85.4 | <0.001 |
| Female | 8 | 1.26 (0.99–1.60) | 0.06 | 68.2 | 0.003 |
| Both | 7 | 1.34 (1.13–1.59) | 0.001 | 61.8 | 0.015 |
| Age (years) | |||||
| ≤60 | 17 | 1.11 (0.96–1.28) | 0.16 | 69.0 | <0.001 |
| >60 | 4 | 1.84 (1.30–2.60) | 0.001 | 75.5 | 0.007 |
| Location | |||||
| North America | 10 | 1.38 (1.19–1.59) | <0.001 | 69.2 | 0.001 |
| Europe | 2 | 1.46 (1.22–1.75) | <0.001 | 7.1 | 0.30 |
| Asia | 9 | 0.82 (0.57–1.18) | 0.28 | 65.5 | 0.003 |
| Follow‐up duration (years) | |||||
| ≥10 | 12 | 0.94 (0.74–1.21) | 0.64 | 72.1 | <0.001 |
| <10 | 9 | 1.50 (1.26–1.79) | <0.001 | 72.7 | <0.001 |
| Original measurement of weight fluctuation | |||||
| Deviations degree | 7 | 1.38 (1.18–1.60) | <0.001 | 65.4 | 0.008 |
| Weight cycle | 14 | 1.08 (0.84–1.38) | 0.55 | 77.3 | <0.001 |
| Study quality | |||||
| Score >7 | 12 | 0.94 (0.77–1.16) | 0.58 | 66.9 | <0.001 |
| Score ≤7 | 9 | 1.59 (1.32–1.91) | <0.001 | 72.8 | <0.001 |
| Method for weight ascertainment | |||||
| Self‐reported | 5 | 1.61 (1.22–2.12) | 0.001 | 67.4 | 0.17 |
| Measured at each visit | 8 | 1.33 (1.17–1.53) | <0.001 | 63.3 | 0.008 |
| Missing information | 8 | 0.72 (0.54–0.95) | 0.019 | 32.8 | 0.17 |
| Weight loss | |||||
| Intentional | 3 | 1.23 (1.16–1.31) | <0.001 | 0.0 | 0.85 |
| Unintentional | None | ||||
| No discrimination | 18 | 1.21 (0.99–1.47) | 0.069 | 77.7 | <0.001 |
| BMI at baseline (kg/m2) | |||||
| BMI <25 | 10 | 0.89 (0.62–1.29) | 0.20 | 68.9 | 0.001 |
| 25 ≤ BMI < 30 | 3 | 1.62 (1.31–2.00) | <0.001 | 0.0 | 0.64 |
| BMI ≥30 | 3 | 1.47 (0.96–2.24) | 0.55 | 88.8 | <0.001 |
| Missing information | 5 | 1.30 (1.16–1.46) | <0.001 | 41.6 | 0.14 |
| Follow‐up rate | |||||
| ≥80% | 7 | 1.34 (1.13–1.59) | 0.001 | 61.8 | 0.015 |
| <80% | 14 | 1.12 (0.90–1.40) | 0.33 | 78.4 | <0.001 |
P 1 for the significance of association of weight fluctuation and the risk of diabetes in each subgroup. P 2 for heterogeneity within each subgroup.
BMI, body mass index; CI, confidence interval; RR, relative risk for developing diabetes.
Publication bias
Egger’s (P = 0.53) and Begg’s (P = 0.81) tests suggested that there was no significant publication bias. Visual inspection of the funnel plot is shown in Figure S2.
Discussion
A total of 253,766 participants and 8,904 new‐onset diabetes patients were included from the 14 studies in the pooled analysis. In general, individuals with weight cycling had a significant 23% increased risk of developing diabetes. The meta‐regression provided the specific potential sources of heterogeneity, such as age, location, follow‐up duration and method used for the weight ascertainment.
Although most large studies, such as the Rancho Bernardo cohort, Iowa Women’s Health Study and the Alpha‐Tocopherol, Beta‐Carotene Cancer Prevention Study 14 , 19 , 31 suggested that weight cycling is associated with the risk of developing diabetes, the findings are not consistent 10 , 12 , 16 , 30 . The discrepancy in the results might be due to the lack of a standardized definition or measurement of weight cycling, differentiation between intentional and unintentional weight cycling, the participants’ age or baseline BMI. Therefore, we carried out subgroup analysis based on these variables. Various reasons could explain the variation in body weight. Several studies showed that individuals with intentional weight cycling had a higher risk of diabetes. However, the association between weight cycling and the risk of developing diabetes was not observed in several subgroup analyses, such as in participants aged ≤60 years, individuals with normal weight or obesity and in studies using weight cycle as the original measurement for weight cycling. It might be due to the null association in eight reports from the Yokomichi et al. study 12 among 21 reports, which might have a potential influence on the association in subgroup analyses. After omission of the Yokomichi et al. study, the results of most subgroups were consistent with the original findings. The Yokomichi et al. study involved middle‐aged employees of private companies who underwent medical checkups. Furthermore, in the study, the point estimate regarding the weight cycling was gain/loss >4% of their baseline weight, which was lower than that in other studies 13 , 30 . This magnitude of weight cycling might not have shown any adverse effect. In addition, the prevalence of diabetes in Japan was at a relative low level compared with that in other countries 37 . The disparity between this result and other Western studies might also be related to ethnicity, which is associated with genetic constitution, living conditions, lifestyle factors and anthropometry 38 , 39 . However, the null association was also detected in obese individuals (BMI ≥30 kg/m2) even after removing the Yokomichi et al. study, suggesting that obesity might have an effect on the risk of diabetes similar to that of weight cycling.
Potential mechanisms for these findings are unclear; intriguing evidence suggests that weight cycling was associated with metabolic disturbance, such as insulin resistance 40 , elevations in triglycerides 41 and abdominal fat accumulation 42 , all of which might contribute to metabolic disease. Another possibility is that weight cycling might be a marker of potential illnesses that have worse prognoses in these participants 9 . In this case, weight cycling could be the early sign and consequence, and not the cause, of the health endpoints, including diabetes and related diseases. It was reported that the risk of developing depression is increased in people with diabetes 43 . Previous research also suggested that participants with a psychological disorder, such as bipolar disorder 44 and depression 45 , are more likely to present with weight cycling. In this case, the cycling in weight could be a consequence rather than the cause of the diabetes. A previous meta‐analysis also showed that weight cycling was associated with higher CVD mortality and morbidity 46 , which is the most common cause of morbidity and mortality among individuals with type 2 diabetes 47 .
The present analysis also had several limitations. First, the findings of this meta‐analysis are based on observational data; therefore, the present study could not identify the causal mechanisms driving the observed association between weight cycling and the risk of developing diabetes, which limits the generalizability of the findings. Second, the different definitions and measurements of weight cycling in the included studies might have an important confounding effect, although we carried out detailed subgroup, sensitivity and meta‐regression analyses to confirm the robustness of our results. Furthermore, other anthropometric measurements (e.g., waist circumference and waist‐to‐hip ratio), which were closely related to diabetes, were beyond the scope of our present analyses. Finally, significant heterogeneity was detected among studies, although we carried out detailed subgroup and meta‐regression analyses to detect potential sources of heterogeneity.
In summary, the pooled estimates from available cohort studies showed that individuals with weight cycling had a higher risk of developing diabetes. Future physiological studies are required to show the causal links and underlying mechanisms between weight cycling and diabetes.
Disclosure
The authors declare no conflict of interest.
Supporting information
Appendix S1 | Search terms.
Appendix S2 | Additional methods.
Table S1 | Descriptive characteristics of 14 included articles.
Table S2 | Quality assessment of individual studies using the Newcastle–Ottawa Scale.
Table S3 | Meta‐regression of factors affecting heterogeneity.
Table S4 | Sensitivity analysis based on study level for association of weight cycling and risk of diabetes.
Table S5 | Subgroup analyses for the association between weight cycling and the risk of diabetes after omission of the Yokomichi et al. study.
Figure S1 | Sensitivity analysis based on report level for association of weight cycling and risk of diabetes.
Figure S2 | Funnel plot for assessment of publication bias.
Acknowledgments
This study was supported by a grant for Integrated Innovative Team for Major Human Diseases Program of Tongji Medical College, HUST, grants from the National Key R&D Program of China (2016YFC0901203) and the National Natural Science Foundation of China (81974109, 81570740).
J Diabetes Investig. 2021
References
- 1. Roberto CA, Swinburn B, Hawkes C, et al. Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. Lancet 2015; 385 :2400–2409. [DOI] [PubMed] [Google Scholar]
- 2. Article O. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017; 377: 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lyall DM, Celis‐Morales C, Ward J, et al. Association of body mass index with cardiometabolic disease in the UK Biobank: a Mendelian randomization study. JAMA Cardiol 2017; 2: 882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Phillips WG. Obesity and weight cycling. J Med Assoc Ga 1993; 82: 537–540. [PubMed] [Google Scholar]
- 5. Teixeira PJ, Going SB, Sardinha LB, et al. A review of psychosocial pre‐treatment predictors of weight control. Obes Rev 2005; 6: 43–65. [DOI] [PubMed] [Google Scholar]
- 6. Papas MA, Alberg AJ, Ewing R, et al. The built environment and obesity. Epidemiol Rev 2007; 29: 129–143. [DOI] [PubMed] [Google Scholar]
- 7. Field AE, Wing RR, Manson JE, et al. Relationship of a large weight loss to long‐term weight change among young and middle‐aged US women. Int J Obes Relat Metab Disord 2001; 25: 1113–1121. [DOI] [PubMed] [Google Scholar]
- 8. National Task Force on the Prevention and Treatment of Obesity . Weight cycling. JAMA 1994; 272: 1196–1202. [PubMed] [Google Scholar]
- 9. Delahanty LM, Pan Q, Jablonski KA, et al. Effects of weight loss, weight cycling, and weight loss maintenance on diabetes incidence and change in cardiometabolic traits in the diabetes prevention program. Diabetes Care 2014; 37: 2738–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Field AE, Manson JE, Laird N, et al. Weight cycling and the risk of developing type 2 diabetes among adult women in the United States. Obes Res 2004; 12: 267–274. [DOI] [PubMed] [Google Scholar]
- 11. Neamat‐Allah J, Barrdahl M, Hüsing A, et al. Weight cycling and the risk of type 2 diabetes in the EPIC‐Germany cohort. Diabetologia 2015; 58: 2718–2725. [DOI] [PubMed] [Google Scholar]
- 12. Yokomichi H, Ohde S, Takahashi O, et al. Weight cycling and the subsequent onset of type 2 diabetes mellitus: 10‐year cohort studies in urban and rural Japan. BMJ Open 2017; 7: e014684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taing KY, Ardern CI, Kuk JL. Effect of the timing of weight cycling during adulthood on mortality risk in overweight and obese postmenopausal women. Obesity 2012; 20: 407–413. [DOI] [PubMed] [Google Scholar]
- 14. French SA, Folsom AR, Jeffery RW, et al. Weight variability and incident disease in older women: the Iowa Women’s Health Study. Int J Obes 1997; 21: 217–223. [DOI] [PubMed] [Google Scholar]
- 15. French SA, Jeffery RW, Folsom AR, et al. Weight variability in a population‐based sample of older women: reliability and intercorrelation of measures. Int J Obes Relat Metab Disord 1995; 19: 22–29. [PubMed] [Google Scholar]
- 16. Hanson RL, Narayan KMV, McCance DR, et al. Rate of weight gain, weight fluctuation, and incidence of NIDDM. Diabetes 1995; 44: 261–266. [DOI] [PubMed] [Google Scholar]
- 17. Rhee EJ, Cho JH, Kwon H, et al. Increased risk of diabetes development in individuals with weight cycling over 4 years: the Kangbuk Samsung Health study. Diabetes Res Clin Pract 2018; 139: 230–238. [DOI] [PubMed] [Google Scholar]
- 18. Dekkers OM, Vandenbroucke JP, Cevallos M, et al. COSMOS‐E: Guidance on conducting systematic reviews and meta‐analyses of observational studies of etiology. PLoS Medicine 2019; 16: e100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kataja‐Tuomola M, Sundell J, Männistö S, et al. Short‐term weight change and fluctuation as risk factors for type 2 diabetes in Finnish male smokers. Eur J Epidemiol 2010; 25: 333–339. [DOI] [PubMed] [Google Scholar]
- 20. Rong Y, Chen L, Zhu T, et al. Egg consumption and risk of coronary heart disease and stroke: dose‐response meta‐analysis of prospective cohort studies. BMJ 2013; 346: e8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hanson RL, Jacobsson LT, McCance DR, et al. Weight fluctuation, mortality and vascular disease in Pima Indians. Int J Obes Relat Metab Disord 1996; 20: 463–471. [PubMed] [Google Scholar]
- 22. Lissner L, Odell PM, D’Agostino RB, et al. Variability of body weight and health outcomes in the Framingham population. N Engl J Med 1991; 324: 1839–1844. [DOI] [PubMed] [Google Scholar]
- 23. Wells G, Shea B, O’Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐analyses. Ottawa, ON: Ottawa Health Research Institute, 2012. [Google Scholar]
- 24. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088. [PubMed] [Google Scholar]
- 25. Egger M, Smith GD, Schneider M, et al. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins JP, Thompson SG, Deeks JJAD. Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Samprit Chatterjee ASH. Regression Analysis by Example, 4th edn. New York, NY: Wiley‐Interscience, 2006. [Google Scholar]
- 28. Thompson SG, Higgins JPT. How should meta‐regression analyses be undertaken and interpreted? Stat Med 2002; 21: 1559–1573. [DOI] [PubMed] [Google Scholar]
- 29. Brancati FL, Wang N‐Y, Mead LA, et al. Body weight patterns from 20 to 49 years of age and subsequent risk for diabetes mellitus. Arch Intern Med 1999; 159: 957. [DOI] [PubMed] [Google Scholar]
- 30. Waring ME, Eaton CB, Lasater TM, et al. Incident diabetes in relation to weight patterns during middle age. Am J Epidemiol 2010; 171: 550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holbrook TL, Barrett‐Connor E, Wingard DL. The association of lifetime weight and weight control patterns with diabetes among men and women in an adult community. Int J Obes 1989; 13: 723–729. [PubMed] [Google Scholar]
- 32. Morris RD, Rimm AA. Long‐term weight fluctuation and non‐insulin‐dependent diabetes mellitus in white women. Ann Epidemiol 1992; 2: 657–664. [DOI] [PubMed] [Google Scholar]
- 33. Bangalore S, Fayyad R, Laskey R, et al. Body‐weight fluctuations and outcomes in coronary disease. N Engl J Med 2017; 376: 1332–1340. [DOI] [PubMed] [Google Scholar]
- 34. Oh TJ, Moon JH, Choi SH, et al. Body‐weight fluctuation and incident diabetes mellitus, cardiovascular disease, and mortality: a 16‐year prospective cohort study. J Clin Endocrinol Metab 2019; 104: 639–646. [DOI] [PubMed] [Google Scholar]
- 35. Zhang Y, Yatsuya H, Li Y, et al. Long‐term weight‐change slope, weight fluctuation and risk of type 2 diabetes mellitus in middle‐aged Japanese men and women: findings of Aichi Workers’ Cohort Study. Nutr Diabetes 2017; 7: e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moore LL, Visioni AJ, Wilson PWF, et al. Can sustained weight loss in overweight individuals reduce the risk of diabetes mellitus? Epidemiology 2000; 11: 269–273. [DOI] [PubMed] [Google Scholar]
- 37. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 2017; 128: 40–50. [DOI] [PubMed] [Google Scholar]
- 38. Jacobs S, Boushey CJ, Franke AA, et al. A priori‐defined diet quality indices, biomarkers and risk for type 2 diabetes in five ethnic groups: the Multiethnic Cohort. Br J Nutr 2017; 118: 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nakagami T, Borch‐Johnsen K, Carstensen B, et al. Age, body mass index and Type 2 diabetes – associations modified by ethnicity. Diabetologia 2003; 46: 1063–1070. [DOI] [PubMed] [Google Scholar]
- 40. Anastasiou CA, Yannakoulia M, Pirogianni V, et al. Fitness and weight cycling in relation to body fat and insulin sensitivity in normal‐weight young women. J Am Diet Assoc 2010; 110: 280–284. [DOI] [PubMed] [Google Scholar]
- 41. Cereda E, Malavazos AE, Caccialanza R, et al. Weight cycling is associated with body weight excess and abdominal fat accumulation: a cross‐sectional study. Clin Nutr 2011; 30: 718–723. [DOI] [PubMed] [Google Scholar]
- 42. Rodin J, Radke‐Sharpe N, Rebuffé‐Scrive M, et al. Weight cycling and fat distribution. Int J Obes 1990; 14: 303–310. [PubMed] [Google Scholar]
- 43. Roy T, Lloyd CE. Epidemiology of depression and diabetes: a systematic review. J Affect Disord 2012; 142(Suppl.): S8–S21. [DOI] [PubMed] [Google Scholar]
- 44. Reininghaus EZ, Lackner N, Fellendorf FT, et al. Weight cycling in bipolar disorder. J Affect Disord 2015; 171: 33–38. [DOI] [PubMed] [Google Scholar]
- 45. Messier L, Elisha B, Schmitz N, et al. Weight cycling and depressive symptoms in diabetes: a community‐based study of adults with type 2 diabetes mellitus in Quebec. Can J Diabetes 2014; 38: 456–460. [DOI] [PubMed] [Google Scholar]
- 46. Zou H, Yin P, Liu L, et al. Body‐weight fluctuation was associated with increased risk for cardiovascular disease, all‐cause and cardiovascular mortality : a systematic review and meta‐analysis. Front Endocrinol (Lausanne) 2019; 10: 728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lorber D. Importance of cardiovascular disease risk management in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes Targets Ther 2014; 7: 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 | Search terms.
Appendix S2 | Additional methods.
Table S1 | Descriptive characteristics of 14 included articles.
Table S2 | Quality assessment of individual studies using the Newcastle–Ottawa Scale.
Table S3 | Meta‐regression of factors affecting heterogeneity.
Table S4 | Sensitivity analysis based on study level for association of weight cycling and risk of diabetes.
Table S5 | Subgroup analyses for the association between weight cycling and the risk of diabetes after omission of the Yokomichi et al. study.
Figure S1 | Sensitivity analysis based on report level for association of weight cycling and risk of diabetes.
Figure S2 | Funnel plot for assessment of publication bias.


